Utilization of arsenic trioxide as a treatment of cisplatin-resistant non-small cell lung cancer PC-9/CDDP and PC-14/CDDP cells
- Authors:
- Published online on: June 10, 2015 https://doi.org/10.3892/ol.2015.3352
- Pages: 805-809
-
Copyright: © Suzuki et al. This is an open access article distributed under the terms of Creative Commons Attribution License.
Abstract
Introduction
Since the late 20th century, a large number of molecular targeted therapeutic drugs, such as epidermal growth factor receptor tyrosine kinase inhibitors, have been developed for cancer chemotherapy. However, platinum-based combination chemotherapy continues to be important for the treatment of various cancers, including lung cancer. Cisplatin is a commonly used drug for combination chemotherapy (1). Although cisplatin is a potent anticancer drug, various malignant tumors frequently acquire resistance to cisplatin, limiting the clinical application of this agent (2,3).
Arsenite is a toxic metalloid that is widely distributed in the environment (4). Despite the toxicity, arsenic-containing compounds have been used in traditional Chinese medicine for >2,000 years (5). In addition, arsenic trioxide (ATO) has been approved in numerous countries for the treatment of acute promyelocytic leukemia (APL) (6). In vitro studies have revealed that a combination of ATO and cisplatin exerts a synergistic inhibitory effect on the growth of ovarian cancer and small cell lung cancer cells (7–9).
It is well known that adenosine triphosphate-binding cassette (ABC) transporters are important molecules for the resistance of cancer cells to chemotherapy agents and the detoxification of toxic metals in xenobiotic metabolism (10). Leslie et al previously reported that ABC subfamily C, member 1 (ABCC1) and ABC, subfamily C, member 2 (ABCC2) each play an important role in multidrug resistance and arsenic detoxification in cells, through the cellular efflux of the glutathione (GSH) conjugate (11).
The present study aimed to examine the sensitivity of the non-small cell lung cancer (NSCLC) PC-9 cell line and cisplatin-resistant PC-9/CDDP subline to toxic metals. The cisplatin-resistant PC-9/CDDP cell line demonstrated hypersensitivity to arsenite and arsenate. Furthermore, the accumulation of arsenite was decreased in PC-9/CDDP cells relative to the parental cells. Notably, the sensitivity to arsenite and accumulation of arsenite were markedly increased compared with the cisplatin-resistant cell line. Therefore, it was hypothesized that the GS-X pump systems were downregulated in the cisplatin resistant NSCLC cell line. In addition, the arsenic-containing chemotherapeutic agent ATO was applied to the cisplatin-sensitive and -resistant NSCLC cells, and it was found that the cisplatin-resistant NSCLC cell lines PC-9/CDDP and PC-14/CDDP were hypersensitive to ATO.
The results of the present study suggest that arsenite accumulation depends on the GS-X pump and that treatment with ATO may overcome certain cases of cisplatin resistance in NSCLC.
Materials and methods
Cell lines and materials
The NSCLC PC-9 and PC-14 cell lines, which were derived from untreated patients with pulmonary adenocarcinoma, were provided by Professor Y. Hayata (Tokyo Medical College, Tokyo, Japan). The cisplatin-resistant PC-9/CDDP and PC-14/CDDP sublines were established at the National Cancer Center Research Institute (Tokyo, Japan) (12).
The mouse anti-human ABCC1 monoclonal antibody (clone number, QCRL1; ALX-801-010) and mouse anti-human ABCC2 monoclonal antibody (clone number, M2I-4; ALX-801-015) were obtained from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). The mouse anti-human GST-π monoclonal antibody (clone number, GST-π/3; 610718) was obtained from BD Biosciences (Franklin Lakes, NJ, USA). The Vectastain ABC kit and horseradish peroxidase-conjugated anti-mouse immunoglobulin G secondary antibody were obtained from Vector Laboratories (Burlingame, CA, USA). Enhanced chemiluminescence (ECL) reagent and Hyperfilm were obtained from GE Healthcare Biosciences (Pittsburgh, PA, USA). Sodium serenate, sodium selenite, sodium arsenite and sodium arsenate were obtained from Sigma-Aldrich (St. Louis, MO, USA). Antimony and cadmium chloride were obtained from Nacalai Tesque, Inc. (Kyoto, Japan). Arsenic trioxide (Trisenox) was obtained from Nippon Shinyaku Co. Ltd. (Kyoto, Japan). All other chemicals were obtained from Nacalai Tesque, Inc. or Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Cellular cytotoxicity assay to determine sensitivity to metals
Cell counting kit-8 was obtained from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan) and, with MTT, was used for colorimetric assays to determine the cell viability, as previously described (13). The cells were plated on 96-well microplates at a density of 2×103 cells per well, incubated overnight, and then treated with various concentrations of arsenate, arsenite, selenate, selenite, mercury, cadmium or antimony for 72 h.
Determination of arsenite accumulation
The cells were plated on 6-cm dishes at a density of 1×106 cells per dish, incubated overnight, and then treated with 100 µM sodium arsenite for 4 h. Subsequent to washing with ice-cold PBS (–), the cells were immediately harvested using a cell scraper. The cells were pelleted by centrifugation, and the cell pellets were extracted with 20 mM Tris-HCl (pH 7.4) containing 1% Triton-X 100 and 0.1% SDS. The arsenite concentrations in the cells were determined by atomic absorption spectrometry (AAS; Z-9000; Hitachi, Ltd., Tokyo, Japan).
Analysis of the GSH concentration and activity of GSH-associated enzymes
The intracellular GSH concentration was measured using 5,5-dithiobis(2-nitrobenzoic acid) (DTNB). Cell lysates were diluted in 100 mM sodium phosphate buffer (pH 7.4) containing 2.5 mM EDTA, 0.25 mM NADPH and 1 unit/ml glutathione reductase (Oriental Yeast, Co., Ltd., Tokyo, Japan). The reaction was then initiated by adding 200 µM DTNB, and the absorbance at 415 nm was subsequently measured. The amount of total GSH was determined by comparing the absorbance of the cell lysate with a GSH standard curve.
The activity of γ-glutamyl cysteine synthetase (γ-GCS) was measured using a previously reported high-performance liquid chromatography (HPLC) method (14), with slight modification. A 10-µl aliquot of cell lysate was diluted in 100 µl of assay mixture containing 75 mM KCl, 10 mM MgCl2, 0.2 mM EDTA, 10 mM adenosine triphosphate, 10 mM cysteine, 5 mM glutamate and 100 mM Tris-HCl buffer (pH 7.4). Following 20 min of incubation at 37°C, the reaction was terminated by the addition of 20 µl of 15% trichloroacetic acid, and the assay mixture was then chilled on ice. Subsequent to centrifugation, quantitation of Glu-Cys was conducted by post-labeled fluorescence detection HPLC using the ortho-phthalaldehyde reagent (Nacalai Tesqe, Inc., Kyoto, Japan).
Glutathione-S-transferase (GST) activity was determined using standard spectrophotometric assays by monitoring the formation of the conjugate of 1-chloro-2,4-dinitrobenzene (CDNB) and reduced glutathione (GSH) (15).
GST-π protein expression was determined using immunoblotting. In total, 20 µg of protein was loaded onto 12.5% (w/v) SDS-polyacrylamide gels and subjected to electrophoresis. Subsequent to electrotransfer onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA, USA), the membranes were blocked with Block Ace (Yukijirushi-Nyugyo, Co., Sapporo, Japan) according to the manufacturer's instructions. Probing was performed overnight at 4°C with the monoclonal mouse anti-GST-π antibody diluted with PBS containing 0.1% Tween-20 (1:200; clone number, 3; BD Biosciences, Franklin Lakes, NJ, USA). Subsequent to washing, specific signals were detected using horseradish peroxidase-conjugated anti-mouse immunoglobulin G secondary antibody diluted with PBS containing 0.1% Tween-20 (1:10,000) and ECL reagent according to the manufacturer's instructions (GE Healthcare Biosciences, Pittsburgh, PA, USA).
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR) for mRNA
Total RNA was isolated using an RNeasy Mini kit (Qiagen, Hilden, Germany). Reverse transcription was performed on 1 µg of total RNA using High Capacity RNA-to-cDNA Master Mix (Applied Biosystems, Foster City, CA, USA). RT-qPCR was performed using converted cDNA and the following reagents: TaqMan Gene Expression Assays for human ABCC1 (assay ID, Hs00219905_m1), ABCC2 (assay ID, Hs00166123_m1), ABCC3 (assay ID, Hs00978473_m1), ABCC4 (assay ID, Hs00988717_m1), ABCC5 (assay ID, Hs00981087_m1) and GAPDH (assay ID, Hs99999901_s1) genes, and the TaqMan Universal PCR Master Mix, no AmpErase UNG (Thermo Fisher Scientific Inc., Waltham, MA). An ABI PRISM 7300 Sequence Detector System (Thermo Fisher Scientific Inc.) was used to collect the signals according to the manufacturer's instructions. The relative gene expression was determined using the comparative cycle threshold (CT) method based on the normalization of the target gene against the GAPDH reference gene. ΔCT was calculated by subtracting the CT of GAPDH from the CT of the target genes.
Immunoblotting of ABCC1 and ABCC2
Plasma membrane fractions were prepared by ultracentrifugation, as previously described (16). Plasma membrane fractions (50 µg protein) were loaded onto 7.5% (w/v) SDS-polyacrylamide gels and resolved by electrophoresis. Following electrotransfer onto PVDF membranes, probing was performed overnight at 4°C with the anti-ABCC1 antibody or anti-ABCC2 antibody. Subsequent to washing, specific signals were detected using the Vectastain ABC system and ECL chemiluminescence reagent.
Statistical analysis
The results are expressed as the mean ± standard deviation. Statistical evaluations were performed using Student's t-tests with Microsoft Excel 2013 (Microsoft Corporation, Redmond, WA, USA) for the analysis.
Results
Cross-resistance to metals
To elucidate the mechanism underlying cisplatin resistance, an analysis of the sensitivity of the PC-9 and PC-9/CDDP cell lines to various metals was performed. The IC50 values of various metal compounds in the parental PC-9 and cisplatin-resistant PC-9/CDDP cell lines are reported in Table I. Only selenate (VI) demonstrated an increased cross-resistance in PC-9/CDDP cells compared with parental PC-9 cells, with a 1.90-fold increase. By contrast, antimony (IV), arsenate (V), arsenite (III) and mercury (II) were revealed to be more potent inhibitors of PC-9/CDDP cells compared with the effect on the parental PC-9 cell line. No difference in sensitivity was observed for the other metals between the PC-9/CDDP and parental PC-9 cells.
Arsenite accumulation
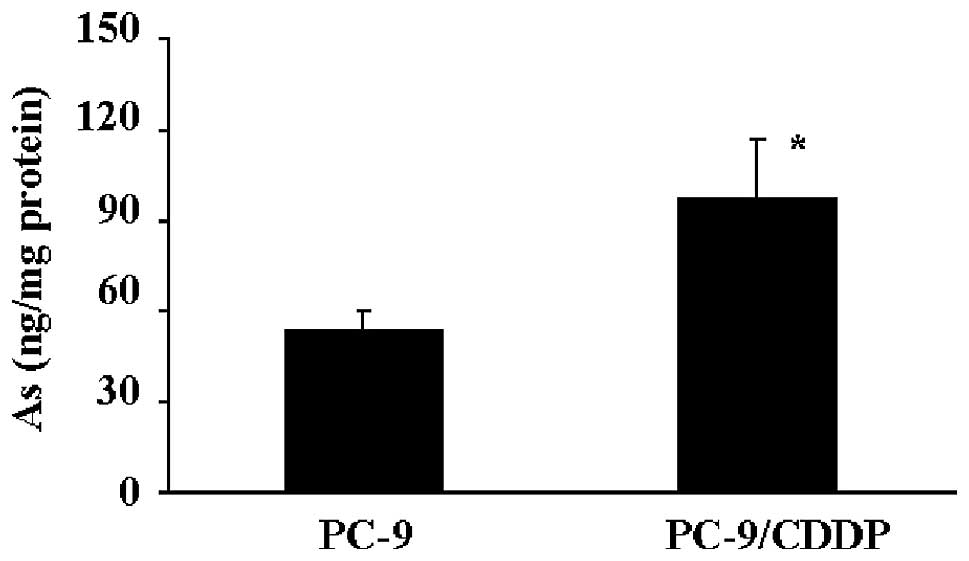
The PC-9/CDDP cells demonstrated hypersensitivity to arsenite and arsenate. To elucidate the mechanisms of arsenite sensitivity in the cisplatin-resistant PC-9/CDDP cell line, the accumulation of arsenite was measured. When the cells were incubated in medium containing 100 mM sodium arsenite for 4 h, the accumulation of arsenite in PC-9/CDDP cells was increased ~1.7-fold compared with the accumulation demonstrated by PC-9 cells (Fig. 1). When the cisplatin accumulation in the cells was determined under the same conditions, the cisplatin concentration in the PC-9/CDDP cells was found to be markedly decreased, demonstrating an accumulation of ~20% of the cisplatin found in the parental PC-9 cells (data not shown).
Analysis of the GSH concentration and activity of GSH-associated enzymes
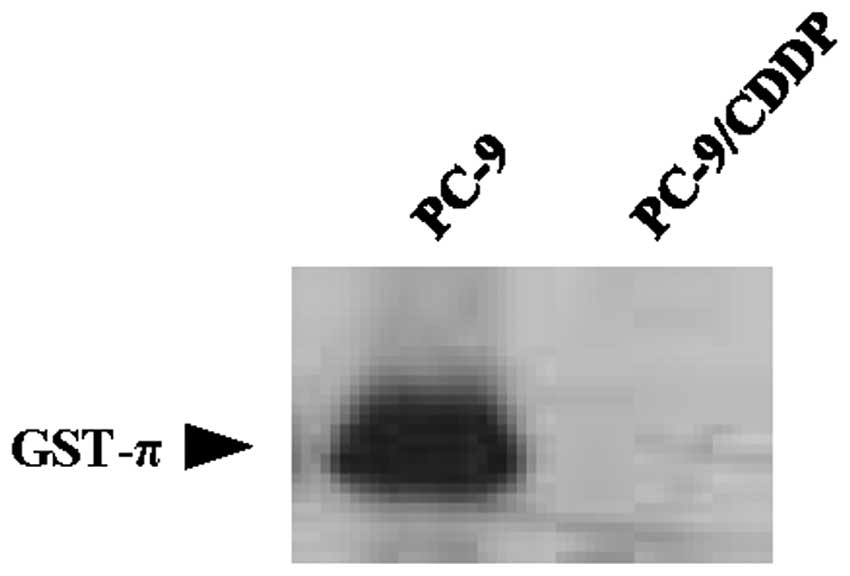
GS-X pump systems are an important mechanism by which metals, including cisplatin and arsenite, are transported (17,18). To investigate the molecular basis for the decreased accumulation of arsenite, the GS-X pump systems were analyzed, including cellular GSH levels, activity of the GSH synthesis rate-limiting enzyme γ-GCS, GST activity and expression of the ABC transporters. Cellular GSH levels in PC-9/CDDP cells were 65% of those measured in the parental PC-9 cell line. γ-GCS activity was also downregulated in PC-9/CDDP cells, where it was 67% of the activity measured in PC-9 cells. GST activity was also extremely decreased to ~4.0% of the activity observed in the parental cell line (Table II). The protein expression of GST-π was also downregulated in PC-9/CDDP cells compared with the parental cell line (Fig. 2).
Table II.Concentration of GSH and activity of associated enzymes in the cisplatin-resistant PC-9/CDDP cell subline. |
Analysis of the expression of ABCC transporters
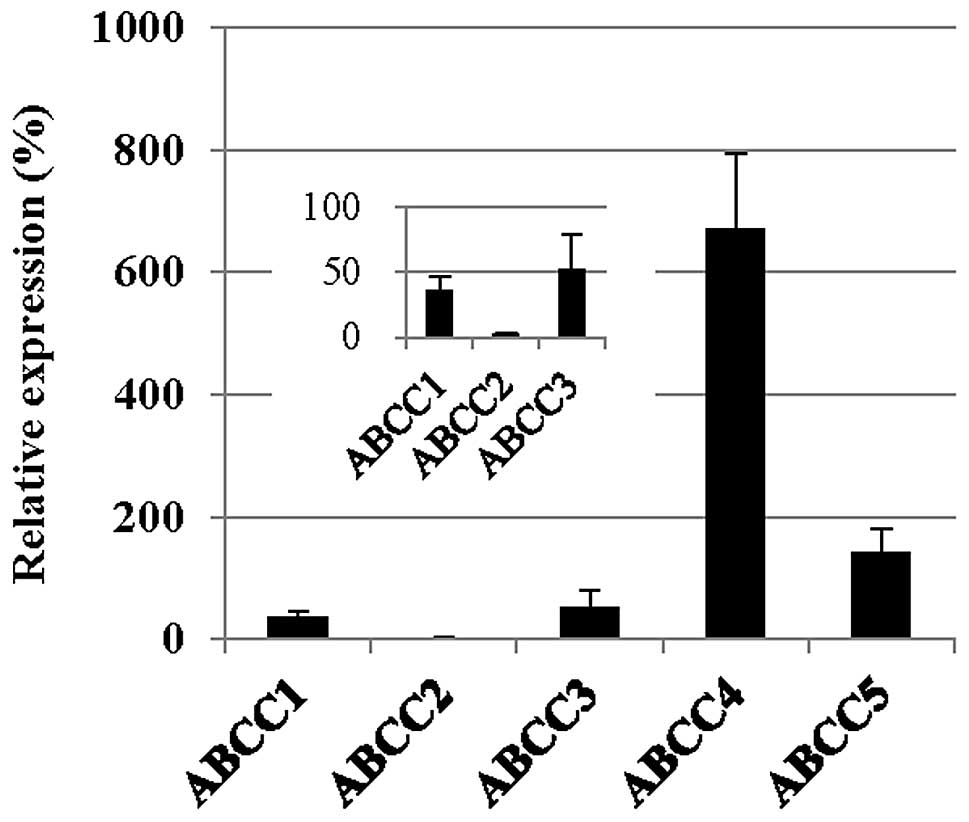
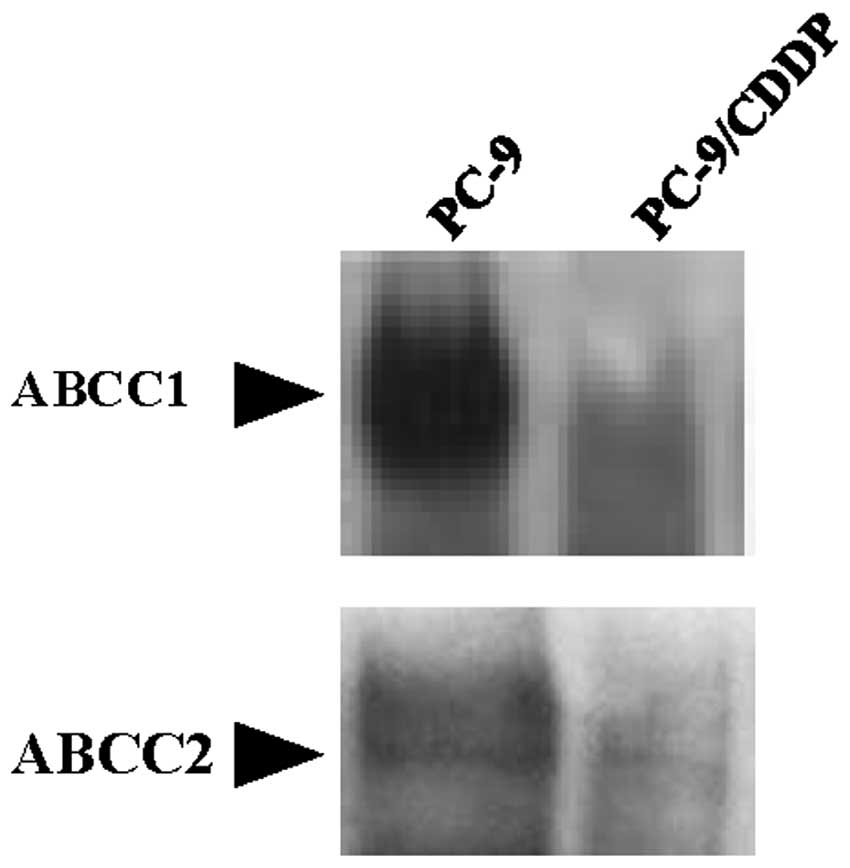
It has previously been indicated that arsenite is a substrate for the GS-X pump, as a GS-As conjugate (11). As shown in Fig. 3, the expression of ABCC1, 2 and 3 was downregulated in the PC-9/CDDP cells, with mRNA expression levels of 36.0, 2.7 and 51.9% of the level measured in the parental PC-9 cell line, respectively. The protein levels of ABCC1 and 2 in the plasma membrane were also decreased in the PC-9/CDDP cells compared with the parental cells. In western blot analysis, only faint bands corresponding to ABCC1 and 2 were obtained from the fractions derived from the PC-9/CDDP plasma membrane (Fig. 4).
Sensitivity of the cisplatin-resistant lung cancer PC-9/CDDP and PC-14/CDDP cell lines to ATO
As aforementioned, the cisplatin-resistant PC-9/CDDP cell line was revealed to demonstrate hypersensitivity to arsenite. Additionally, the cytotoxic effect of the arsenite-containing chemotherapeutic agent ATO was examined in the cisplatin-resistant NSCLC PC-9/CDDP and PC-14/CDDP cell lines.
The findings revealed that the PC-9/CDDP and PC-14/CDDP cell lines each demonstrated a 3-fold increase in sensitivity to ATO compared with the PC-9 and PC-14 parental cell lines (Table III).
Table III.Sensitivity to arsenic trioxide in the parent and cisplatin-resistant non-small cell lung cancer PC-9 and PC-14 cell lines. |
Discussion
Cisplatin is a coordination compound of platinum. Certain cisplatin-resistant cell lines demonstrate cross-resistance to metals that include cadmium, antimony and arsenite (19). The identification of cross-resistant metals aids the evaluation of the mechanism underlying cisplatin resistance and metal transport systems. Previously, cisplatin-resistant cell lines were developed from metallothionine knockout (MT-KO) cells, and these cell lines demonstrated cross-resistance to arsenite and decreased arsenite accumulation (20). These results indicate the presence of a mechanism by which arsenite and cisplatin resistance in MT-KO cell lines depends on the same transport systems.
By contrast, an in vitro study has revealed that the combined administration of cisplatin and ATO demonstrates a synergistic inhibitory effect on the growth of various cancer cell lines, including squamous carcinoma, ovarian cancer and small cell lung cancer cells (7–9,21).
In the present study, the cisplatin-resistant PC-9/CDDP cell line was revealed to be hypersensitive to arsenite. A marked decrease in the accumulation of arsenite was also observed in the PC-9/CDDP cells. These results indicate that the transport systems for cisplatin and arsenite are independent in PC-9 cells. Consequently, hypersensitivity to arsenite in PC-9/CDDP cells appears to be dependent on the cellular accumulation of arsenite.
It has previously been reported that the mechanisms of arsenite accumulation are mediated by transporter systems, influx transporters, including the aquaglyceroporins aquaporin (AQP)7 and AQP9 (22), and efflux transporters, such as ABCC1 and ABCC2 (11). Although the cisplatin uptake transporter was not identified in the present cell lines, the transporter of cisplatin in PC-9/CDDP cells may not contribute to the uptake of arsenite.
The present results clearly reveal that GSH and GS-X pump-associated factors, including GST activity and ABC transporter expression, were downregulated in PC-9/CDDP cells. GSH and GST are important for the formation of the arsenic-glutathione conjugate, which is a good substrate for ABCC1 and ABCC2.
In conclusion, ATO, an approved drug, potentially possesses sensitivity for cisplatin-resistant NSCLC cell lines. The mechanism of ATO hypersensitivity is dependent on the downregulation of GS-X pump systems, particularly GSH, GST, ABCC1 and ABCC2.
The aforementioned findings indicate that ATO has the potential to treat cisplatin resistance in NSCLC and that ABCC1 and ABCCC2 are important biomarkers when considering a chemotherapeutic regimen containing ATO for the treatment of cisplatin-resistant NSCLC.
Acknowledgements
This study was supported by the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research KAKENHI (grant nos., 20790147, 22790173 and 24590218).
References
|
Dasari S and Tchounwou PB: Cisplatin in cancer therapy: Molecular mechanisms of action. Eur J Pharmacol. 740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Nishio K, Nakamura T, Koh Y, Suzuki T, Fukumoto H and Saijo N: Drug resistance in lung cancer. Curr Opin Oncol. 11:109–115. 1999. View Article : Google Scholar : PubMed/NCBI | |
|
Suzuki T, Nishio K and Tanabe S: The MRP family and anticancer drug metabolism. Curr Drug Metab. 2:367–377. 2001. View Article : Google Scholar : PubMed/NCBI | |
|
Nordstrom DK: Public health. Worldwide occurrences of arsenic in ground water. Science. 296:2143–2145. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Rao Y, Li R and Zhang D: A drug from poison: How the therapeutic effect of arsenic trioxide on acute promyelocytic leukemia was discovered. Sci China Life Sci. 56:495–502. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Lengfelder E, Hofmann WK and Nowak D: Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia. 26:433–442. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Zhang N, Wu ZM, McGowan E, Shi J, Hong ZB, Ding CW, Xia P and Di W: Arsenic trioxide and cisplatin synergism increase cytotoxicity in human ovarian cancer cells: Therapeutic potential for ovarian cancer. Cancer Sci. 100:2459–2464. 2009. View Article : Google Scholar : PubMed/NCBI | |
|
Helm CW and States JC: Enhancing the efficacy of cisplatin in ovarian cancer treatment-could arsenic have a role. J Ovarian Res. 2:22009. View Article : Google Scholar : PubMed/NCBI | |
|
Zheng CY, Lam SK, Li YY, Fong BM, Mak JC and Ho JC: Combination of arsenic trioxide and chemotherapy in small cell lung cancer. Lung Cancer. 82:222–230. 2013. View Article : Google Scholar : PubMed/NCBI | |
|
Szakács G, Paterson JK, Ludwig JA, Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI | |
|
Leslie EM: Arsenic-glutathione conjugate transport by the human multidrug resistance proteins (MRPs/ABCCs). J Inorg Biochem. 108:141–149. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Hong WS, Saijo N, Sasaki Y, Minato K, Nakano H, Nakagawa K, Fujiwara Y, Nomura K and Twentyman PR: Establishment and characterization of cisplatin-resistant sublines of human lung cancer cell lines. Int J Cancer. 41:462–467. 1988. View Article : Google Scholar : PubMed/NCBI | |
|
Uemura M, Suzuki T, Nishio K, Chikuma M and Komeda S: An in vivo highly antitumor-active tetrazolato-bridged dinuclear platinum (II) complex largely circumvents in vitro cisplatin resistance: Two linkage isomers yield the same product upon reaction with 9-ethylguanine but exhibit different cytotoxic profiles. Metallomics. 4:686–692. 2012. View Article : Google Scholar : PubMed/NCBI | |
|
Mukai Y, Togawa T, Suzuki T, Ohata K and Tanabe S: Determination of homocysteine thiolactone and homocysteine in cell cultures using high-performance liquid chromatography with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 767:263–268. 2002. View Article : Google Scholar : PubMed/NCBI | |
|
Habig WH, Pabst MJ, Fleischner G, Gatmaitan Z, Arias IM and Jakoby WB: The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc Natl Acad Sci USA. 71:3879–3882. 1974. View Article : Google Scholar : PubMed/NCBI | |
|
Yoshikawa M, Ikegami Y, Hayasaka S, Ishii K, Ito A, Sano K, Suzuki T, Togawa T, Yoshida H and Soda H: Novel camptothecin analogues that circumvent ABCG2-associated drug resistance in human tumor cells. Int J Cancer. 110:921–927. 2004. View Article : Google Scholar : PubMed/NCBI | |
|
Kurokawa H, Ishida T, Nishio K, Arioka H, Sata M, Fukumoto H, Miura M and Saijo N: Gamma-glutamylcysteine synthetase gene overexpression results in increased activity of the ATP-dependent glutathione S-conjugate export pump and cisplatin resistance. Biochem Biophys Res Comm. 216:258–264. 1995. View Article : Google Scholar : PubMed/NCBI | |
|
Ishikawa T, Bao JJ, Yamane Y, Akimaru K, Frindrich K, Wright CD and Kuo MT: Coordinated induction of MRP/GS-X pump and gamma-glutamylcysteine synthetase by heavy metals in human leukemia cells. The Journal of biological chemistry. 271:14981–14988. 1996. View Article : Google Scholar : PubMed/NCBI | |
|
Naredi P, Heath DD, Enns RE and Howell SB: Cross-resistance between cisplatin, antimony potassium tartrate and arsenite in human tumor cells. J Clin Invest. 95:1193–1198. 1995. View Article : Google Scholar : PubMed/NCBI | |
|
Suzuki T, Ohata S, Togawa T, Himeno S and Tanabe S: Arsenic accumulation decreased in metallothionein null Cisplatin-resistant cell lines. J Toxicol Sci. 32:321–328. 2007. View Article : Google Scholar : PubMed/NCBI | |
|
Nakaoka T, Ota A, Ono T, Karnan S, Konishi H, Furuhashi A, Ohmura Y, Yamada Y, Hosokawa Y, Kazaoka Y, et al: Combined arsenic trioxide-cisplatin treatment enhances apoptosis in oral squamous cell carcinoma cells. Cell Oncol (Dordr). 37:119–129. 2014. View Article : Google Scholar : PubMed/NCBI | |
|
Liu Z, Shen J, Carbrey JM, Mukhopadhyay R, Agre P and Rosen BP: Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. Proc Natl Acad Sci USA. 99:6053–6058. 2002. View Article : Google Scholar : PubMed/NCBI |