Introduction
The cephalosporin nucleus has proved to be
significantly amenable to modification, allowing more derivatives
with different properties. The fourth-generation cephalosporins
have been noted for their stability to β-lactamase enzymes, with a
markedly reduced affinity for β-lactamase and increased outer
membrane permeability compared to third-generation cephalosporins,
thus making them widely used in clinical infectious diseases. A
review of clinical studies indicates that fourth-generation
cephalosporins are potentially useful as a first-line empiric
therapy for serious infections, including severe community-acquired
and nosocomial pneumonia, bacteremia, febrile episodes in
neutropenic patients and meningitis (1). Among them, cefepime and cefpirome were
most available due to their well-balanced antibacterial spectrum
(2).
Previous studies mostly focused on the efficacy of
fourth-generation cephalosporins; however, few clinical studies
reported their associated renal function adverse effects (3–5). Cefpirome
caused nephrotoxic symptoms in a rabbit model and the results
suggested that cefpirome is potentially nephrotoxic compared to
cefazolin, particularly in a single administration (6,7).
Additionally, for the clinical patients at the top three hospitals
in Nanjing, the serum creatinine (SCr) level was observed to
augment during treatment of cefepime and cefpirome, which indicated
the influence associated with fourth-generation cephalosporins on
renal function. Therefore, the present study carried out an
investigation on the nephrotoxic potential of cefepime and
cefpirome in vitro and in a clinical cohort study.
Materials and methods
In vitro cytotoxicity assay
Renal mesangial cells were used in the cell
viability assay. Cells were seeded 12–16 h before drug treatment at
densities of 10,000 cells per well. Six drug dilutions were
prepared as follows: 1,000, 100, 10, 1, 0.1 and 0.01 µmol/l. The
growth medium without drugs was set as the control group. Each
dilution group was tested in five duplicates. The drug solution was
added into the wells and cells were cultured at 37°C in 5%
CO2. A total of 24 h later, the drug solution was
removed and each well was fed with fresh medium containing
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and incubated for another 4 h. The medium and MTT were removed and
the MTT-formazan crystals were dissolved by dimethyl sulfoxide. The
optical values were tested at 570 nm. Data were analyzed by SPSS
version 18.0 (SPSS, Inc., Chicago, IL, USA) and Graphpad prism 5.0
software (GraphPad Software, Inc., La Jolla, CA, USA).
Clinical cohort study
A total of 944 hospitalized patients who were
randomly selected from a third-grade class-A teaching hospital (the
Second Affiliated Hospital of Nanjing Medical University, of
Nanjing Medical University, Nanjing, China) between January 2009
and December 2012 were included in the cohort study. The inclusion
criteria were patients who needed to receive conventional treatment
(2.0 g every 12 h) of fourth-generation cephalosporin (cefepime or
cefpirome) according to clinical diagnosis, additionally with an
originally normal renal function and creatinine clearance rate.
Excluded criteria included chronic renal insufficiency, nephrotic
syndrome, renal transplantation, previous use of aminoglycoside
antibiotic drugs, combined-use of antimicrobial agents and previous
treatment of nephrotoxic drugs. Patients were divided by drug
treatment and among each drug group, patients were divided into two
age groups: <65 and ≥65 years. The clinical characteristics of
patients included are described in Table
I.
 | Table I.Clinical characteristics of the
patients included in the cohort study. |
Table I.
Clinical characteristics of the
patients included in the cohort study.
| Features | Cefepime (n=472) | Cefpirome
(n=472) | P-value |
|---|
| Age, years |
|
| >0.05 |
|
<65 | 165 | 158 | >0.05 |
| ≥65 | 307 | 314 | >0.05 |
| Male | 254 | 263 | >0.05 |
| Underlying
diseases |
|
| >0.05 |
|
AECOPD | 213 | 217 | >0.05 |
| CAP | 85 | 87 | >0.05 |
|
Bloodstream infections | 19 | 17 | >0.05 |
| Abdominal
infection | 47 | 51 | >0.05 |
| Skin and
soft tissue infection | 21 | 20 | >0.05 |
| Urinary
system infection | 38 | 41 | >0.05 |
| HAP | 49 | 39 | >0.05 |
| Complications |
|
| >0.05 |
|
Diabetes | 37 | 35 | >0.05 |
|
Hypertension | 41 | 42 | >0.05 |
| Coronary
heart disease | 51 | 51 | >0.05 |
| Malignant
tumor | 19 | 21 | >0.05 |
| Cerebral
stroke | 31 | 34 | >0.05 |
|
Cases treated
according to drug-sensitivity test | 179 | 181 | >0.05 |
|
Cases treated
according to clinical experience | 293 | 291 | >0.05 |
|
Cases with
satisfactory bacterial eradication | 157 | 161 | >0.05 |
|
Cases with
controlled infection | 370 | 367 | >0.05 |
The renal function of patients was tested by
examining the SCr level before, and on days 3 and 7 during the
therapy. Following this, the average levels of SCr in each group
were calculated; the incidence of SCr >445 µmol/l, as an
indicator for potential renal failure, was recorded.
Statistical analysis
P<0.05 was considered to indicate a statistically
significant difference.
Results
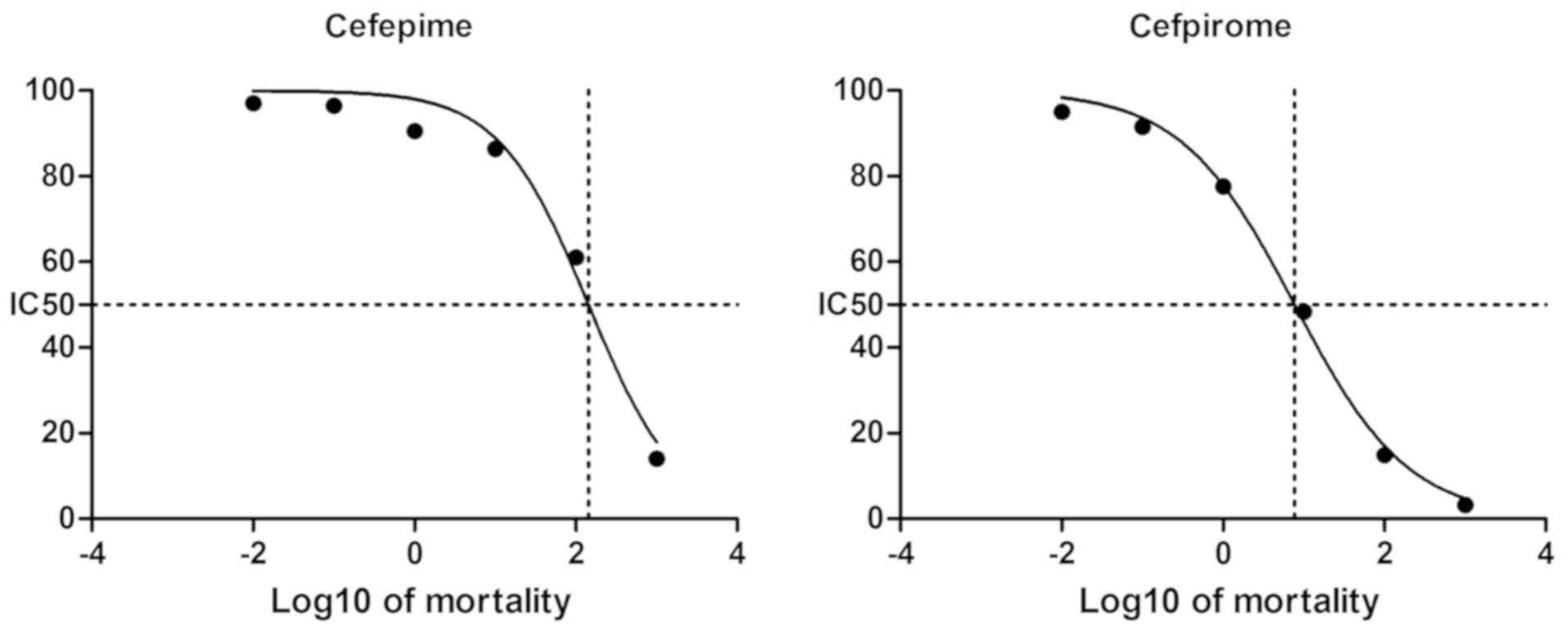
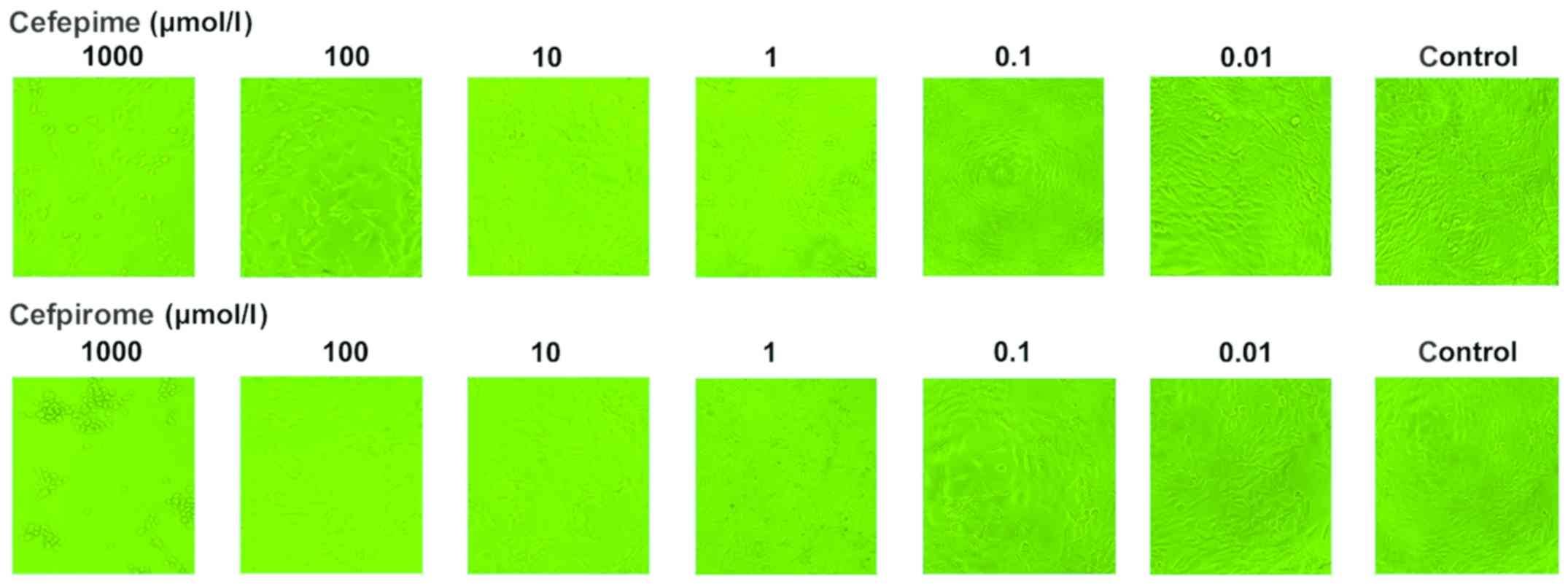
Comparison of the effects on cell
viability of cefepime and cefpirome on renal mesangial cells
As depicted in Table
II and Fig. 1, cefepime and
cefpirome showed significant inhibition on cell activity compared
to the control group and the inhibition rate was dependent on the
drug concentration. In each dilution group, cefpirome exhibited a
higher inhibition on cell viability than cefepime, as shown in
Table II and Fig. 2. Additionally, the half maximal
inhibitory concentration (IC50) of the drugs on renal
mesangial cells for 24 h was 143.5 and 7.702 µmol/l for cefepime
and cefpirome, respectively.
 | Table II.Optical value (OD) at 570 nm and
inhibition rate of drugs on renal mesangial cells treated with
cephalosporins. |
Table II.
Optical value (OD) at 570 nm and
inhibition rate of drugs on renal mesangial cells treated with
cephalosporins.
|
| Cefepime | Cefpirome |
|---|
|
|
|
|
|---|
| Concentration,
µmol/l | Mean ± SD (n=5) | P-value vs.
control | Inhibition rate,
% | Mean ± SD (n=5) | P-value vs.
control | Inhibition rate,
% |
|---|
| 1,000 | 0.295±0.056 | <0.05 | 85.99±2.66 | 0.067±0.007 | <0.05 | 96.83±0.34 |
| 100 | 1.285±0.052 | <0.05 | 38.94±2.45 | 0.313±0.013 | <0.05 | 85.13±0.62 |
| 10 | 1.818±0.046 | <0.05 | 13.62±2.21 | 1.021±0.015 | <0.05 | 51.58±0.71 |
| 1 | 1.906±0.026 | <0.05 |
9.42±1.22 | 1.636±0.029 | <0.05 | 22.38±1.39 |
| 0.1 | 2.030±0.082 | <0.05 |
3.53±3.91 | 1.930±0.011 | <0.05 |
8.46±0.53 |
| 0.01 | 2.042±0.014 | >0.05 |
2.97±0.67 | 2.003±0.013 | <0.05 |
4.98±0.60 |
| Control | 2.105±0.015 |
|
| 2.108±0.009 |
|
|
| IC50,
µmol/l | 143.5 |
|
| 7.702 |
|
|
Changes of serum creatine level
following treatment with cefepime or cefpirome
As shown in Table
III, treatment of cefepime resulted in a greater increase of
SCr in the two age groups compared to cefpirome. In patients aged
<65 years, cefepime rose from an average SCr level of 67±7.4 to
83±11.1 µmol/l on day 3 and 87±10.3 µmol/l on day 7, while
cefpirome augmented an average SCr level from 65±8.7 to 95±10.9
µmol/l on day 3 and 98±14.2 µmol/l on day 7. On day 3, none of the
patients suffered from SCr >445 µmol/l in the cefepime group,
but one patient suffered in the cefpirome group (incidence, 0.6%).
On day 7, one case in the cefepime group (incidence, 0.6%) and two
additional cases in the cefpirome group (incidence, 1.9%) exhibited
SCr >445 µmol/l, as shown in Table
IV.
 | Table III.Comparison of the changes of serum
creatinine levels during treatment of fourth-generation
cephalosporins. |
Table III.
Comparison of the changes of serum
creatinine levels during treatment of fourth-generation
cephalosporins.
|
| Levels of serum
creatinine (mean ± SD, µmol/l) |
|---|
|
|
|
|---|
|
| Cefepime | Cefpirome |
|---|
|
|
|
|
|---|
| Days during
therapy | <65 years old
(n=165) | ≥65 years old
(n=307) | P-value | <65 years old
(n=158) | ≥65 years old
(n=314) | P-value |
|---|
| Before | 67±7.4 | 67±7.4 | >0.05 | 65±8.7 | 65±8.7 | >0.05 |
| Day 3 |
83±11.1 |
91±11.7 | >0.05 |
95±10.9 | 130±12.4 | >0.01,
<0.05 |
| Day 7 |
87±10.3 | 102±11.8 | >0.05 |
98±14.2 | 155±13.1 | <0.01 |
 | Table IV.Comparison of the serum creatinine
(SCr) >445 µmol/l incidences in the two fourth-generation
cephalosporins groups. |
Table IV.
Comparison of the serum creatinine
(SCr) >445 µmol/l incidences in the two fourth-generation
cephalosporins groups.
|
| Incidence of SCr
>445 µmol/l |
|---|
|
|
|
|---|
|
| Cefepime | Cefpirome |
|---|
|
|
|
|
|---|
| Days during
therapy | <65 years old, n
(%) (n=165) | ≥65 years old, n (%)
(n=307) | <65 years old, n
(%) (n=158) | ≥65 years old, n (%)
(n=314) |
| Before | 0 | 0 | 0 | 0 |
| Day 3 | 0 (0.0) | 1 (0.3) | 1 (0.6) | 4
(1.3) |
| Day 7 | 1 (0.6) | 2 (0.7) | 3 (1.9) | 10 (3.2) |
In patients aged ≥65 years, a higher increase of SCr
was observed for the two drugs. In the cefepime group, the average
SCr level was 91±11.7 µmol/l on day 3 and 102±11.8 µmol/l on day 7,
while for the cefepime group the level was 130±12.4 and 155±13.1
µmol/l on days 3 and 7, respectively. Among the ≥65 age group, the
incidence of SCr >445 µmol/l increased, which was 0.3% (one
case) on day 3 and 0.7% (two cases) on day 7 for the cefepime
group, and 1.3% (four cases) on day 3 and 3.2% (10 cases) on day 7
for the cefpirome group, as depicted in Tables III and IV.
Discussion
As a large group of associated β-lactam
antimicrobial agents, the cephalosporins possess various
advantages, including low rates of toxicity, relatively broad
activity spectrum and ease of administration on clinical treatment
against infectious diseases. Currently, the fourth-generation
cephalosporins are used more widely as the drug-resistant condition
grows more severe, among which cefpirome and cefepime are the most
available and used. Cephalosporins cause renal toxicity and
cephaloridine was noted as the most nephrotoxic (8). Corresponding mechanisms include lipid
peroxidation, competitive inhibition of mitochondrial carnitine
transport, fatty acid oxidation and acylation, and inactivation of
tubular cell proteins. The fourth-generation cephalosporins were
revealed to cause renal damage in animal models (9–11).
Therefore, the potential influence on renal function of
fourth-generation cephalosporins should be considered as a matter
of concern.
In the present study, cefepime and cefpirome caused
significant cytotoxicity on renal mesangial cells and cefpirome
with a higher IC50 on cell viability may be more renal
cytotoxic than cefepime. Consistent results were observed in the
clinical cohort study. Cefpirome was observed to cause a greater
elevation of the average SCr level, as well as the incidence of SCr
>445 µmol/l compared to the cefepime group. Within each drug
group, the ≥65 years subgroup was more susceptive to nephrotoxicity
caused by drugs, with a larger increase of the average SCr levels
and higher incidence of SCr >445 µmol/l. Cefpirome had more
potential to induce renal damage compared to cefepime. Future
studies should focus on renal interstitial cells, as the majority
of observed renal damage caused by cephalosporins are interstitial
nephritis. In addition, further clinical indicators associated with
renal function are required for a deeper insight of the adverse
effects of fourth-generation cephalosporins. Animal models should
be designed to well expound the mechanisms of potential
nephrotoxicity of fourth-generation cephalosporins.
In conclusion, the two fourth-generation
cephalosporins have nephrotoxic potential and cefpirome has a
greater tendency to cause renal damage. Additional attention should
be paid to elder patients. Cefpirome should be a more cautious
choice in clinical treatment.
Acknowledgements
The present study was sponsored by the Health
Department of Jiangsu province (grant no. H200911).
References
|
1
|
Garau J, Wilson W, Wood M and Carlet J:
Fourth-generation cephalosporins: A review of in vitro activity,
pharmacokinetics, pharmacodynamics and clinical utility. Clin
Microbiol Infect. 3(Suppl 1): s87–s101. 1997. View Article : Google Scholar
|
|
2
|
Gould IM: Do we need fourth-generation
cephalosporins? Clin Microbiol Infect. 5:S1–S5. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saito T, Ichinohe T, Kanda J, et al:
Historical cohort study of the efficacy and safety of
piperacillin/tazobactam versus fourth-generation cephalosporins for
empirical treatment of febrile neutropenia in patients with
hematological malignancies. Int J Clin Med. 2:18–22. 2011.
View Article : Google Scholar
|
|
4
|
Hoffman JM, Frediani J, Herr M, Flynn PM
and Adderson EE: The safety of cefepime and ceftazidime in
pediatric oncology patients. Pediatr Blood Cancer. 60:806–809.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gonçalves-Pereira J and Póvoa P:
Antibiotics in critically ill patients: A systematic review of the
pharmacokinetics of β-lactams. Crit Care. 15:R2062011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deki T, Matsuoka A, Marutani K, et al:
Nephrotoxicity of cefpirome sulfate in rabbits - single and
multiple intravenous administration. J Toxicol Sci. 15(Suppl 3):
173–200. 1990.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cojocel C: Biochemical aspects of the
renal tolerance for cefpirome and other cephalosporins.
Arzneimittelforschung. 40:1140–1144. 1990.PubMed/NCBI
|
|
8
|
Tune BM: Nephrotoxicity of β-lactam
antibiotics: Mechanisms and strategies for prevention. Pediatr
Nephrol. 11:768–772. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rokushima M, Fujisawa K, Furukawa N, et
al: Transcriptomic analysis of nephrotoxicity induced by
cephaloridine, a representative cephalosporin antibiotic. Chem Res
Toxicol. 21:1186–1196. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soni A, Chaudhary M and Dwivedi VK:
Ceftriaxone-vancomycin drug toxicity reduction by VRP 1020 in Mus
musculus mice. Curr Clin Pharmacol. 4:95–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yılmaz N, Ilhan S, Nazıroğlu M, Oktar S,
Nacar A, Arıca V and Tutanç M: Ceftriaxone ameliorates cyclosporine
A-induced oxidative nephrotoxicity in rat. Cell Biochem Funct.
29:102–107. 2011. View
Article : Google Scholar : PubMed/NCBI
|