Organic cation transporter 6 directly confers resistance to anticancer platinum drugs
- Authors:
- Published online on: October 4, 2016 https://doi.org/10.3892/br.2016.772
- Pages: 639-643
Abstract
Introduction
Although the development of molecular-targeted therapies has improved the prognoses for many types of cancer (1,2), anticancer platinum drugs, such as cisplatin (CDDP) and oxaliplatin (L-OHP), remain key cancer chemotherapeutics (3). Nonetheless, the effectiveness of platinum drugs is limited by the development of resistance in many cases (3). Intracellular active drug concentration is very important for cytotoxicity in cancer cells (4). Additionally, various mechanisms, including drug inactivation by glutathione and overexpression of drug efflux pumps, facilitate platinum drug resistance (5–8).
Organic cation transporters (OCTs) are influx transporters belonging to the solute carrier family 22 (SLC22), which contains four subtypes, OCT1 (SLC22A1), OCT2 (SLC22A2), OCT3 (SLC22A3), and OCT6 (SLC22A16). OCTs have characteristic patterns of tissue expression and transport-specific substrates (9). Previously, OCTs were shown to play a critical role in the cellular uptake of platinum drugs in cancer cells (10). OCT1 and OCT2 are expressed in some colon cancer cell lines, and mediate the cellular uptake and cytotoxicity of L-OHP (9,10). OCT2 transports CDDP, and is responsible for CDDP-induced nephrotoxicity in cancer patients (11). Recently, we found that a decreased OCT6 expression is associated with resistance to CDDP, mediated by a decreased intracellular uptake of the drug (12).
In the present study, we established L-OHP-resistant sublines to further investigate the relationship between the resistance to anticancer platinum drugs and OCTs.
Materials and methods
Cell lines and culture conditions
The parental lung adenocarcinoma cell line (PC-14) and CDDP-resistant (PC-14/CDDP) and L-OHP resistant (PC-14/L-OHP) derivatives, as well as the human small cell lung cancer cell line (SBC-3) and an L-OHP resistant derivative (SBC-3/L-OHP) were used in the present study. The L-OHP-resistant cells were created by continuous exposure to stepwise-increasing concentrations of L-OHP. Intermediate-resistant variants were cloned and cultured again in the presence of the maximum concentration of L-OHP at which they remained viable. The CDDP-resistant cell line, PC-14/CDDP, was engineered to overexpress SLC22A16, and was described previously (12). The cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated FBS and 1% (v/w) penicillin/streptomycin in a humidified chamber (37°C, 5% CO2). CDDP and L-OHP were purchased from Nippon Kayaku (Tokyo, Japan).
Concentration of anticancer agents for 50% cell survival (IC50)
Cells were cultured at 5×103 cells/well in 96-well tissue culture plates. To assess cell viability, stepwise 10-fold dilutions of the anticancer drugs (10–100 µM) were added 2 h after plating, and the cultures were incubated at 37°C for 96 h. At the end of the culture period, 20 µl of MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium] solution (CellTiter 96® AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI, USA) was added, the cells were incubated for a further 4 h, and the absorbance was measured at 490 nm using an ELISA plate reader. Mean values were calculated from three independent experiments performed in quadruplicate. Chemosensitivity was expressed as the IC50 drug concentration, which was determined from the concentration-effect relationship by using Graph Pad Prism version 5.0 (GraphPad Software, La Jolla, CA, USA).
Protein extraction and western blotting
Equal amounts of total cell lysates were solubilized in sample buffer (50 mM Tris-HCl (pH 6.8), 2% SDS, 1 mM EDTA, 10% glycerol) with cOmplete™ Mini Protease Inhibitor Cocktail Tablets, and PhosSTOP Phosphatase Inhibitor Cocktail (Roche Applied Science, Mannheim, Germany). Subsequently, the lysates were electrophoresed on 7.5% Ready Gel Tris-HCl Precast Gels (Bio-Rad Laboratories, Hercules, CA, USA) and transferred to Immobilon-P filters (Merck Millipore, Billerica, MA, USA). The filters were then incubated with primary antibodies for 2 h at room temperature, followed by horseradish peroxidase (HRP)-conjugated secondary antibodies for 1 h. The following antibodies were used: anti-OCT1 (polyclonal antirabbit ab15112, 1:500), anti-OCT2 (monoclonal antirabbit ab33919, 1:10,000), anti-OCT3 (monoclonal antirabbit ab124826, 1:3,000), anti-multidrug and toxin extrusion 1 (MATE1) (polyclonal antirabbit ab104016, 1:500), anti-MATE2 (polyclonal antirabbit ab105050, 1:1,000) (these antidodies fromm Abcam, Cambridge, MA, USA), anti-OCT6 (polyclonal antigoat sc-132395, 1:1,000, Santa Cruz Biotechnology, Dallas, TX, USA), and HRP-conjugated secondary antibody (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA). Anti-tubulin (monoclonal antimouse T6199, 1:10,000) and anti-vinculin antibodies (monoclonal antimouse V9131, 1:20,000) (Sigma Aldrich, St. Louis, MO, USA) were used as loading controls. The density of each visualized immunoreactive band associated with the protein expression levels was calculated using a densitometer relative to those associated with loading control bands. The Human Tumor/Normal Tissue Protein Lysate Assay Strip Arrays [ST1-6X-1 (lung cancer) and ST7-6X1 (colon cancer)] from Proteus BioSciences, Inc. (Ramona, CA, USA) were screened to examine OCT6 protein expression in human lung and colon cancer tissues, and their respective matched normal tissues using western blot analysis.
Cellular accumulation of platinum
PC-14 and PC-14/L-OHP cells (1×107) were incubated for 1 or 4 h at 37°C with 40 or 100 µM L-OHP. After incubation, the cells were washed three times with ice-cold PBS and resuspended in 1.5 ml PBS. After centrifugation for 5 min at 1,300 × g, the cell pellets were homogenized and re-centrifuged for 10 min at 3,000 × g. The supernatants were collected and ultrafiltered using Centrifree tubes (Amicon Inc, Beverly, MA, USA). The platinum concentration in the supernatants was measured by an atomic absorption spectrometry assay.
Statistical analysis
Differences between the samples were evaluated for statistical significance using the Student's unpaired t-test. The level of significance was set at 5% with two-sided analysis.
Results
IC50 values for L-OHP and CDDP in L-OHP-resistant cell lines
We measured the IC50 for CDDP and L-OHP in the PC-14/L-OHP and SBC-3/L-OHP cell lines and their parental cells (Table I). The IC50 for L-OHP was 16.49- and 32.29-fold higher in the PC-14/L-OHP and SBC-3/L-OHP cells, respectively, compared with the parental cell lines. Similarly, the IC50 for CDDP was 4.69- and 2.26-fold higher in the PC-14/L-OHP and SBC-3/L-OHP cells, respectively, compared with the parental cell lines. The L-OHP-resistant cancer cells were cross-resistant to CDDP.
Protein expression levels of OCTs and MATE transporters in L-OHP-resistant cells
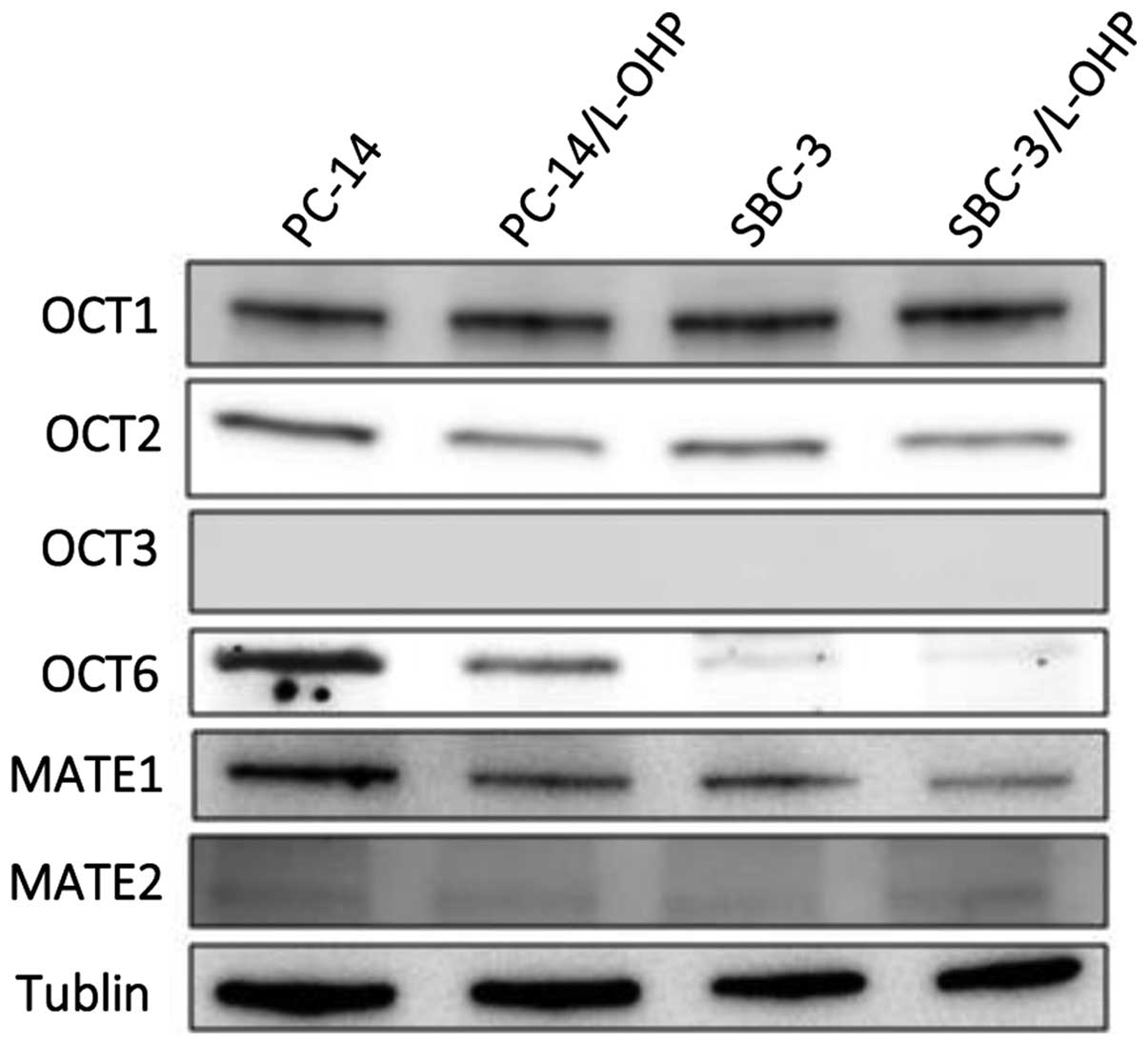
We examined the protein expression levels of OCTs and MATE proteins in parental and L-OHP-resistant cells (Fig. 1). Each of the visualized immunoreactive bands associated with the protein expression levels was calculated using a densitometer relative to those associated with the loading control bands. We found that the OCT6 protein expression levels in PC-14/L-OHP and SBC-3/L-OHP were downregulated compared with the parental cells, PC-14 and SBC-3. By contrast, OCT1 and OCT2 expression levels were similar in the parental and L-OHP-resistant cells, and OCT3 expression was not detected. The expression levels of MATE1 and MATE2 were not different in the parental and L-OHP-resistant cells.
Cellular accumulation of platinum
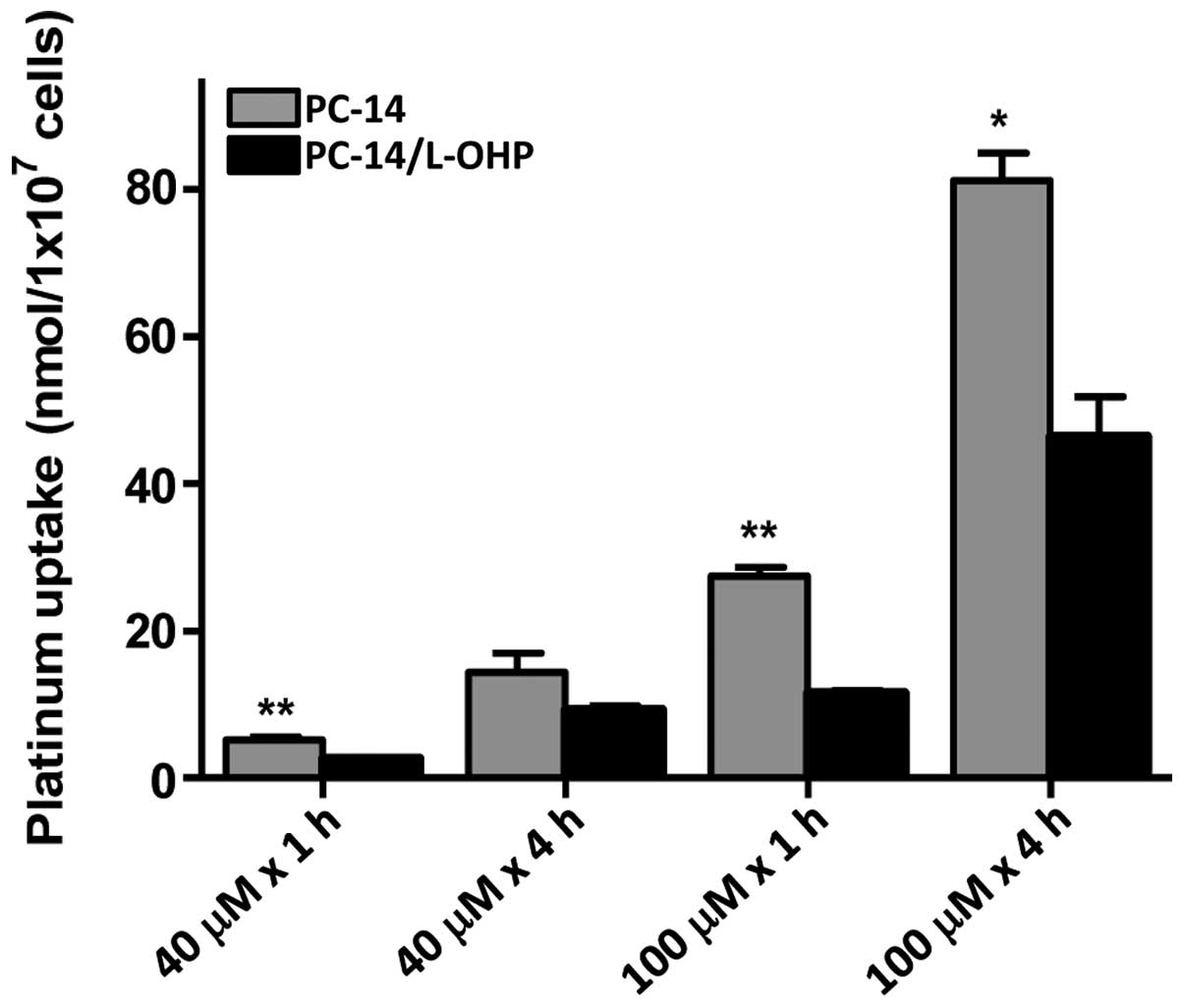
The intracellular accumulation of platinum was measured in PC-14 and PC-14/L-OHP cells to determine whether a lower expression of OCT6 in the drug-resistant cells resulted in a reduced influx of drugs. After treatment with 40 and 100 µM L-OHP over periods of 1 and 4 h, the intracellular platinum accumulation was significantly lower in the PC-14/L-OHP cells compared with the parental PC-14 cells (Fig. 2).
Increased platinum accumulation in cells overexpressing OCT6
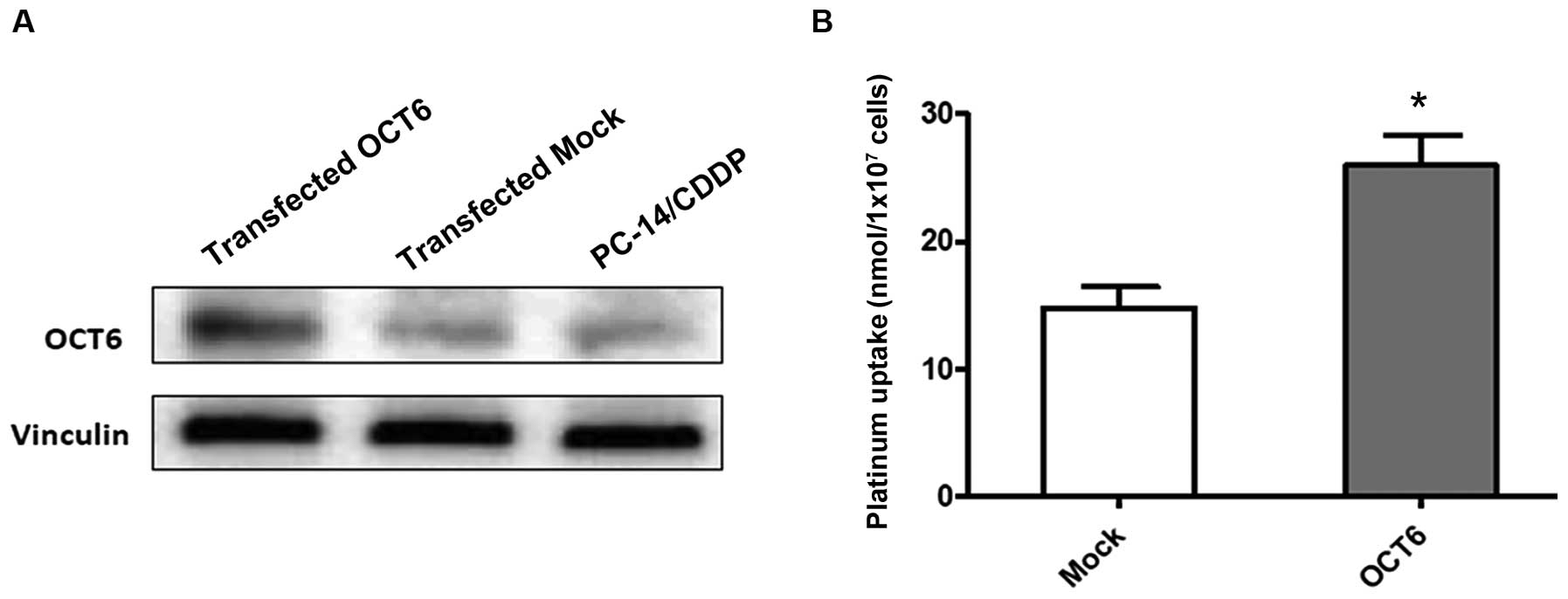
To further investigate the role of OCT6 in intracellular platinum drug accumulation, we used the previously established OCT6 overexpressing PC-14/CDDP cell line, which was engineered to constitutively express the OCT6 gene, SLC22A16. The expression level of the OCT6 protein was markedly increased in OCT6 overexpressing PC-14/CDDP cells compared with the parental and mock-transfected cells (Fig. 3A). The intracellular platinum accumulation after treatment with 100 µM L-OHP for 4 h was significantly increased in the OCT6-overexpressing PC-14/CDDP cells compared with the mock-transfected control cells (P<0.05) (Fig. 3B).
OCT6 expression in human tissues
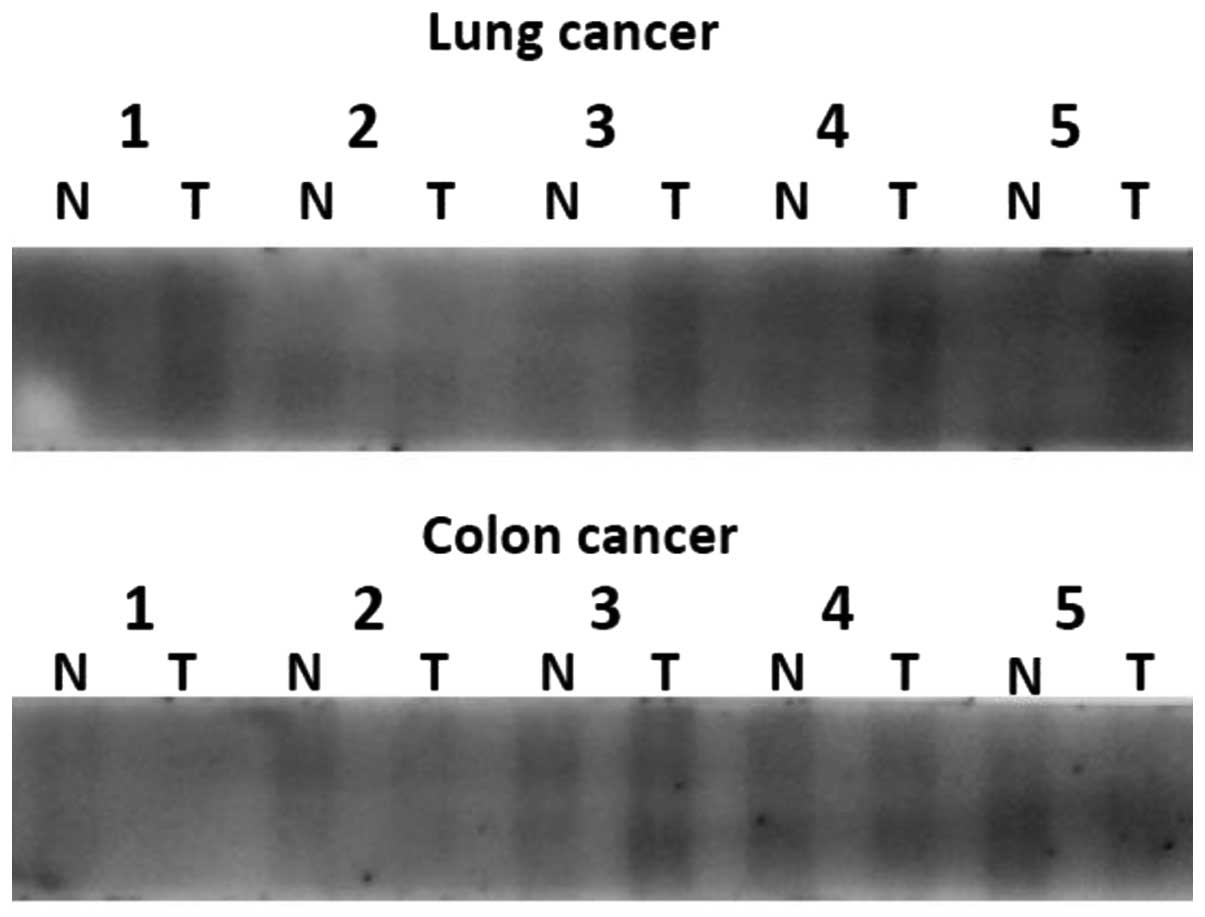
We investigated OCT6 protein expression in a panel of human lung and colon cancer tissue protein lysates, and their respective matched normal ones using western blot analysis. We found that OCT6 was detected in a screen panel of cancer and matched normal tissue protein lysates. In the lung cancers, the expression of OCT6 in cancer tissues was higher than that in the matched normal tissues. In the colon cancers, the expression of OCT6 was variable (Fig. 4).
Discussion
Recently, we reported that a decreased OCT6 expression is associated with resistance to CDDP, mediated by decreased intracellular uptake of the drug (12). In the present study, we established L-OHP-resistant cell lines and found that similar to CDDP, a decreased expression of OCT6 in these cells resulted in resistance to L-OHP, associated with the decreased intracellular uptake of L-OHP. Thus, OCT6 expression directly confers resistance to anticancer platinum drugs in cancer cells.
The efficacy of anticancer drugs in tumor cells is determined in part by the effective intracellular concentration of the drugs. Indeed, tissue platinum concentration is significantly associated with tumor response and survival in non-small cell lung cancer patients (13). Although OCTs play a critical role in the cellular uptake of platinum drugs, the relationship between OCTs and drug resistance is not fully understood. More specifically, although the characteristic substrate specificities for platinum drugs are known for some OCTs (4,9), the specificities of OCT6 have not been comprehensively investigated. In this regard, the results from this, and our previous study, indicate that both CDDP and L-OHP are transported by OCT6. By contrast, the expression levels of OCT1, OCT2 and OCT3 were not altered in either CDDP or L-OHP resistant cells compared with the parental cells, suggesting that in this context, these OCT family members are not mainly involved in resistance to CDDP or L-OHP.
Drug efflux systems are also considered to be another important determinant of the effective intracellular concentration of platinum drugs. Recently, the MATE multidrug efflux transporters were found to play crucial roles in platinum drug efflux from the kidney. L-OHP was transported intracellularly by OCT2, and MATE-mediated efflux of L-OHP from renal tubules protected against L-OHP nephrotoxicity. Notably, although CDDP was also transported by OCT2, MATE-mediated CDDP efflux was weak (14,15). The pharmacological characteristics of L-OHP, which are different from those of CDDP, may also be a major reason for the low nephrotoxicity of L-OHP in the kidney. With these findings in mind, we investigated MATE1 and MATE2 expression in L-OHP-resistant lung cancer cells and found that expression of both did not differ between parental and L-OHP-resistant cells. We believe that these data are the first to indicate that MATEs expression is not a major determinant of resistance to platinum drugs in cancer cells.
A limitation of the present study was the exclusive use of lung cancer cell lines. Indeed, OCTs have characteristic patterns of tissue expression (9), and L-OHP is now mainly used in colon cancer chemotherapy (3). Previously, OCT1 and OCT2 were reported to be expressed in colon cancer samples, where OCT2 plays a critical role in the cellular uptake and consequent cytotoxicity of L-OHP (9,10). In addition, recent clinical studies revealed that OCT2 expression is an important independent predictor of good outcome in 5-fluorouracil/leucovorin/L-OHP-treated metastatic colon cancers (16). OCT2 can also transport CDDP (9,11), and OCT2 expression was reported to be a potential predictor of response to neoadjuvant chemotherapy with S-1/CDDP in gastric cancer (17). We detected OCT6 expression in primary colon cancer tissues and matched normal tissues, which is consistent with a previous report showing a strong expression of OCT6 in some colon and liver cancer cell lines (18). These data raise the possibility that OCT6 expression may influence the efficacy of L-OHP treatment for colon cancer.
The anticancer platinum drugs are necessary to improve survival even for those lung cancer patients treated with molecularly-targeted therapy (19). It may be noteworthy to determine whether the expression of OCT6 in lung cancer tissues, and potentially hematopoietic cells where OCT6 is also expressed (20), is an important predictor of efficacy and chemotoxicity after anticancer platinum drug chemotherapy.
Previous findings demonstrated the potential for drug-drug interactions when certain tyrosine kinase inhibitors are used concomitantly with drugs such as L-OHP, which are transported by OCTs (21). As the OCTs have an established role in regulating the pharmacokinetic profile of several anticancer drugs, as well as mediating the intrinsic cellular sensitivity of healthy organs and cancer cells, further studies are required to clarify the potential role of OCTs for reducing treatment related toxicity and improving the outcomes for cancer patients.
Acknowledgements
The present study was supported by a Grant-in-Aid from Kidani Memorial Trust and a Grant-in-Aid for Scientific Research (c) from the Japan Society for the Promotion of Science (MEXT/JSPSKAKENHI 15K08593).
References
|
Sculier JP, Berghmans T and Meert AP: Advances in target therapy in lung cancer. Eur Respir Rev. 24:23–29. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Heinemann V, Douillard JY, Ducreux M and Peeters M: Targeted therapy in metastatic colorectal cancer - an example of personalised medicine in action. Cancer Treat Rev. 39:592–601. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Kelland L: The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 7:573–584. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Dasari S and Tchounwou PB: Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol. 740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Oguri T, Fujiwara Y, Isobe T, Katoh O, Watanabe H and Yamakido M: Expression of γ-glutamylcysteine synthetase (γ-GCS) and multidrug resistance-associated protein (MRP), but not human canalicular multispecific organic anion transporter (cMOAT), genes correlates with exposure of human lung cancers to platinum drugs. Br J Cancer. 77:1089–1096. 1998. View Article : Google Scholar : PubMed/NCBI | |
|
Oguri T, Fujiwara Y, Miyazaki M, Takahashi T, Kurata T, Yokozaki M, Ohashi N, Isobe T, Katoh O and Yamakido M: Induction of γ-glutamylcysteine synthetase gene expression by platinum drugs in peripheral mononuclear cells of lung cancer patients. Ann Oncol. 10:455–460. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Oguri T, Isobe T, Suzuki T, Nishio K, Fujiwara Y, Katoh O and Yamakido M: Increased expression of the MRP5 gene is associated with exposure to platinum drugs in lung cancer. Int J Cancer. 86:95–100. 2000. View Article : Google Scholar : PubMed/NCBI | |
|
Oguri T, Isobe T, Fujitaka K, Ishikawa N and Kohno N: Association between expression of the MRP3 gene and exposure to platinum drugs in lung cancer. Int J Cancer. 93:584–589. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Koepsell H, Lips K and Volk C: Polyspecific organic cation transporters: Structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 24:1227–1251. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang S, Lovejoy KS, Shima JE, Lagpacan LL, Shu Y, Lapuk A, Chen Y, Komori T, Gray JW, Chen X, et al: Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res. 66:8847–8857. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Ciarimboli G, Ludwig T, Lang D, Pavenstädt H, Koepsell H, Piechota HJ, Haier J, Jaehde U, Zisowsky J and Schlatter E: Cisplatin nephrotoxicity is critically mediated via the human organic cation transporter 2. Am J Pathol. 167:1477–1484. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Kunii E, Oguri T, Kasai D, Ozasa H, Uemura T, Takakuwa O, Ohkubo H, Takemura M, Maeno K and Niimi A: Organic cation transporter OCT6 mediates cisplatin uptake and resistance to cisplatin in lung cancer. Cancer Chemother Pharmacol. 75:985–991. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Kim ES, Lee JJ, He G, Chow CW, Fujimoto J, Kalhor N, Swisher SG, Wistuba II, Stewart DJ and Siddik ZH: Tissue platinum concentration and tumor response in non-small-cell lung cancer. J Clin Oncol. 30:3345–3352. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Yokoo S, Yonezawa A, Masuda S, Fukatsu A, Katsura T and Inui K: Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol. 74:477–487. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Yonezawa A and Inui K: Organic cation transporter OCT/SLC22A and H(+)/organic cation antiporter MATE/SLC47A are key molecules for nephrotoxicity of platinum agents. Biochem Pharmacol. 81:563–568. 2011. View Article : Google Scholar : PubMed/NCBI | |
|
Tashiro A, Tatsumi S, Takeda R, Naka A, Matsuoka H, Hashimoto Y, Hatta K, Maeda K and Kamoshida S: High expression of organic anion transporter 2 and organic cation transporter 2 is an independent predictor of good outcomes in patients with metastatic colorectal cancer treated with FOLFOX-based chemotherapy. Am J Cancer Res. 4:528–536. 2014.PubMed/NCBI | |
|
Naka A, Takeda R, Shintani M, Ogane N, Kameda Y, Aoyama T, Yoshikawa T and Kamoshida S and Kamoshida S: Organic cation transporter 2 for predicting cisplatin-based neoadjuvant chemotherapy response in gastric cancer. Am J Cancer Res. 5:2285–2293. 2015.PubMed/NCBI | |
|
Okabe M, Unno M, Harigae H, Kaku M, Okitsu Y, Sasaki T, Mizoi T, Shiiba K, Takanaga H, Terasaki T, et al: Characterization of the organic cation transporter SLC22A16: A doxorubicin importer. Biochem Biophys Res Commun. 333:754–762. 2005. View Article : Google Scholar : PubMed/NCBI | |
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, et al: Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol. 26:1877–1883. 2015. View Article : Google Scholar : PubMed/NCBI | |
|
Gong S, Lu X, Xu Y, Swiderski CF, Jordan CT and Moscow JA: Identification of OCT6 as a novel organic cation transporter preferentially expressed in hematopoietic cells and leukemias. Exp Hematol. 30:1162–1169. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Minematsu T and Giacomini KM: Interactions of tyrosine kinase inhibitors with organic cation transporters and multidrug and toxic compound extrusion proteins. Mol Cancer Ther. 10:531–539. 2011. View Article : Google Scholar : PubMed/NCBI |