CD14, a novel surface marker of esophageal cancer stem cells
- Authors:
- Published online on: November 23, 2022 https://doi.org/10.3892/or.2022.8450
- Article Number: 13
Abstract
Introduction
Esophageal cancer (EC), which is mainly classified into esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC) based on histopathology, remains one of the major global healthcare challenges due to its poor prognosis (1,2). Cancer stem cells (CSCs), a small subset of cells within solid tumors, have the capacity to self-renew and differentiate into several types of cells that constitute the tumor (3,4). Esophageal CSCs (ECSCs) directly regulate cancer initiation, progression, metastasis, resistance to therapy and recurrence in both EAC and ESCC (5,6). Therapeutic strategies aimed at targeting CSCs may be among the most promising ones for the comprehensive treatment of tumors (7). Various trials have achieved satisfactory efficacy in the treatment of certain hematopoietic malignancies, e.g. the trials on chimeric antigen receptor T-cell therapy (8–10). However, studies on the treatment of solid tumors have not yielded satisfactory results. One of the reasons for this is the difficulty involved in identifying a relatively specific antigen in solid tumors (11).
In the tumor microenvironment, immune cells and tumor cells are in close contact and are mutually influenced (12), which may result in a change in phenotype. For instance, CD70, which is generally expressed on the surface of activated T-lymphocytes, B-lymphocytes and a portion of dendritic cells, is also ectopically expressed in certain solid tumors (13,14). CD14, a specific surface marker of monocytes, macrophages and neutrophils, in combination with lipopolysaccharide, induces pro-inflammatory responses to invading pathogens via the Toll-like receptor 4 signaling pathway (15,16). CD14 has also been indicated to be associated with tumor recurrence, growth, metastasis and resistance to treatment (17–19), which is in conformity with the characteristics of CSCs. It may thus be hypothesized that there is an inevitable connection between CD14 and CSCs.
In the present study, paraffin-embedded sections of human EC (HEC) and tissues adjacent to the tumor (AT) were examined to qualitatively determine the expression of CD14 in ECSCs using immunofluorescence double staining with CD14 and aldehyde dehydrogenase-1 (ALDH1), which is expressed in EAC and ESCC CSCs as an ECSC marker (20–22). CD14+ cells were then isolated and stemness properties were examined by the detection of proliferation, migration, invasion and tumorigenicity.
Materials and methods
Tissue samples
Paraffin-embedded sections of HEC (12 well-differentiated, 11 moderately differentiated and 9 poorly differentiated ESCC tissues; 9 well-differentiated, 9 moderately differentiated and 7 poorly differentiated EAC tissues) and 9 AT tissues were acquired from Mudanjiang Tumour Hospital (Mudanjiang, China) between January 2017 and May 2022, and all tissues of patients were pathologically verified by the Department of Pathology. The HEC tissue specimens were obtained from the surgical specimens of patients with EC undergoing surgery without pre-operative chemotherapy or radiotherapy at the Affiliated Hongqi Hospital of Mudanjiang Medical University (Mudanjiang, China) for cell culture. One patient was a 62-year-old woman with well differentiated ESCC, another patient was a 71-year-old man with moderately differentiated ESCC, and the third patient was a 74-year-old man with moderately differentiated EAC. The clinical and pathological data were collected (Table I) and written informed consent was obtained from all patients. The present study was approved by the Research Ethics Committee of Mudanjiang Medical University (approval no. 2022-MYGZR06).
Reagents
The following main reagents were utilized: Collagenase Ӏ (Coolaber); D-MEM/F12 powder (Gibco; Thermo Fisher Scientific, Inc.); basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), leukemia inhibitory factor (LIF) (PeproTech, Inc.); ALDH1 (cat. no. 60171-1-Ig) and CD14 polyclonal antibodies (cat. no. CL647-65056; ProteinTech Group, Inc.); the EasySep™ Human CD14 Positive Selection Kit II (EasySep™; Stemcell Technologies, Inc.); the Cell Counting Kit-8 (CCK-8; Shanghai Yeasen Biotechnology Co., Ltd.); and the EdU Assay/EdU Staining Proliferation kit (Abcam).
Immunofluorescence staining
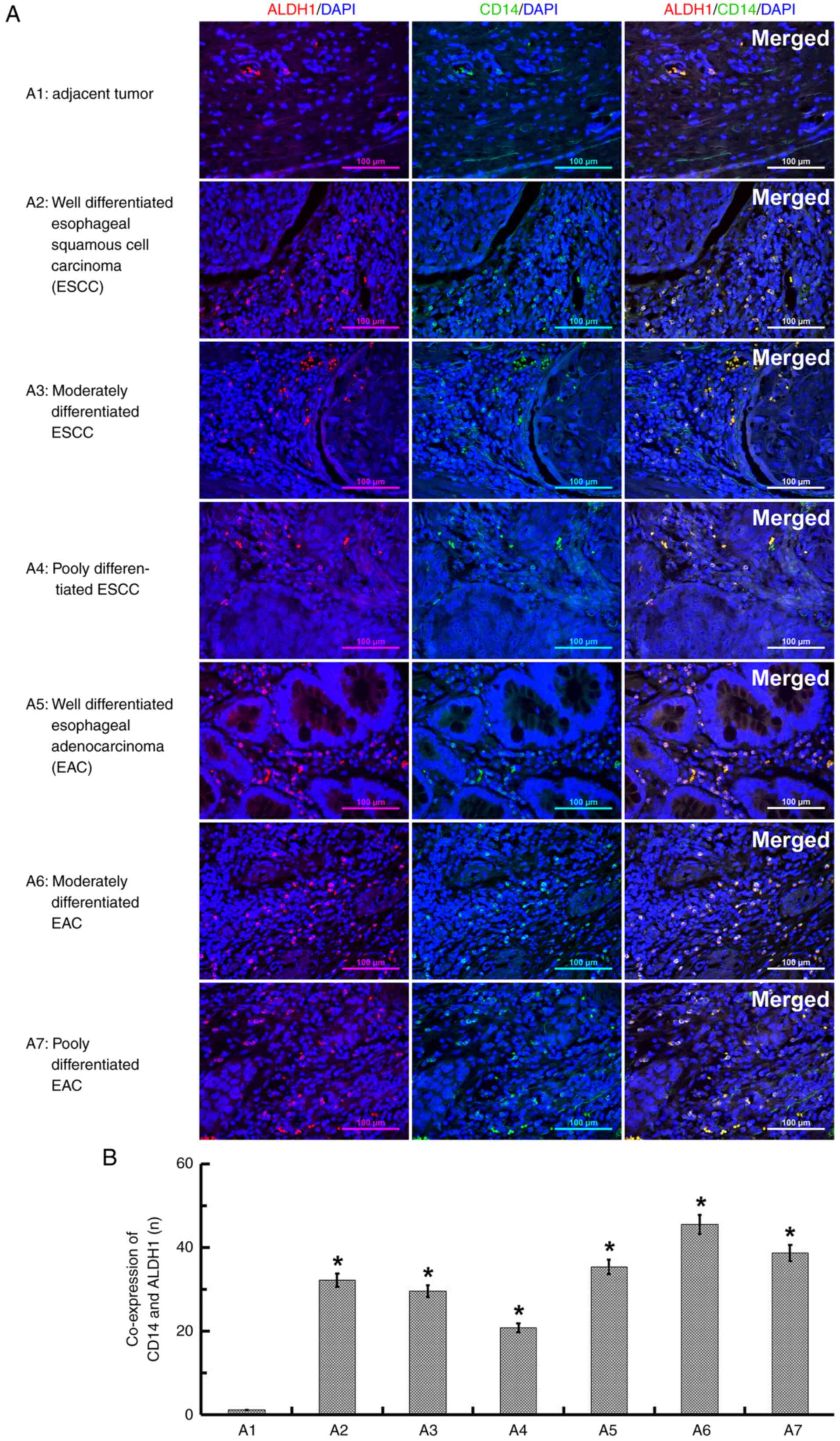
The paraffin-embedded sections (5 µm thickness) were used to determine the co-expression of CD14 and ALDH1 in ECSCs. Following conventional deparaffinization and rehydration, antigen retrieval was performed using 0.01 mol/l sodium citrate buffer at 100°C for 15 min. The sections were then permeabilized with 0.3% Triton X-100 for 15 min and blocked with 3% methanol-H2O2 solution for 15 min at room temperature. To label the ECSCs, the sections were incubated with ALDH antibody (1:100 dilution) at 37°C for 100 min and stained with the secondary antibody IgG Texas Red (cat. no. ab6800; 1:100 dilution; Abcam, Inc.) at 37°C for 40 min. For the analysis of CD14, the sections were incubated again with CD14 antibody (1:100 dilution) at 37°C for 100 min and stained with another secondary antibody IgG FITC (cat. no. Abs20004-500 ul; 1:100 dilution; Absin Inc.) at 37°C for 40 min. For the cellular count, the sections were counterstained with DAPI (1:100 dilution) at 37°C for 20 min. A total of 7 images per section were captured at a magnification of ×200 (n=3). Quantified results are presented as a percentage of positive staining (red and green) out of the total number of cells, as visualized using DAPI nuclear staining (blue).
Primary culture of human EC
When obtained from surgical specimens, the HEC tissue was transferred to the laboratory as soon as possible. After being washed with PBS three times, the tissue was cut into small sections with a maximum size of 4 mm. The sections were then cultured in serum-free medium (DMEM/F12 containing 10 ng/ml bFGF, 20 ng/ml EGF and 20 ng/ml LIF) under a constant temperature of 37°C in a humidified atmosphere containing 5% CO2. The culture solution was replaced every other day for ~2 weeks until the cells stopped migrating out from the tissue specimens.
Purification of CD14+ cells
CD14+ cells were isolated using the EasySep™ Human CD14 Positive Selection Kit II according to the protocol provided by the manufacturer, as follows: The cells were washed, detached using 0.25% trypsin (Solarbio, Inc.; without EDTA), centrifuged at 800 × g for 4 min at room temperature and suspended with 1 ml PBS in a 5-ml polystyrene round-bottom tube. The cells were incubated with 50 µl EasySep™ Human CD14 Positive Selection Cocktail II for 40 min at 37°C; they were then further incubated with 25 µl EasySep™ RapidSpheres™ for 10 min at 37°C. After 1.5 ml PBS was added to the tube, the Stemcell 18,000 EasySep™ Magnet was used to hold the tube for 5 min at room temperature. The solution which then contained CD14− cells in the tube was poured into another tube, and the CD14+ cells remained.
CCK-8 assay
Cell proliferation was detected using a CCK-8 assay kit according to the manufacturer's protocol. The CD14+ and control CD14− cells were seeded in 96-well plates in 100 µl serum-free medium at a density of 2×103 cells/well and cultured for 24, 48 or 72 h. At the designated time-points, 10 µl CCK-8 reagent was added separately to a well of each corresponding group and the optical density values were measured at 450 nm after the cells were continually cultured for 4 h.
EdU assay
Cell proliferation was also detected using the EdU Assay/EdU Staining Proliferation kit. The cells were seeded in 24-well plates in 500 µl serum-free medium at a density of 5×103 cells/well and cultured for 24 h. The cells were then treated with EdU medium (10 µM final concentration) at 37°C for 21 h, and were then fixed with 4% paraformaldehyde at room temperature for 30 min and permeabilized with 0.5% Triton X-100 in PBS at room temperature for 10 min. Subsequently, the cells were thoroughly washed with PBS three times to remove the residual paraformaldehyde before being incubated with DAPI at 37°C for 20 min. Quantified results are presented as the percentage of positive staining (red) out of the total number of cells, as visualized using DAPI nuclear staining (blue).
Colony-formation assay
The CD14+ and control CD14− cells were seeded in 6-well plates at a density of 200 cells/well and maintained in 2.5 ml serum-free medium in an incubator at 37°C with 5% CO2 for 14 days. During this period, the medium was changed on the 11th day. Subsequently, the cells were washed with PBS three times, fixed with 4% paraformaldehyde at room temperature for 10 min and stained with 0.2% crystal violet solution at room temperature for 10 min. Colonies (number of cells, >50) were counted under an optical microscope (Olympus Corporation).
Transwell assay
Cell invasion was detected using Nunc™ Polycarbonate Cell Culture Inserts in Multidishes (cat. no. 140644; 8 µm pore size, 6-well plates; Thermo Fisher Scientific Inc.). Matrigel® (Solarbio, Inc.) was diluted to a 1 mg/ml concentration using DMEM/F12 and added at 100 µl to the chamber, followed by incubation overnight at 37°C for gelling. A total of 1×104 cells in DMEM/F12 (without serum or nutrient factors) were placed in the upper chamber, while 2 ml serum-free medium was added to the lower chamber. Following incubation at 37°C with 5% CO2 for 24 h, the cells at the top side were wiped off and the cells at the lower side of the membrane were fixed with 4% paraformaldehyde at room temperature for 10 min and dyed using 0.1% crystal violet at room temperature for 10 min. Cells were visualized and counted using an optical microscope.
The Transwell migration assay was performed in the same manner but without Matrigel coating.
Wound-healing assay
Cell migration was also detected using a wound-healing assay. The cells were seeded in 6-well plates and cultured in serum-free medium until reaching ~98% confluency. A 100 ul pipette (Thermo Fisher Scientific Inc.) tip was used to create a straight scratch on the monolayer of cells. Following culture at 37°C with 5% CO2 for 24 h, images of the wounded areas were obtained using an optical microscope (model TH4-200; Olympus Corp.).
Transplantation assay
The cell tumorigenicity was assessed in vivo using nu/nu nude mice (male; age, 4–5 weeks; median body weight, 20 g) obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. They were housed with three or four animals per cage under specific pathogen-free conditions at a controlled temperature of 24±2°C with 50±10% relative humidity, a 12-h light/dark cycle, ventilation (15 times/h) and free access to food and water. Animal health and behavior were monitored every day. The experiment would be terminated in advance when the weight of the mouse was reduced by 20–25% of the body weight, the tumor weight exceeded 10% of the body weight of the mouse, the tumor was ulcerated or damaged or the animal had poor appetite (50% less than normal; food intake <2g/d) for 3 days. The animals were divided into 2 groups (CD14+ and CD14−) with 7 animals in each group. After being resuspended with PBS, ~5×106 cells were subcutaneously injected in a single flank on the dorsal surface of the mice. The tumors were measured using calipers every other day and once the length of a tumor reached a maximum size of 10 mm, all of the mice were euthanized by excess CO2 in the euthanasia chamber for ~5 min at 45 days after injection. When the animals were euthanized, the EZ SmartBox Prodigy (E-Z Systems, Inc.) was used during the process in compliance with the American Veterinary Medical Association guidelines. The controlled displacement rate of CO2 was 30% volume of the euthanasia chamber per minute. Death was verified by the mice being motionless with absence of breathing and dilated pupils for 5 min. The xenograft tumors were extracted and their weight was determined with an electronic balance (SARTORIUS Corp.; model TH4-200). The present study was approved by the Institutional Animal Care and Use Committee of Mudanjiang Medical University (approval no. 20220228-26).
Statistical analysis
Statistical analysis was performed using SPSS 14.0 software (SPSS, Inc.) and Origin 2021b SR1 v9.8.5.204 (Origin Software, Inc.) statistical software. The data are expressed as the mean ± standard deviation. One-way ANOVA followed by Dunnett's post-hoc test was used to compare the means among multiple groups, whereas statistical comparison of only two groups was performed by Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of CD14 in ALDH1-labeled ECSCs
Immuno-fluorescence staining was performed to examine whether CD14 is expressed in ECSC tissues. The tissue donors (n=60) had a median age of 60.3 years (range, 45–78 years) and 58.3% were male. Squamous cell carcinoma was the pathology of 56.7% of cases, while the remaining ones were adenocarcinoma (Table I). The ECSC marker ALDH1 was first used to label the ECSCs, and CD14 was then detected to determine its expression in the same EC tissues. In all types of EC tissues, it was observed that the ALDH1-labeled ECSCs had small cell bodies and exhibited more nuclear division, mainly located in the areas of relatively loose tissues and were rich in blood vessels around cancer nests. In addition, the tissues significantly expressed CD14 relative to the AT tissues (Fig. 1).
Morphological characterization of primary cells
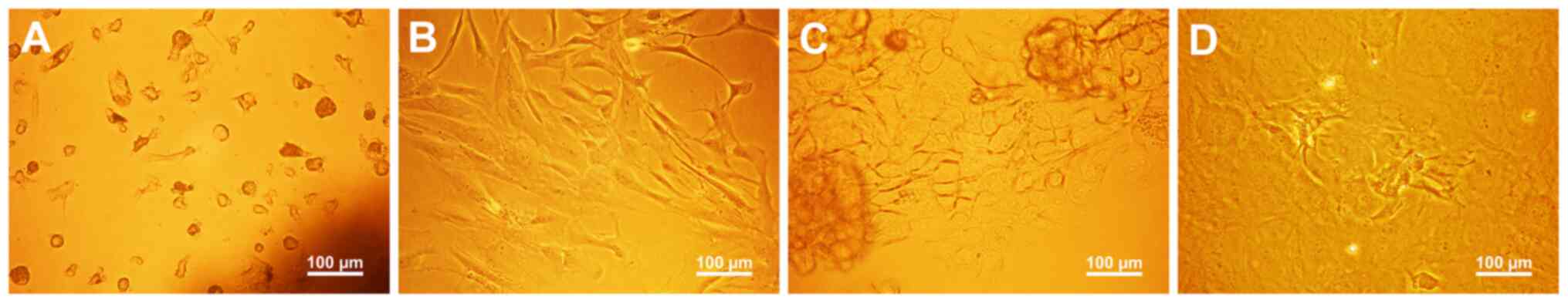
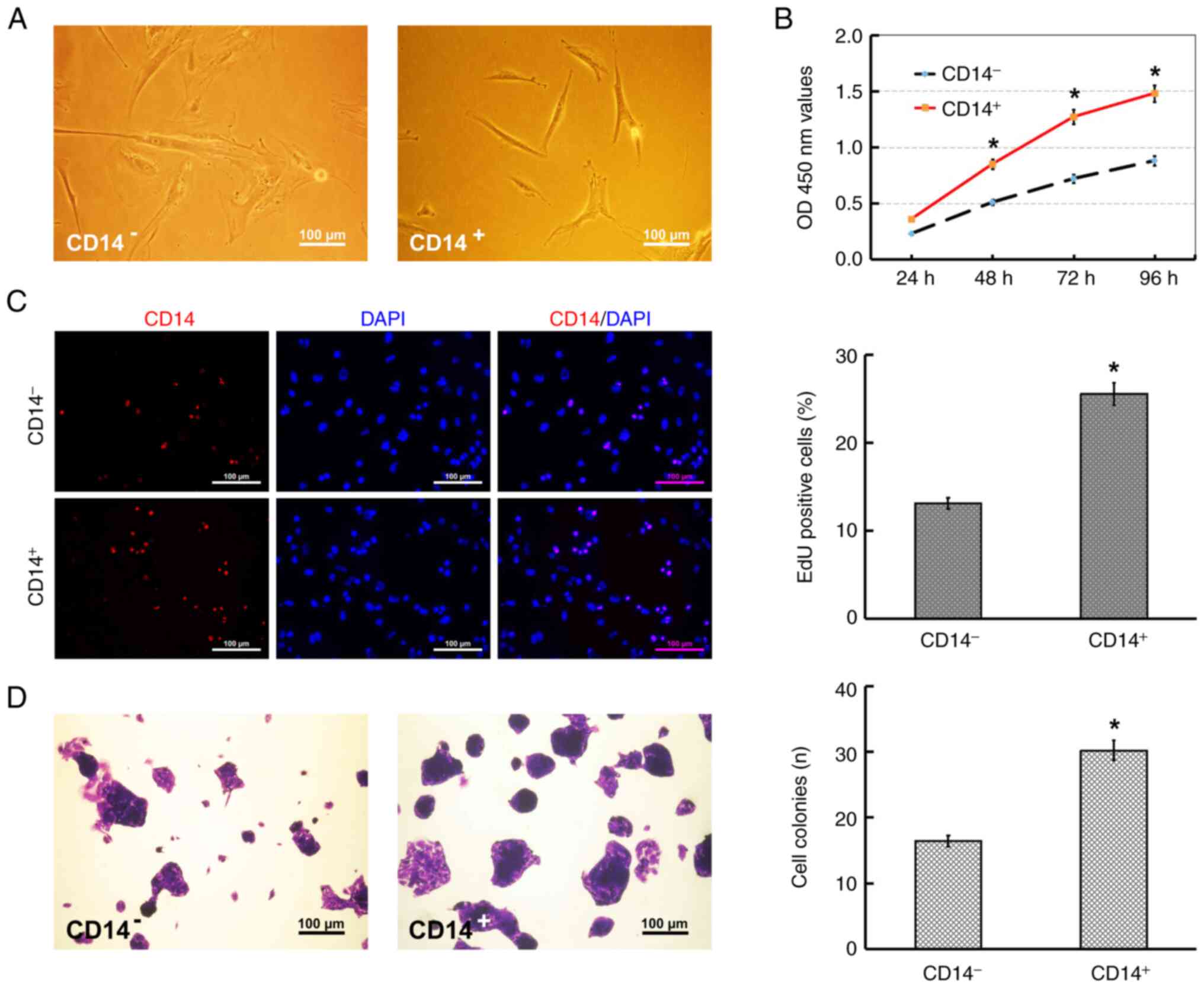
Primary cells were isolated and cultured from HEC tissues by the method of explanted tissue culture. During the cultivation for ~2 weeks, multiple shapes or types of cells were observed and selection bias was not found. As indicated in Fig. 2A, after 4-day culture, viable and adherent cells, which infiltrated from fragments and were uneven in shape, were observed to surround fragments. Subsequently, the adherent cells surrounding fragments developed into multiple shapes and predominantly tended to form parallel spindle-shaped morphology (Fig. 2B). When the fragments were removed from the plate after 12-day culture, the cells under fragments, which were uneven in size and shape, grew side by side with an untypical cobblestone-like morphology (Fig. 2C) and certain cells formed amorphous cell clusters due to overproliferation (Fig. 2D). Following immunomagnetic separation and culture, the CD14+ cells exhibited a spindle-like cell shape, with a smaller cell body relative to the CD14− cells, and the CD14− cells exhibited a predominantly polyhedral or irregular spindle-shaped morphology (Fig. 3A).
Proliferative abilities of CD14+ cells
Cell proliferation was evaluated using CCK-8, EdU and colony-formation assays. The results of the CCK-8 assay revealed that the cellular growth of CD14+ cells occurred more rapidly than that of the CD14− cells (Fig. 3B). In addition, the results of the EdU and colony formation assays further confirmed that the proliferative ability of the CD14+ cells was higher than that of the CD14− cells, which was consistent with the results of the CCK-8 assay (Fig. 3C and D).
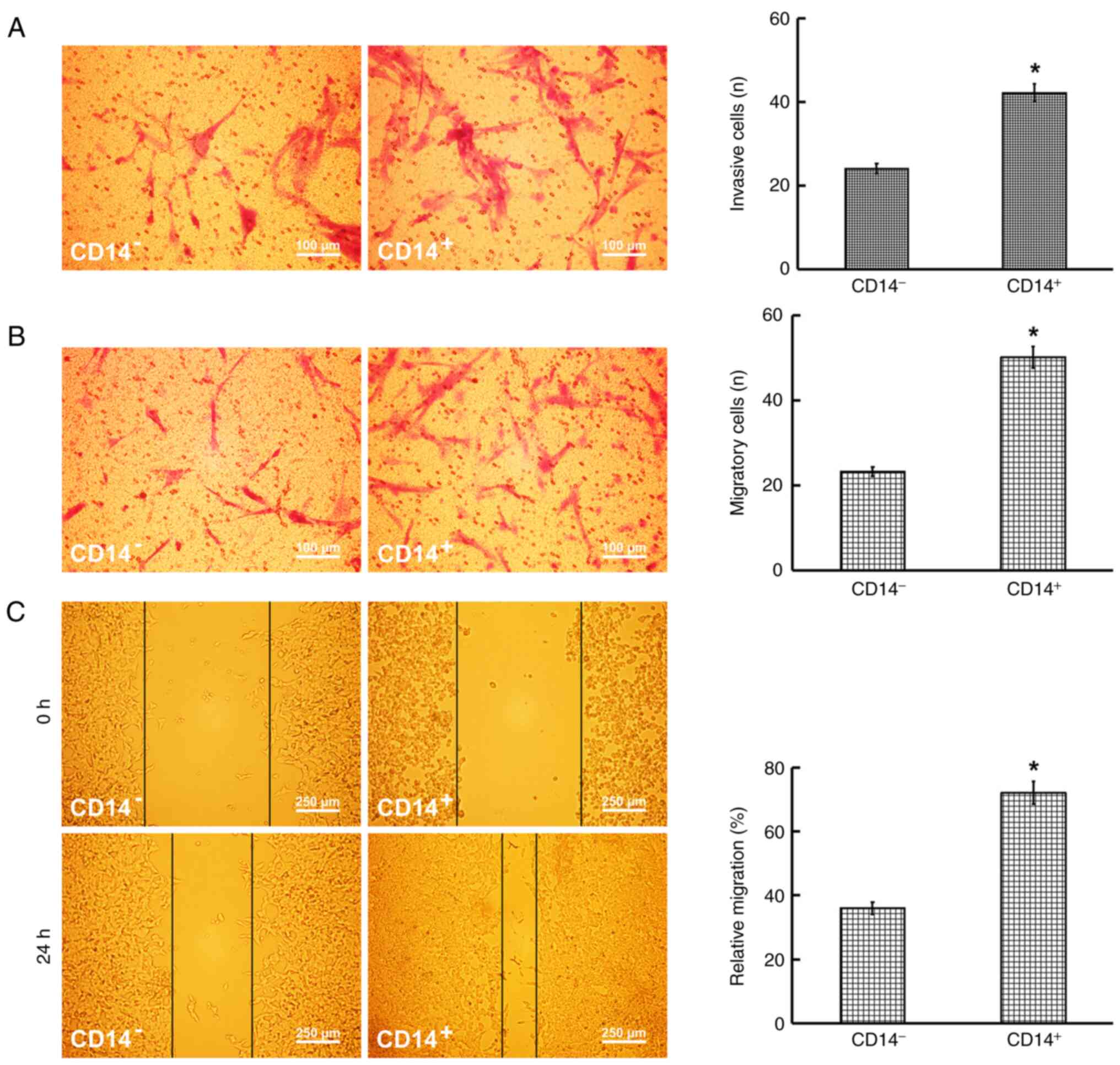
Metastatic and invasive abilities of CD14+ cells
The results of the Transwell assays revealed that the number of CD14+ cells with an invasive and migratory ability was significantly higher than that of the CD14− cells (Fig. 4A and B). Furthermore, the wound-healing assay demonstrated that the migratory ability of the CD14+ cells was higher than that of the CD14− cells (Fig. 4C).
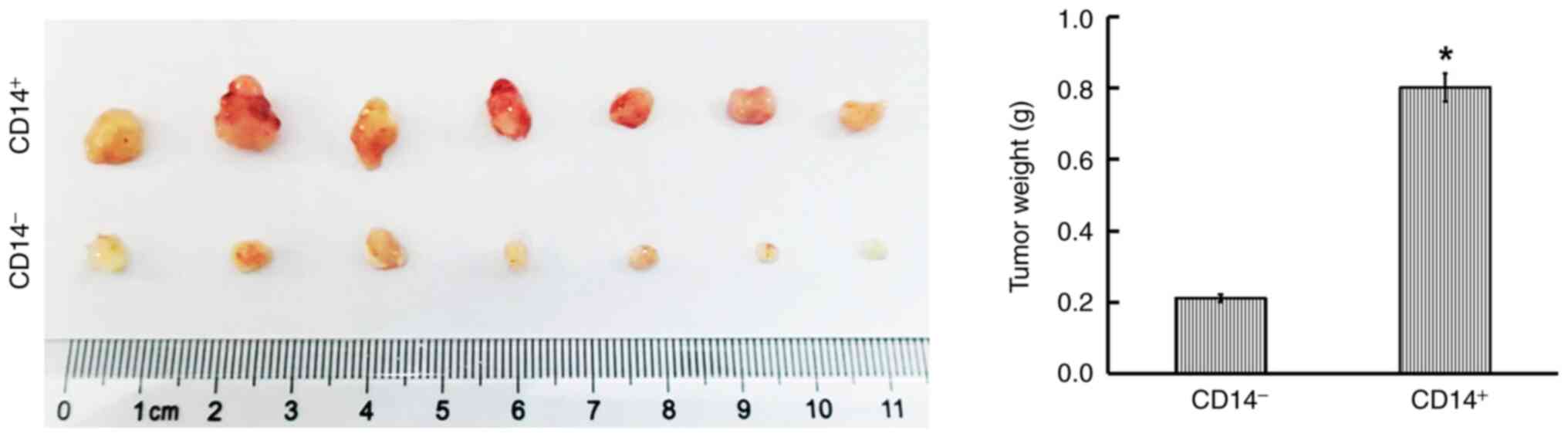
Tumorigenic ability of CD14+ cells in vivo
Tumor xenografts were established using nude mice to examine the tumorigenic ability of CD14+ cells. At 45 days following implantation, the tumors in the CD14+ cell group were distinctly heavier than those in the CD14− cell group (Fig. 5).
Discussion
The understanding of CSC morphology in tumor tissues may contribute to the observation, identification and localization of CSCs and may also avoid interference by false-positive staining in staining experiments related to CSCs. However, there is frequently no clear morphological distinction between tumorigenic and non-tumorigenic cancer cells (23). According to the data presented in the current study, the size of the labeled ECSCs was similar to that of the neutrophils, based on the size of the false-positive erythrocytes, and was evidently smaller than that of the cells of tumor lobes, particularly in moderately and poorly differentiated tissues; it was also possible to observe the nuclear division of the ECSCs, and the karyoplasmic ratio was relatively larger compared to the cells of tumor lobes. Of note, reports on CSC distribution in tumor tissues are controversial. It has been reported that CSCs are preferentially located in the core of the tumor lobes (a hypoxic environment) in vivo (24), and Ji et al (25) found diffuse CSCs in ESCCs. The present study demonstrated that the diffusive ECSCs were mainly located in areas that were relatively loose and rich in blood vessels, around the cancer nests. Regarding the quantification of CSCs, the statistical proportion of CSCs varies markedly in tumor tissues, even among primary cell lines, due to differences in sampling site, sample size, tumor grade, patient age and detection methods in different studies (26–29). The main aim of the present study was to qualitatively detect the expression of CD14 in ECSCs.
The three major characteristics of CSCs are an unlimited proliferative ability, self-renewal ability and an ability for strong tumorigenesis; these abilities were thus examined to identify and verify the stemness of isolated cells (30). CCK-8 and EdU assays are two common methods used to investigate the proliferation and/or self-renewal ability of cancer cells (31). In addition, two main methods have been applied to identify tumorigenesis characteristics in published studies. One approach is the colony formation assay, which is also used to detect the proliferation of cancer cells, which is considered the most appropriate in vitro (32); the other approach is the xenograft assay, an in vivo method involving the implantation of cancer cells into immunodeficient mice (33). However, several issues are associated with the transplantation assay. A total of 105 CSCs, which may not be indicative of a rare tumor-initiating cell, are frequently used in transplantation experiments, which poses difficulties in inducing efficient tumorigenesis due to species barriers, host strains, developmental stages and even sex (34). On the other hand, the majority of tumor cells, even those not associated with stem cell markers, result in tumor initiation due to CSC plasticity by the host microenvironments (35–37). However, the majority of studies to date using CSCs still utilize the transplantation assay to prove the existence of CSCs for a particular tumor, as a better alternative is not yet available. In addition, the metastatic ability of CSCs is usually examined using Transwell and wound-healing assays in vitro (38). Using the aforementioned methods, the present study determined that CD14+ cells of EC possessed the characteristics of ECSCs.
In solid tumors, membrane-associated proteins are usually utilized for research into CSCs. The membrane-associated biomarkers associated with ECSCs include the following: CD34 (39), CD44 (40), CD90 (41), CD133 (42), CD271 (43), CD326 (44), LgR5 (45), integrinα7 (46) and podoplanin (47). However, these membrane-associated proteins are similarly found in normal tissue cells; thus, the availability of CSC-specific biomarkers is limited (48). As a type of stromal cells, telocytes express CD34 (49); CD44 and CD90 are the positive markers of mesenchymal stem cells (50) and CD44 is equally expressed in normal head and neck epithelium (51,52); CD133 has been used as a marker to identify prostate and neural stem cells, and is also expressed in differentiated epithelial cells in certain organs, such as the pancreas, liver, colon, gastric contents, as well as sweat, salivary and lacrimal glands (53); CD271 has been found to be expressed in human adipose-derived mesenchymal stem cells (54) and the glial cells of the central and peripheral nervous systems (55); CD326 is expressed in certain epithelial cells (56) and various stem and progenitor cells (57,58); LGR5 has been suggested to be a marker of adult stem cells of the intestine, stomach, skin and hair follicles (59,60); integrin α7 is a key adhesion receptor that is highly expressed in vascular smooth, skeletal and cardiac muscle (61,62); podoplanin expression has been detected in a variety of normal tissues, including glomerular podocytes, lymphatic endothelial cells, heart cells, type I alveolar cells and skeletal muscle (63). With regard to CD14, it is mainly expressed on myeloid-derived cells, such as monocytes, polymorphonuclear leucocytes and macrophages (15,16). In the present study, while CD14 was not found to be expressed in immunocytes in the tissues of esophageal cancer by immunofluorescence double staining, possibly due to incomplete detection, it remains to be further elucidated whether CD14 is a highly selective marker for ECSCs.
In conclusion, the present study demonstrated that the ALDH1-labeled ECSCs expressed the surface marker CD14; in vitro and in vivo experiments further confirmed that the primary CD14+ cells possessed the characteristics of CSCs. Regarding the distribution of CD14 in adult tissues, CD14 may be considered a novel surface marker of ECSCs; thus, this may provide a novel therapeutic target against ECSCs, which may be used in the treatment of EC. However, the lack of knockdown and CD14 expression rescue experiments was one limitation of the present study, and the lack of experiments on chemoresistance both in vivo and in vitro was another limitation. Further research is required to confirm the role of CD14 in the maintenance of ECSC characteristics.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Start-up Fund for Doctor Scientific Research of Mudanjiang Medical University (grant no. 2021-MYBSKY-024).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Authors' contributions
JuL, YoL and JD conceived and designed the study. YoL, WY, YuL, ZL, JiL and LZ performed the experiments. CW and JQ analyzed the data. YoL wrote the first draft of the manuscript. LuJ and YoL confirm the authenticity of all the raw data. All authors contributed to the article and approved the submitted version, and all authors have read and approved the final manuscript.
Ethics approval and consent to participate
The experiments involving human participants were reviewed and approved by the Research Ethics Committee of Mudanjiang Medical University (approval no. 2022-MYGZR06). The patients provided their written informed consent to participate in the study. The animal experiment was reviewed and approved by the Institutional Animal Care and Use Committee of Mudanjiang Medical University (approval no. 20220228-26).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
Lagergren J, Smyth E, Cunningham D and Lagergren P: Oesophageal cancer. Lancet. 390:2383–2396. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou C, Fan N, Liu F, Fang N, Plum PS, Thieme R, Gockel I, Gromnitza S, Hillmer AM, Chon SH, et al: Linking cancer stem cell plasticity to therapeutic resistance-mechanism and novel therapeutic strategies in esophageal cancer. Cells. 9:14812020. View Article : Google Scholar : PubMed/NCBI | |
|
Chang JC: Cancer stem cells: Role in tumor growth, recurrence, metastasis, and treatment resistance. Medicine (Baltimore). 95 (1 Suppl 1):S20–S25. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Islam F, Qiao B, Smith RA, Gopalan V and Lam AK: Cancer stem cell: Fundamental experimental pathological concepts and updates. Exp Mol Pathol. 98:184–191. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Islam F, Gopalan V, Law S, Tang JC and Lam AK: FAM134B promotes esophageal squamous cell carcinoma in vitro and its correlations with clinicopathologic features. Hum Pathol. 87:1–10. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Islam F, Gopalan V and Lam AK: Detention and identification of cancer stem cells in esophageal squamous cell carcinoma. Methods Mol Biol. 2129:177–191. 2020. View Article : Google Scholar : PubMed/NCBI | |
|
La Noce M, Paino F, Mele L, Papaccio G, Regad T, Lombardi A, Papaccio F, Desiderio V and Tirino V: HDAC2 depletion promotes osteosarcoma's stemness both in vitro and in vivo: A study on a putative new target for CSCs directed therapy. J Exp Clin Cancer Res. 37:2962018. View Article : Google Scholar : PubMed/NCBI | |
|
Xu Y, Yang Z, Horan LH, Zhang P, Liu L, Zimdah B, Green S, Lu J, Morales JF, Barrett DM, et al: A novel antibody-TCR (AbTCR) platform combines Fab-based antigen recognition with gamma/delta-TCR signaling to facilitate T-cell cytotoxicity with low cytokine release. Cell Discov. 4:622018. View Article : Google Scholar : PubMed/NCBI | |
|
Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al: T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet. 385:517–528. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Li S, Tao Z, Xu Y, Liu J, An N, Wang Y, Xing H, Tian Z, Tang K, Liao X, et al: CD33-specific chimeric antigen receptor T cells with different co-stimulators showed potent anti-leukemia efficacy and different phenotype. Hum Gene Ther. 29:626–639. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Z, Chen W, Zhang X, Cai Z and Huang W: A long way to the battlefront: CAR T cell therapy against solid cancers. J Cancer. 10:3112–3123. 2019. View Article : Google Scholar : PubMed/NCBI | |
|
Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L, Pasche B, Lee C and Grippo PJ: TGF-β: Duality of function between tumor prevention and carcinogenesis. J Natl Cancer Inst. 106:djt3692014. View Article : Google Scholar : PubMed/NCBI | |
|
Jin L, Ge H, Long Y, Yang C, Chang YE, Mu L, Sayour EJ, De Leon G, Wang QJ, Yang JC, et al: CD70, a novel target of CAR T-cell therapy for gliomas. Neuro Oncol. 20:55–65. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Pich C, Sarrabayrouse G, Teiti I, Mariamé B, Rochaix P, Lamant L, Favre G, Maisongrosse V and Tilkin-Mariamé AF: Melanoma-expressed CD70 is involved in invasion and metastasis. Br J Cancer. 114:63–70. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Janova H, Böttcher C, Holtman IR, Regen T, van Rossum D, Götz A, Ernst AS, Fritsche C, Gertig U, Saiepour N, et al: CD14 is a key organizer of microglial responses to CNS infection and injury. Glia. 64:635–649. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Ciesielska A, Matyjek M and Kwiatkowska K: TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 78:1233–1261. 2021. View Article : Google Scholar : PubMed/NCBI | |
|
Zhao D, Sun T, Zhang X, Guo Y, Yu D, Yang M, Tan W, Wang G and Lin D: Role of CD14 promoter polymorphisms in Helicobacter pylori infection-related gastric carcinoma. Clin Cancer Res. 13:2362–2368. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, Nakamoto Y and Kaneko S: Increase in CD14 + HLA-DR-/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 62:1421–1430. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Huang A, Zhang B, Wang B, Zhang F, Fan KX and Guo YJ: Increased CD14(+)HLA-DR (−/low) myeloid-derived suppressor cells correlate with extrathoracic metastasis and poor response to chemotherapy in non-small cell lung cancer patients. Cancer Immunol Immunother. 62:1439–1451. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
AjaniJ A, Wang X, Song S, Suzuki A, Taketa T, Sudo K, Wadhwa R, Hofstetter WL, Komaki R, Maru DM, et al: ALDH-1 expression levels predict response or resistance to preoperative chemoradiation in resectable esophageal cancer patients. Mol Oncol. 8:142–149. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Yang L, Ren Y, Yu X, Qian F, Bian BSJ, Xiao HL, Wang WG, Xu SL, Yang J, Cui W, et al: ALDH1A1 defines invasive cancer stem-like cells and predicts poor prognosis in patients with esophageal squamous cell carcinoma. Mod Pathol. 27:775–783. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Chen MF, Chen PT, Lu MS and Chen WC: Role of ALDH1 in the prognosis of esophageal cancer and its relationship with tumor microenvironment. Mol Carcinog. 57:78–88. 2018. View Article : Google Scholar : PubMed/NCBI | |
|
Plaks V, Kong N and Werb Z: The cancer stem cell niche: How essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 16:225–238. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Sekino Y, Imaizumi A, Komune N, Ono M, Sato K, Masuda S, Fujimura A, Koike K, Hongo T, Uchi R, et al: Establishment and characterization of a primary cell culture derived from external auditory canal squamous cell carcinoma. FEBS Open Bio. 11:2211–2224. 2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI | |
|
Ji Y, Li X, Li Y, Zhong Y, Cao J, Xu R, Wang J, Zhou F, Li X, Yu D, et al: Aldehyde dehydrogenase-1 expression predicts unfavorable outcomes in patients with esophageal squamous cell carcinoma. Anticancer Res. 36:343–349. 2016.PubMed/NCBI | |
|
Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B and Herold-Mende CC: Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 14:123–129. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Tanei T, Morimoto K, Shimazu K, Kim SJ, Tanji Y, Taguchi T, Tamaki Y and Noguchi S: Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 15:4234–4241. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA and Katz RL: Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 7:330–338. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Grube S, Freitag D, Kalff R, Ewald C and Walter J: Characterization of adherent primary cell lines from fresh human glioblastoma tissue, defining glial fibrillary acidic protein as a reliable marker in establishment of glioblastoma cell culture. Cancer Rep (Hoboken). 4:e13242021.PubMed/NCBI | |
|
Akbarzadeh M, Maroufi NF, Tazehkand AP, Akbarzadeh M, Bastani S, Safdari R, Farzane A, Fattahi A, Nejabati HR, Nouri M and Samadi N: Current approaches in identification and isolation of cancer stem cells. J Cell Physiol. Feb 11–2019.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI | |
|
Han GD, Sun Y, Hui HX, Tao MY, Liu YQ and Zhu J: MiR-1224 acts as a prognostic biomarker and inhibits the progression of gastric cancer by targeting SATB1. Front Oncol. 11:7488962021. View Article : Google Scholar : PubMed/NCBI | |
|
Vargo-Gogola T and Rosen JM: Modelling breast cancer: One size does not fit all. Nat Rev Cancer. 7:659–672. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Tsai RYL: Balancing self-renewal against genome preservation in stem cells: How do they manage to have the cake and eat it too? Cell Mol Life Sci. 73:1803–1823. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
LaBarge MA: The difficulty of targeting cancer stem cell niches. Clin Cancer Res. 16:3121–3129. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM and Morrison SJ: Efficient tumour formation by single human melanoma cells. Nature. 456:593–598. 2008. View Article : Google Scholar : PubMed/NCBI | |
|
Quintana E, Piskounova E, Shackleton M, Weinberg D, Eskiocak U, Fullen DR, Johnson TM and Morrison SJ: Human melanoma metastasis in NSG mice correlates with clinical outcome in patients. Sci Transl Med. 4:159ra1492012. View Article : Google Scholar : PubMed/NCBI | |
|
Kreso A and Dick JE: Evolution of the cancer stem cell model. Cell Stem Cell. 14:275–291. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Q, Cui X, Yu X, Bian BS, Qian F, Hu XG, Ji CD, Yang L, Ren Y, Cui W, et al: Cripto-1 acts as a functional marker of cancer stem-like cells and predicts prognosis of the patients in esophageal squamous cell carcinoma. Mol Cancer. 16:812017. View Article : Google Scholar : PubMed/NCBI | |
|
Perry C, Soomro I, Kaye P, Hardy E, Parsons SL, Ragunath K, Lobo DN, Martin SG and Madhusudan S: Analysis of lymphatic and blood vessel invasion biomarkers in T1 esophagogastric adenocarcinomas for improved patient prognostication. Dis Esophagus. 28:262–268. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Schizas D, Moris D, Kanavidis P, Michalinos A, Sioulas A, Pavlakis K, Machairas A and Liakakos T: The prognostic value of CD44 expression in epithelial-mesenchymal transition: Preliminary data from patients with gastric and esophageal cancer. In Vivo. 30:939–944. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Wang Y, Zhang C, Zhu H, Tang J, Zhang S, Luo J and Sun X: CD90 positive cells exhibit aggressive radioresistance in esophageal squamous cell carcinoma. J Thorac Dis. 9:610–620. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Okamoto K, Ninomiya I, Ohbatake Y, Hirose A, Tsukada T, Nakanuma S, Sakai S, Kinoshita J, Makino I, Nakamura K, et al: Expression status of CD44 and CD133 as a prognostic marker in esophageal squamous cell carcinoma treated with neoadjuvant chemotherapy followed by radical esophagectomy. Oncol Rep. 36:3333–3342. 2016. View Article : Google Scholar : PubMed/NCBI | |
|
Li S, Yue D, Chen X, Wang L, Li J, Ping Y, Gao Q, Wang D, Zhang T, Li F, et al: Epigenetic regulation of CD271, a potential cancer stem cell marker associated with chemoresistance and metastatic capacity. Oncol Rep. 33:425–432. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Matsuda T, Takeuchi H, Matsuda S, Hiraiwa K, Miyasho T, Okamoto M, Kawasako K, Nakamura R, Takahashi T, Wada N, et al: EpCAM, a potential therapeutic target for esophageal squamous cell carcinoma. Ann Surg Oncol. 21 (Suppl 3):S356–S364. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Lv Z, Yu JJ, Zhang WJ, Xiong L, Wang F, Li LF, Zhou XL, Gao XY, Ding XF, Han L, et al: Expression and functional regulation of stemness gene Lgr5 in esophageal squamous cell carcinoma. Oncotarget. 8:26492–26504. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Ming XY, Fu L, Zhang LY, Qin YR, Cao TT, Chan KW, Ma S, Xie D and Guan XY: Integrin α7 is a functional cancer stem cell surface marker in oesophageal squamous cell carcinoma. Nat Commun. 7:135682016. View Article : Google Scholar : PubMed/NCBI | |
|
Li JC, Li Y, Ai JY, Chen K, Zhu YH, Fu L, Qin YR, Wang LJ and Guan XY: Podoplanin-positive cancer cells at the edge of esophageal squamous cell carcinomas are involved in invasion. Mol Med Rep. 10:1513–1518. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Kim WT and Ryu CJ: Cancer stem cell surface markers on normal stem cells. BMB Rep. 50:285–298. 2017. View Article : Google Scholar : PubMed/NCBI | |
|
Díaz-Flores L, Gutiérrez R, García MP, González-Gómez M, Carrasco JL, Alvarez-Argüelles H and Díaz-Flores L Jr: Telocytes/CD34+ stromal cells in pathologically affected white adipose tissue. Mol Sci. 21:96942020. View Article : Google Scholar | |
|
Lin HD, Fong CY, Biswas A and Bongso A: Allogeneic human umbilical cord Wharton's jelly stem cells increase several-fold the expansion of human cord blood CD34+ cells both in vitro and in vivo. Stem Cell Res Ther. 11:5272020. View Article : Google Scholar : PubMed/NCBI | |
|
Chen C, Wei Y, Hummel M, Hoffmann TK, Gross M, Kaufmann AM and Albers AE: Evidence for epithelial-mesenchymal transition in cancer stem cells of head and neck squamous cell carcinoma. PLoS One. 6:e164662011. View Article : Google Scholar : PubMed/NCBI | |
|
Mack B and Gires O: CD44s and CD44v6 expression in head and neck epithelia. PLoS One. 3:e33602008. View Article : Google Scholar : PubMed/NCBI | |
|
Glumac PM and LeBeau AM: The role of CD133 in cancer: A concise review. Clin Transl Med. 7:182018. View Article : Google Scholar : PubMed/NCBI | |
|
Smith RJP, Faroni A, Barrow JR, Soul J and Reid AJ: The angiogenic potential of CD271+ human adipose tissue-derived mesenchymal stem cells. Stem Cell Res Ther. 12:1602021. View Article : Google Scholar : PubMed/NCBI | |
|
Chen Y, Zeng J, Cen L, Chen Y, Wang X, Yao G, Wang W, Qi W and Kong K: Multiple roles of the p75 neurotrophin receptor in the nervous system. J Int Med Res. 37:281–288. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Balzar M, Winter MJ, de Boer CJ and Litvinov SV: The biology of the 17-1A antigen (Ep-CAM). J Mol Med (Berl). 77:699–712. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Schmelzer E, Zhang L, Bruce A, Wauthier E, Ludlow J, Yao H, Moss N, Melhem A, McClelland R, Turner W, et al: Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 204:1973–1987. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Kamimoto K, Kaneko K, Kok CYY, Okada H, Miyajima A and Itoh T: Heterogeneity and stochastic growth regulation of biliary epithelial cells dictate dynamic epithelial tissue remodeling. Elife. 5:e150342016. View Article : Google Scholar : PubMed/NCBI | |
|
Barker N and Clevers H: Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 138:1681–1696. 2010. View Article : Google Scholar : PubMed/NCBI | |
|
Haegebarth A and Clevers H: Wnt signaling, lgr5, and stem cells in the intestine and skin. Am J Pathol. 174:715–721. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Welser JV, Lange ND, Flintoff-Dye N, Burkin HR and Burkin DJ: Placental defects in alpha7 integrin null mice. Placenta. 28:1219–1228. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Nunes AM, Barraza-Flores P, Smith CH and Burkin DJ: Integrin α7: A major driver and therapeutic target for glioblastoma malignancy. Stem Cell Investig. 4:972017. View Article : Google Scholar : PubMed/NCBI | |
|
Wang X, Wang X, Carvalho V, Wang Q, Li T, Wang J, Chen Y, Ni C, Liu S and Zhang J: Prognostic value of podoplanin in various tumors. Technol Cancer Res Treat. 20:153303382110381422021. View Article : Google Scholar : PubMed/NCBI |