A wound of the skin is generally described as the
interruption of the epithelial surface caused by a physical or
thermal challenge (1). Skin wounds
have been extensively studied as their healing represents a
critical step in achieving homeostasis following a traumatic event.
Dependent on the severity of the damage, wounds are categorized
into either acute or chronic (2). To
date, chronic wounds have the highest economic impact as long term
treatment increases wound care costs (3). It is estimated that 1-2% of the
population of the developing world will experience a chronic wound
in their lifetime (4). According to
Brem et al (5), in 2007
chronic wounds had affected 6.5 million patients in the United
States, with an annual estimated health care expense of $25 billion
(6). However, to date, the actual
cost of chronic wound care in the United States is unknown
(7). There has been a relatively high
increase in the incidence of chronic wounds, and this may be
closely associated with the increase in factors which impair wound
healing, such as diabetes, obesity, or therapeutics such as
chemotherapy, steroids and non-steroidal anti-inflammatory drugs
(6).
The cost of chronic wound care represents a
complicated scenario for patients and health care systems, leading
to a necessity for the development of healing solutions which are
both quicker and more cost-effective. To date, the available wound
treatment therapeutics are: dressings, such as antimicrobial, films
and alginate; hydrocolloids, collagen products, gauze composites
and hydrogels; and active wound care (8). Active wound care represents the fastest
growth category (20.6% compound annual growth rate between
2016-2022) as it is an alternative that has a more specific action
and is more cost-effective (9).
Within the active wound care category, proteases from a range of
sources have been employed as successful agents in debridement
(10), enhancing wound healing
(11), coagulation (12) and keloid scar treatments (13). Of these, debridement comprises the
principal dermatological application in enzymatic wound care, a
proven and well-established principle (14).
The wound healing process is predominantly mediated
by matrix metalloproteinases (MMPs) (15-17).
Dysregulation of MMPs results in defective wound healing, which has
made them targets of study in cases of chronic wounds, diabetic
foot injury, keloid healing and burned skin (10). The topical application of non-human
proteases has demonstrated beneficial therapeutic effects in events
where MMPs fail due to dysregulation, for example in hemostasis
(18), wound closure (19) and debridement (20).
Debridement is the most widely explored enzymatic
wound care application, in which the most frequently used proteases
are collagenases, serine proteases and cysteine proteases. The
therapeutic activity of animal secretions from fish epithelial
mucus (21), maggot (Lucilia
sericata) secretory products (22) and snake venom (23) have also been demonstrated. These
secretions contain different types of proteases capable of
degrading the same substrates as MMPs. Besides these, no further
use cases, sources or types of proteases for wound healing were
found based on the currently available literature.
Through the present review, the context of enzymatic
wound care alternatives will be discussed, along with a comparison
of substrate homology of secreted proteases (SPs) and human MMPs.
This review will aid in the identification of which stages of the
wound healing process SPs may be used as therapeutic agents.
Debridement is the first step to enhance repair of
chronic wounds. According to the European Wound Management
Association, this procedure is considered a basic necessity to
induce the physiological process of tissue repair (24). Through debridement, necrotic tissue is
removed by external means to create a stable and healthy scaffold
for re-epithelialization (25). In
healthy individuals under normal circumstances, debridement is
performed naturally following clot formation by neutrophil-derived
MMPs and other components (26).
However, when the MMP machinery fails, there is an accumulation of
devitalized tissue. As a consequence, the steadiness of prolonged
catabolism diminishes re-epithelialization and results in chronic
wounding (27).
This failure represents an important baseline to
treat chronic wounds, as devitalized epithelium builds up a
physical barrier that precludes the healing process by interfering
with the repair machinery, mimicking signs of infection, providing
nutrients to anaerobic pathogenic agents, such as Clostridium
perfringens or Bacteroides sp., and promoting cytokine
production that in severe cases generates a septic response
(28).
Debridement can be performed through autolytic,
surgical, biological or enzymatic means (28). Of these, autolytic debridement is the
most conservative treatment strategy. It enhances the action of
endogenous phagocytic cells and proteases such as MMPs through
dressings that provide the ideal catalytic conditions for removal
of necrotic tissue (29). Among the
dressings available for autolysis, films (polydimethylsiloxane),
gauzes, hydrocolloids, hydrogels, alginates, hydrofibers and foams
have been proposed (25,30). This strategy is selective, painless,
inexpensive and suitable for most types of wounds (31). However, this process is slow,
dependent on suitable reaction conditions and on the physiological
response of the patient, and carries the risk of skin degradation
due to prolonged exposure to moisture (maceration) (32) within the surrounding skin (28).
Surgical debridement strategies are performed by
excising necrotic tissue until only healthy skin regions are
exposed (33). Available variants of
surgical debridement include ultrasound debridement,
plasma-mediated bipolar radio-frequency ablation, versa-jet (fluid
jet technology) and hydrosurgery (34,35).
Surgical debridement is the fastest and most effective route of
treatment, but is an expensive method that requires a sterile
surgical environment, trained practitioners, and specific
instruments, and is contraindicated for patients with clotting
disorders (28,36).
By contrast, biological debridement promotes the
removal of devitalized epithelium through the digestive action of
Lucilia sericata sterile maggots (31). Maggots are caged in wound-sized
hydrocolloid dressings that are placed in the affected area
(37). The secretion of several
components including proteolytic enzymes, such as trypsin and
chymotrypsin serine proteases, then catalyze non-viable skin into a
liquid feedstock that facilitates maggot feed (38). This alternative has proved to be
efficient in several types of chronic wounds (39) and ulcers (40,41) by
providing quick wound debridement, reduction in the use of
biofilms, disinfection from bacteria (40,42-45)
and improved pain control (46).
However, due to the negative image several societies impose on
maggots, this alternative has not been well accepted by patients
and practitioners (47). Furthermore,
it is contraindicated for the treatment of fistulae, exposed
vessels and wounds in proximity to vital organs (42).
A potential compromise is enzymatic debridement, in
which proteases from different sources (bacterial, vegetal or
animal) is applied to the wounded area to remove necrotic tissue
(48,49). Enzymatic debridement is selective and
suitable for infected wounds (36),
without the need for complex equipment or application procedures.
This alternative also takes less time and requires fewer
applications to accomplish debridement compared with dressings used
for autolytic treatments (50). Other
reported enzymatic wound healing approaches are anti- or
pro-coagulation through venom toxins from Bothrops sp.
(51,52). These enzymes may frequently be
inhibited by salts, temperature and hydrogen peroxide, which are
common elements of aseptic solutions. A stinging sensation and
exudate may also be observed as an after-effect of enzymatic
treatment (36).
From these four mentioned alternatives, three are
directly dependent on proteases to perform the debriding activity.
The direct or indirect use of proteases is therefore the second
most commonly used tool after surgical debridement. In the current
literature, the most commonly used proteases in direct enzymatic
debridement are bromelain, papain and bacterial collagenases
(53). Other enzymes have been
demonstrated to intervene as anti- or pro-coagulation agents and in
non-specific wound healing from animal secretions. The most common
commercially and non-commercially available proteases associated
with wound healing are listed in Table
I.
Animal secretions with high quantities of protease
content, including fish epithelial mucus and snake venom, have been
reported to enhance wound healing. Wound healing properties were
reported for the secreted mucus of the fish species Netuma
barba (54), Channa
striatus (55) and Clarias
gariepinus (56). A reduction in
healing time of almost 60% was achieved following the topical
application of mucus preparations in the wounds of mice, rats,
guinea pigs and humans (57). For
snake venom, anti- or pro-coagulation and epithelial cell migration
properties were observed with the toxins from the venom of
Bothrops moojeni, B. atrox (51), B. alternatus (18) and B. jararaca (58).
Thus far, the primary application of proteases in
wound treatment has been debridement. Information regarding the use
of proteases being used for other wound healing treatments is
scarce, suggesting that relatively little attempt has been made to
propose the use of proteases in different stages of the wound
healing process (57,59). Several therapeutic benefits have been
described from animal secretions, but studies on their possible use
in wound healing stages are limited. It may be beneficial to
determine whether the existing types of SPs present in animal
secretions with reported therapeutic effects (maggots, fish and
snakes), can mimic human MMPs.
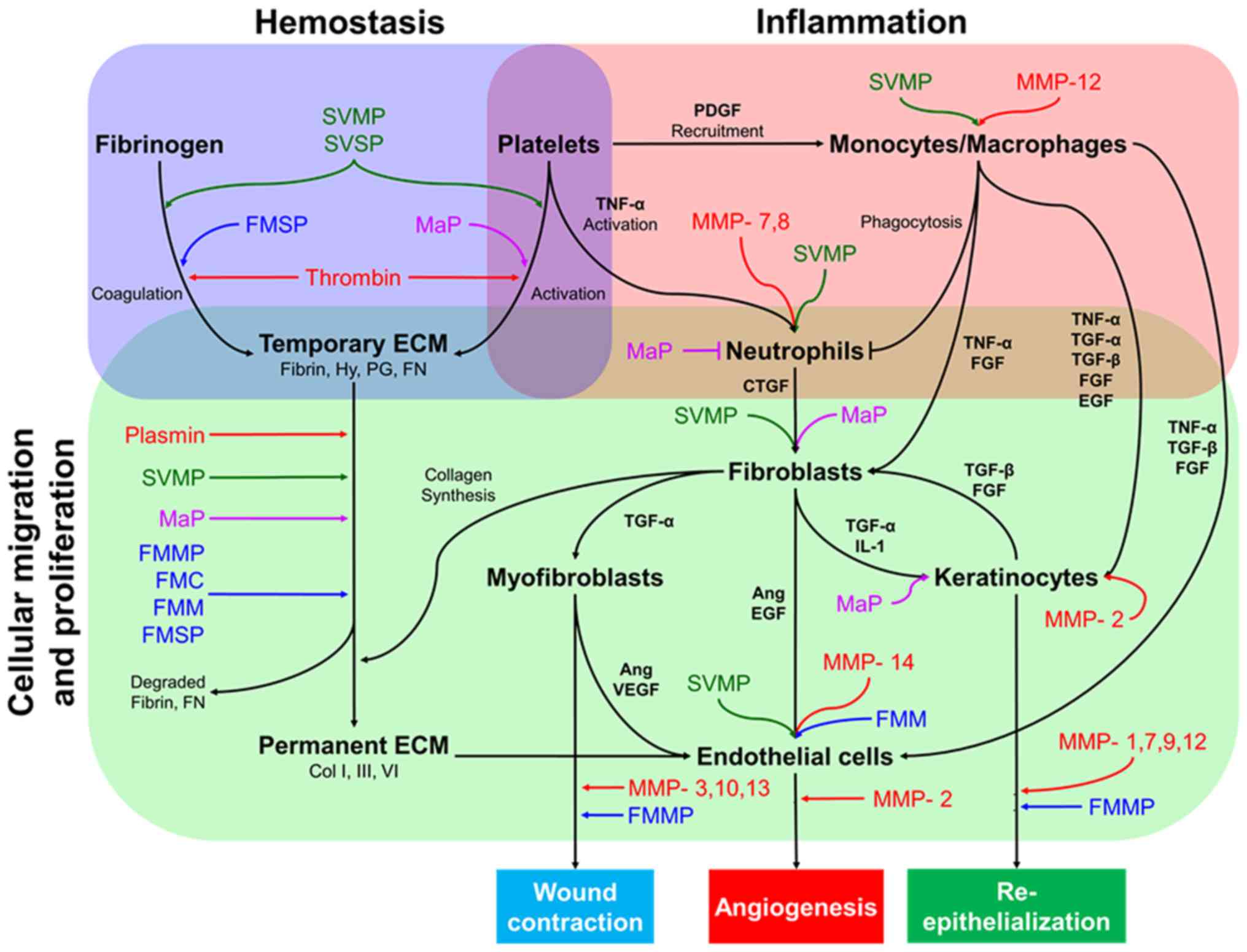
Wound healing is the process by which an epithelial
discontinuity is closed and is divided into four major steps:
Hemostasis, inflammation, cell migration-proliferation and skin
remodeling (60,61). The interaction and co-ordination of
several elements such as cytokines, growth factors, coagulation
elements, extracellular matrix (ECM) components, parenchymal cells
and MMPs (62,63) enable the correct progression of these
major steps (Fig. 1).
It has been reported that MMPs predominantly mediate
the wound healing process and are involved in several events in
each stage, including ECM degradation (64), cell proliferation/migration,
mesenchymal cell differentiation (65), wound contraction, angiogenesis and
re-epithelialization (66-68).
At present, 25 different MMP variants have been identified in the
human genome (64,69). Of these, 11 are responsible for skin
remodeling and wound healing (Table
II).
Maggot therapy efficiency in the treatment of
necrotic, infected chronic wounds is due to the activity of several
SPs. This secretion consists of serine proteases (trypsin-like and
chymotrypsin-like) and metalloproteases (71,72). As a
secretion, maggot proteases (MaPs) contribute to the wound healing
process, primarily in fibroblast stimulation and bacterial
disinfection. MaPs degrade fibrin clots and fibronectin (74), enhancing fibroblast metabolism and
migration (22,75). In addition, MaPs increase TGF-β
(transforming growth factor-β) signaling in wounds treated with
maggots (76), which enhances
endothelial cell and keratinocyte migration, thus promoting wound
closure. Furthermore, MaPs inhibit neutrophil migration and
decrease the production of pro-inflammatory mediators in
neutrophils and monocytes (44,77),
leading to recruitment of pro-angiogenic growth factors (78) and healthy granulation tissue (79). MaPs are also considered antimicrobial
enzymes (80), capable of eliminating
Staphylococcus aureus and Pseudomonas aeruginosa
(44,81) as well as degradation of biofilms
produced by S. epidermidis and S. aureus (41).
From MaPs, only a chymotrypsin-like protease has
been isolated from maggot secretions, which exhibited clotting and
proteolytic activity in fibronectin, suggesting its use in
hemostasis and for temporary collagen-rich replacement of ECM
(74,82). These proteases also reduce biofilms in
patients with leg ulcers (40,41).
Similar to maggot secretions, fish mucus and snake
venom have been hypothesized as wound healing treatment agents. In
traditional medicine, they have been used as a therapy for skin
burns and hemostasis (51,55,56,83). Fish
epithelial mucus consists primarily of glycoproteins and immune
biomolecules (84). Immune
components, metalloproteases, serine proteases, and cathepsins B, D
and L, have been identified in fish epithelial mucus (85,86).
Enzymatic components from crude secretions contribute to
accelerated clot formation and agglutination of red cells (87).
In the case of metalloproteases, fish matrix
metalloproteinases (FMMPs) 9 and 13 and fish mucus meprins (FMM)
have been described as components of fish mucosal secretions
(88,89). FMMPs 9 and 13 have analogous variants
in human tissue, which participate in wound contraction and
re-epithelialization (66,90). FMMs can degrade collagen IV, fibrillar
procollagen and fibronectin (91-93),
which are also degraded by MMPs 3, 10, 11 and 12 (Table II). These proteases are involved in
wound contraction, monocyte/macrophage metabolism and
re-epithelialization (66).
Cathepsins are a family of proteases that have been
identified in fish epithelial mucus, and these cathepsins in fish
mucus have not been characterized. It is hypothesized that the
cathepsins in fish mucus may exhibit a therapeutic effect on wound
healing based on the available data regarding their properties on
human skin. These proteases are normally present in lysosomal
vesicles, but their presence has also been demonstrated
extracellularly (94). In human
physiology, they participate in wound healing during hemostasis
(95), ECM remodeling (96) and keratinocyte migration (97). Cathepsin-L substrate affinity has been
described for laminins, fibronectin, elastin and collagen (98,99).
Cathepsin-D has affinity for fibronectin, proteoglycans, and
collagens I and II (100), while
substrate affinity of Cathepsin-B has been described primarily for
collagen II, IX and XI (101). These
substrates are also target proteins for MMPs 1, 8, 13 and 14
(66,102), which supports the reported role of
cathepsins in wound contraction and hemostasis.
Additionally, fish mucus serine proteases (FMSPs)
are present in mucosal secretions (103), albeit with only poor substrate
characterization thus far. Nevertheless, this family of proteases
has reported activity on collagen, elastin, fibrin and fibrinogen
(104,105). Thus, this protease may be useful
during hemostasis, generating platelet aggregation and fibrin clot
formation (106). Additionally,
FMSPs degrade fibrin, which may assist in the change of ECM from
temporary to collagen-rich, resulting in cellular proliferation and
migration (107). This family of
enzymes also interferes with the maturation of MMPs (66) and the desquamation processes (108).
SVMPs can intervene in hemostasis, as these
hydrolyze glycoprotein Ib and factor X, which promote coagulation
(110-112)
and platelet aggregation (113,114),
respectively. During inflammation, SVMPs enhance the infiltration
of inflammatory cells (115,116) as well as increasing neutrophil and
macrophage numbers (117-119),
which increases soluble collagen levels and enhances angiogenesis
through increasing vascular endothelial growth factor (VEGF) and
TGF-β1 release (58). During cell
migration and proliferation, it has been demonstrated that SVMPs
degrade fibrin and fibronectin (112,120),
resulting in the change from temporary to collagen-rich ECM. SVMPs
also activate migration of skin fibroblasts (121) and endothelial cells (111,122-124).
In addition, SVSPs exhibit proteolytic activity on Factor V and
fibrinogen, promoting fibrin clot formation (125-127).
SVSPs also promote aggregation of platelets (128).
Following analysis of reported interventions of SPs
in wound healing, it could be presumed that they can intervene as
helpers in several intermediate steps of the wound healing
processes including coagulation, ECM degradation for
re-epithelialization, or wound contraction, among other steps. The
hypothesized mechanisms of SPs during the process of wound healing
are presented in Fig. 1. Study of
these variants may assist in the development of novel specific
alternatives for active chronic wound healing care.
Substrate homology analysis among MMPs and SPs
suggest that animal enzymes may act similarly to the ones
physiologically present in human skin. As presented in Fig. 1, previously compared SPs may be used
to facilitate several steps involved in the process of wound
healing, or to compensate for the physiological variants when they
do not function properly. To understand this from a clearer
perspective, it is important to comprehend in which of the most
common chronic wounds types SPs may serve as suitable
co-adjuvants.
In the current literature, chronic wounds have been
classified into pressure ulcers, venous ulcers or diabetic ulcers
(129,130). Pressure ulcers are caused by
pressure, shear force, friction or a combination of these (131). The prevention and cure of pressure
ulcers is associated with daily movement of extremities and
frequent body positioning during hospitalization (132). In this case, the use of proteases
may serve as palliative care in bed preparation for wounded
patients as opposed to assisting the metabolic processes of wound
healing.
Chronic venous ulcers are associated with
inflammation, mechanical damage and erratic structural remodeling
of the vein. Pathological hemodynamics results in changes to
microcirculation; this produces thrombosis, proinflammatory
activity and impaired MMP-3 activity (133), leading to cell dysfunction and
finally to ulceration (134). For
ulceration and potential necrosis, maggot therapy has shown
efficacy (40,41) by decreasing inflammation and
neutrophil migration (77,135). It also degrades eschar, debrides the
wound and serves as a bacterial disinfectant (40,42-45).
Furthermore, fish mucus proteases have been shown to exhibit
antibacterial activity (55,136), which may be useful for bacterial
disinfection of ulcers.
Diabetic foot ulcers are wounds that manifest after
a cascade of metabolic dysregulations initiated by long-term
hyperglycemia (137). As a result of
prolonged exposure to high blood sugar levels, there is a decrease
in fibrinolytic activity, thus increasing blood viscosity and
coagulation in this type of wound (138). In addition, hyperglycemia results in
a reduction of growth factors and receptor levels (such as TGF-β1),
accompanied by a prolonged inflammatory phase due to upregulation
of MMP-9 (139,140), which interrupts the inflammatory and
proliferative phases of wound healing (141).
As an alternative therapy for diabetic foot ulcers,
maggot treatment has demonstrated improved efficacy and efficiency
compared with conventional methods (142). Furthermore, MaPs (74), FMMPs (91) and a certain type of SVMP (112,120)
have been reported to exhibit fibrinolytic activity which may
ameliorate the characteristic viscosity of diabetic ulcers.
Additionally, it has been reported that TGF-β signaling is
increased in the presence of MaPs (76) and SVMPs (58), and this may also assist wound healing
in this type of ulcer. However, certain SVMPs can promote
coagulation (110-112,120);
thus, meticulous care must be taken to separate and study each
component embedded within the secretion instead of applying it as a
whole.
In another report, fish mucus application enhanced
the healing of laparotomy wounds (143). Therefore, SPs may be used to reduce
the time taken for wound healing or for the removal of necrotic
tissue, depending on the wound pathophysiology.
Despite the positive effects of SPs in wound
healing, further research must be performed to determine the
specific mechanisms of action, regulation, site delivery and
bioavailability of proposed proteases before they may be
recommended as feasible pharmacological candidates for treatment of
chronic wounds. The application of SPs may be limited however, as
its use for treatment of burn wounds exhibits highly variable
results in patients (14).
It is also important to determine how SPs may affect
other wound healing mechanisms when used as an adjuvant with other
healing methods such as skin transplants. In this procedure, lost
skin is covered with healthy tissue or artificial composites
(144,145) that provide the necessary elements
(cells, growth factors, MMPs and scaffolds) for the healing process
(146). The success of a skin
transplant is primarily dependent on angiogenesis between the skin
graft and the injury, which is predominantly mediated by MMP-2, 9
and 14(147). Thus, SPs have been
proposed as potential adjuvants to increase tissue compatibility
during skin transplants.
Nevertheless, studies on SP-aided transplants is
still ambiguous. For example, the use of botulinum toxin A during
skin transplantation in murine models enhances the expression of
VEGF and prolonged the survival of skin grafts (148). By contrast, Kucukkaya et al
(149) demonstrated that the same
toxin reduces wound-graft contraction. Thus, the effects of SPs on
skin transplants requires additional studies to determine its
benefits during skin transplantation.
Studies and development of less expensive wound
healing treatment alternatives must be encouraged. Treatment of all
types of even the most common chronic wounds still incur a high
cost, and the reported care expenses are $50,000 for a diabetic
ulcer (25), $500-$70,000 dollars for
a pressure ulcer (150) and
$390-$50,967 dollars per venous ulcer (151). The proposal of proteases obtained
from animal secretions is a promising area to explore, as these act
on specific substrates involved in the wound healing process.
Furthermore, it is important to determine the molecular events
specific to each chronic wound case, as these may represent key
tags on how the proposed SPs may intervene. Under these conditions,
active wound care represents a viable solution if its use is based
on specific requirements. Importantly, SP characterization is
crucial to dispense with the use of secretions in wound repair, and
instead use only the SPs. This may also allow heterologous
production, immobilization or improvement of the therapeutic
properties of the characterized SPs through mutagenesis. In
addition, time-efficient diagnostic tests on for detection of
molecular targets in skin wound healing may be developed to guide
practitioners on which tool to use for chronic wound care,
resulting in improved wound healing and thus restoration of
homeostasis.
Not applicable.
The present study was funded by CONACYT (grant nos.
886264 and 548216).
Not applicable.
All authors (MIAR, DMM, CLC, JMAY, JB and MLS)
contributed to writing, editing and revising the manuscript. All
authors approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Dhivya S, Padma VV and Santhini E: Wound
dressings-a review. Biomedicine (Taipei). 5(22)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nicoli Aldini N, Fini M and Giardino R:
From Hippocrates to tissue engineering: Surgical strategies in
wound treatment. World J Surg. 32:2114–2121. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Sen CK: Human wounds and its burden: An
updated compendium of estimates. Adv Wound Care (New Rochelle).
8:39–48. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Järbrink K, Ni G, Sönnergren H,
Schmidtchen A, Pang C, Bajpai R and Car J: Prevalence and incidence
of chronic wounds and related complications: A protocol for a
systematic review. Syst Rev. 5(152)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Brem H, Stojadinovic O, Diegelmann RF,
Entero H, Lee B, Pastar I, Golinko M, Rosenberg H and Tomic-Canic
M: Molecular markers in patients with chronic wounds to guide
surgical debridement. Mol Med. 13:30–39. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Anderson K and Hamm RL: Factors that
impair wound healing. J Am Coll Clin Wound Spec. 4:84–91.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nussbaum SR, Carter MJ, Fife CE, DaVanzo
J, Haught R, Nusgart M and Cartwright D: An economic evaluation of
the impact, cost, and medicare policy implications of chronic
nonhealing wounds. Value Health. 21:27–32. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Avila Rodríguez MI, Rodríguez Barroso LG
and Sánchez ML: Collagen: A review on its sources and potential
cosmetic applications. J Cosmet Dermatol. 17:20–26. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Malik M: Advanced wound care market by
product type (Infection Management, Exudate Management, Active
Wound Care, Therapy Devices), application (Chronic Wounds and Acute
Wounds), end user (Hospitals and Community Centers)-global
opportunity analysis and industry forecast, 2014-2022. 2016.
|
|
10
|
Khan W and Morgan-Jones R: Debridement:
Defining something we all do. J Trauma Orthop. 4(48)2016.
|
|
11
|
Kwan SH and Ismail MN: Identification of
the potential bio-active proteins associated with wound healing
properties in snakehead fish (Channa striata) mucus. Curr
Proteomics. 15:299–312. 2018. View Article : Google Scholar
|
|
12
|
Fatima L and Fatah C: Pathophysiological
and pharmacological effects of snake venom components: Molecular
targets. J Clin Toxicol. 4(190)2014.
|
|
13
|
Fierro-Arias L, Campos-Cornejo NG,
Contreras-Ruiz J, Espinosa-Maceda S, López-Gehrke I,
Márquez-Cárdenas R, Ramírez-Padilla M, Veras-Castillo E and
Rodríguez-Alcocer AN: Productos enzimáticos (hialuronidasa,
colagenasa y lipasa) y su uso en dermatología. Dermatol Rev Mex.
61:206–219. 2017.
|
|
14
|
Klasen HJ: A review on the nonoperative
removal of necrotic tissue from burn wounds. Burns. 26:207–222.
2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gill SE and Parks WC: Metalloproteinases
and their inhibitors: Regulators of wound healing. Int J Biochem
Cell Biol. 40:1334–1347. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ayuk SM, Abrahamse H and Houreld NN: The
role of matrix metalloproteinases in diabetic wound healing in
relation to photobiomodulation. J Diabetes Res.
2016(2897656)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mclennan SV, Min D and Yue DK: Matrix
metalloproteinases and their roles in poor wound healing in
diabetes. Wound Pract Res. 16:116–120. 2008.
|
|
18
|
De Marco Almeida F, de Castro Pimenta AM,
Oliveira MC and De Lima ME: Venoms, toxins and derivatives from the
Brazilian fauna: Valuable sources for drug discovery. Sheng Li Xue
Bao. 67:261–270. 2015.PubMed/NCBI
|
|
19
|
Riley KN and Herman IM: Collagenase
promotes the cellular responses to injury and wound healing in
vivo. J Burns Wounds. 4(e8)2005.PubMed/NCBI
|
|
20
|
Muhammad I, Shaikh SA and Rashid HU: Role
of papaya dressings in the management of diabetic foot ulcers. J
Rawalpindi Med College. 18:87–89. 2014.
|
|
21
|
Esteban MÁ: An overview of the
immunological defenses in fish skin. ISRN Immunol.
2012(853470)2012. View Article : Google Scholar
|
|
22
|
Horobin AJ, Shakesheff KM and Pritchard
DI: Maggots and wound healing: an investigation of the effects of
secretions from Lucilia sericata larvae upon the migration of human
dermal fibroblasts over a fibronectin-coated surface. Wound Repair
Regen. 13:422–433. 2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rajesh R, Raghavendra Gowda CD, Nataraju
A, Dhananjaya BL, Kemparaju K and Vishwanath BS: Procoagulant
activity of Calotropis gigantea latex associated with
fibrin(ogen)olytic activity. Toxicon. 46:84–92. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
White R: The costs of wound debridement
and exudate management. Br J Health Care Manag. 21:172–175. 2015.
View Article : Google Scholar
|
|
25
|
Han G and Ceilley R: Chronic wound
healing: A review of current management and treatments. Adv Ther.
34:599–610. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sinclair RD and Ryan TJ: Proteolytic
enzymes in wound healing: The role of enzymatic debridement.
Australas J Dermatol. 35:35–41. 1994.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Glyantsev SP, Savvina TV and Zayets TL:
Comparative study of proteolytic enzymes used for debridement of
purulent wounds. Bull Exp Biol Med. 121:646–650. 1996. View Article : Google Scholar
|
|
28
|
Gray D, Acton C, Chadwick P, Fumarola S,
Leaper D, Morris C, Stang D, Vowden K, Vowden P and Young T:
Consensus guidance for the use of debridement techniques in the UK.
Wounds UK. 7:77–84. 2011.
|
|
29
|
Atkin L: Understanding methods of wound
debridement. Br J Nurs. (23)S10-S12, S14-S15:2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Dabiri G, Damstetter E and Phillips T:
Choosing a wound dressing based on common wound characteristics.
Adv Wound Care (New Rochelle). 5:32–41. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Manna B and Morrison CA: Wond debridement.
StatPearls. 2019.
|
|
32
|
Cutting K and White R: Maceration of the
skin and wound bed. 1: Its nature and causes. J Wound Care.
11:275–278. 2002. View Article : Google Scholar
|
|
33
|
Mahoney J and Ward J: Surgical
debridement. In: Surgery in wounds. Téot L, Banwell PE and Ziegler
UE (eds.) Springer Berlin Heidelberg, Berlin, Heidelberg. 67–71.
2004.
|
|
34
|
Bekara F, Vitse J, Fluieraru S, Masson R,
Runz A, Georgescu V, Bressy G, Labbé JL, Chaput B and Herlin C: New
techniques for wound management: A systematic review of their role
in the management of chronic wounds. Arch Plast Surg. 45:102–110.
2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Liu W, Ma K, Kwon SH, Garg R, Patta YR,
Fujiwara T and Gurtner GC: The abnormal architecture of healed
diabetic ulcers is the result of FAK degradation by calpain 1. J
Invest Dermatol. 137:1155–1165. 2017. View Article : Google Scholar
|
|
36
|
Ayello EA and Cuddigan JE: Debridement:
Controlling the necrotic/cellular burden. Adv Skin Wound Care.
17:66–75. quiz:76–78. 2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Whitaker IS, Twine C, Whitaker MJ, Welck
M, Brown CS and Shandall A: Larval therapy from antiquity to the
present day: Mechanisms of action, clinical applications and future
potential. Postgrad Med J. 83:409–413. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gray M: Is larval (maggot) debridement
effective for removal of necrotic tissue from chronic wounds? J
Wound Ostomy Continence Nurs. 35:378–384. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jordan A, Khiyani N, Bowers SR, Lukaszczyk
JJ and Stawicki SP: Maggot debridement therapy: A practical review.
Int J Acad Med. 4:21–34. 2018. View Article : Google Scholar
|
|
40
|
Brown A, Horobin A, Blount DG, Hill PJ,
English J, Rich A, Williams PM and Pritchard DI: Blow fly Lucilia
sericata nuclease digests DNA associated with wound slough/eschar
and with Pseudomonas aeruginosa biofilm. Med Vet Entomol.
26:432–439. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Harris LG, Nigam Y, Sawyer J, Mack D and
Pritchard DI: Lucilia sericata chymotrypsin disrupts protein
adhesin-mediated staphylococcal biofilm formation. Appl Environ
Microbiol. 79:1393–1395. 2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Parnés A and Lagan KM: Larval therapy in
wound management: A review. Int J Clin Pract. 61:488–493.
2007.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Cazander G, Pritchard DI, Nigam Y, Jung W
and Nibbering PH: Multiple actions of Lucilia sericata larvae in
hard-to-heal wounds: Larval secretions contain molecules that
accelerate wound healing, reduce chronic inflammation and inhibit
bacterial infection. Bioessays. 35:1083–1092. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
van der Plas MJ, Jukema GN, Wai SW,
Dogterom-Ballering HC, Lagendijk EL, van Gulpen C, van Dissel JT,
Bloemberg GV and Nibbering PH: Maggot excretions/secretions are
differentially effective against biofilms of Staphylococcus aureus
and Pseudomonas aeruginosa. J Antimicrob Chemother. 61:117–122.
2008.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Pritchard DI and Brown AP: Degradation of
MSCRAMM target macromolecules in VLU slough by Lucilia sericata
chymotrypsin 1 (ISP) persists in the presence of tissue gelatinase
activity. Int Wound J. 12:414–421. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Arabloo J, Grey S, Mobinizadeh M,
Olyaeemanesh A, Hamouzadeh P and Khamisabadi K: Safety,
effectiveness and economic aspects of maggot debridement therapy
for wound healing. Med J Islam Repub Iran. 30(319)2016.PubMed/NCBI
|
|
47
|
Evans H: A treatment of last resort. Nurs
Times. 93:62–65. 1997.PubMed/NCBI
|
|
48
|
Ramundo J and Gray M: Enzymatic wound
debridement. J Wound Ostomy Continence Nurs. 35:273–280.
2008.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Madhok BM, Vowden K and Vowden P: New
techniques for wound debridement. Int Wound J. 10:247–251.
2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ziegler B, Hundeshagen G, Cordts T, Kneser
U and Hirche C: State of the art in enzymatic debridement. Plast
Aesthet Res. 5(33)2018. View Article : Google Scholar
|
|
51
|
Waheed H, Moin SF and Choudhary MI: Snake
venom: From deadly toxins to life-saving therapeutics. Curr Med
Chem. 24:1874–1891. 2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chan YS, Cheung RCF, Xia L, Wong JH, Ng TB
and Chan WY: Snake venom toxins: Toxicity and medicinal
applications. Appl Microbiol Biotechnol. 100:6165–6181.
2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Smith RG: Enzymatic debriding agents: An
evaluation of the medical literature. Ostomy Wound Manage.
54:16–34. 2008.PubMed/NCBI
|
|
54
|
Costa-Neto EM: Implications and
applications of folk zootherapy in the state of Bahia, Northeastern
Brazil. Sust Dev. 12:161–174. 2004. View
Article : Google Scholar
|
|
55
|
Manan Mat Jais A: Pharmacognosy and
pharmacology of Haruan (Channa striatus), a medicinal fish with
wound healing properties. Bol Latinoam Caribe Plant Med Aromaticas.
6:52–60. 2007.
|
|
56
|
Akunne TC, Okafor SN, Okechukwu DC,
Nwankwor SS, Emene JO and Okoro BN: Catfish (Clarias gariepinus)
slime coat possesses antimicrobial and wound healing activities. UK
J Pharm Biosci. 4:81–87. 2016. View Article : Google Scholar
|
|
57
|
Al-Hassan J, Thomson M and Griddle RS:
Accelerated wound healing by a preparation from skin of the Arabian
gulf catfish. Lancet. 321:1043–1044. 1983.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ferreira BA, Deconte SR, de Moura FBR,
Tomiosso TC, Clissa PB, Andrade SP and Araújo FA: Inflammation,
angiogenesis and fibrogenesis are differentially modulated by
distinct domains of the snake venom metalloproteinase jararhagin.
Int J Biol Macromol. 119:1179–1187. 2018.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ferreira RS Jr, de Barros LC, Abbade LPF,
Barraviera SRCS, Silvares MRC, de Pontes LG, Dos Santos LD and
Barraviera B: Heterologous fibrin sealant derived from snake venom:
From bench to bedside-an overview. J Venom Anim Toxins Incl Trop
Dis. 23(21)2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wang PH, Huang BS, Horng HC, Yeh CC and
Chen YJ: Wound healing. J Chin Med Assoc. 81:94–101.
2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Sorg H, Tilkorn DJ, Hager S, Hauser J and
Mirastschijski U: Skin wound healing: An update on the current
knowledge and concepts. Eur Surg Res. 58:81–94. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Clark RAF: Wound repair: Overview and
general considerations. In: Clark RAF (ed): The molecular, cellular
biology of wound repair, Plenum Press, New York. 3–55. 1996.
|
|
63
|
Martin P and Nunan R: Cellular and
molecular mechanisms of repair in acute and chronic wound healing.
Br J Dermatol. 173:370–378. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Cui N, Hu M and Khalil RA: Biochemical and
biological attributes of matrix metalloproteinases. Prog Mol Biol
Transl Sci. 147:1–73. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Greaves NS, Ashcroft KJ, Baguneid M and
Bayat A: Current understanding of molecular and cellular mechanisms
in fibroplasia and angiogenesis during acute wound healing. J
Dermatol Sci. 72:206–217. 2013.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Caley MP, Martins VL and O'Toole EA:
Metalloproteinases and wound healing. Adv Wound Care (New
Rochelle). 4:225–234. 2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Krampert M, Bloch W, Sasaki T, Bugnon P,
Rülicke T, Wolf E, Aumailley M, Parks WC and Werner S: Activities
of the matrix metalloproteinase stromelysin-2 (MMP-10) in matrix
degradation and keratinocyte organization in wounded skin. Mol Biol
Cell. 15:5242–5254. 2004.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Matziari M, Dive V and Yiotakis A: Matrix
metalloproteinase 11 (MMP-11; stromelysin-3) and synthetic
inhibitors. Med Res Rev. 27:528–552. 2007.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Gomis-Rüth FX: Structural aspects of the
metzincin clan of metalloendopeptidases. Mol Biotechnol.
24:157–202. 2003.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Subramanian S, MacKinnon SL and Ross NW: A
comparative study on innate immune parameters in the epidermal
mucus of various fish species. Comp Biochem Physiol B Biochem Mol
Biol. 148:256–263. 2007.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Franta Z, Vogel H, Lehmann R, Rupp O,
Goesmann A and Vilcinskas A: Next generation sequencing identifies
five major classes of potentially therapeutic enzymes secreted by
Lucilia sericata medical maggots. Biomed Res Int.
2016(8285428)2016. View Article : Google Scholar
|
|
72
|
Valachova I, Majtan T, Takac P and Majtan
J: Identification and characterisation of different proteases in
Lucilia sericata medicinal maggots involved in maggot debridement
therapy. J Appl Biomed. 12:171–177. 2014. View Article : Google Scholar
|
|
73
|
Tasoulis T and Isbister GK: A review and
database of snake venom proteomes. Toxins (Basel). 9(pii:
E290)2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Chambers L, Woodrow S, Brown AP, Harris
PD, Phillips D, Hall M, Church JC and Pritchard DI: Degradation of
extracellular matrix components by defined proteinases from the
greenbottle larva Lucilia sericata used for the clinical
debridement of non-healing wounds. Br J Dermatol. 148:14–23.
2003.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Polakovicova S, Polák Š, Kuniaková M,
Čambal M, Čaplovičová M, Kozánek M, Danišovič L and Kopáni M: The
effect of salivary gland extract of Lucilia sericata maggots on
human dermal fibroblast proliferation within collagen/hyaluronan
membrane in vitro: Transmission electron microscopy study. Adv Skin
Wound Care. 28:221–226. 2015.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Li PN, Li H, Zhong LX, Sun Y, Yu LJ, Wu
ML, Zhang LL, Kong QY, Wang SY and Lv DC: Molecular events
underlying maggot extract promoted rat in vivo and human in vitro
skin wound healing. Wound Repair Regen. 23:65–73. 2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
van der Plas MJA, van der Does AM, Baldry
M, Dogterom-Ballering HC, van Gulpen C, van Dissel JT, Nibbering PH
and Jukema GN: Maggot excretions/secretions inhibit multiple
neutrophil pro-inflammatory responses. Microbes Infect. 9:507–514.
2007.PubMed/NCBI View Article : Google Scholar
|
|
78
|
van der Plas MJ, van Dissel JT and
Nibbering PH: Maggot secretions skew monocyte-macrophage
differentiation away from a pro-inflammatory to a pro-angiogenic
type. PLoS One. 4(e8071)2009.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Honda K, Okamoto K, Mochida Y, Ishioka K,
Oka M, Maesato K, Ikee R, Moriya H, Hidaka S, Ohtake T, et al: A
novel mechanism in maggot debridement therapy: Protease in
excretion/secretion promotes hepatocyte growth factor production.
Am J Physiol Cell Physiol. 301(C1423-C1430)2011.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Andersen AS, Sandvang D, Schnorr KM, Kruse
T, Neve S, Joergensen B, Karlsmark T and Krogfelt KA: A novel
approach to the antimicrobial activity of maggot debridement
therapy. J Antimicrob Chemother. 65:1646–1654. 2010.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Margolin L and Gialanella P: Assessment of
the antimicrobial properties of maggots. Int Wound J. 7:202–204.
2010.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Pöppel AK, Kahl M, Baumann A, Wiesner J,
Gökçen A, Beckert A, Preissner KT, Vilcinskas A and Franta Z: A
Jonah-like chymotrypsin from the therapeutic maggot Lucilia
sericata plays a role in wound debridement and coagulation. Insect
Biochem Mol Biol. 70:138–147. 2016.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Mukherjee S, Gomes A and Dasgupta S: Zoo
therapeutic uses of snake body parts in folk & traditional
medicine. J Zool Res. 1:1–9. 2017.
|
|
84
|
Shephard KL: Functions for fish mucus. Rev
Fish Biol Fisheries. 4:401–429. 1994. View Article : Google Scholar
|
|
85
|
Dash S, Das SK, Samal J and Thatoi HN:
Epidermal mucus, a major determinant in fish health: A review. Iran
J Vet Res. 19:72–81. 2018.PubMed/NCBI
|
|
86
|
Sveen L, Timmerhaus GF, Torgersen J,
Ytteborg E, Jørgensen SM, Handeland SO, Stefansson SO, Nilsen TO,
Calabrese S, Ebbesson LOE, et al: Impact of fish density and
specific water flow on skin properties in Atlantic salmon (Salmo
salar L.) post-smolts. Aquaculture. 464:629–637. 2016. View Article : Google Scholar
|
|
87
|
Al-Hassan JM, Thomson M, Criddle KR,
Summers B and Criddle RS: Catfish epidermal secretions in response
to threat or injury. Marine Biol. 88:117–123. 1985. View Article : Google Scholar
|
|
88
|
Krasnov A, Skugor S, Todorcevic M, Glover
KA and Nilsen F: Gene expression in Atlantic salmon skin in
response to infection with the parasitic copepod Lepeophtheirus
salmonis, cortisol implant, and their combination. BMC Genomics.
13(130)2012.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Schütte A, Lottaz D, Sterchi EE, Stöcker W
and Becker-Pauly C: Two alpha subunits and one beta subunit of
meprin zinc-endopeptidases are differentially expressed in the
zebrafish Danio rerio. Biol Chem. 388:523–531. 2007.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Nguyen TT, Mobashery S and Chang M: Roles
of Matrix Metalloproteinases in Cutaneous Wound Healing. Wound
Healing-New insights into Ancient Challenges. 2016.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Sterchi EE, Stöcker W and Bond JS:
Meprins, membrane-bound and secreted astacin metalloproteinases.
Mol Aspects Med. 29:309–328. 2008.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Bertenshaw GP, Turk BE, Hubbard SJ,
Matters GL, Bylander JE, Crisman JM, Cantley LC and Bond JS: Marked
differences between metalloproteases meprin A and B in substrate
and peptide bond specificity. J Biol Chem. 276:13248–13255.
2001.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Kruse MN, Becker C, Lottaz D, Köhler D,
Yiallouros I, Krell HW, Sterchi EE and Stöcker W: Human meprin
alpha and beta homo-oligomers: Cleavage of basement membrane
proteins and sensitivity to metalloprotease inhibitors. Biochem J.
378:383–389. 2004.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Sun H, Lou X, Shan Q, Zhang J, Zhu X,
Zhang J, Wang Y, Xie Y, Xu N and Liu S: Proteolytic characteristics
of cathepsin D related to the recognition and cleavage of its
target proteins. PLoS One. 8(e65733)2013.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Wolters BK: Cathepsin L and V in human
keratinocytes. J Univ. 2006.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Vidak E, Javoršek U, Vizovišek M and Turk
B: Cysteine cathepsins and their extracellular roles: Shaping the
microenvironment. Cells. 8(pii: E264)2019.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Reinheckel T, Hagemann S, Dollwet-Mack S,
Martinez E, Lohmüller T, Zlatkovic G, Tobin DJ, Maas-Szabowski N
and Peters C: The lysosomal cysteine protease cathepsin L regulates
keratinocyte proliferation by control of growth factor recycling. J
Cell Sci. 118:3387–3395. 2005.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Mason RW: Interaction of lysosomal
cysteine proteinases with α2-macroglobulin: Conclusive evidence for
the endopeptidase activities of cathepsins B and H. Arch Biochem
Bioph. 273:367–374. 1989.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Maciewicz RA, Etherington DJ, Kos J and
Turk V: Collagenolytic cathepsins of rabbit spleen: A kinetic
analysis of collagen degradation and inhibition by chicken
cystatin. Coll Relat Res. 7:295–304. 1987. View Article : Google Scholar
|
|
100
|
Benes P, Vetvicka V and Fusek M: Cathepsin
D-many functions of one aspartic protease. Crit Rev Oncol Hematol.
68:12–28. 2008.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Cavallo-Medved D, Moin K and Sloane B:
Cathepsin B: Basis sequence: Mouse. AFCS Nat Mol Pages. 2011(pii:
A000508)2011.PubMed/NCBI
|
|
102
|
Krejner A, Litwiniuk M and Grzela T:
Matrix metalloproteinases in the wound microenvironment:
Therapeutic perspectives. Chronic Wound Care Manag Res. 3:29–39.
2016. View Article : Google Scholar
|
|
103
|
Kim GY, Kim HY, Kim HT, Moon JM, Kim CH,
Kang S and Rhim H: HtrA1 is a novel antagonist controlling
fibroblast growth factor (FGF) signaling via cleavage of FGF8. Mol
Cell Biol. 32:4482–4492. 2012. View Article : Google Scholar
|
|
104
|
Meyer-Hoffert U and Schröder JM: Epidermal
proteases in the pathogenesis of rosacea. J Investig Dermatol Symp
Proc. 15:16–23. 2011.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Kim SK, Park PJ, Kim JB and Shahidi F:
Purification and characterization of a collagenolytic protease from
the filefish, Novoden modestrus. J Biochem Mol Biol. 35:165–171.
2002.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Coughlin SR: Thrombin signalling and
protease-activated receptors. Nature. 407:258–264. 2000.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Perona JJ and Craik CS: Structural basis
of substrate specificity in the serine proteases. Protein Sci.
4:337–360. 1995.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Rawlings AV and Voegeli R: Stratum corneum
proteases and dry skin conditions. Cell Tissue Res. 351:217–235.
2013.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Gutiérrez JM, Escalante T, Rucavado A,
Herrera C and Fox JW: A Comprehensive view of the structural and
functional alterations of extracellular matrix by snake venom
metalloproteinases (SVMPs): Novel perspectives on the
pathophysiology of envenoming. Toxins (Basel). 8(pii:
E304)2016.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Kini RM and Koh CY: Metalloproteases
affecting blood coagulation, fibrinolysis and platelet aggregation
from snake venoms: Definition and nomenclature of interaction
sites. Toxins (Basel). 8(pii: E284)2016.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Silva MB, Schattner M, Ramos CR,
Junqueira-de-Azevedo IL, Guarnieri MC, Lazzari MA, Sampaio CA,
Pozner RG, Ventura JS, Ho PL and Chudzinski-Tavassi AM: A
prothrombin activator from Bothrops erythromelas (jararaca-da-seca)
snake venom: Characterization and molecular cloning. Biochem J.
369:129–139. 2003.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Sanchez EF, Richardson M, Gremski LH,
Veiga SS, Yarleque A, Niland S, Lima AM, Estevao-Costa MI and Eble
JA: Data for a direct fibrinolytic metalloproteinase,
barnettlysin-I from Bothrops barnetti (barnett(,)s pitviper) snake
venom with anti-thrombotic effect. Data Brief. 7:1609–1613.
2016.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Kamiguti AS: Platelets as targets of snake
venom metalloproteinases. Toxicon. 45:1041–1049. 2005.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Howes JM, Kamiguti AS, Theakston RD,
Wilkinson MC and Laing GD: Effects of three novel
metalloproteinases from the venom of the West African saw-scaled
viper, Echis ocellatus on blood coagulation and platelets. Biochim
Biophys Acta. 1724:194–202. 2005.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Fernandes CM, Zamuner SR, Zuliani JP,
Rucavado A, Gutiérrez JM and Teixeira Cde F: Inflammatory effects
of BaP1 a metalloproteinase isolated from Bothrops asper snake
venom: Leukocyte recruitment and release of cytokines. Toxicon.
47:549–559. 2006.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Silva A, Gunawardena P, Weilgama D,
Maduwage K and Gawarammana I: Comparative in-vivo toxicity of
venoms from South Asian hump-nosed pit vipers (Viperidae:
Crotalinae: Hypnale). BMC Res Notes. 5(471)2012.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Mariano-Oliveira A, Coelho ALJ, Terruggi
CH, Selistre-de-Araújo HS, Barja-Fidalgo C and De Freitas MS:
Alternagin-C, a nonRGD-disintegrin, induces neutrophil migration
via integrin signaling. Eur J Biochem. 270:4799–4808.
2003.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Silva CA, Zuliani JP, Assakura MT, Mentele
R, Camargo ACM, Teixeira CFP and Serrano SMT: Activation of
αMβ2-mediated phagocytosis by HF3, a P-III class metalloproteinase
isolated from the venom of Bothrops jararaca. Biochem Biophys Res
Commun. 322:950–956. 2004. View Article : Google Scholar
|
|
119
|
Tseng YL, Lee CJ and Huang TF: Effects of
a snake venom metalloproteinase, triflamp, on platelet aggregation,
platelet-neutrophil and neutrophil-neutrophil interactions:
Involvement of platelet GPIbalpha and neutrophil PSGL-1. Thromb
Haemost. 91:315–324. 2004.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Bernardes CP, Menaldo DL, Camacho E, Rosa
JC, Escalante T, Rucavado A, Lomonte B, Gutiérrez JM and Sampaio
SV: Proteomic analysis of Bothrops pirajai snake venom and
characterization of BpirMP, a new P-I metalloproteinase. J
Proteomics. 80:250–267. 2013.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Zigrino P, Kamiguti AS, Eble J, Drescher
C, Nischt R, Fox JW and Mauch C: The reprolysin jararhagin, a snake
venom metalloproteinase, functions as a fibrillar collagen agonist
involved in fibroblast cell adhesion and signaling. J Biol Chem.
277:40528–40535. 2002.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Costa ÉP and Santos MF: Jararhagin, a
snake venom metalloproteinase-disintegrin, stimulates epithelial
cell migration in an in vitro restitution model. Toxicon.
44:861–870. 2004.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Cominetti MR, Terruggi CH, Ramos OH, Fox
JW, Mariano-Oliveira A, De Freitas MS, Figueiredo CC, Morandi V and
Selistre-de-Araujo HS: Alternagin-C, a disintegrin-like protein,
induces vascular endothelial cell growth factor (VEGF) expression
and endothelial cell proliferation in vitro. J Biol Chem.
279:18247–18255. 2004.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Schattner M, Fritzen M, Ventura Jde S, de
Albuquerque Modesto JC, Pozner RG, Moura-da-Silva AM and
Chudzinski-Tavassi AM: The snake venom metalloproteases
berythractivase and jararhagin activate endothelial cells. Biol
Chem. 386:369–374. 2005.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Siigur E, Tõnismägi K, Trummal K, Samel M,
Vija H, Subbi J and Siigur J: Factor X activator from Vipera
lebetina snake venom, molecular characterization and substrate
specificity. Biochim Biophys Acta. 1568:90–98. 2001.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Markland FS, Kettner C, Schiffman S, Shaw
E, Bajwa SS, Reddy KN, Kirakossian H, Patkos GB, Theodor I and
Pirkle H: Kallikrein-like activity of crotalase, a snake venom
enzyme that clots fibrinogen. Proc Natl Acad Sci USA. 79:1688–1692.
1982.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Zhang Y, Wisner A, Xiong Y and Bon C: A
novel plasminogen activator from snake venom. Purification,
characterization, and molecular cloning. J Biol Chem.
270:10246–10255. 1995.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Serrano SM, Matos MF, Mandelbaum FR and
Sampaio CA: Basic proteinases from Bothrops moojeni (caissaca)
venom-I. Isolation and activity of two serine proteinases, MSP 1
and MSP 2, on synthetic substrates and on platelet aggregation.
Toxicon. 31:471–481. 1993.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Frykberg RG and Banks J: Challenges in the
treatment of chronic wounds. Adv Wound Care (New Rochelle).
4:560–582. 2015.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Telgenhoff D and Shroot B: Cellular
senescence mechanisms in chronic wound healing. Cell Death Differ.
12:695–698. 2005.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Lumbers M: Pressure ulcers: An overview of
risk. Br J Nurs. 26(S49-S50)2017.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Secretariat MA: Management of chronic
pressure ulcers: An evidence-based analysis. Ont Health Technol
Assess Ser. 9:1–203. 2009.PubMed/NCBI
|
|
133
|
Comerota A and Lurie F: Pathogenesis of
venous ulcer. Semin Vasc Surg. 28:6–14. 2015.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Mannello F and Raffetto JD: Matrix
metalloproteinase activity and glycosaminoglycans in chronic venous
disease: The linkage among cell biology, pathology and
translational research. Am J Transl Res. 3:149–158. 2011.PubMed/NCBI
|
|
135
|
van der Plas MJ, Baldry M, van Dissel JT,
Jukema GN and Nibbering PH: Maggot secretions suppress
pro-inflammatory responses of human monocytes through elevation of
cyclic AMP. Diabetologia. 52:1962–1970. 2009.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Wei OY, Xavier R and Marimuthu K:
Screening of antibacterial activity of mucus extract of snakehead
fish, Channa striatus (Bloch). Eur Rev Med Pharmacol Sci.
14:675–681. 2010.PubMed/NCBI
|
|
137
|
Jhamb S, Vangaveti VN and Malabu UH:
Genetic and molecular basis of diabetic foot ulcers: Clinical
review. J Tissue Viability. 25:229–236. 2016.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Su N, Tong N, Du L, Wu B and Xu T: Heparin
and related substances for treating diabetic foot ulcers. Cochrane
Database Syst Rev. 2017(CD011087)2017. View Article : Google Scholar
|
|
139
|
Bruhn-Olszewska B, Korzon-Burakowska A,
Gabig-Ciminska M, Olszewski P, Wegrzyn A and Jakóbkiewicz-Banecka
J: Molecular factors involved in the development of diabetic foot
syndrome. Acta Biochim Pol. 59:507–513. 2012.PubMed/NCBI
|
|
140
|
Blakytny R and Jude EB: Altered molecular
mechanisms of diabetic foot ulcers. Int J Low Extrem Wounds.
8:95–104. 2009.PubMed/NCBI View Article : Google Scholar
|
|
141
|
Patel S, Srivastava S, Singh MR and Singh
D: Mechanistic insight into diabetic wounds: Pathogenesis,
molecular targets and treatment strategies to pace wound healing.
Biomed Pharmacother. 112(108615)2019.PubMed/NCBI View Article : Google Scholar
|
|
142
|
Sherman RA: Maggot therapy for treating
diabetic foot ulcers unresponsive to conventional therapy. Diabetes
Care. 26:446–451. 2003.PubMed/NCBI View Article : Google Scholar
|
|
143
|
Pasha M, Husin RA and Hassan S: The
influence of oral and topical Channa striatus on laparotomy wound
healing in malnourished wistar rats. Int J Pharm Pharm Sci Invent.
4:37–41. 2015. View Article : Google Scholar
|
|
144
|
Anish S: Skin substitutes in dermatology.
Indian J Dermatol Venereol Leprol. 81:175–178. 2015.PubMed/NCBI View Article : Google Scholar
|
|
145
|
Kordestani SS: Chapter 5-wound care
management. In: Atlas of wound healing. Kordestani SS (ed).
Elsevier. 31–47. 2019.
|
|
146
|
Sun BK, Siprashvili Z and Khavari PA:
Advances in skin grafting and treatment of cutaneous wounds.
Science. 346:941–945. 2014.PubMed/NCBI View Article : Google Scholar
|
|
147
|
Knapik A, Hegland N, Calcagni M, Althaus
M, Vollmar B, Giovanoli P and Lindenblatt N: Metalloproteinases
facilitate connection of wound bed vessels to pre-existing skin
graft vasculature. Microvasc Res. 84:16–23. 2012.PubMed/NCBI View Article : Google Scholar
|
|
148
|
Park YJ, Lee JW, Chong Y and Park TH:
Botulinum toxin A increases allograft tolerance in an experimental
transplantation model: A preliminary study. Biosci Rep. 38(pii:
BSR20171721)2018.PubMed/NCBI View Article : Google Scholar
|
|
149
|
Kucukkaya D, Irkoren S, Ozkan S and
Sivrioglu N: The effects of botulinum toxin A on the wound and skin
graft contraction. J Craniofac Surg. 25:1908–1911. 2014.PubMed/NCBI View Article : Google Scholar
|
|
150
|
Boyko TV, Longaker MT and Yang GP: Review
of the current management of pressure ulcers. Adv Wound Care (New
Rochelle). 7:57–67. 2018.PubMed/NCBI View Article : Google Scholar
|
|
151
|
Ma H, O'Donnell TF Jr, Rosen NA and
Iafrati MD: The real cost of treating venous ulcers in a
contemporary vascular practice. J Vasc Surg Venous Lymphat Disord.
2:355–361. 2014.PubMed/NCBI View Article : Google Scholar
|
|
152
|
Ford CN, Reinhard ER, Yeh D, Syrek D, De
Las Morenas A, Bergman SB, Williams S and Hamori CA: Interim
Analysis of a Prospective, Randomized Trial of Vacuum-Assisted
Closure Versus the Healthpoint System in the Management of Pressure
Ulcers. Ann Plast Surg. 49(1):55–61. 2002.PubMed/NCBI View Article : Google Scholar
|
|
153
|
Yaakobi T, Cohen-Hadar N, Yaron H,
Hirszowicz E, Simantov Y, Bass A and Freeman A: Wound debridement
by continuous streaming of proteolytic enzyme solutions: Effects on
experimental chronic wound model in porcin. Wounds. 19:192–200.
2007.PubMed/NCBI
|
|
154
|
Smith & Nephew, Inc.: Enzymatic
debridement with collagenase SANTYL® Ointment,. 2014.
|
|
155
|
Giudice G, Filoni A, Maggio G, Bonamonte D
and Vestita M: Cost analysis of a novel enzymatic debriding agent
for management of burn wounds. Biomed Res Int. 2017(9567498)2017.
View Article : Google Scholar
|
|
156
|
Gorecki M and Toren A: Debriding
composition from bromelain and methods of production thereof,
Patent Appl Publ. 2005.
|
|
157
|
Klein GKV and Houck JC: Hydrolytic enzyme
material. 1980.
|
|
158
|
Niehaus F, Eck J, Schulze R and Krohn M:
Proteasa para el acondicionamiento de heridas y el cuidado de la
piel. Brain Biotechnol Res Inf Netw. 2012.
|
|
159
|
Niehaus F, Eck J, Schulze R and Krohn M:
Protease for wound conditioning and skin care. Brain Biotechnol Res
Inf Netw. 2012.
|
|
160
|
Rosenberg L: Aparato y procedimientos para
su uso en escarotomía enzimática en síndrome de compartimento
inducido por quemaduras. MediWound. 2012.
|
|
161
|
Freeman A, Hirszowicz E and
Be'eri-lipperman M: Apparatus and method for the enzymatic
debridement of skin lesions, Ramot At Tel-Aviv Univ. 2012.
|
|
162
|
Yaakobi T, Roth D, Chen Y and Freeman A:
Streaming of proteolytic enzyme solutions for wound debridement: A
feasibility study. Wounds. 16:201–205. 2004.
|
|
163
|
Rodeheaver G, Edgerton MT, Elliott MB,
Kurtz LD and Edlich RF: Proteolytic enzymes as adjuncts to
antibiotic prophylaxis of surgical wounds. Am J Surg. 127:564–572.
1974. View Article : Google Scholar
|
|
164
|
Gao M, Nguyen TT, Suckow MA, Wolter WR,
Gooyit M, Mobashery S and Chang M: Acceleration of diabetic wound
healing using a novel protease-anti-protease combination therapy.
Proc Natl Acad Sci USA. 112:15226–15231. 2015.PubMed/NCBI View Article : Google Scholar
|
|
165
|
Gutiérrez-Fernández A, Fueyo A, Folgueras
AR, Garabaya C, Pennington CJ, Pilgrim S, Edwards DR, Holliday DL,
Jones JL, Span PN, et al: Matrix metalloproteinase-8 functions as a
metastasis suppressor through modulation of tumor cell adhesion and
invasion. Cancer Res. 68:2755–2763. 2008.PubMed/NCBI View Article : Google Scholar
|
|
166
|
Hartenstein B, Dittrich BT, Stickens D,
Heyer B, Vu TH, Teurich S, Schorpp-Kistner M, Werb Z and Angel P:
Epidermal development and wound healing in matrix metalloproteinase
13-deficient mice. J Invest Dermatol. 126:486–496. 2006.PubMed/NCBI View Article : Google Scholar
|
|
167
|
Kudo Y, Iizuka S, Yoshida M, Tsunematsu T,
Kondo T, Subarnbhesaj A, Deraz EM, Siriwardena SB, Tahara H,
Ishimaru N, et al: Matrix metalloproteinase-13 (MMP-13) directly
and indirectly promotes tumor angiogenesis. J Biol Chem.
287:38716–38728. 2012.PubMed/NCBI View Article : Google Scholar
|
|
168
|
Rohani MG and Parks WC: Matrix remodeling
by MMPs during wound repair. Matrix Biol. 44–46. 113–121.
2015.PubMed/NCBI View Article : Google Scholar
|
|
169
|
Thirkettle S, Decock J, Arnold H,
Pennington CJ, Jaworski DM and Edwards DR: Matrix Matrix
metalloproteinase 8 (collagenase 2) induces the expression of
interleukins 6 and 8 in breast cancer cells. J Biol Chem.
288:16282–16294. 2013.PubMed/NCBI View Article : Google Scholar
|
|
170
|
Utz ER, Elster EA, Tadaki DK, Gage F,
Perdue PW, Forsberg JA, Stojadinovic A, Hawksworth JS and Brown TS:
Metalloproteinase expression is associated with traumatic wound
failure. J Surg Res. 159:633–639. 2010.PubMed/NCBI View Article : Google Scholar
|
|
171
|
Yamamoto K, Okano H, Miyagawa W, Visse R,
Shitomi Y, Santamaria S, Dudhia J, Troeberg L, Strickland DK,
Hirohata S and Nagase H: MMP-13 is constitutively produced in human
chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the
endocytic receptor LRP1. Matrix Biol. 56:57–73. 2016.PubMed/NCBI View Article : Google Scholar
|
|
172
|
Motrescu ER, Blaise S, Etique N, Messaddeq
N, Chenard MP, Stoll I, Tomasetto C and Rio MC: Matrix
metalloproteinase-11/stromelysin-3 exhibits collagenolytic function
against collagen VI under normal and malignant conditions.
Oncogene. 27:6347–6355. 2008.PubMed/NCBI View Article : Google Scholar
|
|
173
|
Pittayapruek P, Meephansan J, Prapapan O,
Komine M and Ohtsuki M: Role of matrix metalloproteinases in
photoaging and photocarcinogenesis. Int J Mol Sci. 17(pii:
e868)2016.PubMed/NCBI View Article : Google Scholar
|
|
174
|
Saarialho-Kere UK, Pentland AP,
Birkedal-Hansen H, Parks WC and Welgus HG: Distinct populations of
basal keratinocytes express stromelysin-1 and stromelysin-2 in
chronic wounds. J Clin Invest. 94:79–88. 1994.PubMed/NCBI View Article : Google Scholar
|
|
175
|
Sato T, Nomura K and Hashimoto I:
Expression of collagenase and stromelysin in skin fibroblasts from
recessive dystrophic epidermolysis bullosa. Arch Dermatol Res.
287:428–433. 1995.PubMed/NCBI View Article : Google Scholar
|
|
176
|
Kren L, Goncharuk V, Krenová Z, Stratil D,
Hermanová M, Skricková J, Sheehan CE and Ross JS: Expression of
matrix metalloproteinases 3, 10 and 11 (stromelysins 1, 2 and 3)
and matrix metalloproteinase 7 (matrilysin) by cancer cells in
non-small cell lung neoplasms. Clinicopathologic studies. Cesk
Patol. 42:16–19. 2006.PubMed/NCBI
|
|
177
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007.PubMed/NCBI View Article : Google Scholar
|
|
178
|
Purcell WT and Hidalgo M: Matrix
metalloproteinase inhibitors in cancer therapy. In: Proteases in
tissue remodelling of lung and heart. Lendeckel U and Hooper NM
(eds). Springer US, Boston, MA. pp75–118. 2003.
|
|
179
|
Herouy Y: The role of matrix
metalloproteinases (MMPs) and their inhibitors in venous leg ulcer
healing. Phlebolymphology. 44:231–243. 2004.
|
|
180
|
Lagente V, Manoury B, Nenan S, Le Quement
C, Martin-Chouly C and Boichot E: Role of matrix metalloproteinases
in the development of airway inflammation and remodeling. Braz J
Med Biol Res. 38:1521–1530. 2005.PubMed/NCBI View Article : Google Scholar
|
|
181
|
van Marion MMH: Matrix metalloproteinases
and collagen remodeling. A Literature Review. 2006.
|
|
182
|
Tewari A, Grys K, Kollet J, Sarkany R and
Young AR: Upregulation of MMP12 and its activity by UVA1 in human
skin: potential implications for photoaging. J Invest Dermatol.
134:2598–2609. 2014.PubMed/NCBI View Article : Google Scholar
|