Introduction
Natural antisense ribonucleic acids (RNAs) are
transcripts that contain sequences complementary to other
endogenous RNAs including messenger RNAs (mRNAs). Kiyosawa et
al (1,2) identified ~2,500 complementary
deoxyribonucleic acid (cDNA) pairs originating from opposite
strands of the same genomic loci by mapping these cDNAs to the
draft mouse genome sequence. The functional annotation of the
mammalian genome (FANTOM) cDNA dataset contained numerous
non-coding RNAs (ncRNAs) (1–3). More than 70% of the antisense pairs
identified by Kiyosawa et al (1,2) included
ncRNAs as one of the members in the pair. To date, a number of
mammalian antisense ncRNAs have been found, indicating that
antisense transcription may be a commonly employed mechanism to
regulate gene expression in human cells (1–5). Faghihi
et al (6) reviewed the
functions of known antisense ncRNAs, such as transcriptional
interference, genomic imprinting, X chromosome inactivation and
alternative splicing. Certain investigators have reported that the
gene expression of antisense ncRNAs was up- or downregulated under
conditions of physiological change, such as the development of
various types of cancer (7,8). However, it is difficult to elucidate the
function of antisense ncRNAs via gene expression analyses, such as
microarrays.
The neural cell adhesion molecule 1 (Ncam1,
also known as CD56) gene encodes a cell adhesion protein and is a
member of the immunoglobulin superfamily. The encoded protein is
involved in cell-to-cell and cell-matrix interactions during
development and differentiation, as well as nervous system
development (9,10). Antisense ncRNAs from opposite strands
of the Ncam1 loci in mice have been registered in the NCBI
database (http://www.ncbi.nlm.nih.gov/gene/100036537) and
antisense viewer (http://genome.gsc.riken.jp/m/antisense). In addition,
the expression of antisense ncRNAs from Ncam1 loci in the
brain has been proven using microarray analysis by Kiyosawa et
al (2). However, the expression
and localization of this ncRNAs in other tissues remains
unclear.
It is necessary to examine the cells and/or tissues
from which the ncRNAs are expressed in order to understand the role
of Ncam1 antisense ncRNAs. In the present study, the
expression of Ncam1 antisense ncRNAs was examined in mice
tissues at different developmental stages by in situ
hybridization.
Materials and methods
Sections of the embryo and
tissues
C57BL/6J mice at different developmental stages (at
day 14 of mouse embryo development, E14; day 17 of development,
E17; and newborn mice; 8-week-old mice) were obtained from the
RIKEN BioResource Center (Ibaraki, Japan).
For in situ hybridization, tissues from mice
at different developmental stages were first fixed in situ
by perfusion with 4% (w/v) ice-cold paraformaldehyde solution in
phosphate-buffered saline (PBS). The resulting tissues were excised
and further fixed overnight in the paraformaldehyde solution. The
fixed tissues were embedded in paraffin and 4 µm sections were cut.
Sections were placed on glass slides and subjected to in
situ hybridization.
Complementary RNA (cRNA) probe
For Ncam1 antisense ncRNAs and mRNAs, probes
with a specific sequence:
5′-ATCTGGTCAAGTACAGAGCGCTCGCCTCTGAGTGGAAACCGGAAATCAGGCTCCCATCCGGCAGTGACGACCACGTCATGCTCAAGTCCCTGGACTGGAACGCAGAGTATGAAGTCTATG
T-3′; and a size of 118 nucleotides were designed in the mRNAs of
Ncam1. As a control RNA probe, a 120-nucleotide λ-phage
sequence that had no similarity with any of the mammalian sequences
registered in the DNA Data Bank of Japan (www.ddbj.nig.ac.jp/index-j.html) was used in all the
in situ hybridization experiments to verify that the
hybridization system did not emit any non-specific hybridization
signals. Digoxigenin (DIG)-labeled cRNA probes were provided by
Tsukuba GeneTech Laboratories (Ibaraki, Japan).
In situ hybridization
Sections of embryos and tissues on glass slides were
deparrafinized with xylene and ethanol, washed in PBS and incubated
in PBS containing 1.0 µg/ml proteinase K, at 37°C for 15 min. The
slides were washed in PBS and treated with 0.1 M triethanolamine in
0.25% acetic anhydride for 15 min. Slides were subsequently washed
with 0.1 M triethanolamine and 4X standard saline citrate (SSC),
and incubated in prehybridization solution (50% formamide, 2X SSC)
at 42°C for 30 min. Hybridization was performed in a solution
containing 50% formamide, 2X SSC, 1.0 mg/ml transfer RNA (tRNA),
1.0 mg/ml salmon DNA, 1.0 mg/ml bovine serum albumin, 1.0% sodium
dodecyl sulfate and 3.0 µg/ml DIG-labeled sense or antisense cRNA
probes, at 42°C for 16 h.
The resulting slides were successively washed with
prehybridization solution, 0.2X SSC and 0.1X SSC, at 42°C, followed
by treatment with NT buffer [150 mmol/l NaCl and 100 mmol/l
Tris-HCl (pH 7.5)] containing 10% fetal bovine serum, 1% blocking
reagent (Roche Diagnostics, Basel, Switzerland) and 1 mg/ml tRNA,
for 30 min. Subsequently, hybridization signals were visualized
using the alkaline phosphatase-labeled anti-DIG antibody/nitro blue
tetrazolium chloride 5-bromo-4-chloro-3-indolyl-phosphate system
(Roche Diagnostics).
Results and Discussion
Recently, antisense ncRNAs from a number of genes
have been identified in mice and humans using bioinformatic and
microarray analyses. Antisense ncRNAs have been inferred to be
involved in the control of trait expression in mammals, including
humans, mice and livestock. Study of the sites of antisense ncRNA
expression in tissues would provide important information regarding
the functions of antisense ncRNAs in mice. Therefore, mice were
used in the present study to investigate the expression sites of
previously described antisense ncRNAs from Ncam1.
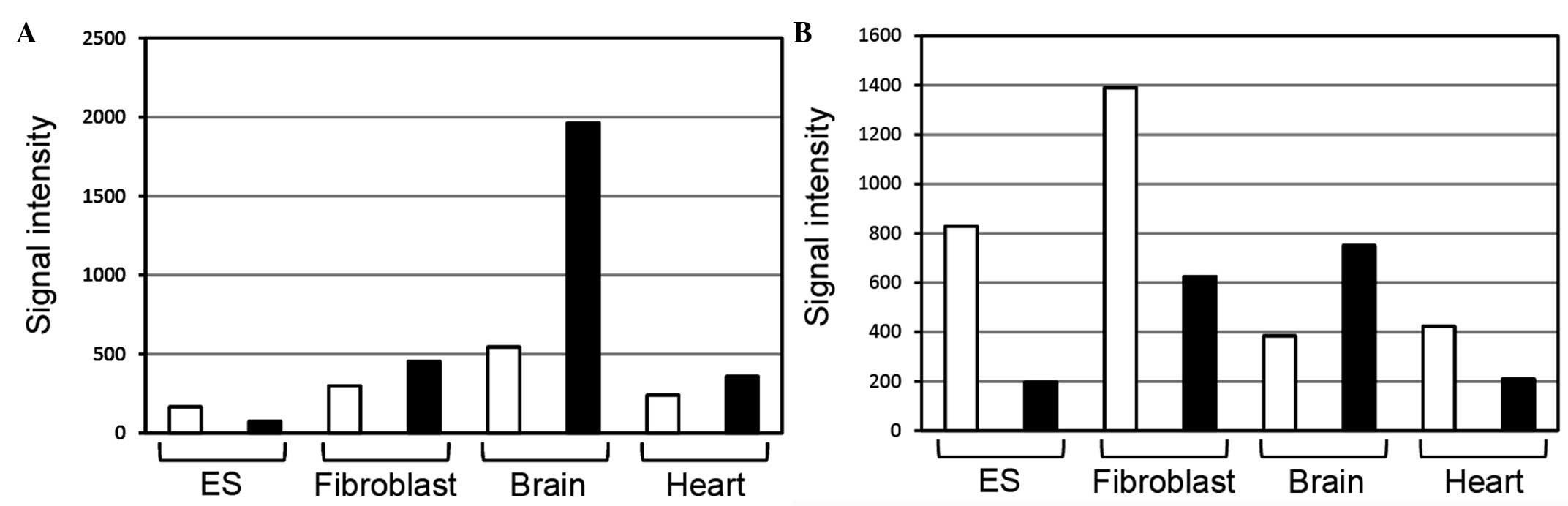
Kiyosawa et al (2) examined sense/antisense expression using a
custom microarray with different cDNA priming methods. Ncam1
antisense ncRNAs in the brain were more strongly expressed than
those mRNAs in the microarray analysis using oligo(dT) or random
nonamer primers (Fig. 1). Microarray
analysis using oligo(dT) primers is assumed to detect RNAs
(transcripts) with poly(A) tails, whereas microarray analysis using
random nonamer primers is assumed to detect RNAs with and without
poly(A) tails. This information suggests that Ncam1
antisense ncRNAs may have poly(A) tails.
To date, the open reading frame, or protein-coding
region, of Ncam1 antisense RNAs has not been confirmed in
several databases, including FANTOM. Therefore, Ncam1
antisense RNAs are ncRNAs. To examine the expression and
localization of Ncam1 antisense ncRNAs at different
developmental stages, in situ hybridization was performed.
In situ hybridization is known to detect RNAs with and
without poly(A) tails. Therefore, it is generally considered that
the signal intensity patterns of in situ hybridization are
the same as those of microarray analysis using random nonamer
primers.
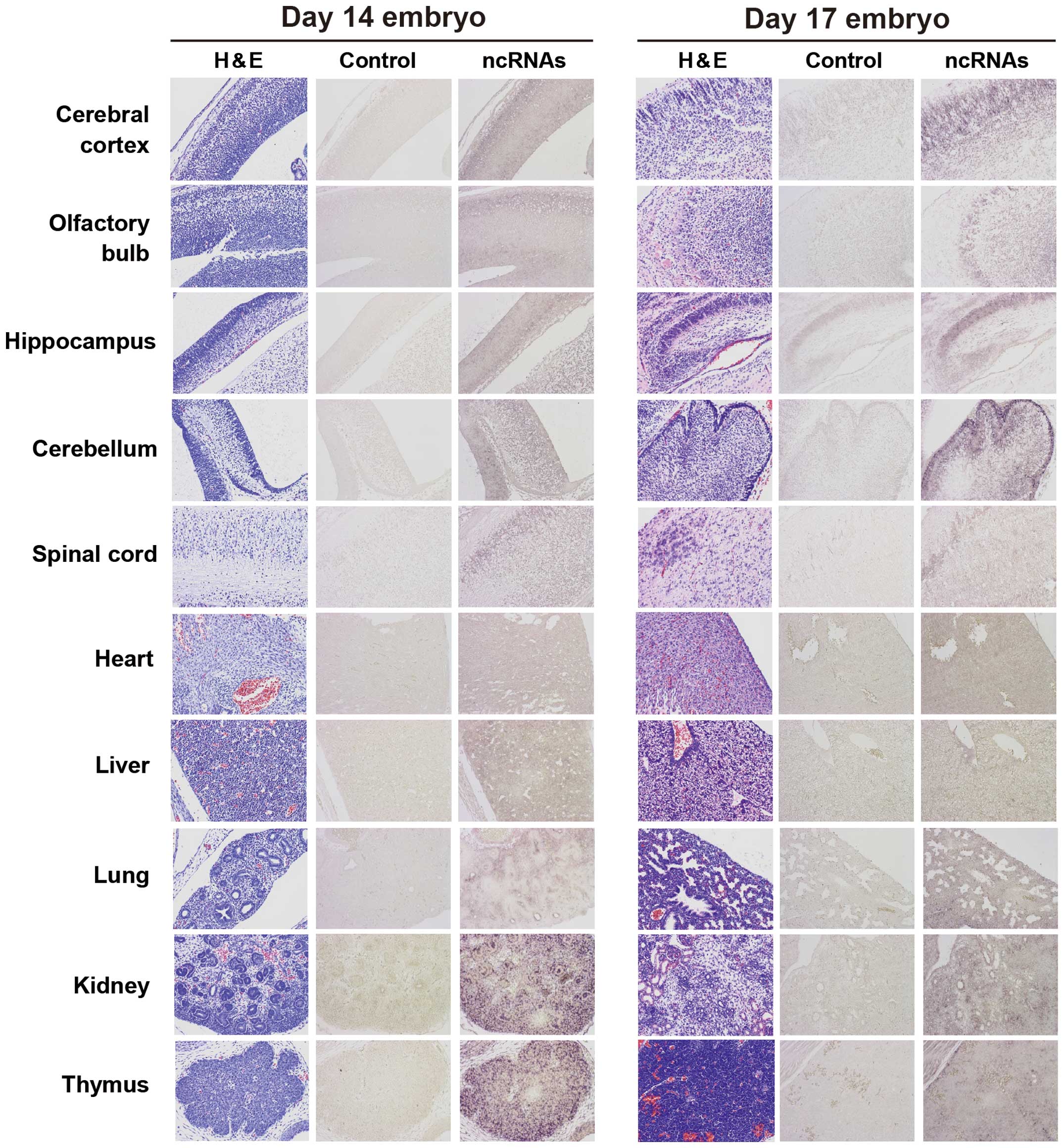
In E14 embryos, Ncam1 antisense ncRNAs were
uniformly detected in the heart, liver and nervous system regions,
including the brain, and were detected at high levels in certain
regions of the lung, kidney and thymus (Fig. 2). In E17 embryos, Ncam1
antisense ncRNAs were detected at high levels in specific cells of
the brain, whereas they were uniformly detected in other tissues
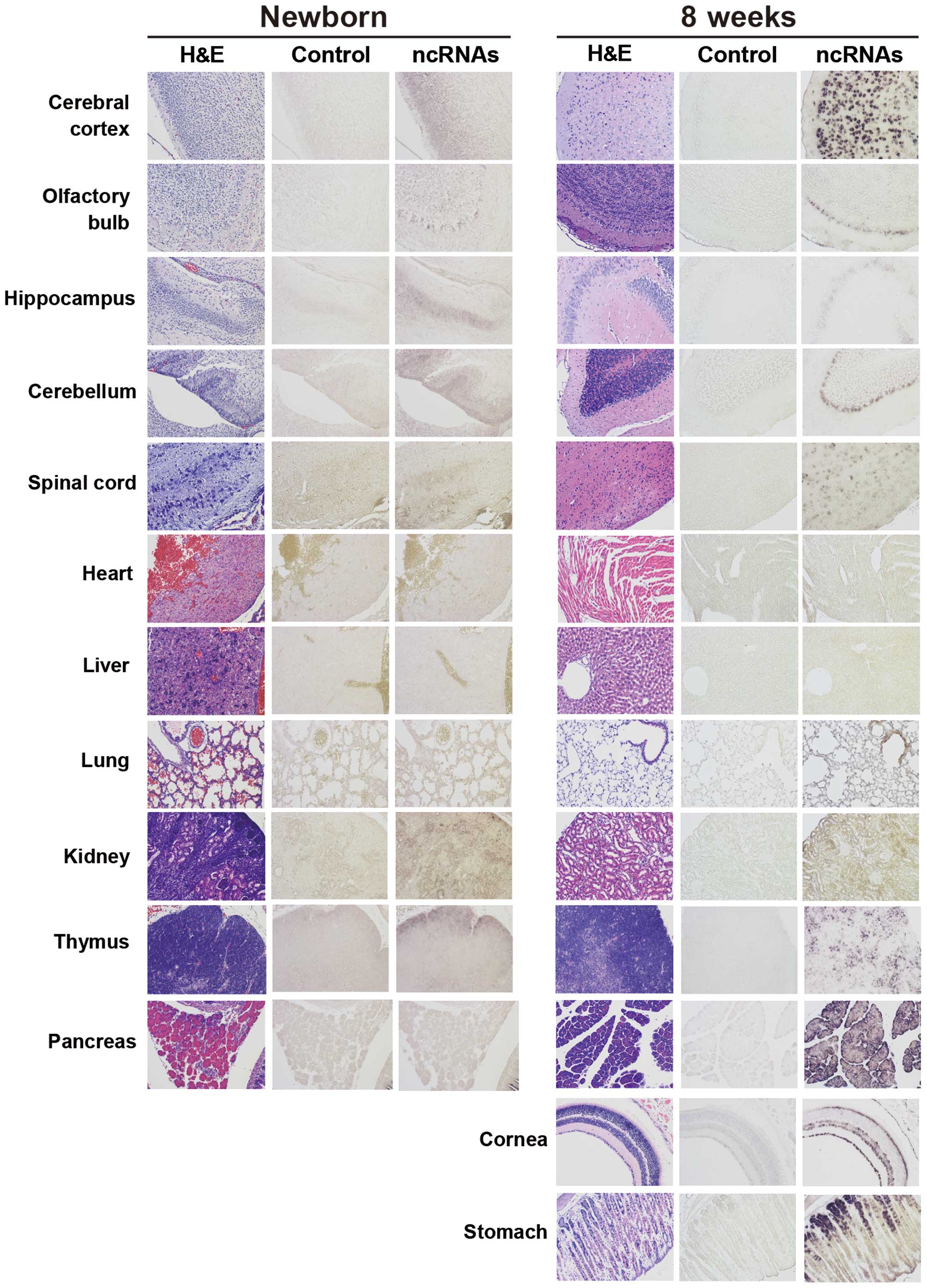
(heart, liver, lung, kidney and thymus). In newborn mice,
Ncam1 antisense ncRNAs were weakly detected in the brain,
kidney and thymus. The transcription pattern of NB mice was similar
to that of E17 embryos, except that the signals in organs other
than the brain were weaker than those of the E17 embryos (Figs. 2 and 3).
In the 8-week-old mice, Ncam1 antisense ncRNAs were detected
at high levels in limited regions of the brain, stomach, cornea and
all the regions of the pancreas. Contrastingly, they were detected
at low levels in the remaining organs that were examined, excluding
the liver. With regards to the liver, negligible amounts of
Ncam1 antisense ncRNA expression was detected (Fig. 3). These results indicate that
Ncam1 antisense ncRNAs are expressed in mice tissues.
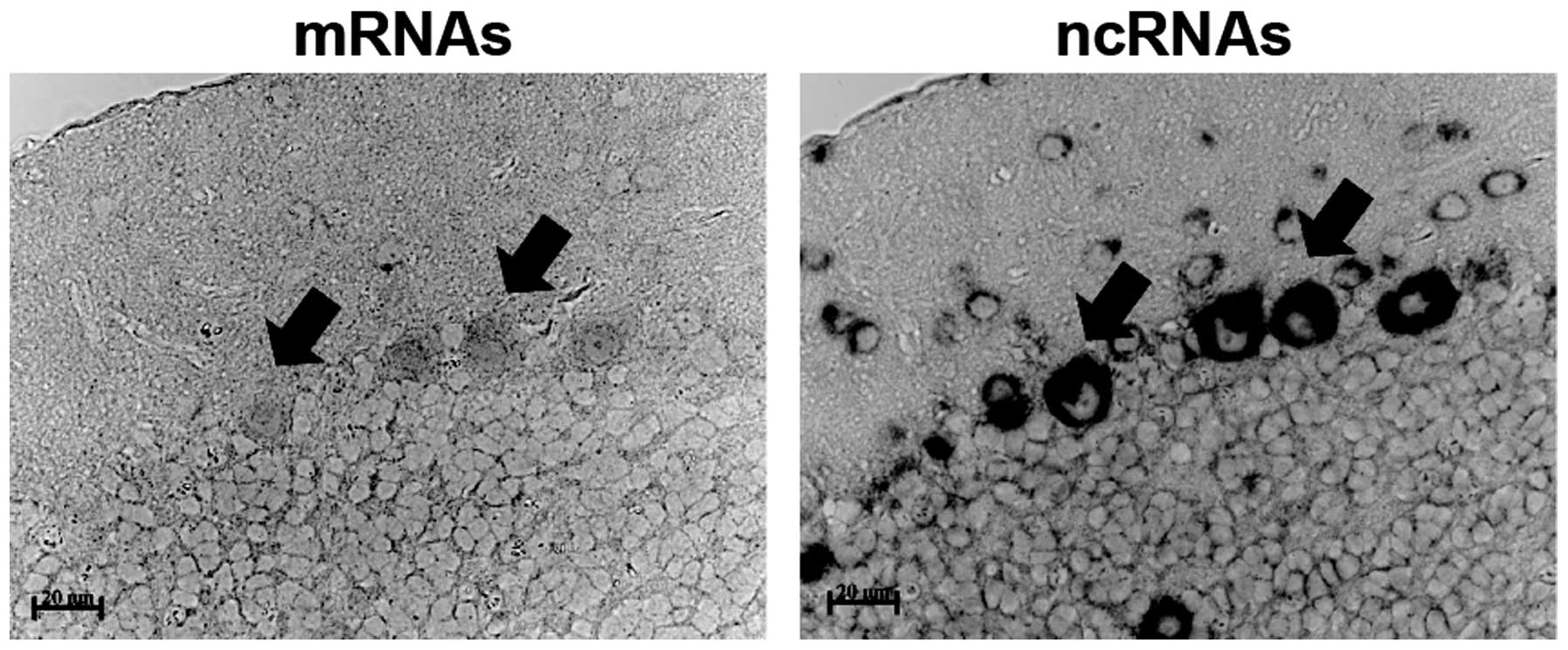
Of note, Ncam1 mRNAs and antisense ncRNAs
co-localized in Purkinje cells of the cerebellum and the levels of
antisense ncRNAs appeared to be higher than those of mRNAs
(Fig. 4). Based on the patterns of the
in situ hybridization signals for Ncam1 antisense
ncRNAs detected in tissues, some potential functions were
identified for Ncam1 antisense ncRNAs. In the analysis of
the adjacent sections, Ncam1 mRNAs were detected in some of
the brain cells found to produce Ncam1 antisense ncRNAs.
This indicates that Ncam1 antisense ncRNAs may control the
function of Ncam1 mRNAs, as previously proposed in reviews
on other antisense ncRNAs (6,11). Currently, the exact function of
Ncam1 antisense ncRNAs in each tissue is unknown. However,
the expression patterns of Ncam1 antisense ncRNAs may
provide information for understanding their function. In future
studies, the interactions between Ncam1 mRNAs and antisense
ncRNAs should be examined.
Acknowledgements
The author would like to thank Dr Hiroshi Yasue of
Tsukuba GeneTech Laboratories (Ibaraki, Japan) for the provision of
DIG-labeled cRNA probes and Ms. Noriko Hiraiwa of RIKEN BioResource
Center for offering the samples. The present study was supported in
part by the Grants in Aid from the Ministry of Education, Culture,
Sports, Science and Technology of Japan and the Hirosaki University
Grant for Exploratory Research by Young Scientists.
References
|
1
|
Kiyosawa H, Yamanaka I, Osato N, Kondo S
and Hayashizaki YRIKEN GER Group: Role: GSL MembersAntisense
transcripts with FANTOM2 clone set and their implications for gene
regulation. Genome Res. 13((6B)): 1324–1334. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kiyosawa H, Mise N, Iwase S, Hayashizaki Y
and Abe K: Disclosing hidden transcripts: Mouse natural
sense-antisense transcripts tend to be poly(A) negative and nuclear
localized. Genome Res. 15:463–474. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Okazaki Y, Furuno M, Kasukawa T, Adachi J,
Bono H, Kondo S, Nikaido I, Osato N, Saito R, Suzuki H, et al
FANTOM Consortium; RIKEN Genome Exploration Research Group Phase I
& II Team: Analysis of the mouse transcriptome based on
functional annotation of 60,770 full-length cDNAs. Nature.
420:563–573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katayama S, Tomaru Y, Kasukawa T, Waki K,
Nakanishi M, Nakamura M, Nishida H, Yap CC, Suzuki M, Kawai J, et
al FANTOM Consortium: Antisense transcription in the mammalian
transcriptome. Science. 309:1564–1566. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lehner B, Williams G, Campbell RD and
Sanderson CM: Antisense transcripts in the human genome. Trends
Genet. 18:63–65. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Faghihi MA and Wahlestedt C: Regulatory
roles of natural antisense transcripts. Nat Rev Mol Cell Biol.
10:637–643. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kohno K, Chiba M, Murata S, Pak S, Nagai
K, Yamamoto M, Yanagisawa K, Kobayashi A, Yasue H and Ohkohchi N:
Identification of natural antisense transcripts involved in human
colorectal cancer development. Int J Oncol. 37:1425–1432.
2010.PubMed/NCBI
|
|
8
|
Grigoriadis A, Oliver GR, Tanney A,
Kendrick H, Smalley MJ, Jat P and Neville AM: Identification of
differentially expressed sense and antisense transcript pairs in
breast epithelial tissues. BMC Genomics. 10:3242009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cremer H, Lange R, Christoph A, Plomann M,
Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, et al:
Inactivation of the N-CAM gene in mice results in size reduction of
the olfactory bulb and deficits in spatial learning. Nature.
367:455–459. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dityatev A, Dityateva G, Sytnyk V, Delling
M, Toni N, Nikonenko I, Muller D and Schachner M: Polysialylated
neural cell adhesion molecule promotes remodeling and formation of
hippocampal synapses. J Neurosci. 24:9372–9382. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lavorgna G, Dahary D, Lehner B, Sorek R,
Sanderson CM and Casari G: In search of antisense. Trends Biochem
Sci. 29:88–94. 2004. View Article : Google Scholar : PubMed/NCBI
|