Introduction
The articular cartilage of the temporomandibular
joint (TMJ) is composed of fibrocartilage, and undergoes
endochondral ossification during the active growth period (1). TMJ osteoarthritis (OA) is typically a
slowly progressive, asymmetric disease resulting in the destruction
of articular tissues, and presents as functional pain, crepitus or
multiple joint noises. Recent histological and biochemical studies
of TMJ OA have provided evidence of breakdown of the fibrocartilage
covering the articular surfaces of the mandibular condyle and
temporal bone; however, the pathogenesis and natural progression of
TMJ OA remain unclear. Several in vivo experimental animal
models have been developed to study TMJ OA, including
age-accelerated mice (2) and
transgenic mice (3); however, there
are no studies of TMJ OA in naturally occurring mice.
The STR/ort mouse often develops spontaneous OA of
the medial tibial cartilage of the knee joint, and is a useful
model for studying the pathogenesis of knee OA (4). The histopathological lesions of knee OA
in STR/ort mice are progressive and closely resemble those of human
knee OA. Eighty-five percent of STR/ort mice have histological OA
lesions in the medial tibial cartilage by 35 weeks of age. However,
there have been no studies regarding OA lesions in the TMJ of
STR/ort mice to date.
To investigate the occurrence of OA and histological
changes in the TMJ of STR/ort mice, the changes in the articular
cartilage were examined using histological and microcomputed
tomography (micro-CT) analyses.
Materials and methods
Mice
All the experimental procedures in the animal
experiment were approved by the ethical committee for the
guidelines on animal experiments of Tsurumi University, School of
Dental Medicine (Yokohama, Kanagawa, Japan), Sagamihara National
Hospital (Sagamihara, Kanagawa, Japan) and Nippon Dental
University, School of Dentistry (Tokyo, Japan).
Thirty-two male STR/ort mice aged between 4 and 60
weeks were obtained from Charles River Laboratories, Inc.
(Yokohama, Japan). The STR/ort mice were 30 (n=3), 40 (n=10), 50
(n=15) and 60 (n=4) weeks of age. Six age-matched male CBA/JN mice,
which showed no evidence of histological OA lesions, were used as
controls. The mice were housed ≤5/cage, with a 12:12-h light:dark
cycle and with ad libitum access to standard mouse chow and
water, ≤15 months of age. STR/ort mice were euthanized at 30, 40,
50, 60 or 70 weeks of age under diethyl ether anesthesia. The body
weights of all the mice were recorded prior to euthanasia.
Histology
After euthanasia at 30 to 60 weeks of age, the
STR/ort (n=32) and CBA/JN (n=6) mice were bisected at the level of
the mandibular symphysis and stored in 70% ethanol. The mandible
was fixed in 4% paraformaldehyde in 0.1 M phosphate-buffered saline
(PBS) at pH 7.4 for 24 h at 4°C. Samples were rinsed with PBS,
followed by decalcification in 10% EDTA for 8 weeks. Following
dehydration with a graded ethanol series, the samples were passed
through xylene and embedded in paraffin. The embedded condylar head
samples were serially sectioned in frontal, sagittal and horizontal
planes to produce 4-µm sections, which were deparaffinized. The
serial sections were stained with hematoxylin and eosin (H&E),
toluidine blue and tartrate-resistant acid phosphatase (TRAP) to
observe the osteoid existence produced or newly produced by the
osteoblasts.
Micro-CT
Three-dimensional morphometric analysis of the
subchondral bone in the mandibular condylar heads was performed
using two micro-CTs; R-mCT (Rigaku Co., Tokyo, Japan) and Latheta
LCT-200 (Hitachi-Aloka, Tokyo, Japan). Three-dimensional
reconstruction of the mandibular condyles was performed using a
TRI-BON system (Ratoc System Engineering, Co., Ltd., Tokyo,
Japan).
Results
Histological evaluation of mandibular
condyles in CBA/JN and STR/ort mice
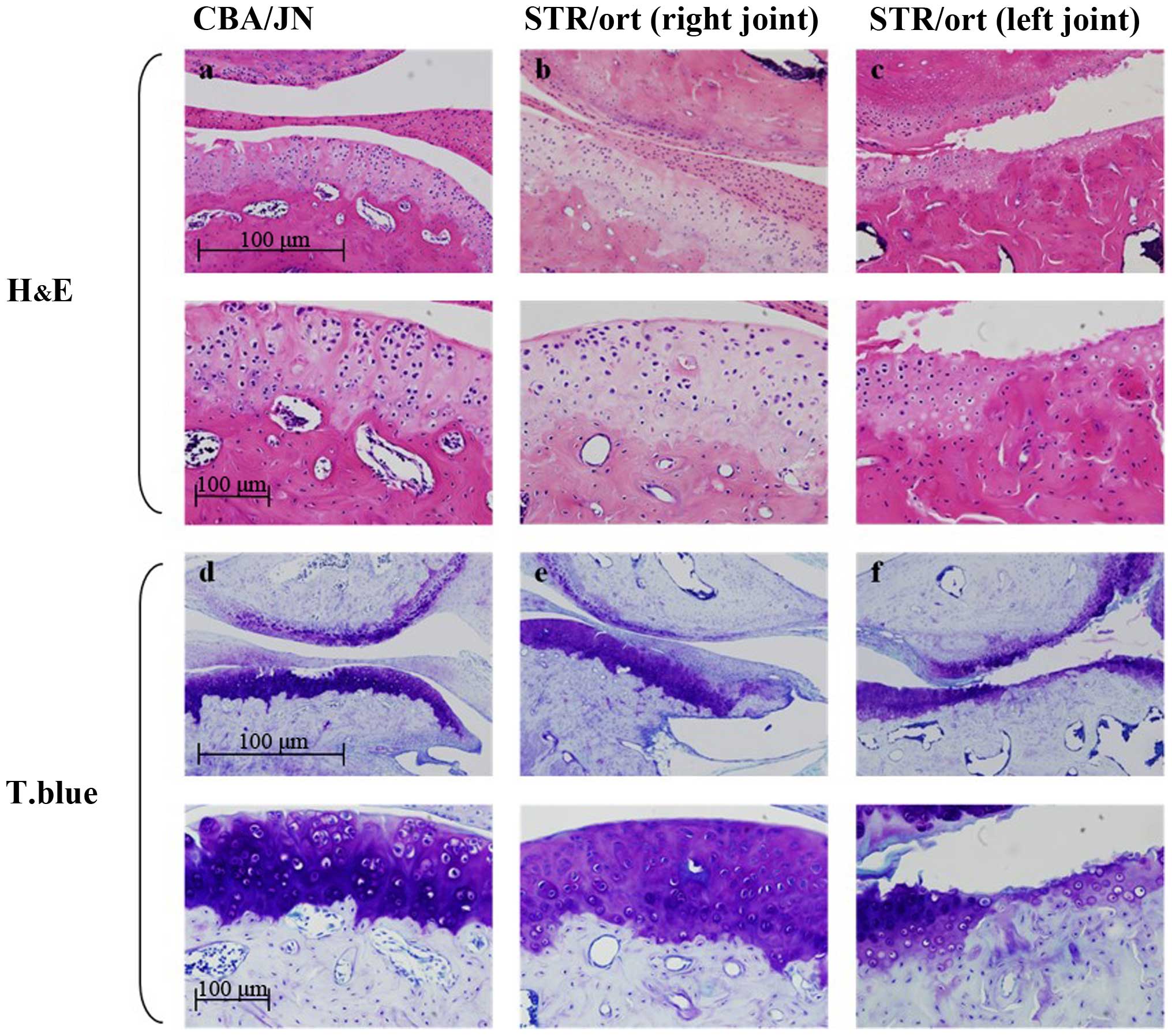
The control CBA mouse condyles were covered by
fibrocartilage with distinct cellular layers: Fibrous,
prechondroblastic and cartilaginous layers, and subchondral bone
(Fig. 1a). By contrast, 17 of the 25
(68%) edematous 40- and 50-week-old STR/ort mice had unilateral
changes in the mandibular condyles. There was an exposure of the
differentiation and proliferation cartilage layer, the
fibrocartilage layer was lost, and the contour of the subchondral
bone was irregular (Fig. 1b).
Furthermore, the articular cartilage layer was damaged, clear
boundaries between cartilage layers were lost, and the arrangement
of chondrocytes was more irregular compared to the control mice.
There were no signs of inflammation or granular tissues in any
tissue specimen from either the CBA/JN or the STR/ort mice
(Fig. 1b).
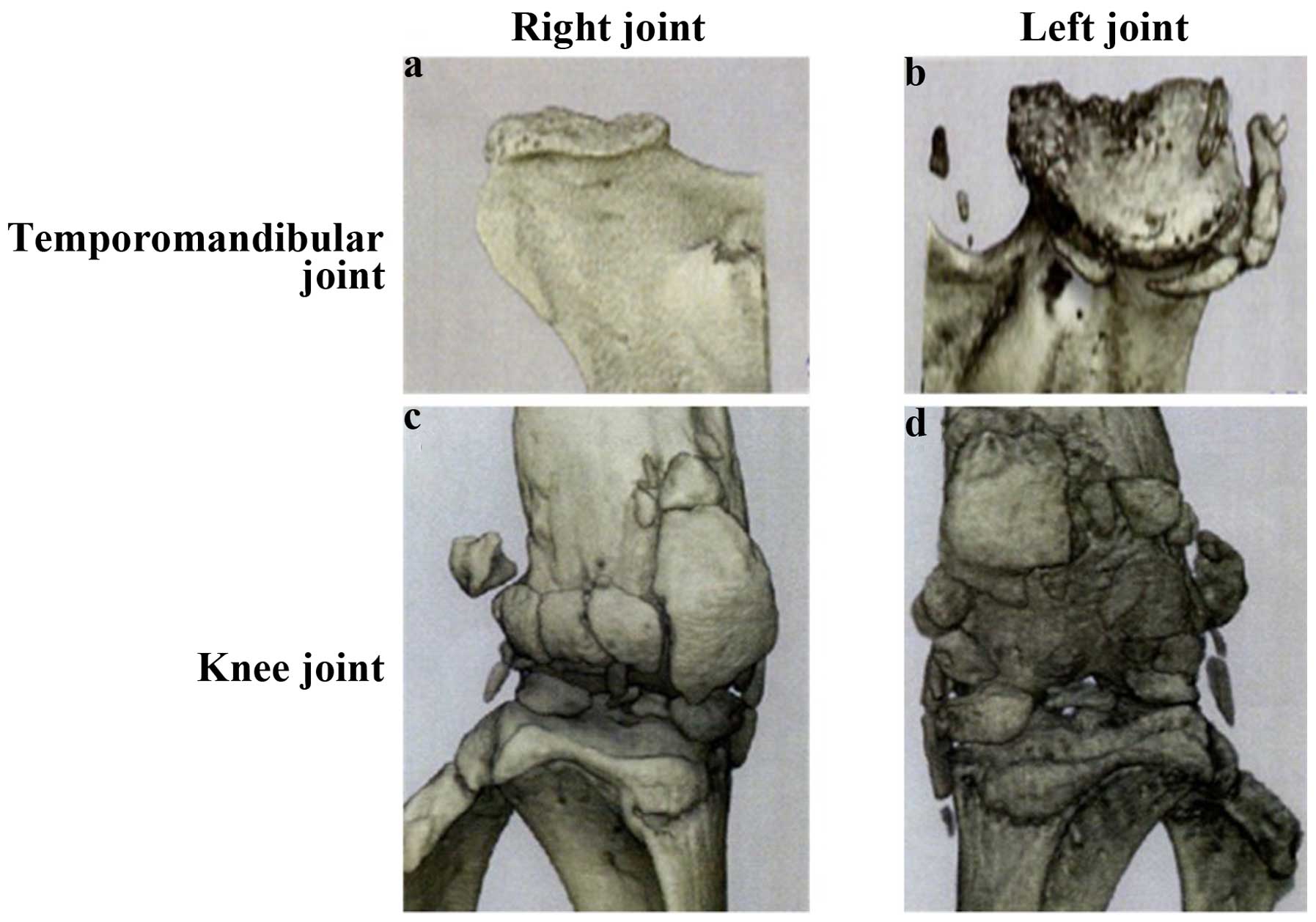
Changes in the microarchitecture of
subchondral TMJ bone with OA
Osteoarthritis-induced changes over time in the
microarchitecture of the subchondral bone of the mandibular condyle
were evaluated by micro-CT analysis. No significant differences
were identified at 30 weeks between CBA and STR/ort mice in any of
the micro-CT parameters examined. However, at 40 weeks,
osteoarthritic morphological changes and structural alterations
were observed in the STR/ort mice compared with the CBA controls
(Fig. 2). The articular cartilage was
thinner in STR/ort mice than that in the CBA mice. There were no
significant differences in the fibrous or prechondroblastic layers
between CBA and STR/ort mice.
Toluidine blue and TRAP staining of
knee joints and mandibular condyles in STR/ort mice
The TMJ and knee joint sections obtained from 40-
and 50-week-old STR/ort mice were stained with toluidine blue. In
the STR/ort mice, there was less staining in the affected OA
condyles due to the fewer chondrocyte numbers. Some metachromasia
in the free bodies with toluidine blue staining in the superior
joint space were also identified (Fig.
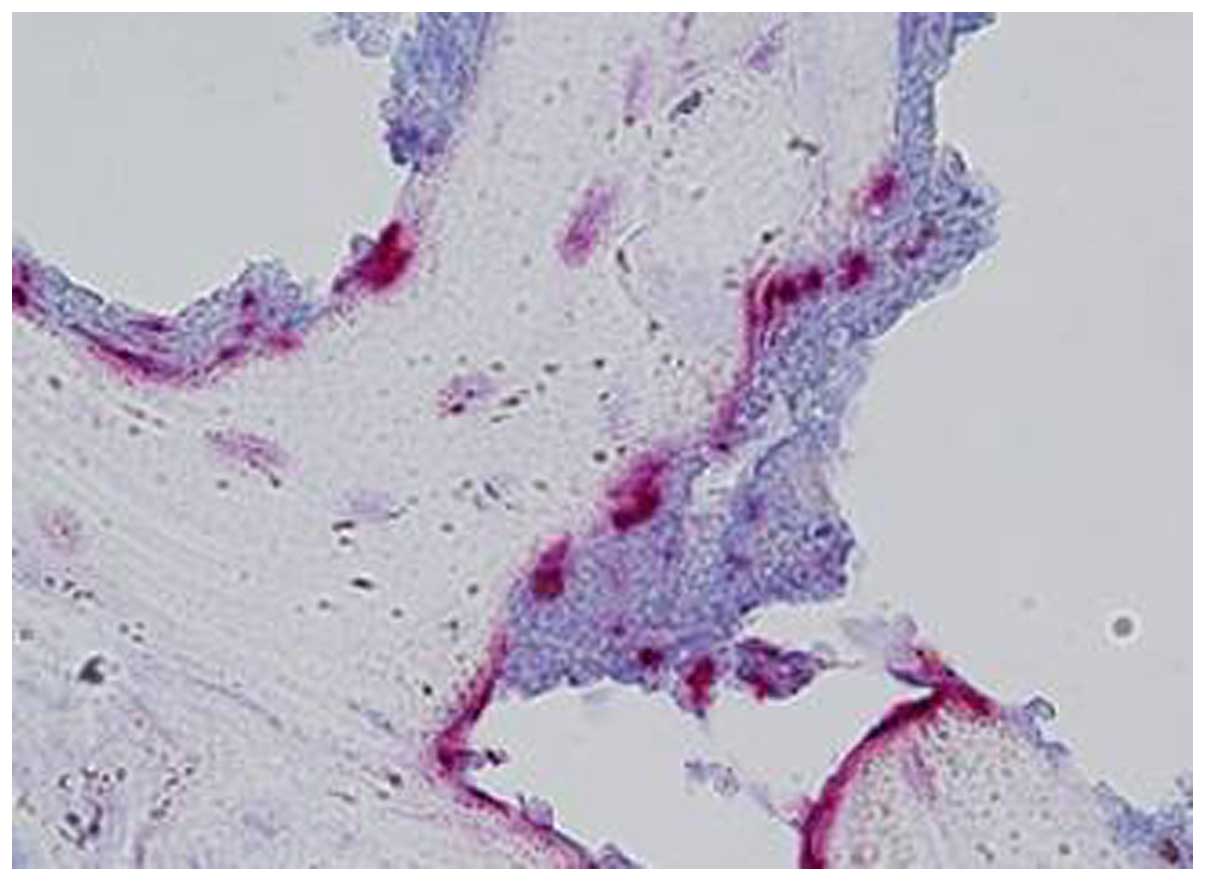
1). Additionally, multinucleated TRAP+
osteoclast-like cells were detected at the erosive front of the
condyle, which is localized between the pannus and the bone surface
(Fig. 3).
Discussion
The STR/ort mouse strain appears to develop
spontaneous OA-like lesions in the TMJ. To the best of our
knowledge, this is the first study of the histomorphometric
characteristics of OA changes in the TMJ of STR/ort mice. At the
age of 40 weeks, STR/ort mice often have loss of articular
cartilage on histology, with cavitation and erosion of the exposed
bone and gradual changes in condylar shape.
The initial joint pathology caused by OA is
difficult to characterize in humans as OA is often diagnosed in the
late stages of the disease. Animal models of OA can facilitate the
understanding of the early pathology of OA, including loss of
proteoglycans and fibrillations. Animal models of induced OA
(5–8) of
genetically modified mice (9,10) and of spontaneously occurring OA
(11,12)
have potential physiological relevance to the OA that develops
spontaneously in humans.
STR/ort mice, an inbred sub-strain of STR/N mice,
are a valuable animal model of spontaneous OA (13). Previous studies have suggested two
possible etiologies for OA in the knee joints of male STR/ort mice:
Biomechanically-induced OA (due to patellar luxation) and
spontaneous OA caused by a lack of keratin sulfate, a change in
proteoglycans. The spontaneous obesity of the strain suggests that
excess body weight, or a factor associated with it, may play a role
in the accelerated development of OA in this strain. However, the
lack of correlation between OA and naturally varying or
diet-altered body weight suggests that body weight alone is not a
causal factor in the strain's spontaneous development of OA
(13–15). The presence of degenerative changes in
the TMJ of STR/ort mice suggests that the basis of the disease is
systemic (16).
The TMJ is a diarthrodial joint similar to other
load-bearing articular joints. Unlike the majority of diarthodial
joints, in which the articular surfaces are covered by hyaline
cartilage, those of the mandibular condyles are covered by
fibrocartilage. Among the earliest molecular events involved in the
pathogenesis of TMJ OA are the disruption of the collagenous
components of the fibrocartilage and the subsequent loss of
proteoglycans and glycosaminoglycans. The loss of these molecules
eventually leads to articular tissues that lack resiliency to the
compressive and shearing loads generated during jaw movements. As a
result, the affected joint becomes increasingly susceptible to
structural damage with repetitive mechanical loading. The cause of
TMJ OA is thought to be the mechanical loading to the TMJ developed
by parafunction. It has been reported that an imbalance between
host factors and excessive mechanical stress to the TMJ may be
associated with condylar resorption. Arnett et al (17) suggested that parafunctions may produce
compression capable of initiating condylar resorption or enhancing
resorption caused by other factors that have already initiated this
process. However, certain cases often spontaneously develop TMJ OA
without mechanical stress (18),
therefore, there remain numerous unknown factors in association
with the causes and progress of TMJ OA, and more information
regarding its etiology and its natural course in untreated patients
is desirable.
It has also been suggested that the cartilage of TMJ
in normal mice is replaced by fibrocartilage, and is histologically
established at 30 weeks of age (19).
The articular cartilage of the condyle in STR/ort mice appears to
develop spontaneous OA-like lesions that resemble human OA;
therefore, the STR/ort mouse strain would be a useful model to
study the pathogenesis of TMJ OA. Furthermore, as TMJ OA occurred
concurrently with knee OA in STR/ort mice, the specific gene
expression associated with cartilage degeneration may be involved
in the pathogenesis of spontaneous TMJ OA. The development of TMJ
OA may be associated with genetic factors in inbred laboratory
mice. STR/ort mouse genetics are thought to affect vascular factors
or synovial responses, enhancing the degeneration of the TMJ
tissue. Further studies are required to clarify the mechanism of
TMJ OA in STR/ort mice.
The underlying mechanisms of cartilage degeneration
in TMJ OA have not been clearly identified. Determining the
biomarkers for TMJ OA would lead to the elucidation of the initial
changes in the disease. The identification of an experimental
animal model that undergoes spontaneous TMJ OA degeneration is
essential to help understand the disease pathogenesis. Thus, the
STR/ort mice appear to be a promising research tool for an improved
understanding of the pathogenic mechanisms involved in OA of the
TMJ OA.
Acknowledgements
The authors would like to thank Dr Tsuyoshi Amemiya
and Dr Hiroaki Shigematsu from Tsurumi University for their help in
parts of the experiments. The authors also thank the Applied
Medical Research Laboratory for the immunohistochemisty. The
present study was supported by the JSPS KAKENHI (grant no.
25861985) for Young Scientists B, and (grant no. 23593004) from
Scientific Research C.
References
|
1
|
Shen G and Darendeliler MA: The adaptive
remodeling of condylar cartilage - a transition from chondrogenesis
to osteogenesis. J Dent Res. 84:691–699. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen WH, Hosokawa M, Tsuboyama T, Ono T,
Iizuka T and Takeda T: Age-related changes in the temporomandibular
joint of the senescence accelerated mouse. SAM-P/3 as a new murine
model of degenerative joint disease. Am J Pathol. 135:379–385.
1989.PubMed/NCBI
|
|
3
|
Embree M, Ono M, Kilts T, Walker D,
Langguth J, Mao J, Bi Y, Barth JL and Young M: Role of subchondral
bone during early-stage experimental TMJ osteoarthritis. J Dent
Res. 90:1331–1338. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chambers MG, Cox L, Chong L, Suri N, Cover
P, Bayliss MT and Mason RM: Matrix metalloproteinases and
aggrecanases cleave aggrecan in different zones of normal cartilage
but colocalize in the development of osteoarthritic lesions in
STR/ort mice. Arthritis Rheum. 44:1455–1465. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mehraban F, Lark MW, Ahmed FN, Xu F and
Moskowitz RW: Increased secretion and activity of matrix
metalloproteinase-3 in synovial tissues and chondrocytes from
experimental osteoarthritis. Osteoarthritis Cartilage. 6:286–294.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van de Loo FA, Joosten LA, van Lent PL,
Arntz OJ and van den Berg WB: Role of interleukin-1, tumor necrosis
factor alpha, and interleukin-6 in cartilage proteoglycan
metabolism and destruction. Effect of in situ blocking in murine
antigen- and zymosan-induced arthritis. Arthritis Rheum.
38:164–172. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kammermann JR, Kincaid SA, Rumph PF, Baird
DK and Visco DM: Tumor necrosis factor-alpha (TNF-alpha) in canine
osteoarthritis: Immunolocalization of TNF-alpha, stromelysin and
TNF receptors in canine osteoarthritic cartilage. Osteoarthritis
Cartilage. 4:23–34. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Janusz MJ, Hookfin EB, Heitmeyer SA,
Woessner JF, Freemont AJ, Hoyland JA, Brown KK, Hsieh LC, Almstead
NG, De B, et al: Moderation of iodoacetate-induced experimental
osteoarthritis in rats by matrix metalloproteinase inhibitors.
Osteoarthritis Cartilage. 9:751–760. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Puzas JE, Landeau JM, Tallents R, Albright
J, Schwarz EM and Landesberg R: Degradative pathways in tissues of
the temporomandibular joint. Use of in vitro and in vivo models to
characterize matrix metalloproteinase and cytokine activity. Cells
Tissues Organs. 169:248–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Flannelly J, Chambers MG, Dudhia J, Hembry
RM, Murphy G, Mason RM and Bayliss MT: Metalloproteinase and tissue
inhibitor of metalloproteinase expression in the murine STR/ort
model of osteoarthritis. Osteoarthritis Cartilage. 10:722–733.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blumenfeld I, Laufer D and Livne E:
Effects of transforming growth factor-beta 1 and interleukin-1
alpha on matrix synthesis in osteoarthritic cartilage of the
temporo-mandibular joint in aged mice. Mech Ageing Dev. 95:101–111.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huebner JL, Otterness IG, Freund EM,
Caterson B and Kraus VB: Collagenase 1 and collagenase 3 expression
in a guinea pig model of osteoarthritis. Arthritis Rheum.
41:877–890. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mason RM, Chambers MG, Flannelly J, Gaffen
JD, Dudhia J and Bayliss MT: The STR/ort mouse and its use as a
model of osteoarthritis. Osteoarthritis Cartilage. 9:85–91. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sokoloff L, Mickelsen O, Silverstein E,
Jay GE Jr and Yamamoto RS: Experimental obesity and osteoarthritis.
Am J Physiol. 198:765–770. 1960.PubMed/NCBI
|
|
15
|
Walton M: Obesity as an aetiological
factor in the development of osteoarthrosis. Gerontology. 25:36–41.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dreessen D and Halata Z: Age-related
osteo-arthrotic degeneration of the temporomandibular joint in the
mouse. Acta Anat (Basel). 139:91–96. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Arnett GW, Milam SB and Gottesman L:
Progressive mandibular retrusion - idiopathic condylar resorption.
Part I. Am J Orthod Dentofacial Orthop. 110:8–15. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamada K, Saito I, Hanada K and Hayashi T:
Observation of three cases of temporomandibular joint
osteoarthritis and mandibular morphology during adolescence using
helical CT. J Oral Rehabil. 31:298–305. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen J, Sorensen KP, Gupta T, Kilts T,
Young M and Wadhwa S: Altered functional loading causes
differential effects in the subchondral bone and condylar cartilage
in the temporomandibular joint from young mice. Osteoarthritis
Cartilage. 17:354–361. 2009. View Article : Google Scholar : PubMed/NCBI
|