Introduction
Paclitaxel (PTX) is commonly used for the treatment
of various malignancies, including breast, lung, ovarian and other
cancers (1). The major adverse
reactions of this drug include alopecia, bone marrow suppression,
polyneuropathy and cardiovascular toxicities (2). The incidence of cardiovascular
toxicities in patients receiving PTX is 12–13% worldwide (3). Manifestations of the cardiovascular
toxicities include atrial arrhythmias, asymptomatic bradycardia,
left bundle branch block, ventricular tachycardia, congestive
cardiac failure and atrial fibrillation (AF) (3,4). AF is
among the most critical adverse effects, though is relatively rare
with an incidence rate of 1.0–1.7% worldwide (5). The main mechanisms underlying
PTX-induced AF are considered to be adrenergic or vagal
stimulation, changing atrial conduction, refractoriness,
automaticity, coronary vasoconstriction or ischemia, local
electrolyte disturbances, and direct cardiotoxicity (5).
According to the literature, PTX may cause AF,
particularly in elderly or patients with a history of
cardiovascular disease, but also in patients with no cardiac risk
factors (5,6). Therefore, the possibility of AF should
be considered in patients who develop arrhythmia or other symptoms
following receipt of PTX. This is indicated in the present report,
which presents a case of AF induced by PTX in a patient with
non-small-cell carcinoma.
Case report
A 51-year-old Chinese male ex-smoker with stage IIIB
(T4N2M0) non-small-cell carcinoma (7)
presenting with right hilar and carina lymph node metastasis,
diagnosed on August 2, 2016 at the Third Hospital of Mianyang
(Mianyang, China). The patient had no history of diabetes,
hypertension or cardiac illness, and his base line
electrocardiogram (ECG) was normal (Fig.
1). The patient's heart rate was 82 beats per minute (bpm), and
the QRS duration, and QT and PR intervals were 80, 384 and 151
msec, respectively. He started the first cycle of combination
chemotherapy with PTX and cisplatin (TP; PTX, 135 mg/m2
on day 1 and cisplatin, 25 mg/m2 on days 1–3) on
September 30, 2016.
Three weeks later, the patient underwent the second
cycle of chemotherapy with TP. Dexamethasone (20 mg per os) was
administered ~12 and 6 h before PTX, and diphenhydramine [50 mg
intravenous (iv)] and cimetidine [300 mg (iv)] were administered
30–60 min prior to PTX. At 2 days after administration of PTX, the
patient's heart rate increased to 160 bpm (normal range 60–90 bpm),
which was accompanied by mild dizziness and shortness of breath,
but with no obvious heart palpitations. The ECG indicated a rapid
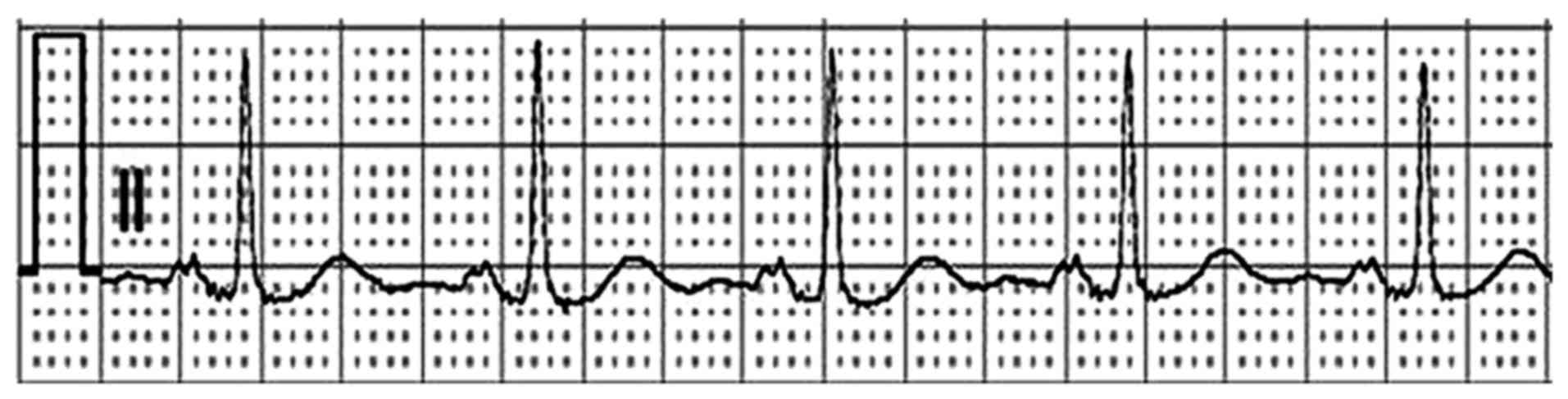
AF with rapid ventricular rate (Fig.
2). A diagnosis of AF was made. The patient was immediately
administered amiodarone (150 mg bolus then 300 mg continuous
infusion). Two hours later, the symptom of shortness of breath had
disappeared, and the heart rate had decreased to 106 bpm.
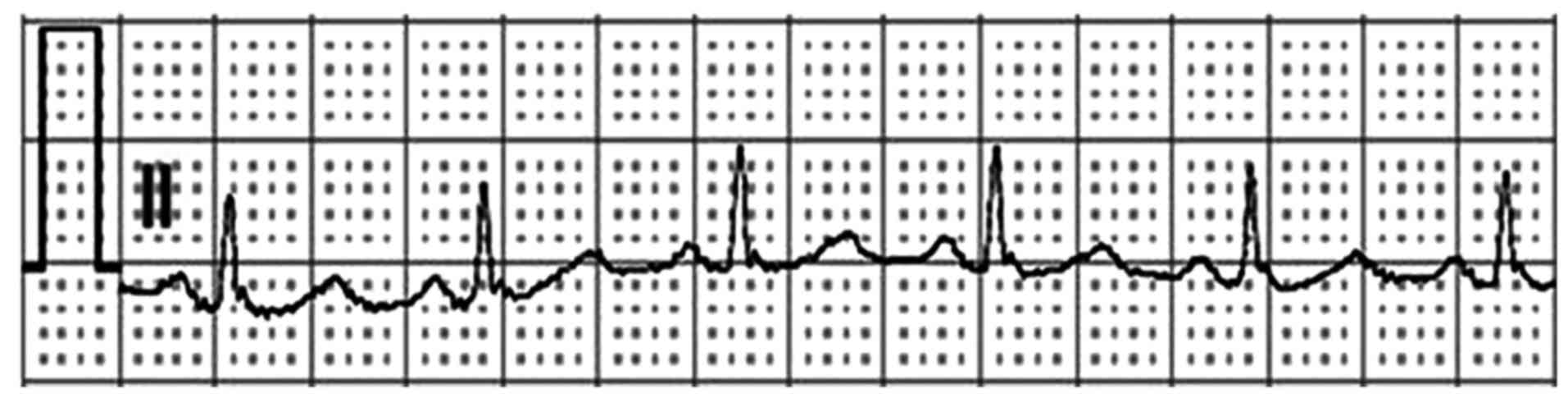
Subsequently, metoprolol was administered to the patient to reduce
heart rate, and three days later, the ECG was normalized and
indicated normal heart rate and rhythm (Fig. 3).
To confirm the association between PTX and AF in the
present case, the Naranjio algorithm (8,9) was used
to evaluate the potential causal relationship between PTX and AF.
According to the Naranjio algorithm, the score was 6 points
(Table I), indicating that the
occurrence of AF was likely to be associated with use of PTX.
Scoring was based on the following: i) There have been previous
conclusive reports on this reaction (10–12), and
therefore a score of 1 point was given; ii) 2 days after
administration of PTX, the patient developed AF, and therefore 2
points were given; iii) in addition to PTX, there were no other
drugs that may cause AF, and therefore 2 points were given; and iv)
the ECG indicated a rapid AF with rapid ventricular rate, and
therefore 1 score point was given.
 | Table I.Naranjio algorithm score
assignment. |
Table I.
Naranjio algorithm score
assignment.
| Question | Yes | No or Do not
know | Score |
|---|
| 1. Are there previous
conclusive reports on this reaction? | +1 | 0 | +1 |
| 2. Did the adverse
events appear after the suspected drug was given? | +2 | −1 | +2 |
| 3. Did the adverse
reaction improve when the drug was discontinued? Or when a specific
antagonist was given? | +1 | 0 | 0 |
| 4. Did the adverse
reaction appear when the drug was readministered? | +2 | −1 | 0 |
| 5. Are there
alternative causes that could have caused the reaction? | −1 | +2 | +2 |
| 6. Did the reaction
reappear when a placebo was given? | −1 | +1 | 0 |
| 7. Was the drug
detected in any body fluid in toxic concentrations? | +1 | 0 | 0 |
| 8. Was the reaction
more severe when the dose was increased, or less severe when the
dose was decreased? | +1 | 0 | 0 |
| 9. Did the patient
have a similar reaction to the same or similar drugs in any
previous exposure? | +1 | 0 | 0 |
| 10. Was the adverse
event confirmed by any objective evidence? | +1 | 0 | +1 |
| Total | – | – | 6 |
During the second chemotherapy cycle, except for
mild nausea, no other adverse reactions were noted in the patient.
Hematological and biochemical parameters prior to and following
termination of the chemotherapy are listed in Table II. Except for hemoglobin (pre- and
post-chemotherapy, 97 and 91 g/l, respectively; normal range
120–160 g/l), all of these parameters were normal. PTX was replaced
with docetaxel in the next four chemotherapy cycles, and no AF
occurred in the patient. However at 1 year on, the patient
succumbed due to disease progression.
 | Table II.Hematological and biochemical
parameters prior to and following chemotherapy. |
Table II.
Hematological and biochemical
parameters prior to and following chemotherapy.
|
| Measured values |
|
|---|
|
|
|
|
|---|
| Auxiliary
examination | Pre-chemotherapy |
Post-chemotherapy | Normal
rangea |
|---|
| Routine |
|
Neutrophils
(109/l) | 5.8 | 5.2 | 1.8–6.3 |
| Blood test |
|
Hemoglobin (g/l) | 97 | 91 | 120–160 |
| Platelets
(109/l) | 239 | 214 | 100–300 |
| Hepatic function |
| Alanine
aminotransferase (U/l) | 42 | 45 | 9–50 |
| Aspartate
aminotransferase (U/l) | 33 | 24 | 15–40 |
| Total
bilirubin (µmol/l) | 9.2 | 11.1 | 5–21 |
| Renal function |
|
Creatinine clearance
(ml/min) | 85.4 | 83.2 | >80 |
| Blood
urea nitrogen (mmol/l) | 3.6 | 3.4 | 2.9–7.1 |
| Cardiac function |
|
N-terminal pro-B-type
natriuretic peptide (pg/ml) | 268 | 282 | 0–300 |
| Creatine
kinase MB (U/l) | 10 | 9 | ≤25 |
| Cardiac
troponin I (µg/ml) | 0.01 | 0.02 | 0–0.3 |
Discussion
AF is the most prevalent cardiac arrhythmia and a
major cause of hospitalization, morbidity and mortality (5). PTX, an antimicrotubule agent, induces AF
infrequently (10). In an analysis of
~3,400 cancer patients in the National Cancer Institute Adverse
Drug Reactions database (13), atrial
arrhythmias occurred in >0.2% of patients who received PTX
(5). Meanwhile, an increasing number
of cardiovascular and non-cardiovascular drugs have been reported
to induce AF (6). In the present
case, the Naranjio algorithm was used to confirm the association
between PTX and AF, which indicated that the occurrence of AF was
likely to be associated with use of PTX.
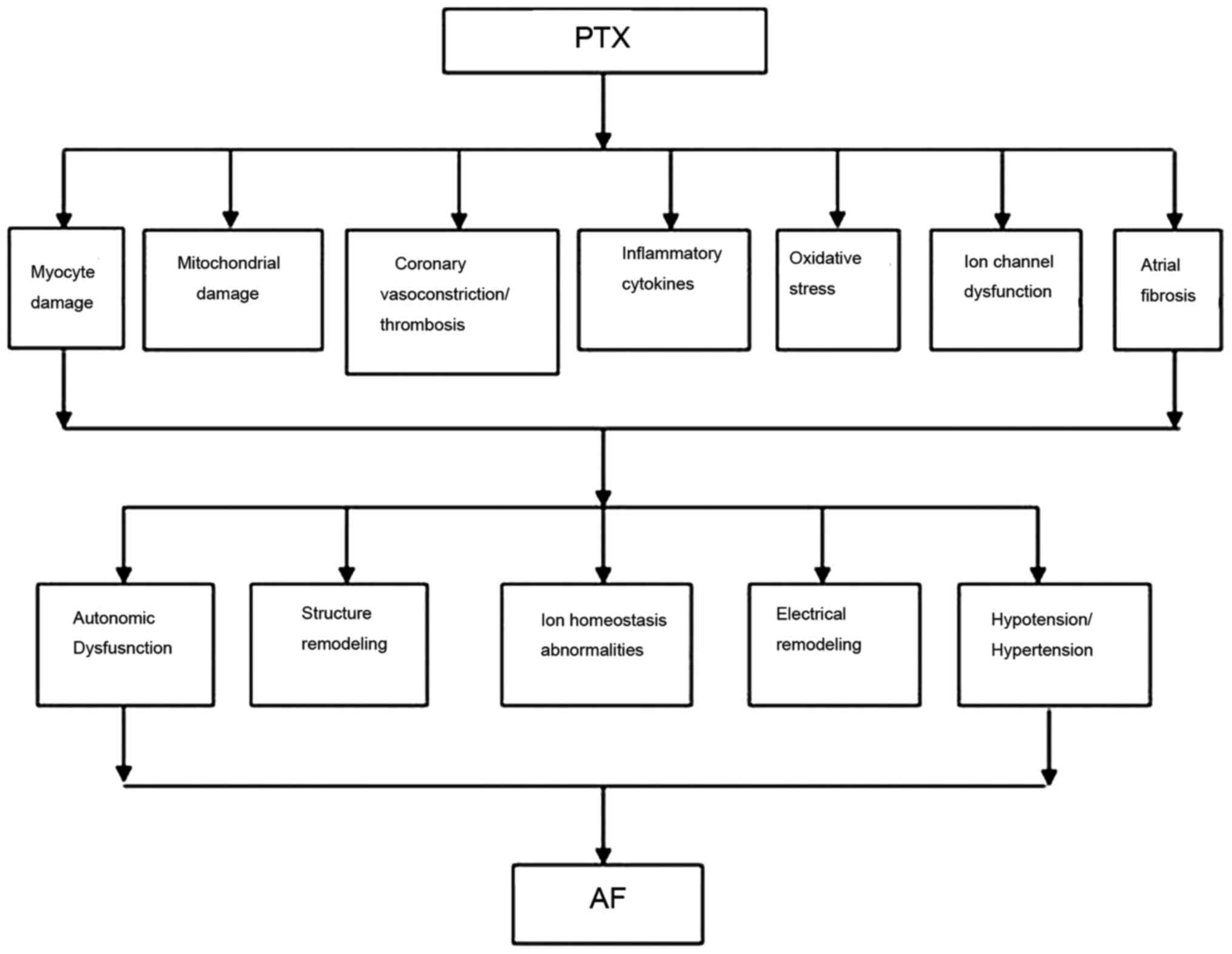
The exact mechanism of AF induced by PTX is not well
described or understood in the literature (10–12);
however there are a number of hypotheses, including a stimulation
of the sympathetic nervous system or parasympathetic nervous system
to influence the function of the atrionector, or a stimulation of
histamine 1 and 2 receptors to cause myocardial oxygen demand,
coronary vasoconstriction and chronotropic effects (5,6). A
schematic flow chart of the potential mechanisms underlying
PTX-induced AF is provided in Fig. 4.
Considering the critical nature of this adverse reaction, attention
should been paid when administering adjuvant chemotherapy with PTX,
particularly in patients with possible or known cardiac disease
(14).
AF typically lasts for a few minutes or hours in
patients when the suspected drug is stopped (5). If AF persists for several hours or days
following drug discontinuation (5,6), the
treatment is the same as that recommended for paroxysmal/persistent
AF (5), with the common treatment
procedures being drug withdrawal and control of rhythm and rate.
However, the effectiveness of rhythm and rate control therapies on
drug-induced AF has not been adequately studied, and guidelines are
based exclusively on patients with cancer diseases (6). Certain studies (5,6) have
documented that amiodarone and β1 blocker may be effective for
rhythm and rate control. If the causative drug is necessary for the
patient, the treatment can be started at a lower dose with
continuous ECG monitoring to detect the recurrence of AF (5,6).
Occasionally, it is possible to replace the causative agent for
another drug of the same family (i.e., PTX replaced by docetaxel)
(6).
In conclusion, AF is a rare, albeit critical adverse
effect induced by PTX, and thorough observation should be performed
during PTX treatment, even for patients with no previous
presentation of cardiac risk factors. Further studies are required
to establish the underlying mechanisms of PTX-induced AF in cancer
patients, though preventive steps can be taken in the meantime.
Acknowledgements
The authors would like to thank Dr Shang Jingchuan
(Department of Pharmaceutical Analysis, School of Pharmacy,
Chongqing Medical University, Chongqing, China) for performing
language editing of the manuscript.
Funding
No funding was received.
Availability of data and materials
All data described in the current report are
available from the corresponding author on reasonable request.
Authors' contributions
DZ and XL were responsible for clinical evaluation
and therapeutic management of the patient. DZ, JC and XL were
responsible for the literature search. DZ, LC and JW were
responsible for manuscript writing and provided corrections to the
manuscript and figures. All authors reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written consent for case
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cesca M, Morosi L, Berndt A, Fuso Nerini
I, Frapolli R, Richter P, Decio A, Dirsch O, Micotti E, Giordano S,
et al: Bevacizumab-induced inhibition of angiogenesis promotes a
more homogeneous intratumoral distribution of paclitaxel, improving
the antitumor response. Mol Cancer Ther. 15:125–135. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brotto L, Brundage M, Hoskins P, Vergote
I, Cervantes A, Casado HA, Poveda A, Eisenhauer E and Tu D;
Gynecologic Cancer Intergroup Study of NCIC Clinical Trials Group
(NCIC CTG); European Organization for Research and Treatment of
Cancer - Gynecologic Cancer Group (EORTC-GCG); Grupo de
Investigación de Cáncer de Ovario (GEICO), . Randomized study of
sequential cisplatin-topotecan/carboplatin-paclitaxel versus
carboplatin-paclitaxel: Effects on quality of life. Support Care
Cancer. 24:1241–1249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shah K, Gupta S, Ghosh J, Bajpai J and
Maheshwari A: Acute non-ST elevation myocardial infarction
following paclitaxel administration for ovarian carcinoma: A case
report and review of literature. J Cancer Res Ther. 8:442–444.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rawal G, Yadav S and Kumar R: Paclitaxel
induced acute ST elevation myocardial infarction: A Rare Case
Report. J Clin Diagn Res. 10:XD01–XD02. 2016.PubMed/NCBI
|
|
5
|
Kaakeh Y, Overholser BR, Lopshire JC and
Tisdale JE: Drug-induced atrial fibrillation. Drugs. 72:1617–1630.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tamargo J, Caballero R and Delpón E:
Drug-induced atrial fibrillation: Does it matter? Discov Med.
14:295–299. 2012.PubMed/NCBI
|
|
7
|
Tsim S, O'Dowd CA, Milroy R and Davidson
S: Staging of non-small cell lung cancer (NSCLC): A review. Respir
Med. 104:1767–1774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Théophile H, André M, Miremont-Salamé G,
Arimone Y and Bégaud B: Comparison of three methods (an updated
logistic probabilistic method, the Naranjo and Liverpool
algorithms) for the evaluation of routine pharmacovigilance case
reports using consensual expert judgement as reference. Drug Saf.
36:1033–1044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Belhekar MN, Taur SR and Munshi RP: A
study of agreement between the Naranjo algorithm and WHO-UMC
criteria for causality assessment of adverse drug reactions. Indian
J Pharmacol. 46:117–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lombardi D, Crivellari D, Scuderi C, Magri
MD, Spazzapan S, Sorio R, Di Lauro V, Scalone S and Veronesi A:
Long-term, weekly one-hour infusion of paclitaxel in patients with
metastatic breast cancer: A phase II monoinstitutional study.
Tumori. 90:285–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palma M, Mancuso A, Grifalchi F, Lugini A,
Pizzardi N and Cortesi E: Atrial fibrillation during adjuvant
chemotherapy with docetaxel: A case report. Tumori. 88:527–529.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moscetti L, Ramponi S, Maccaglia C,
Villanti P and Cortesi E: Atrial fibrillation in a patient with
non-small-cell carcinoma of the lung in the course of paclitaxel
therapy. Clin Ter. 149:377–379. 1998.(In Italian). PubMed/NCBI
|
|
13
|
National Cancer Institute: National Cancer
Institute Adverse Drug Reactions database. https://www.cancer.gov/.1993
|
|
14
|
Zamorano JL, Lancellotti P, Muñoz DR,
Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GY,
Lyon AR, et al: ESC Position Paper on cancer treatments and
cardiovascular toxicity developed under the auspices of the ESC
Committee for Practice Guidelines. Kardiol Pol. 74:1193–1233.
2016.(In Polish). View Article : Google Scholar : PubMed/NCBI
|