Introduction
Atrial fibrillation (AF) is one of the most commonly
observed arrhythmias in clinical practice. Epidemiological surveys
have shown that its incidence is increasing annually (1,2). Patients
with AF, in particular those with a disease duration >6 months
(chronic AF), are at an increased risk of stroke and other
thrombotic events (TEs), which are significant causes of mortality
and morbidity in these patients (1,2). Although
the treatment options for AF are continually improving, the
prevention of TEs remains one the primary goals for the long-term
treatment of patients with chronic AF. Therefore, improving the
clinical risk stratification of TEs, early evaluation of the risk
of developing TE among patients with AF, and the development of
individualized antiplatelet or anticoagulant therapies are
important strategies. However, the development of TEs involves a
number of factors. Recent studies in this area have hypothesized
that platelet activation is one of the most important factors.
Activated platelets have larger volumes and contain larger
quantities of vasoactive substances and prothrombotic factors.
Therefore, mean platelet volume (MPV) is a marker of platelet
activation and function, and may also be a response to inflammation
and thrombosis (3,4,22). Recent
studies (5,6,22) have shown
that smoking, hypertension, diabetes mellitus, dyslipidemia and
abdominal obesity are associated with a raised MPV. An increased
MPV is associated with overall cerebrovascular and cardiovascular
mortality rates, including those due to myocardial infarction,
transient ischemic attack (TIA) and cerebral infarction. However,
few studies have been conducted on the association between MPV and
AF, or their effect on the presence of TEs. The present study aimed
to evaluate the association between MPV and chronic AF, and their
effect on the presence of TEs. To the best of our knowledge, the
association between MPV and inflammatory markers, such as
high-sensitivity C-reactive protein (hsCRP)or markers of
thrombosis, such as D-dimer, in patients with chronic AF has not
been studied to date.
Patients and methods
Study design and patients
Consecutive patients who were hospitalized at the
Department of Cardiology and Neurology of the Third Xiangya
Hospital at Central South University (Changsha, China) were
referred to our center between November 2012 to July 2014. A total
of 172 consecutive participants (males, 51.2%; females, 48.8%; mean
age, 67.05±9.35) were enrolled. Study individuals were divided into
three groups: The AF+TE group (n=57, 33.1%):, which comprised
patients in AF complicated by the presence of TEs; The AF group
(n=57, 33.1%), which comprised patients in AF with no identifiable
TEs, as confirmed by brain computed tomography (CT),
transesophageal echocardiography (TEE), ultrasonic cardiogram,
magnetic resonance imaging with diffusion weighted imaging
(MRI+DWI), pulmonary vein imaging, or a combination of these
techniques; and a control group (58, 33.7%), which comprised
patients in sinus rhythm. The AF+TE group included 45 patients with
cerebral infarctions, seven patients with left atrium or left
atrial appendage thrombosis, three patients with both conditions,
and two patients with cerebral infarction and a venous thrombosis
in a lower extremity. These 114 patients were confirmed to be in
chronic AF by electrocardiography on at least two separate
occasions prior to recruitment and met the diagnostic criteria used
in the 2011 ACCF/AHA/HRS Guidelines for the management of patients
with AF (1).
All patients with cerebral infarction and TIA had
symptoms of cerebral ischemia for the first time, occurring within
6 months. The exclusion criteria were i) the presence of other
organic heart diseases, such as myocardial infarction, rheumatic
valvular heart disease, valvular heart disease, dilated
cardiomyopathy, chronic heart failure (grade III or IV according to
the New York Heart Association heart failure classification)
(7) and pulmonary heart disease; ii)
AF due to hyperthyroidism or alcoholic cardiomyopathy; iii)
hemorrhagic disease, such as cerebral hemorrhage and
gastrointestinal hemorrhage; iv) hematological disease, such as
severe anemia, thrombocytopenia or diseases of hematopoiesis; or v)
others factors, such as hepatic insufficiency (alanine transaminase
>3x normal upper limit), renal insufficiency (glomerular
filtration rate <30 ml/min/1.73 m2), an acute or
chronic systemic inflammatory state, connective tissue disease,
autoimmune disease or malignant tumors. Our Ethics Committee
approved the present study and all patients provided informed
consent.
Collection of clinical
information
Patient gender, age, smoking history, medication, as
well as history of hypertension, diabetes mellitus, hematological
disease and other related diseases, were recorded.
Blood collection and measurement of
biomarkers
Fasting blood samples of the patients were collected
within 2 h of admission. A total of 2–3 ml venous blood was
collected in an EDTA-K2 tube (Sysmex Corporation, Kobe, Japan), and
MPV and platelet counts were analyzed using a Sysmex XE5100
hematology analyzer (XE5100; Sysmex Corporation) within 2 h of
sample collection. The instrument accompanied the reagents:
Hemolytic agent, dilution solution and washing solution. The
manipulation was performed according to the manufacturer's
instructions. The normal values for MPV in our laboratory range
from 7.6 to 13.2 femtoliter (fl).
A total of 2 ml non-coagulated fasting blood samples
were collected, and low-density lipoprotein-cholesterol, high
sensitivity C-reactive protein (hsCRP) and creatinine were measured
using a Hitachi 7600-020 automatic biochemistry analyzer (Hitachi
Ltd, Tokyo, Japan). Serum hsCRP was determined using
immune-enhanced nephelometry. Hitachi provided all reagents.
D-dimer and fibrinogen (Fbg) quantification was performed using the
Sysmex CA-1500 via latex-enhanced nephelometry. Sysmex Corporation
provided all reagents.
Iconography analysis
Specialist physicians determined left ventricular
ejection fraction using standard methods with an HP-5500 color
Doppler ultrasound scanner at a 25-MHz probe frequency. The left
atrial diameter (LAD) and the presence of atrial thrombosis were
examined using TEE. Cloudy shadows in the left atrium or left
atrial appendage were assumed to represent thrombi.
TIA and cerebral infarction were determined
according to the 2013 AHA/ASA guidelines for the early management
of patients with acute ischemic stroke (8) and confirmed using brain CT (64-detector
CT; Siemens, Munich, Germany), MRI+DWI (Siemens) and cerebral
angiography at our hospital.
Calculation of CHADS2 score (9) and CHADS2 scoring (10)
All 114 patients who met the inclusion criteria
underwent CHADS2 scoring, where one point was assigned for
congestive heart failure, hypertension, age >75 years or
diabetes mellitus, and two points indicated cerebral infarction or
a history of TIA. The CHA2DS2-VASc score was calculated based on a
point system in which two points were assigned for a history of
stroke or TIA, or age ≥75 years. One point was assigned for age
between 65 and 74 years; a history of hypertension, diabetes,
recent cardiac failure or vascular disease (myocardial infarction,
complex aortic plaque or peripheral arterial disease); or female
gender.
Statistical analysis
Statistical analysis was performed using SPSS 18.0
(SPSS, Inc., Chicago, IL, USA). Continuous variables with a normal
distribution were expressed as the mean ± standard deviation, and
group comparisons were performed by one-way analysis of variance
(the F-test) and Kruskal-Wallis tests, depending on whether the
data was normally or non-normally distributed, respectively.
Non-normally distributed data are presented as the median
(interquartile range) and discrete variables are presented as
frequencies and percentages. Group comparisons were performed using
the χ2 test, Fisher's exact test or Wilcoxon rank-sum
test. Correlation analyses of non-normally distributed data were
performed using Spearman's rank correlation. To assess for
significant differences between the three groups, Tukey's post hoc
test was used to determine the intergroup differences, using
log-transformed data where appropriate. The diagnostic performance
of each indicator was evaluated using the receiver operating
characteristic (ROC) curve. The correlation of clinical variables
associated with TEs were analyzed using univariate and multivariate
logistic regression analysis. The P-value for entry stepwise
multivariate linear regression analysis was set at 0.05, while the
P-value for removal was set 0.10. P<0.05 was considered to
indicate a statistically significant difference.
Results
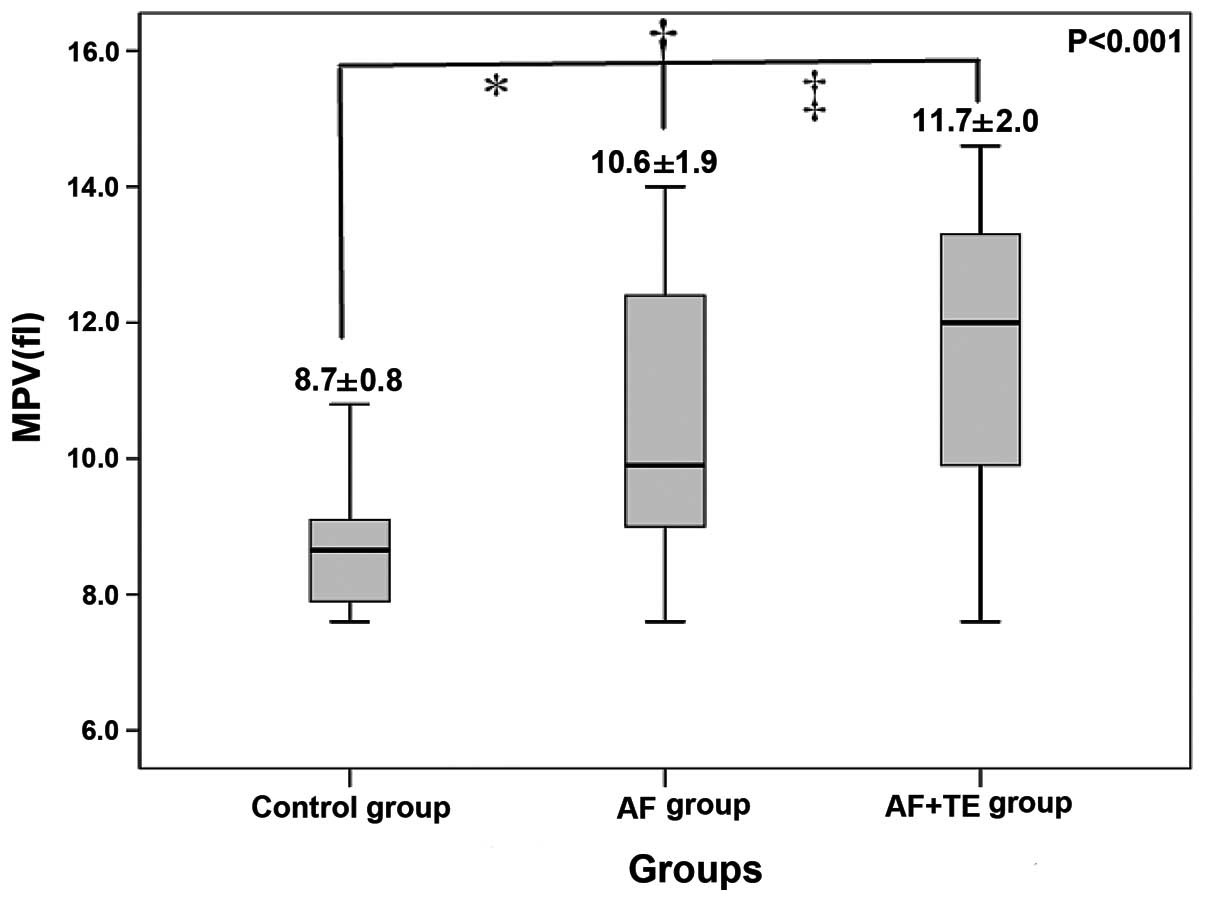
Baseline clinical characteristics, laboratory
examination results and MPV levels are shown in Tables I and II,
and Fig. 1. The levels of hsCRP, Fbg
and D-dimer in the AF+TE group were significantly higher than those
in the AF and the control groups. In addition, patients in the
AF+TE group exhibited a higher MPV and left atrial volume than
patients in the other two groups (Fig.
1).
 | Table I.Baseline characteristic of patients
with AF and control subjects. |
Table I.
Baseline characteristic of patients
with AF and control subjects.
| Variable | Control group,
n=58 | AF group, n=57 | AF+TE group,
n=57 | P-value |
|---|
| Age, years | 67.00±8.62 |
65.19±10.00 |
68.95±9.17 | 0.100 |
| LVEF (%) | 65.00±4.66 |
64.58±5.00 |
63.95±4.43 | 0.484 |
| Male (%) |
29
(50) | 29 (50.9) | 30 (52.6) | 0.960 |
| Smoking (%) | 22 (37.9) | 22 (38.6) | 14 (24.6) | 0.201 |
| Hypertension (%) | 28 (48.3) | 33 (57.9) | 38 (66.7) | 0.136 |
| DM (%) | 23 (39.7) | 20 (35.1) | 19 (33.3) | 0.766 |
| Medications |
|
|
|
| Aspirin
(%) | 9
(15.5) | 29 (50.9) | 28 (49.1) | 0.012a,b |
| Statins
(%) | 17 (29.3) | 17 (29.8) | 13 (22.8) | 0.560 |
| ACEI/ARB
(%) | 26 (44.8) | 23 (40.4) | 21 (36.8) | 0.683 |
| CCB
(%) | 22 (37.9) | 15 (26.3) | 18 (31.6) | 0.409 |
| β-Blocker
(%) | 18 (31.0) | 32 (56.1) | 29 (50.9) | 0.017a -c |
| Warfarin
(%) | 0 (0) | 14 (24.6) | 6 (10.5) | 0.000a -c |
| CHADS2 score | – | 1 (0–2) | 3 (3–4) | 0.000c |
| CHA2DS2-VASc
score | – | 2 (1–4) | 4 (2–6) | 0.000c |
| AF duration
(months) | – | 40.0 (12.0–68.0) | 42.0 (14.0–72.0) | 0.712 |
 | Table II.Comparison of laboratory examination
results between the three groups. |
Table II.
Comparison of laboratory examination
results between the three groups.
| Variable | Control group,
n=58 | AF group, n=57 | AF+TE group,
n=57 | P-value |
|---|
| Hb
(x1012/l) | 146±11 | 145±14 | 146±13 | 0.983 |
| PLT
(x109/l) | 209±41 | 205±31 | 206±42 | 0.907 |
| Cr (µmol/l) | 83±11 | 83±8 | 84±10 | 0.822 |
| hsCRP (mg/l) | 1.66±0.89 | 2.39±0.75 | 2.88±0.66 | 0.000a -c |
| LDL-c (mmol/l) | 2.55±0.64 | 2.66±0.57 | 2.64±0.67 | 0.604 |
| Fbg (g/L) | 2.62±0.50 | 3.64±0.89 | 3.68±0.62 | 0.000a,b |
| D-dimer (µg/l) | 97 (90–110) | 374 (289–481) | 402 (285–504) | 0.000a -c |
| LAD (mm) | 31.0
(29.0–34.0) | 36.0
(32.0–42.5) | 44.0
(37.0–50.0) | 0.000a -c |
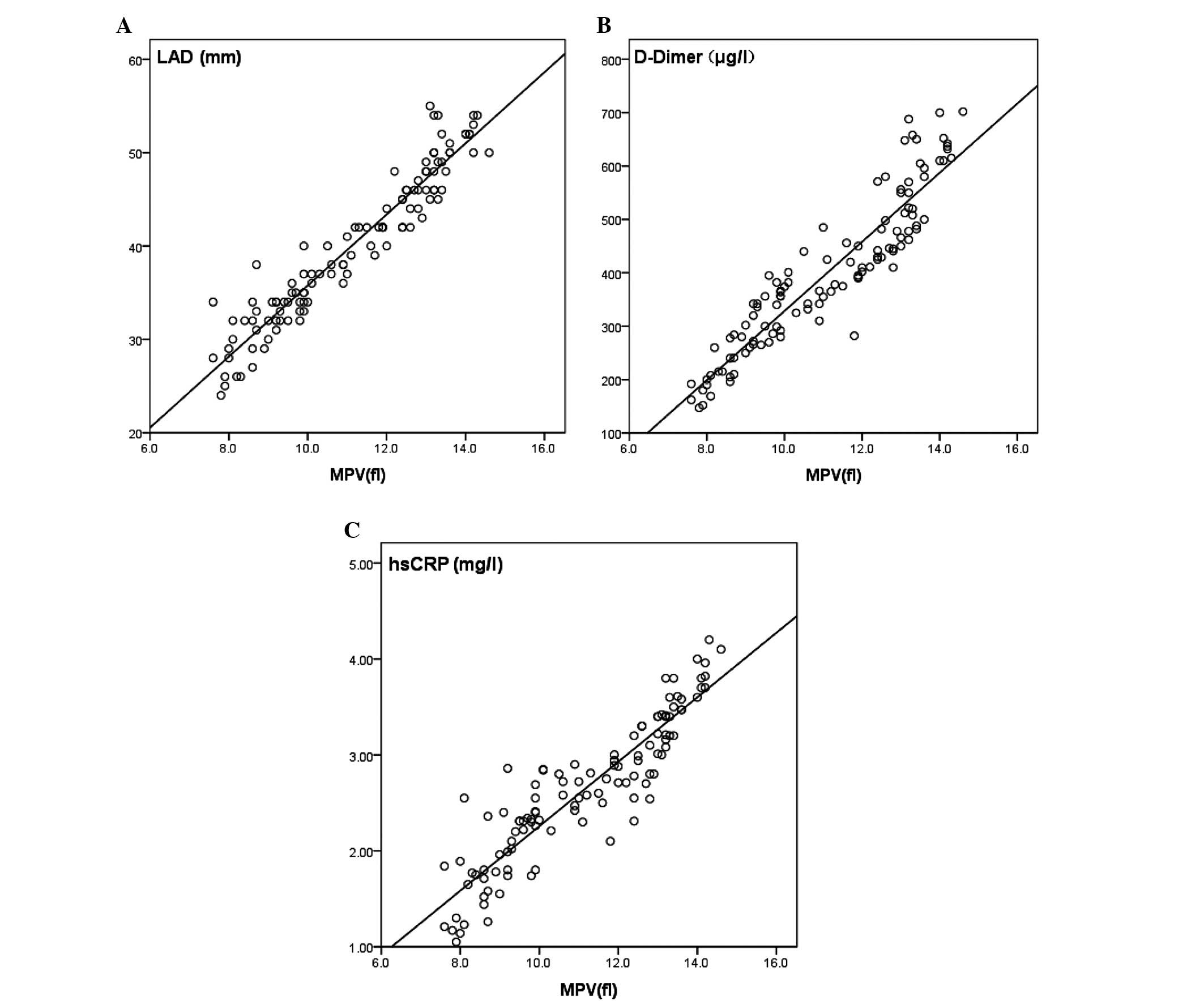
Spearman's correlation coefficients between MPV
levels, and LAD, D-dimer levels and hsCRP levels, among patients
with AF were r=0.960 (P<0.05), r=0.896 (P<0.05) and r=0.924
(P<0.01), respectively. MPV was positively correlated with LAD,
D-dimer and hsCRP [the standardized partial regression coefficient
(β) was 0.926, 0.905 and 0.762, respectively; the correlation
coefficient (b) was 3.589, 80.966 and 0.341, respectively]. Using
MPV as the independent variable, the regression equations between
MPV levels, and LAD, D-dimer and hsCRP are shown in Fig. 2.
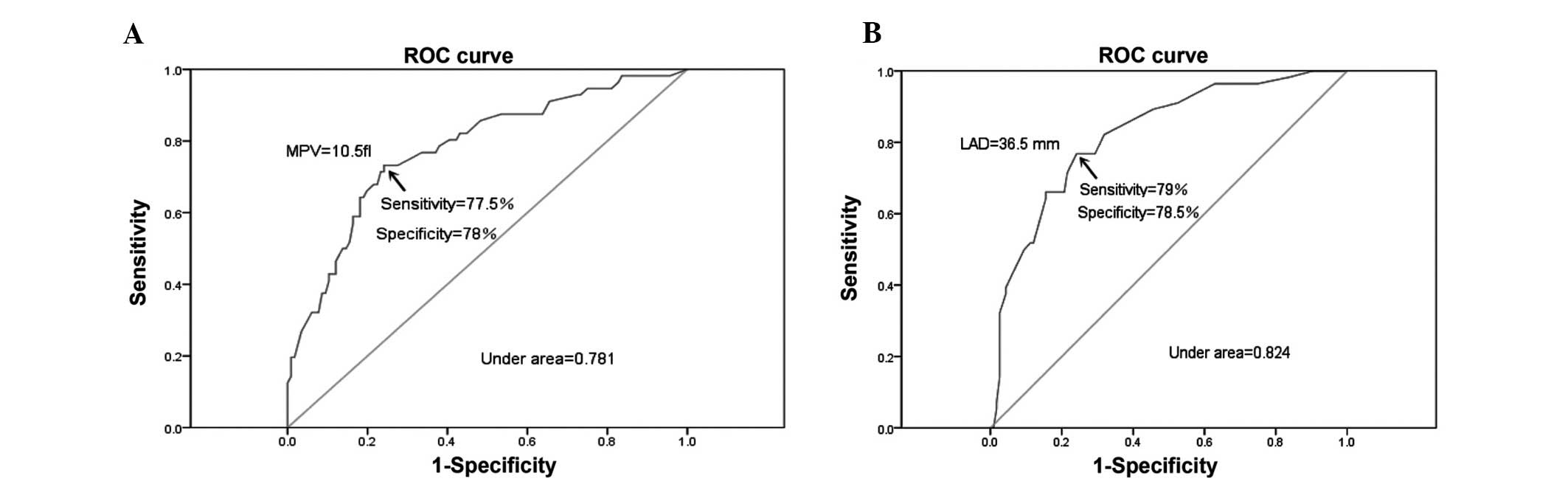
The ROC analysis demonstrated a cut-off value of
MPV>10.5 f1 to predict the presence of TEs. At this level,
sensitivity was 77.5% [95% confidence interval (CI), 58.7–91.2] and
specificity was 78% (95% CI: 60.2–89.5). Area under the curve
(AUC)=0.781; 95% CI, 0.706–0.855; P=0.005. The value for LAD
required to detect TEs with a sensitivity of 79% (95% CI,
52.6–91.7) and a specificity of 78.5% (95% CI, 54.7–90.1) was 36.5
mm. AUC=0.824; 95% CI, 0.701–0.868; P=0.018 (Fig. 3A and B).
Univariate analysis demonstrated that age ≥75 years,
MPV >10.5 fl, LAD >36.5 mm, hsCRP >2.5 mg/l and D-Dimer
>306 µg/l, as well as CHA2DS2-VASc score ≥2 were significantly
correlated with the presence of TEs (Table III). These variables were entered
into a multivariate logistic regression model and MPV was found to
be significantly associated with the presence of TEs (odds ratio
3.1; 95% CI, 1.6–5.1; P=0.000; Table
III).
 | Table III.Univariate and multivariate analysis
of risk factors for thrombotic events in chronic atrial
fibrillation. |
Table III.
Univariate and multivariate analysis
of risk factors for thrombotic events in chronic atrial
fibrillation.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value |
|---|
| Age ≥75 years | 2.9 | 1.7∼4.7 | 0.000 | 2.1 | 1.2∼3.7 | 0.011 |
| Male gender | 1.4 | 0.7∼2.4 | 0.187 | – | – | – |
| Smoking | 0.67 | 0.08∼3.42 | 0.649 | – | – | – |
| DM | 2.35 | 0.74∼6.81 | 0.106 | – | – | – |
| Hypertension | 1.12 | 0.31∼2.87 | 0.844 | – | – | – |
| Using warfarin | 1.55 | 1.02∼2.35 | 0.039 | 1.04 | 0.96∼1.13 | 0.281 |
| MPV >10.5
fl | 3.70 | 2.2∼6.3 | 0.000 | 3.1 | 1.6∼5.1 | 0.000 |
| LAD >36.5
mm | 3.3 | 1.8∼5.7 | 0.000 | 3.2 | 1.8∼5.4 | 0.000 |
| hsCRP >2.5
mg/l | 2.9 | 1.7∼4.8 | 0.000 | 2.1 | 1.2∼3.7 | 0.011 |
| D-dimer >306
µg/L | 2.7 | 1.5∼4.5 | 0.000 | 1.9 | 1.1∼3.4 | 0.039 |
| CHA2DS2-VASc
score≥2 | 4.21 | 1.4∼9.6 | 0.039 | 5.33 | 1.3∼14.7 | 0.011 |
Discussion
As an indicator measured in routine blood samples,
MPV is a low-cost, rapid and low-risk test. Bath et al
(11)studied 3,134 patients who had
experienced a stroke, with an average follow-up time of 3.9 years.
The authors showed that MPV levels were positively correlated with
the risk of stroke. When MPV levels increased by 1 fl, the risk of
stroke increased by 11% (95% CI, 3–19%). The results of the current
study showed that the difference among the three groups of patients
was significant (P<0.05). Specifically, pairwise comparisons
between these groups revealed a significant difference (P<0.05).
These results are similar to those of Yuce et al (12), which indicates that the MPV levels of
patients in AF are higher than those of individuals in sinus
rhythm. Patients in the AF+TE group were in a hypercoagulable
state. Thus, their platelet volumes were larger, and the platelet
activity and aggregation were stronger. Recent studies (13) have shown that platelets with increased
volume exhibit a significantly increased expression of platelet
surface CD62P and CD63 molecules. As the platelet volume increases,
the dense granules and α-granules within these cells also increase.
The quantity of 5-hydroxytryptamine and β-thromboglobulin released
by the dense granules, and the coagulation factors and von
Willebrand (vW) factor released by the α-granules, are also
increased, which may facilitate the formation of thrombi in blood
vessels and continue to increase the volume of existing thrombi,
thereby eventually occluding the affected blood vessels. In
addition, platelets with large volumes have a relatively larger
contact surface. Therefore, they are able to rapidly interact with
adenosine diphosphate, the collagen receptor and the vW factor
receptor on cell membranes. Furthermore, platelets with increased
volumes express higher quantities of adhesion molecules, such as
P-Selectin, which increases the aggregation and adhesion function
of platelets. AF in combination with thrombosis, may consume
platelets and cause a transient decrease in the numbers of these
cells (14). Through α-granule
proteins, this decrease in platelets may stimulate the
proliferation of megakaryocytes in the bone marrow, increase the
conversion rate of platelets and increase the number of platelets
with large volumes in the peripheral blood via negative feedback
(15). The current study also showed
that the total platelet count of patients in the AF+TE group was
higher than that in patients in the AF group, which may be due to
the high turnover rate of platelets or the transient reduction in
platelets among patients with AF complicated by TEs. These results
are in accordance with those of Varol (16).
Alhaji et al (17) calculated the left atrial volume index
by measuring LAD, and found that it was an independent risk factor
for stroke. Beinart et al (18)
performed studies investigating the prevention of stroke in
patients with AF, and demonstrated that the left atrial appendage
dimension predicted the development of thromboembolic
complications. Atrial wall movement disorders, induced by the
expansion of the left atrium, cause derangement of the direction of
atrial blood flow, decreased flow speed and blood stasis, and
frequent collision between platelets, thereby producing abnormal
hemodynamic characteristics, injuring the myocardium and vascular
endothelial cells, activating the exogenous coagulation system via
the exposure of subendothelial collagen, and activating platelets.
Therefore, Providencia et al (19) hypothesized that the increase in MPV is
associated with the expansion of the left atrium and blood stasis.
The above factors may result in the overactivation of platelets and
enhance the function of coagulation substances. The coagulation
factor 1, Fbg, is directly involved in the coagulation process, and
is associated with platelet aggregation. An increased level of this
molecule in the plasma is therefore an important risk factor for
the development of thromboembolism. D-dimer is produced by the
fibrinolysis of cross-linked fibrin proteins. An increase in
D-dimer concentration also indicates an enhancement of coagulation.
Both molecules are important indicators of a prothrombotic state
(PTS) (20).
In addition to platelet activation and the
hemodynamic response, the development of TEs is also associated
with inflammation. Although the mechanism of inflammation in AF
remains unclear, researchers have found that CRP and interleukin-6
(IL-6) appear to be involved in PTS (21). The results of a study by Gasparyan
et al (22) suggested a
correlation between an increase in MPV and the risk of thrombosis.
Specifically, thrombopoietin and high levels of inflammatory
factors, such as IL-1, IL-6 and tumor necrosis factor-α, may be
involved in thrombosis or a PTS. Conway et al (23) found increased plasma levels of IL-6 and
CRP, and raised plasma viscosity in patients with AF compared with
healthy controls, and showed that plasma IL-6 levels were
significantly higher among patients with AF at high risk of stroke.
Further research (24,25) has demonstrated that an increased CRP,
and an activated platelet and coagulation/fibrinolytic system, are
involved in the pathogenesis of cerebral infarction and are
positively correlated with known risk factors for the development
of emboli in patients with AF. Furthermore, CRP levels were
increased in patients with AF who had a history of embolism. A
number of recent studies have confirmed that statins regulate
lipids and stabilize plaques, and also exhibit anti-inflammatory
and anti-oxidative functions (26).
The measurement of LAD, D-dimer and hsCRP requires additional time
in the assessment of patients with AF. Furthermore, the procedure
is complicated, it incurs a relatively high cost and the
controllability of the results is undesirable. However, the
development of TEs appears to be correlated with MPV levels.
Therefore, MPV may be a useful auxiliary indicator of the risk of
developing TEs in patients with AF.
A number of studies have investigated MPV levels in
stroke patients, such as that conducted by Turfan et al
(27), and have found that high MPV
values are associated with prognosis in patients who have had a
stroke. Numerous studies (17,18) have shown that an increase of LAD is
associated with the development of TEs. Similar results were found
in the present study: Patient in the AF+TE group exhibited higher
MPV values than those in the control group and the AF group. In
patients with elevated MPV values (>10.5 fl), the risk of TEs
was increased by ∼three-fold. The risk of TEs is also increased in
patients with a high MPV value, in particular in those ≥75 years,
in patients with left atrial enlargement (LAD >36.5 mm) and in
those with a CHA2DS2-VASc score ≥2. However, the current guidelines
for primary risk assessment to prevent stroke in patients with
non-valvular AF, that is, CHADS2 or CHA2DS2-VASc scoring, do not
include LAD or MPV as risk factors. The results of the current
study suggest that additional markers, such as MPV and LAD, have a
predictive value for assessing the risk of TEs in patients with
AF.
In conclusion, MPV levels were associated with AF
and TEs. The measurement of MPV is simple, convenient and
inexpensive. Therefore, MPV may be useful for indicating the risk
of TE in patients with AF, and for improving the stratification of
risk factors. MPV detection may also be used to guide the
prescription of anticoagulation treatments in patients with AF.
The present study had a small sample size and was
also a retrospective study. In addition, the effects of
antiplatelet and anticoagulant drugs on MPV remain uncertain. Thus,
it may be that MPV levels increased following the occurrence of TE
events, possibly as a result of the initiation of different
medications. Furthermore, the primary limitation was the long
period (2012–2014) over which patients were recruited, which
diminishes the significance of conclusions. Therefore, additional
clinical studies are required in order to assess the usefulness of
MPV as a predictive factor for the development of TEs in patients
with AF.
Acknowledgements
This study was supported by grants from the Science
and Technology Plan (grant no. 2014FJ3097) of the Science and
Technology Bureau (Hunan, China).
References
|
1
|
Fuster V, Rydén LE, Cannom DS, et al: 2011
ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006
Guidelines for the management of patients with atrial fibrillation:
a report of the American College of Cardiology Foundation/American
Heart Association Task Force on Practice Guidelines developed in
partnership with the European Society of Cardiology and in
collaboration with the European Heart Rhythm Association and the
Heart Rhythm Society. J Am Coll Cardiol. 57:e101–e198. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wilke T, Groth A, Mueller S, et al:
Incidence and prevalence of atrial fibrillation: an analysis based
on 8.3 million patients. Europace. 15:486–493. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ulasli SS, Ozyurek BA, Yilmaz EB and
Ulubay G: Mean platelet volume as an inflammatory marker in acute
exacerbation of chronic obstructive pulmonary disease. Pol Arch Med
Wewn. 122:284–290. 2012.PubMed/NCBI
|
|
4
|
Braekkan SK, Mathiesen EB, Njølstad I, et
al: Mean platelet volume is a risk factor for venous
thromboembolism: the Tromsø study, Tromsø, Norway. J Thromb
Haemost. 8:157–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vizioli L, Muscari S and Muscari A: The
relationship of mean platelet volume with the risk and prognosis of
cardiovascular diseases. Int J Clin Pract. 63:1509–1515. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Papanas N, Symeonidis G, Maltezos E, et
al: Mean platelet volume in patients with type 2 diabetes mellitus.
Platelets. 15:475–478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hunt SA, Baker DW, Chin MH, et al: ACC/AHA
guidelines for the evaluation and management of chronic heart
failure in the adult: executive summary a report of the American
College of Cardiology/American Heart Association Task Force on
Practice Guidelines (Committee to revise the 1995 guidelines for
the evaluation and management of heart failure): Developed in
collaboration with the International Society for Heart and Lung
Transplant; endorsed by the Heart Failure society of America.
Circulation. 104:2996–3007. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jauch EC, Saver JL, Adams HP Jr, et al:
Guidelines for the early management of patients with acute ischemic
stroke: a guideline for healthcare professionals from the American
Heart Association/American Stroke Association. Stroke. 44:870–947.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gage BF, Waterman AD, Shannon W, Boechler
M, Rich MW and Radford MJ: Validation of clinical classification
schemes for predicting stroke: results from the National Registry
of Atrial Fibrillation. JAMA. 285:2864–2870. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lip GY, Nieuwlaat R, Pisters R, Lane DA
and Crijns HJ: Refining clinical risk stratification for predicting
stroke and thromboembolism in atrial fibrillation using a novel
risk factor-based approach: the euro heart survey on atrial
fibrillation. Chest. 137:263–272. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bath P, Algert C, Chapman N and Neal B:
Progress Collaborative Group: Association of mean platelet volume
with risk of stroke among 3134 individuals with history of
cerebrovascular disease. Stroke. 35:622–626. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuce M, Cakici M, Davutoglu V, et al:
Relationship between mean platelet volume and atrial thrombus in
patients with atrial fibrillation. Blood Coagul Fibrinolysis.
21:722–725. 2010.PubMed/NCBI
|
|
13
|
Choudhury A, Chung I, Blann AD and Lip GY:
Platelet surface CD62P and CD63, mean platelet volume and
soluble/platelet P-selectin as indexes of platelet function in
atrial fibrillation: a comparison of healthy control subjects and
disease control subjects in sinus rhythm. J Am Coll Cardiol.
49:1957–1964. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nadar SK, Lip GY and Blann AD: Platelet
morphology, soluble P selectin and platelet P-selectin in acute
ischaemic stroke. The West Birmingham Stroke Project. Thromb
Haemost. 92:1342–1348. 2004.PubMed/NCBI
|
|
15
|
Tekin G, Tekin YK, Sivri N and Yetkin E:
Mean platelet volume in patients with nonvalvular atrial
fibrillation. Blood Coagul Fibrinolysis. 24:537–539. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Varol E: Increased thrombopoietin and mean
platelet volume in patients with ischemic stroke. Clin Appl Thromb
Hemost. 19:342–343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alhaji M, Raju M, Al Darazi F, Kakkar A,
Olstein R, Prabhakaran S and Doukky R: Diastolic dysfunction and
left atrial volume mediates embolic stroke in patients with atrial
fibrillation. Stroke. 45 (Suppl 1):ATP1802014.
|
|
18
|
Beinart R, Heist EK, Newell JB, et al:
Left atrial appendage dimensions predict the risk of stroke/TIA in
patients with atrial fibrillation. J Cardiovasc Electrophysiol.
22:10–15. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Providencia R, Faustino A, Paiva L, et al:
Mean platelet volume is associated with the presence of left atrial
stasis in patients with non-valvular atrial fibrillation. BMC
Cardiovascular Disord. 13:402013. View Article : Google Scholar
|
|
20
|
Erdogan D, Uysal BA, Aksoy F, et al:
Strict heart rate control attenuates prothrombotic state and
platelet activity in patients with non-valvular permanent atrial
fibrillation. Clin Hemorheol Microcirc. 56:219–229. 2014.PubMed/NCBI
|
|
21
|
Chung MK, Martin DO, Sprecher D, et al:
C-reactive protein elevation in patients with atrial arrhythmias:
inflammatory mechanisms and persistence of atrial fibrillation.
Circulation. 104:2886–2891. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gasparyan AY, Ayvazyan L, Mikhailidis DP
and Kitas GD: Mean platelet volume: a link between thrombosis and
inflammation? Curr Pharm Des. 17:47–58. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Conway DS, Buggins P, Hughes E and Lip GY:
Relationship of interleukin-6 and C-reactive protein to the
prothrombotic state in chronic atrial fibrillation. J Am Coll
Cardiol. 43:2075–2082. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tohgi H, Konno S, Takahashi S, et al:
Activated coagulation/fibrinolysis system and platelet function in
acute thrombotic stroke patients with increased C-reactive protein
levels. Thromb Res. 100:373–379. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sohara H, Amitani S, Kurose M and Miyahara
K: Atrial fibrillation activates platelets and coagulation in a
time-dependent manner: a study in patients with paroxysmal atrial
fibrillation. J Am Coll Cardiol. 29:106–112. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pinho-Gomes AC, Reilly S, Brandes RP and
Casadei B: Targeting inflammation and oxidative stress in atrial
fibrillation: role of 3-hydroxy-3-methylglutaryl-coenzyme a
reductase inhibition with statins. Antioxid Redox Signal.
20:1268–1285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Turfan M, Erdogan E, Ertas G, et al:
Usefulness of mean platelet volume for predicting stroke risk in
atrial fibrillation patients. Blood Coagul Fibrinolysis. 24:55–58.
2013. View Article : Google Scholar : PubMed/NCBI
|