1. Introduction
According to the 5th edition of the World Health
Organization Classification of Head and Neck Tumours from 2022,
salivary gland tumours (SGTs) consist of 15 benign and 21 malignant
entities of epithelial origin, one type of benign mesenchymal
tumour and two types of non-neoplastic epithelial lesions (1,2). SGTs
are rare, with the overall incidence of benign and malignant
tumours accounting for 2.5-3.0 cases per 100,000 individuals in
Western countries (3). Most being
benign tumours arise in major glands (~70%) (4). The majority of benign tumours (e.g.
pleomorphic adenomas, Warthin tumours, basal cell adenomas) involve
the parotid gland (70%), while malignant tumours mostly involve the
minor glands (56%), followed by the parotid gland (34%) (4). According to Rousseau and Badoual
(5), 15-32% of parotid tumours are
malignant. Even though parotid gland tumours are not very frequent,
the presence of the facial nerve, a significant functional
structure within the parotid, has raised significant interest
focused on this pathological entity.
Surgery is the standard treatment for parotid cancer
and parotidectomy is a common procedure in parotid tumour entities.
Parotidectomy with facial nerve preservation is a pre-requisite
ever since it was first described by Thomas Carwrdine in
1907(6). Whether the mass lies
above or below the plane of the facial nerve, there is a consensus
that facial nerve branches should not be sacrificed unless there is
clear evidence of tumoral nerve involvement. Close margins do not
necessarily prognosticate an unfavourable result (7). However, advanced neoplasms with facial
nerve involvement require radical parotidectomy in conjunction with
facial nerve resection (8,9). Facial nerve rehabilitation is an
important goal to be achieved for increasing the quality of
patients' life, the priority being corneal protection and
maintenance of oral competence, followed by the restoration of
facial symmetry and dynamic movements (8).
Multiple surgical techniques have been developed for
curing facial paralysis and thus restore the function and
aesthetics of the face. Both the literature and previous clinical
results from our group encourage the idea of performing facial
nerve restoration immediately after the ablative procedure in order
to avoid repeated general anesthesia, to prevent neural scarring
and difficulty identifying nerve endings.
Despite the great number of publications over the
last decade on facial nerve reanimation, a consensus on the timing
of the procedure or the donor graft selection has remained to be
established. Therefore, it is necessary to summarize the research
on facial reanimation in radical parotidectomy, highlighting the
trends over time and the advantages of the new techniques.
Bibliometric analysis is a precise mathematical and
statistical evaluation of publications (such as journal articles,
books and proceedings), using literature databases, to provide new
insight into specific research/clinical fields (10). Using such computer-assisted review
methodology, core research or authors, as well as their
relationship, may be identified by covering all of the publications
related to a given topic of interest (11).
The aim of the present study was to conduct a
bibliometric analysis to identify and analyse scientific
publications on the reconstruction of the facial nerve in patients
who underwent radical parotidectomy with facial nerve sacrifice in
order to improve the understanding of the importance of the timing
of facial reanimation.
2. Literature search and bibliometric
analysis
A bibliometric analysis was performed using the
in-built tool of the Scopus database (Elsevier) and VOSviewer
software (version 1.6.19) developed by van Eck and Waltman at
Leiden University's Centre for Science and Technology Studies, The
Netherlands (12), following the
guidelines proposed by Donthu et al (11).
Search strategy
A Scopus database search was conducted up to June
2023, containing the following keywords, with the Boolean operator
OR: (‘radical parotidectomy’ OR ‘facial nerve reinnervation’ OR
‘facial reanimation’ OR ‘concurrent facial reanimation’ OR ‘facial
reanimation timing’); limited to the English language; study type
limited: Article, review, book chapter.
The full record and cited references were retrieved
and exported in comma-separated values format for further analysis
in VOSviewer software.
Scopus in-built tools were used to assess the
distribution of the studies in terms of publication year,
country/region, journal and top cited articles. VOSviewer software
was used to perform data mining, mapping and clustering of the
retrieved articles.
Reviewing of the most cited articles. The top
100 most cited articles were independently reviewed by two
researchers (IF and CMC) looking for answers to the following
questions, in accordance with the aim of the present study: i)
Tumoral nerve invasion; ii) facial nerve grading systems; iii)
neurophysiological preoperative assessment of facial nerve
function; iv) imaging in ablative and reconstructive surgery of the
facial nerve; v) intraoperative management of tumoral invaded
facial nerve; and vi) timing of facial nerve reanimation.
To complete the literature review, besides the
selected articles, a manual search in the 10 most relevant
journals, revealed from the bibliometric analysis, as well as in
the reference lists of the included papers, was also performed.
Search results. A total of 851 publications
were retrieved from the Scopus database, published from 1970 to
June 2023. These documents were full articles (79%), reviews
(15.2%) and book chapters (5.2%). A constant increase in the number
of articles published was noticed, with a peek in 2021 (72
documents) (Fig. 1A), published
from >57 countries, with a considerably higher rate of
publications coming from the USA (n=361; 42,4%), followed by the
United Kingdom (n=80; 9.4%) and Italy (n=65; 7.6%; Fig. 1B). The network visualization of the
co-authorship relationship between countries obtained in VOSviewer
software is displayed in Fig. 1C.
The size of the circles is proportional to the occurrence in the
country. Only countries with a minimum of 5 articles were included.
The USA is an important center of research on radical parotidectomy
with facial nerve reconstruction, in close collaboration with the
United Kingdom, Canada, Germany and Italy. A recent center is South
Korea, in close connection with Canada.
Among the countries with research on facial
reanimation and parotid neoplasm, the first five representative
institutions (out of the 161 institutions with at least three
publications in the field) are as follows: Massachusetts Eye and
Ear Infirmary (Boston, USA), Harvard Medical School (Boston, USA),
Johns Hopkins School of Medicine (Baltimore, USA), University of
Toronto (Toronto, Canada) and Ospedale San Paolo (Milan, Italy).
The top 10 institutions in terms of productivity in the analyzed
field are presented in Fig. 2.
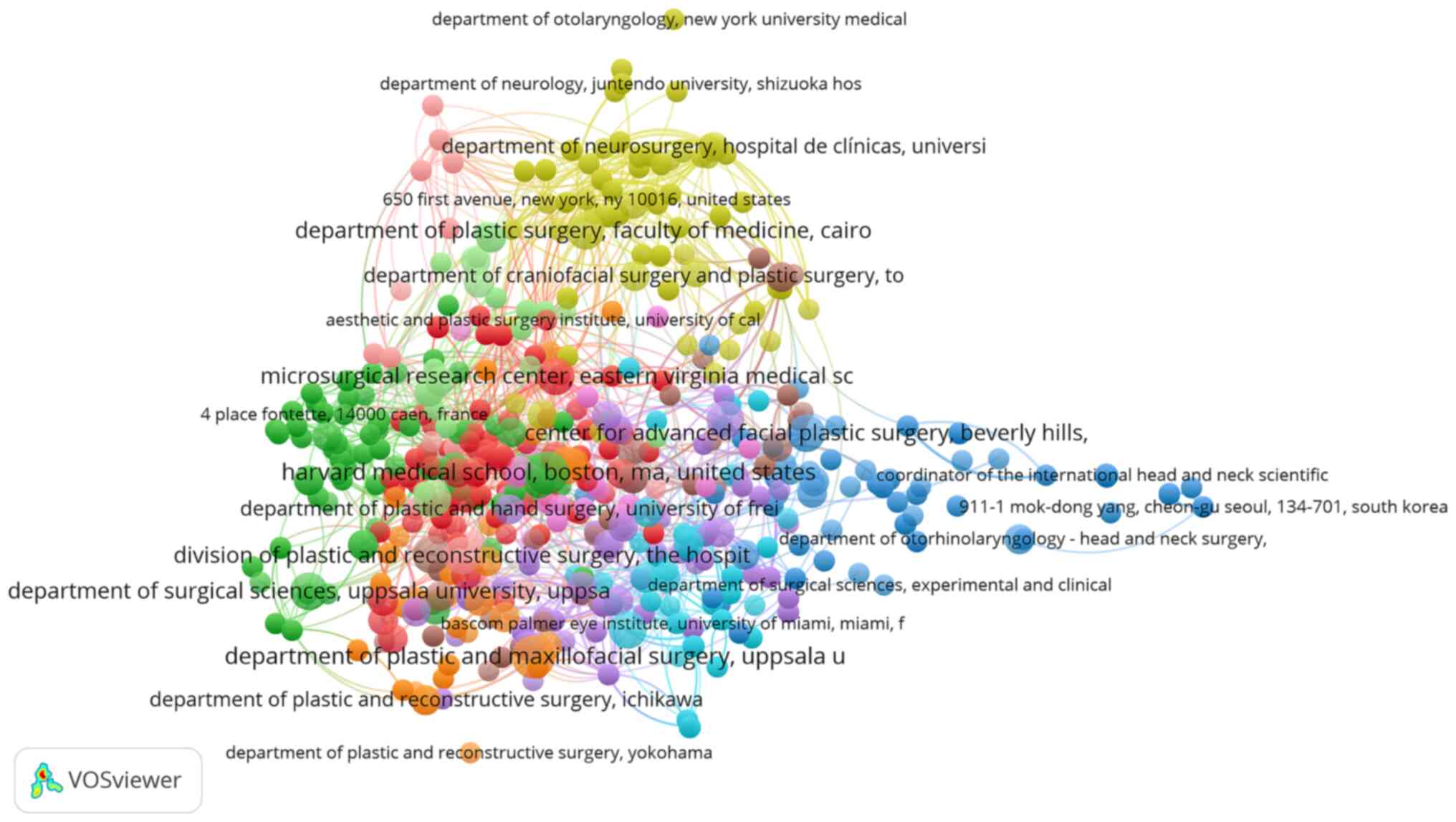
The network map showing the collaboration between
the institutions with research published in the field is
illustrated in Fig. 3. All
organizations with a minimum of one article were included.
The main financial support for the research was
provided by the following 10 institutions: National Institute of
Neurological Disorders and Stroke (USA), Japan Society for the
Promotion of Science (Japan), National Institute on Deafness and
Other Communication Disorders (USA), National Center for Advancing
Translational Sciences (USA), National Institutes of Health (USA),
Deutsche Forschungsgemeinschaft (Germany), National Cancer
Institute (USA), National Institute for Health and Care Research
(Great Britain), National Institute of Dental and Craniofacial
Research (USA) and National Natural Science Foundation of China
(People's Republic of China), with most of the financial support
being from the USA (7 out of 10).
The top 10 journals in terms of the number of
articles published on facial reanimation in parotid neoplasms are
as follows: Plastic and Reconstructive Surgery (70 papers), Journal
of Plastic, Reconstructive and Aesthetic Surgery (67 papers),
Journal of Craniofacial Surgery (35 papers), Annals of Plastic
Surgery (32 papers), Laryngoscope (30 papers), JAMA Facial Plastic
Surgery (22 papers), Journal of Cranio-Maxillo-Facial Surgery (19
papers), Facial Plastic Surgery (17 papers), Head and Neck (17
papers) and Current Opinion in Otolaryngology and Head and Neck
Surgery (16 papers) (Fig. 4). Of
note, the trend of an increased number of publications on the topic
in the last years was observed. The Journal of Plastic,
Reconstructive and Aesthetic Surgery published 8 papers on the
topic in 2015 and the Journal of Cranio-Maxillo-Facial Surgery
published 8 papers on the topic in 2021 and another 8 in 2022.
The authors with the largest number of publications
in the field are displayed in Fig.
5A and the most cited authors are presented in Fig. 5B.
The co-authorship network, including authors with at
least one publication on the topic, clustered on research groups,
is displayed in Fig. 6. The color
code highlights the date of published articles, the most recent
being colored in yellow.
When performing the key-words analysis, the minimum
number of occurrences of an author's key word was set at 5. Out of
1,218 keywords, 88 meet the threshold. Among the most commonly used
keywords by the authors are: Facial reanimation, facial paralysis,
facial palsy, hypoglossal nerve, masseteric nerve and facial nerve
(Fig. 7).
3. Tumoral nerve invasion
The face is one of the most important parts of the
human body and facial expressions represent a critical pillar in
non-verbal communication. Nerve invasion can occur in a variety of
malignant tumours, and together with the tumour grade and stage,
has been reported as a strong prognostic factor for survival
(13). Facial palsy may have
different causes: Nerve invasion, extrinsic compression and
inflammation. Perineural and intraneural nerve invasion may vary
between 7% (14) and 69.1% (for
patients with parotid cancer and preoperative facial weakness)
(13). The tumour diameter has been
determined to be associated with the risk of facial nerve invasion,
with tumours sized >4-5 cm having positive facial nerve margins
in >80% of cases (15,16). Paraesthesia and pain are two
predictive factors of perineural nerve invasion (13).
Whether diagnosed preoperatively or
intraoperatively, facial nerve invasion by the malignant neoplasm
is an indication for radical parotidectomy, a procedure in which
part of the nerve or even the entire nerve branching from the
stylomastoid foramen exit to the parotid margins requires to be
surgically removed. This may cause an impairment for the patient,
both functionally and psychosocially. According to Bovenzi et
al (17), facial nerve
sacrifice was recorded in 3.7% of cases of parotid tumour and only
25.5% of those patients underwent a concurrent reinnervation
procedure. Lu et al (18)
found a similar percentage of patients that underwent concurrent
facial reanimation (31.2%) of any type, after radical
parotidectomy. Since facial weakness may be caused not only by
tumoral invasion, the question arises of how surgeons should make
the decision of whether to spare or sacrifice the facial nerve
(19). Extrinsic tumoral
compression or inflammatory processes (20) may also determine facial weakness and
in this situation, a thorough intraoperative evaluation is a
critical factor in the decision-making process of facial nerve
management. In a comprehensive study, Park et al (13) found that in cases with nerve
reaction, 26.9% had intra-neural tumour invasion, 42.3% had
perineural invasion and 30.8% had no neural invasion of the facial
nerve. This understanding is crucial because facial weakness did
not always indicate tumour invasion of the facial nerve. As a
consequence, the decision to preserve or sacrifice the facial nerve
should be based on intraoperative additional findings.
4. Grading systems
In order to evaluate the status of the facial nerve
either before or after treatment, several facial nerve grading
systems were established. An ideal method for evaluating the facial
nerve has to be easy to use even by inexperienced clinicians,
reproducible, fast, objective, with minimal expenses involved and
clinically relevant. House and Brackmann (21) classified grading systems into 3
categories: Gross, regional and specific. Also, they introduced the
concept of weighted or unweighted regional grading systems.
Weighted means that certain areas of the face are given less
importance within the grading system due to less functional or
aesthetic importance (e.g. the forehead) (22). Specific systems highlight the
existence of certain associated symptoms and signs.
In 1971, Adour and Swanson (23) suggested a weighted grading system,
comparing the frontal, eye and mouth regions of the face on the
paralyzed compared with the normal side. In 1976, Yanagihara
(24) described an unweighted
system that assesses 10 areas of facial function, without the
consideration of secondary effects. Subsequently, in 1977, Stennert
et al (25) described a
double-weighted system: Face at rest, weighted at 40%; and in
motion, weighted at 60%. The different regions of the face were
also weighted. Secondary effects were graded independently
(25).
Since 1985, the House-Brackmann (H-B) scale is the
most frequently used grading scale to evaluate the function of the
facial nerve (26). As it is based
on clinical observational judgment, which may differ from one
clinician to another, this option is a clinical means to assess the
voluntary facial motion and categorize 6 degrees of facial nerve
impairment. There are 6 grades within the H-B grading system: Grade
1 indicates a functionally normal face; grade 2 patients show mild
facial weakness; grade 3 indicates moderate weakness with the
ability to voluntarily close the eye; grade 4 patients show
moderate weakness without volitional eye closure ability; grade 5
indicates severe facial weakness; and grade 6 represents total
facial paralysis.
The Burres-Fisch (B-F) linear measurement system
(26) was introduced in 1986 and it
consists of 3 parts: The patient's global analysis, the physician's
detailed analysis and the physician's global evaluation. In the
patient's analysis, a gross self-evaluation is performed in order
to evaluate the improvement, and it is expressed as a percentage.
In the physician's detailed analysis, a weighted approach is
performed: The scores, with the following significance: 0%, total
asymmetry; 30%, significant asymmetry; 70%, slight asymmetry; and
100%, symmetry, are calculated as the weight of each assessment as
follows: Rest position, 20%; forehead wrinkle, 10%; eyes closed
tight, 30%; smile, 30%; and whistle, 10%. The average calculated
from the results of the three parts gives the final result
(27). The limitations of this
procedure are as follows: It involves a time-consuming process and
there is a lack of evaluation of resting symmetry and secondary
defects.
The Nottingham grading system described by Murty
et al (28) in 1994 is a
more practical and simplified version of the B-F linear measurement
index. It consists of 3 parts: In the first stage, objective
measures are performed at rest and in 3 movement positions: Raise
eyebrows, close eyes tightly and smile. In the second part of the
Nottingham grading system, the clinician records the presence or
absence of secondary defects and the third part consists of a
questionnaire for the patient recording the presence or absence of
crocodile tears, decreased lacrimation or dysgeusia (28).
The Sunnybrook Facial Grading System (SFGS) is a
subjective grading system that assesses face symmetry at rest and
during five standard voluntary movements (29,30).
Furthermore, it allows the assessment of each secondary defect
associated with the voluntary movements and grades the severity of
these, if present. Since it allows quantification of facial
paralysis, it is a convenient tool to be used in monitoring the
changes in time related to facial function.
Evolving imaging technology has facilitated the
development of new clinical tools for the assessment of facial
function. Clinical photography, videos and computer programs have
been used in order to achieve a more universal language between
clinicians and to facilitate the communication between clinicians
and patients. In 1995, el-Naggar et al (31) suggested a method to assess the
recovery of facial functions by using life-size photographs that
were overlapped with previously taken photographs.
As reported in 1997, Yuen et al (32) used the Moiré topography technique to
evaluate facial palsy. The Moiré topography is an optimal
measurement that maybe used to create a 3D map of the face with
high accuracy (33). However, due
to the expense of the equipment and the time-consuming process,
this technique is uncommon in daily clinical practice.
e-FACE is an electronic, observer-graded visual
analogue scale used for unilateral facial paralysis. It is a
16-item instrument structured with 5 static items, 7 dynamic items
and 4 sin kinetic items, which performs a mathematical correlation
to overall disfigurement and offers a graphic output (34). Facial Assessment by Computer
Evaluation (FACE) software is a computer-based clinical tool,
designed for facial analysis and described by Hadlock and Urban
(35). The program involves a
photography-based concept by analysing facial photography at rest
and during 5 standard movements. According to the authors, with the
use of the FACE program, the mean time to complete a set of
measurements was only 1.3 min, which provides a significant benefit
in clinical practice (35).
The Facial Motor Evaluation scale was developed and
validated by Ojha et al (36) to overcome the limitations regarding
the subjectivity and the expertise of the professionals
administering the tests (e.g. H-B, B-F, SFGS tests), the
requirements of specific software, hardware, longer data entry time
such as for the case of e-FACE software and the lack of graphic
representations of scoring criteria.
5. Neurophysiological preoperative
assessment of facial nerve function
Since for patients diagnosed with advanced malignant
parotid tumour, radical parotidectomy is part of the treatment,
there is the need for a clear therapeutic evaluation, in order to
establish whether facial nerve sacrifice is required so that a
negative oncological margin can be achieved. According to the study
by Bendet et al (37) from
1998, facial neurological deficit may become visible after more
than half of the facial nerve fibres are invaded by the malignant
neoplasm. The key implication drawn from this fact is that in
numerous oncological patients, there is a subclinical nerve
degeneration and an objective and reliable method is necessary to
assess the facial nerve integrity. Facial nerve degeneration may be
quantitatively assessed using facial neurography, which is a
reliable method in numerous conditions affecting the facial nerve
(37,38). This examination is based on a
comparative result between stimulation of the facial nerve on the
healthy side and the contralateral side. Facial electroneurography
(ENG) is a subclinical analysis that may influence the
therapeutical decision-making process (39), as it may objectively establish the
degree of nerve damage preoperatively and postoperatively.
In a prospective study on 33 patients with parotid
neoplasms, Aimoni et al (40) found, in 3 out of the 6 patients with
a histologically confirmed malignant tumour, potential
abnormalities of amplitude and latency of the facial movements in
the absence of facial nerve deficits, indicating a strong need for
objective neurophysiological assessment. However, their study was
limited in its application, as the number of patients with parotid
cancer was relatively small (only 6 patients with parotid gland
cancer). Wiertel-Krawczuk et al (39) found a positive correlation between
changes in facial motor fibre transmission, determined on facial
motor nerve fibers (ENG), and the type of tumour. This
understanding is crucial because neurophysiological preoperative
assessment of each facial nerve branch can guide the clinician in
the decision-making process and allows them to decide on surgical
nerve reconstruction at an appropriate time, depending on the
degree of neural degeneration. Timing of surgical nerve
reconstruction can be divided into immediate, early (1 month),
delayed (3 to 6 months) and late (1 to 2 years or more). When
electrodiagnostics reveal a complete lesion of a neural branch
(neurotmesis), immediate nerve reconstruction is desired (39).
6. Imaging in ablative and reconstructive
surgery of the facial nerve
Preoperative prediction of the facial nerve status
and its relation to the parotid neoplasm is of tremendous
importance for surgical planning, since it is a highly vulnerable
structure with a complex course, prone to injury during parotid
surgery. Imaging tools commonly used in preoperative assessment are
ultrasonography, computed tomography (CT) and magnetic resonance
imaging (MRI). The choice of the imaging modality utilized in the
assessment of the facial nerve and relation with other anatomical
or pathological structures is dependent on the differential
diagnosis, patient status, localization of the pathological entity
and purpose of the investigation (41).
Ultrasonography is a dynamic non-invasive diagnostic
tool that has several advantages over other imaging diagnostic
tools, namely: It is fast and cheap, has the ability to be used at
the bedside and does not require radiation. Due to recent
advancements in high-resolution ultrasonography, this investigation
allows the assessment of the extra-temporal course of the facial
nerve in an accurate and reproducible way. High-resolution
ultrasonography is a valuable diagnostic tool, as it has a superior
resolution; it is faster and more dynamic in comparison to
conventional ultrasonography. Wegscheider et al (42) found that the use of a 5-18 MHz
linear array transducer facilitated the identification of the main
facial nerve trunk, parotid plexus and branches innervating the
orbicularis oculi and zygomatic major muscle in 8 cadaveric
hemifaces, as follows: The main trunk was identified in 75% (6 out
of 8) of the cases, parotid plexus was clearly identified in 100%
(8 out of 8) of the cases, the branches innervating the orbicularis
oculi muscle were identified in 7 out of 8 cases (87.5%) and the
branches innervating the zygomatic major muscle in 6 out of 8 cases
(75%). Also, the furcation of the main trunk of the facial nerve
was clearly visible on 4 of 4 sides (100%) in vivo in the
two volunteers included in the study (42). However, the study has limitations,
as it was performed mostly on cadavers and the number of specimens
was small.
Conventional CT and MRI may offer significant
information related to the extent of the parotid neoplasm and the
relation of the tumour to the stylomastoid foramen. As is common
practice in numerous countries, CT scans together with ultrasounds
are the first choice of imaging investigations in cases of patients
clinically diagnosed with parotid tumour. When using CT to evaluate
the facial nerve, high-resolution temporal bone CT may offer
valuable information regarding the intra-fallopian segment of the
facial nerve to the stylomastoid foramen, but the intratemporal
part of the nerve may be indirectly assessed, only if the
pathological entity determined bone erosion or destruction
(41). For the assessment of the
extratemporal segment of the facial nerve, MRI has proven to be a
more accurate imaging examination.
MRI can also be used as a valuable tool in the
assessment of the facial mimetic muscles in long-standing paralysis
cases and in exploring the status of postoperative free flaps, or
facial muscle growth after facial nerve reconstruction (43). However, it has been previously
demonstrated that certain MRI sequences may enable visualizing the
facial nerve from the stylomastoid foramen at least to the level of
the proximal part or event further for both cervico-facial and
temporo-facial trunks in a predictable and repeatable manner.
Guenette et al (44)
assessed 32 facial nerves of 16 healthy patients, with no
exceptions. The same success rate was described for the assessment
of the facial nerve in 4 patients diagnosed with parotid tumours. A
weakness of his study was that in only 3 out of 36 nerve
assessments, an intraoperatory anatomical validation had been made
by the surgeon. In a comprehensive prospective study, Takahashi
et al (45) made the
preoperative assessment of the main trunk of the facial nerve, as
well as the cervico-facial and temporo-facial divisions, using
high-resolution MRI with a surface coil. The imaging results were
then superimposed with the anatomical surgical findings. The
accuracy of the MRI assessment of the facial nerve is reflected by
the following numerical data: The main trunks and cervico-facial
and temporo-facial divisions of the facial nerves were identified
in 100, 84.1 and 53.8%, respectively, in the axial plane of
three-dimensional gradient-recalled acquisition in the steady-state
images and the relationships of the tumours to the facial nerves
were correctly diagnosed in 11 (91.7%) of 12 cases (45). That study highlighted the important
value of high-resolution MRI using specific sequences in the
preoperative evaluation of the facial nerve status and its relation
with the parotid tumour.
7. Intraoperative management of tumoral
invaded facial nerve
There is currently no consensus on the best practice
for immediate facial reanimation. Mild improvement of facial palsy
will tremendously improve the quality of life. Static and dynamic
reanimation may be performed. Non-vascularized nerve grafts are
used for facial nerve reconstruction and there are various
alternatives for donors, e.g. greater auricular nerve, sural nerve,
medial or lateral antebrachial cutaneous nerve, superficial radial
nerve or thoracodorsal nerve.
Quality of life. Even though there is a
subjective component of perception from one individual to another,
a disabled person is likely to have a lower quality of life. Facial
palsy has a tremendous negative impact both on functional and
psychological status of patients affected by this disorder. The
inability to physiologically move the mimetic muscles may
jeopardize numerous functions dependent on these muscles. Speech
disturbance, saliva running out of the mouth, dropping eye or
corner of the mouth, as well as eating difficulties, are common
functional issues specific to facial palsy. The perception of
individuals suffering from facial paralysis by others may also lead
to the deterioration of the quality of life for the affected
individual (46). In a study
published by Walker et al (47), it was indicated that 60% of all
patients showed symptoms suggestive of anxiety and/or depression.
The communication is clearly disturbed due to numerous factors:
Decreased facial movement was the most frequent cause reported by
the affected individuals (74%), followed by synkinesis as a second
reason (48%), dislike of their facial appearance (13%) and facial
asymmetry (3%) (46). Coulson et
al (46) reported that 50% of
the patients classified themselves as not effective at expressing
one or more of the six primary emotions described by Ekman
(48) (happiness, disgust,
surprise, anger, sadness and fear).
Clinicians should be aware of the risk factors
involved in the dysfunction of facial nerves, and proper
preoperatory information related to the management of facial nerves
should be delivered to the patients. Multiple studies have
established the importance of facial palsy treatment in order to
increase the quality of life of the patients who had radical
parotidectomy (49), as well as
pre- and postoperative psychological counseling.
Immediate reanimation options in radical
parotidectomy. Optimal reconstruction following a radical
parotidectomy requires that several aspects should be aesthetically
and functionally restored. Contour deformity, cutaneous defects and
facial reanimation are the main issues to be addressed. For facial
reanimation, the available options may be grossly classified into
cable grafting procedures, regional muscle transfer and suspension
procedures (50,51). One of the main important ablative
sequelae to be managed is the inability to close the eye.
Immediate or recent facial reanimation after radical
parotidectomy has several benefits; two of the most important gains
are the duration of paralysis, which is shortened, and the quality
of life, which is statistically higher in patients whose facial
reanimation was performed in an immediate or recent approach in
comparison to those that had a delayed reanimation (52). Repeated general anesthesia, neural
scarring, Wallerian degeneration and difficulty in identifying
nerve endings are the disadvantages of performing facial
reanimation in a delayed approach. However, due to several aspects,
in most of the oncological ablative procedures that involve
sacrificing the facial nerve, only some of the patients benefit
from facial reanimation or ancillary procedures. Lu et al
(18) found that one-third of the
oncological patients whose main procedure was radical parotidectomy
had concurrent or recent reanimation.
Depending on the nerve resection extent and
availability of the proximal/and distal part of the facial nerve,
several options are available for facial nerve reconstruction:
Direct coaptation/neurorrhaphy [usually when <1 cm is missing
(18)], cable grafting,
mastoidectomy to assess the proximal segment of the facial nerve +
cable grafting, and nerve substitution, when the proximal part of
the nerve is not accessible (53).
Other different ways to make the classification of
the procedures to be performed in facial reanimation are nerve-type
repairs, non-nerve or sling-type repairs and muscle-free flap
procedures (51).
Salivary drooling and incompetence in closing the
eye are common complications after an ablative tumour procedure.
Mixing both static and dynamic facial reanimation is the treatment
of choice for such patients (54).
Frozen sections are supposed to be performed both at
the proximal and distal stump of the resected facial nerve.
However, positive margins do not significantly affect nerve
recovery according to a study published by Wax and Kaylie (55).
In order to facilitate the adaptation between the
caliber of the nerve graft and several distal stumps of the facial
nerve, the stumps can be pulled together and coated to a single
extremity of the nerve graft (56).
Another option is to divide the graft in order to make the
coaptation with multiple distal stumps possible (57).
Cable grafting cases have a larger rate of
synkinesis in comparison to direct coaptation (58,59).
Using 2 different neural inputs for the upper and the lower aspect
of the face also decreases the likelihood of sinkinetic
complication (56).
The distinction between quantitative and qualitative
neural inputs also requires consideration. Tomita et al
(60) described the concept of
double innervation by mixing neural inputs from both the
hypoglossal (quantitative source) and facial nerve (qualitative
input). Bianchi et al (61)
and Biglioli et al (62)
introduced double enervation with masseteric and contralateral
facial input.
The masseteric nerve provides neural input,
decreasing the risk of muscular fibrosis and cross-facial nerve
grafts offer spontaneity to smile. Combining these two options, the
complication of sinkinesis is decreased, since the nerves
corresponding to the upper face have different neural input in
comparison to the muscles of the lower face (63).
The recovery time is also decreased due to the
neural input from the masseteric nerve (53). A technique featuring triple neural
inputs was later on described by Biglioli et al (64). For recent paralysis [cases in which
electromyography (EMG) was used to confirm the presence of
fibrillations], 3 neural sources were used: 2 quantitative neural
inputs (masseteric and 30% of hypoglossal nerve) and one
qualitative source (contralateral facial nerve via two sural nerve
cable grafts). The temporo-facial branch (on the paralyzed side of
the face) is supposed to be identified, sectioned and skeletonized,
and an end-to-end coaptation must be performed to the masseteric
nerve, previously dissected. The cervico-facial branch (on the
paralyzed side of the face) is identified and sectioned in a
similar manner to the one described for the temporo-facial one.
After an epineural window is created on the hypoglossal nerve and
30% of this nerve is incised, an end-to-side coaptation between the
cervico-facial and hypoglossal nerve must be performed. In order to
achieve a tension-free coaptation, a short surreal nerve graft
should be used (64).
When wide excision of malignant neoplasms is
required, in numerous cases, an important part of the mimetic
muscles is sacrificed, either due to direct tumoral invasion or in
order to achieve negative ontological margins. This situation needs
to be addressed by the reconstructive surgeon in order to
reconstruct the missing facial and intraoral soft tissue.
Traditionally, soft tissue and facial nerve reconstruction are
performed in 2 different stages. However, in the literature
one-stage technique has been indicated to provide optimal results
(60-63).
Urken et al (65) was the first to report a concurrent
facial nerve reconstruction after extended radical parotidectomy
with a vascularized radial nerve graft and radial free flap; the
resulting soft-tissue defect after the ablative procedure was
reconstructed with radial free flap and the radial nerve was used
to bridge the defect of the facial nerve.
Teknos et al (66) reported, for the first time, the
reconstruction of complex parotidectomy defects using lateral arm
free tissue transfer, and in the case presented, the posterior
cutaneous nerve of the forearm and the nerve to the lateral arm
were used to reconstruct the facial nerve.
Primary facial nerve reanimation with simultaneous
soft tissue reconstruction using a glacillis myocutaneous free flap
was first described by Lin et al (54). When there was no through-and-through
defect, the cutaneous paddle of the gracilis free flap was used to
restore the intraoral defect followed by inset of the other part of
the muscular flap in order to restore the facial defect. When there
was a through-and-through check resection, two free flaps were used
to restore the ablative defect: The Anterolateral thigh,
antero-medial thigh or fibula flap was used to restore the
intramural defect and the cutaneous paddle of the gracilis free
flap was used to rehabilitate the facial defect (54). The technique, as described by Lin
et al (54), was performed
as follows: A gracilis myocunanteous free flap was harvested in a
conventional manner. After the tumour ablative procedure was done,
the proximal part of the flap was inset between the modiolus and
zygomatic arch (with interosseous wires). The inset was performed
only after the optimal length and vector direction had been
determined. The muscular part of the flap was used to fill either
the retromolar or maxillary antrum dead space and the skin paddle
segment of the flap was used either to reconstruct the intraoral
mucosal defect or the facial skin wound. The coaptation between the
obturator nerve and the proximal facial nerve stumps was performed
together with the microvascular anastomosis to a branch of the
internal jugular vein and a branch of the external carotid artery
(54).
Revenaugh et al (67) described a method for single-stage
reconstruction during radical parotidectomy with the use of
anterolateral thigh fat and fascia flaps for facial contouring,
orthodromic temporalis tendon transfer, cable grafting of the
facial nerve and fascia lata lower lip suspension.
A similar approach was reported by Ch'ng et
al (68). The procedure was a
combination of the anterolateral thigh free flap and cervico-facial
rotation advancement flap, repair of the facial nerve with the
nerve to the vastus lateralis segmental interpositional graft, gold
weight loading of the upper eyelid, lateral canthopexy, temporalis
and digastric muscle transfers, and a delayed brow lift (68).
Donor nerve graft selection. Numerous sources
of nervous tissue have been described for use in facial nerve
reconstruction, such as the sural nerve, lateral antebrachial
cutaneous nerve, medial antebrachial cutaneous nerve, the nerve to
the vastus lateralis and the great auricular nerve. Both sensory
and motor nerve may be used as nerve grafts in facial reanimation
but there is evidence that motor nerve grafts may offer better
results than sensory nerve grafts (69).
The great auricle nerve (10 cm) is a good option as
a nerve graft, since both the donor site and recipient site are
within the same surgical field. The only drawback it brings is the
morbidity associated with the numbness of their lobe, which is a
neglectable aspect in most of the patients (62,70).
However, in parotid cancer, the great auricle nerve is not commonly
used, since it is not uncommon that this nerve is invaded by the
malignant neoplasm as well (71).
The sural nerve is one the most commonly used nerves
as a cable graft due the its available length (40 cm) and its low
morbidity, involving hypoesthesia on the lateral aspect of the foot
(72).
Other nerve graft options are the medial
antebrachial cutaneous nerve and lateral antebrachial cutaneous
nerve of the upper arm (73). The
mean length of this nerve graft is 15-20 cm and its branching is an
important characteristic that fits distal stumps of the sectioned
facial nerve. The most important disadvantage to be taken into
consideration is the risk of injury of the median nerve, brachial
artery or basilica vein (74).
The superficial radial nerve may be a suitable
option, particularly in those clinical situations where the radial
forearm flap is used to reconstruct the soft tissue after ablative
parotid surgery (75).
Thoracodorsal nerve graft used for facial nerve
reconstruction was first described by White et al (76). The limitation of his article is that
there was only one case described using thoracodorsal nerve graft.
A more comprehensive study on this topic was published by Biglioli
et al (77). The main
advantages of the thoracodorsal nerve graft consist of the
branching pattern, similar to the one of the facial nerve, which
facilitates the coaptation between the distal stumps of the facial
nerve and branches of the thoracodorsal nerve.
Cranial nerve selection for transfer.
Selection of the cranial nerves for transfer requires thorough
clinical judgment and a careful understanding of the balance
between advantages and disadvantages of each donor site. The
balance must be individualized for each case scenario, taking into
account several variables, including the type of neural input that
is necessary for an optimal reanimation (quantitative and
qualitative), morbidity associated with the donor site, topographic
characteristics of the donor nerve (fibre type, branching pattern,
diameter and number of axons) and the necessity of a supplemental
nerve graft (78).
Conventional donor nerves for transfer to
reconstruct the facial nerve include the following: Masseteric
branch of the V nerve, contralateral VII nerve with cross-face
graft, the XI nerve and the XII nerve (78), which are described in detail below.
Nerve coaptation may be achieved using suture, tissue glue, nerve
wraps or a combination of these.
i) The masseteric nerve, branching from mandibular
division of the trigeminal nerve (V), for nerve substitution was
first described by Spira (79), who
used it for the reinnervation of the lower division of the facial
nerve. Since then, the use of the masseteric nerve for facial
reanimation gradually evolved due its numerous advantages. From a
surgical point of view, its location is s favourable
characteristic, since the donor location is in the same surgical
field as the recipient site (80).
Related to the anatomical features, the masseteric nerve is quite
similar in diameter to the trunk of the facial nerve and it
provides >1,500-2,500 motor axons for coaptation (81,82).
The morbidity associated with its harvest is low and acceptable in
most patients, since the master muscle and temporalis muscle work
in synergy and there is subsequent chewing dysfunction after nerve
disruption. Since it is located close to the facial nerve
divisions, in most cases, there is no need for an adjunctive nerve
graft to bridge the masseteric to facial nerve. Even though the
masseteric neural input is a quantitative one, it may produce an
effortless smile without biting in most patients (83,84).
This is due to the neuroplasticity of the brain, a mechanism in
which new pathways are created between the trigeminal and facial
nerve (85). Even though the
provided tone is poor, masseteric division of the trigeminal nerve
facilitates significant facial movements (61,62,64).
It provides a reduced time for the reinnervation, ~5 months (range,
2-7 months) (86), varying based on
the facial nerve branch reanimated (increased for the main trunk
and shorter for the zygomatic/buccal branches) and patient's age
(faster in younger patients) (87).
ii) The hypoglossal nerve (XII) has been
traditionally considered the ‘gold standard’ in facial reanimation
using a nerve transfer procedure. The advantage provided by the use
of this nerve in facial reanimation procedures is that it is
relatively easy to dissect without the necessity to extend the
conventional incision performed for reanimation. The nerve transfer
was first described by Korte in 1901, but the morbidity associated
with hypoglossal nerve transection in order to perform an
end-to-end coaptation determined the modification of the surgical
approach. In order to avoid permanent morbidity, a window is
supposed to be created into the hypoglossal nerve and an
end-to-side coaptation to be performed. This neurorrhaphy procedure
is facilitated by the high number of myelinated axons, between
9,200 and 12,594 myelinated axons (88-90).
The neural input is a quantitative one, providing an optimal tone
and movement. Even though the traditional procedure was classified
as one with high morbidity related to tongue atrophy and
deglutition disturbance, using 1/3 of the XII nerve has been
accepted as an optimal alternative. By convention, it is necessary
to use a nerve graft to bridge the VII nerve to the XII nerve.
However, the vertical or mastoid segment of the facial nerve
measures between 15 and 20 cm and mastoidectomy may facilitate the
transposition of the facial nerve in order to avoid the necessity
of an additional cable graft. Another drawback of using the XII
nerve for facial transfer is the synkinesis complication that has a
higher incidence in comparison to other nerve transfer sources.
iii) Contralateral facial nerve (VII) with
cross-face graft: The facial nerve is composed of 6,000-7,000
myelinated motor fibers that innervate between 19 to 24 mimic
muscles (91). Cross-face nerve
grafting with the use of the sural nerve, as a cable nerve graft,
was first described in 1971 by Scaramella, but the result was
suboptimal (92,93). Harii et al (94) described a modification of the
technique by performing the coaptation to a gracilis muscle in a
secondary stage. The neural input is a truly qualitative one,
providing an effortless, natural and spontaneous smile and blinking
ability to the reanimated face (95). Conventionally, buccal or zygomatic
branches of the contralateral facial nerve are selected to transfer
the neurological signal to the affected side. The main
disadvantages of this technique are the low power provided to the
innervated mimetic muscles and the time required to neural input,
with a mean reinnervation time of ~9 months. Statistically, only
half of the axons reach the sural nerve extremity on the affected
side of the face (51). Another
drawback of the cross-face graft is that axons are required to pass
2 suture ‘barriers’ in order to reinnervate the mimetic muscles on
the paralysis side. In order to facilitate a favourable outcome,
the facial reanimation using cross-face nerve graft must be
approached as an adjunctive procedure and not as a standalone one.
A two-stage procedure is the preferred choice as it allows the
axons to reach the extremity of the sural cable graft on the palsy
side, and thereafter, the coaptation to the desired facial stumps
is then performed, usually 12 months later (96). Since it is necessary to wait for a
significant amount of time, in most cases, another nerve transfer
will be performed in order to avoid the atrophy of the mimetic
muscles [‘babysitter’ procedure described by Terzis and Tzafetta
(97), Biglioli et al
(98) in 2012; ‘triple innervation’
concept described by Biglioli (64)
in 2018].
iv) Accessory nerve (XI) transfer: The spinal nerve
is a somatic nerve innervating both the trapezius and
sternocleidomastoid (SCM) muscles. The average number of myelinated
fibres may vary between 817 (at terminal end) to 1,328 axons (at
the proximal end), according to a study published by Vathana et
al (99). Traditionally, the
spinal accessory nerve is not considered as a first-line option for
nerve transfer in facial reanimation due to the significant
morbidity that may result from its transection. An alternative to
spinal nerve transection is to coat only branches that innervate
the SCM muscle, and by doing so, the morbidity is significantly
reduced. Thulin et al (100) reported 15 cases with no
significant atrophy of the trapezius muscle. XI nerve transfer is
an alternative neural source in cases in which masseteric or
hypoglossal nerves are not available, or there is functionality
impairment of speech or swallowing.
Spinal nerve selection for transfer-phrenic
nerve. The phrenic nerve is a mixed nerve, originating from
C3-C5, and is the only source for motor innervation of the
diaphragm. The number of motor nerve fibres in the phrenic nerve is
911±321 to 1,338±467(101).
Phrenic nerves have been widely used in the reconstruction of
brachial plexus injuries with a high success rate. The length, ease
of dissection and relatively high number of motor fibres of the
ipsilateral phrenic nerve make it possible to use it as a donor
motor source for facial reanimation (102). According to Perret (103), unilateral transection of the
phrenic nerve in individuals without any associated pulmonary
disorders does not significantly affect the ventilating capacity.
In the 23 cases with phrenico-facial coaptation performed at the
University of Iowa by Perret (103), good symmetry was achieved in most
of the patients, but the major reported drawbacks were sinkinetic
facial movements with inspiration, coughing and laughing. Of note,
Xu et al (102) reported 6
cases with facial reanimation performed with latissimus dorsi
muscle free flaps and phrenic nerve transfer with no significant
morbidity related to pulmonary ventilation in a single-stage
approach.
8. Timing of facial nerve reanimation
The potential for recovery of any denervated muscle
is highly dependent on the duration of denervation. The time passed
from the moment of nerve functional disruption to the moment of
nerve regeneration is an important aspect influencing the
therapeutic outcome. The mimetic muscles must be reinnervated
before muscular atrophy occurs. The decision-making process of
whether to perform a certain technique or another for reanimation
of a palsy face is directly dependent on the status of the muscular
mimetic muscles. EMG is a preoperative prognostic tool and may
determine whether mimetic muscles are still functional or a
muscle-free flap transfer is necessary for dynamic reanimation. If
muscle tension appears insufficient or there is a hype contraction
at 6 months after the onset of contraction, the flap must be
revised (77).
Another time-dependent aspect is neural
degeneration. The physiopathologic mechanism involved is mainly
explained by Wallerian degeneration. The lag between injury and
axon degeneration is 24-48 h in young rats but it takes several
days for primate (including human) axons to degenerate (104). Motor nerves are more prone to
being impacted by the timing of restoration in comparison to
sensitive ones. Sarhane et al (85) made a solid point highlighting that
delayed nerve repair has a greater deleterious effect on motor than
sensory functional recovery. In a retrospective study, Ozmen et
al (58) found that the most
important variable influencing the final outcome is the time passed
after facial paralysis onset. They concluded that 6 months is the
cutoff point between cases showing an optimal outcome and those
with a suboptimal result; however, no correlation between the size
of the tumour and facial grafting outcome was found. Their results
are supported by those of Zhang et al (105), who found that patients with
complete paralysis who underwent facial nerve transfer 6 months
after denervation achieved postoperative oral commissural excursion
of 11.1 vs. 6.5 mm.
In long-standing facial paralysis cases, muscle
transfer is the treatment of choice in order to achieve dynamic
facial reanimation.
9. Strengths and limitations of the
study
To the best of our knowledge, the present study
provides the first bibliometric analysis on facial nerve
reconstruction following radical parotidectomy.
The present bibliometric analysis focused on the
recommendations and the new trends in facial reanimation after
radical parotidectomy by examining the records from the Scopus
database and did not include any complementary information from
other databases. The Scopus database was chosen due to the fact
that it is a more comprehensive database, reportedly having 20%
unique material compared with Web of Sciences and covering the
entire MEDLINE (PubMed) database (106). In addition, Scopus has built-in
tools to analyze various bibliometric aspects such as published
document by year, authors, territory, affiliation, subject area,
funding sponsor, year and source. Also, the main databases allowing
for direct analysis using VOSviewer software are Web of Science,
Scopus and PubMed.
10. Conclusions
The present bibliometric analysis provides a basic
worldwide analysis of the research publications on facial nerve
reanimation in radical parotidectomy. The interest in the subject
is continuously growing over the years, as facial nerve sacrifice
is a debilitating condition with a dramatic impact on patients'
quality of life.
So far, the United States is the main contributor in
the field and the main research financial support was provided by
the National Institute of Neurological Disorders and Stroke (USA),
closely followed by the Japan Society for the Promotion of
Science.
No consensus was found regarding the recommended
surgical techniques for facial nerve reanimation, nor for the best
timing for surgery, while most of the clinical experience suggests
facial nerve restoration immediately after the ablative
procedure.
The decision to perform immediate facial nerve
grafting directly depends on numerous factors related to the
general health status of the individual, the underlying disease,
the occupational activity, the existing anatomy and patient's
wishes corroborated with the surgical team's recommendations and
should take into consideration the fact that facial reanimation
outcomes are not always predictable, may involve donor site
morbidity and rely on of the complex nature of peripheral nerve
regeneration and reinnervation.
Further research on preoperative prediction of the
facial nerve status and the relation with the tumour in patients
with parotid gland malignancy are mandatory for a conservative
decision or immediate reconstruction.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
Conceptualization: IF, CMC, SD and LC; methodology:
IF, CMC, MS, FB; WOSviewer software: CMC and IF; validation: SD, IF
and CMC; data curation: CMC, LC and SD; writing-original draft
preparation: IF, FB, CMC; writing-review and editing: SD, IF and
CMC; visualization: LC, SD, MS and FB; supervision: CMC. Data
authentication is not applicable. All authors have read and agreed
to the published version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Skalova A and Hyrcza MD: Proceedings of
the North American society of head and neck pathology companion
meeting, new Orleans, LA, March 12, 2023: classification of
salivary gland tumors: Remaining controversial issues? Head Neck
Pathol. 17:285–291. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Skálová A, Hyrcza MD and Leivo I: Update
from the 5th edition of the World Health Organization
classification of head and neck tumors: Salivary glands. Head Neck
Pathol. 16:40–53. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Speight PM and Barrett AW: Salivary gland
tumours. Oral Dis. 8:229–240. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alsanie I, Rajab S, Cottom H, Adegun O,
Agarwal R, Jay A, Graham L, James J, Barrett AW, van Heerden W, et
al: Distribution and frequency of salivary gland tumours: An
international multicenter study. Head Neck Pathol. 16:1043–1054.
2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rousseau A and Badoual C: Head and neck:
Salivary gland tumors: An overview. Atlas Genet Cytogenet Oncol
Haematol. 15:534–541. 2011.
|
|
6
|
Dulguerov P, Marchal F and Lehmann W:
Postparotidectomy facial nerve paralysis: Possible etiologic
factors and results with routine facial nerve monitoring.
Laryngoscope. 109:754–762. 1999.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lombardi D, McGurk M, Vander Poorten V,
Guzzo M, Accorona R, Rampinelli V and Nicolai P: Surgical treatment
of salivary malignant tumors. Oral Oncol. 65:102–113.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
David AP, Seth R and Knott PD: Facial
reanimation and reconstruction of the radical parotidectomy. Facial
Plast Surg Clin North Am. 29:405–414. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sánchez-Burgos R, Otero TG, Lassaletta L,
Arias Gallo J, Cuellar IN and García MB: Facial nerve
reconstruction following radical parotidectomy and subtotal
petrosectomy for advanced malignant parotid neoplasms. Ann
Maxillofac Surg. 5:203–207. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Weng R, Lin DX, Song YK, Guo HW, Zhang WS,
He XM, Li WC, Lin HH, He MC and Wei QS: Bibliometric and visualized
analysis of research relating to minimally invasive spine surgery
reported over the period 2000-2022. Digit Heal.
9(20552076231173562)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Donthu N, Kumar S, Mukherjee D, Pandey N
and Lim WM: How to conduct a bibliometric analysis: An overview and
guidelines. J Bus Res. 133:285–296. 2021.
|
|
12
|
Van Eck N and Waltman L: Software survey:
VOSviewer, a computer program for bibliometric mapping.
Scientometrics. 84:523–538. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park W, Park J, Park SI, Kim H, Bae H, Cho
J, Won H, Park M and Jeong HS: Clinical outcomes and management of
facial nerve in patients with parotid gland cancer and pretreatment
facial weakness. Oral Oncol. 89:144–149. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Spiro RH: Salivary neoplasms: Overview of
a 35-year experience with 2,807 patients. Head Neck Surg.
8:177–184. 1986.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Domenick NA and Johnson JT: Parotid tumor
size predicts proximity to the facial nerve. Laryngoscope.
121:2366–2370. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Preis M, Soudry E, Bachar G, Shufel H,
Feinmesser R and Shpitzer T: Predicting facial nerve invasion by
parotid gland carcinoma and outcome of facial reanimation. Eur Arch
Otorhinolaryngol. 267:107–111. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bovenzi CD, Ciolek P, Crippen M, Curry JM,
Krein H and Heffelfinger R: Reconstructive trends and complications
following parotidectomy: Incidence and predictors in 11,057 cases.
J Otolaryngol Neck Surg. 48(64)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lu GN, Villwock MR, Humphrey CD, Kriet JD
and Bur AM: Analysis of facial reanimation procedures performed
concurrently with total parotidectomy and facial nerve sacrifice.
JAMA Facial Plast Surg. 21:50–55. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Biglioli F: Facial reanimations: Part
I-recent paralyses. Br J Oral Maxillofac Surg. 53:901–906.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huyett P, Duvvuri U, Ferris RL, Johnson
JT, Schaitkin BM and Kim S: Perineural invasion in parotid gland
malignancies. Otolaryngol Head Neck Surg. 158:1035–1041.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
House JW and Brackmann DE: Facial nerve
grading system. Otolaryngol Head Neck Surg. 93:146–147.
1985.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chee GH and Nedzelski JM: Facial nerve
grading systems. Facial Plast Surg. 16:315–324. 2000.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Adour KK and Swanson PJ Jr: Facial
paralysis in 403 consecutive patients: Emphasis on treatment
response in patients with Bell's palsy. Trans Am Acad Ophthalmol
Otolaryngol. 75:1284–1301. 1971.PubMed/NCBI
|
|
24
|
Yanagihara N: Grading of facial palsy. In:
Fisch U (ed). Proceedings of the third international symposium on
facial nerve surgery. Kugler Medical Publications, Zurich,
pp533-535, 1976.
|
|
25
|
Stennert E, Limberg CH and Frentrup KP: An
index for paresis and defective healing-an easily applied method
for objectively determining therapeutic results in facial paresis
(author's transl). HNO. 25:238–245. 1977.PubMed/NCBI(In German).
|
|
26
|
Burres S and Fisch U: The comparison of
facial grading systems. Arch Otolaryngol Head Neck Surg.
112:755–758. 1986.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhai MY, Feng GD and Gao ZQ: Facial
grading system: Physical and psychological impairments to be
considered. J Otol. 3:61–67. 2008.
|
|
28
|
Murty GE, Diver JP, Kelly PJ, O'Donoghue
GM and Bradley PJ: The nottingham system: Objective assessment of
facial nerve function in the clinic. Otolaryngol Head Neck Surg.
110:156–161. 1994.PubMed/NCBI View Article : Google Scholar
|
|
29
|
ten Harkel TC, de Jong G, Marres HAM,
Ingels KJAO, Speksnijder CM and Maal TJJ: Automatic grading of
patients with a unilateral facial paralysis based on the sunnybrook
facial grading system-A deep learning study based on a
convolutional neural network. Am J Otolaryngol.
44(103810)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Neely JG, Cherian NG, Dickerson CB and
Nedzelski JM: Sunnybrook facial grading system: Reliability and
criteria for grading. Laryngoscope. 120:1038–1045. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
el-Naggar M, Rice B and Oswal V: Life-size
photograph transparencies: A method for the photographic detection
and documentation of recovery from facial paralysis. J Laryngol
Otol. 109:748–750. 1995.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yuen K, Inokuchi I, Maeta M, Kawakami SI
and Masuda Y: Evaluation of facial palsy by moiré topography index.
Otolaryngol Head Neck Surg. 117:567–572. 1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Labecka MK and Plandowska M: Moiré
topography as a screening and diagnostic tool-A systematic review.
PLoS One. 16(e0260858)2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Banks CA, Bhama PK, Park J, Hadlock CR and
Hadlock TA: Clinician-graded electronic facial paralysis
assessment: The eFACE. Plast Reconstr Surg. 136:223e–230e.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hadlock TA and Urban LS: Toward a
universal, automated facial measurement tool in facial reanimation.
Arch Facial Plast Surg. 14:277–282. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ojha PT, Nagendra S, Ansari A, Kadam ND,
Mathur A, Gopinathan N, Ojha N, Patel H, Bansode A, Kalel O, et al:
Validation of a new graphic facial nerve grading system: FAME
scale. Ann Indian Acad Neurol. 25:464–472. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bendet E, Talmi YP and Kronenberg J:
Preoperative electroneurography (ENoG) in parotid surgery:
Assessment of facial nerve outcome and involvement by tumor-a
preliminary study. Head Neck. 20:124–131. 1998.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lee DH: Clinical efficacy of
electroneurography in acute facial paralysis. J Audiol Otol.
20:8–12. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wiertel-Krawczuk A, Huber J, Wojtysiak M,
Golusiński W, Pieńkowski P and Golusiński P: Correlations between
the clinical, histological and neurophysiological examinations in
patients before and after parotid gland tumor surgery: Verification
of facial nerve transmission. Eur Arch Otorhinolaryngol.
272:1219–1229. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Aimoni C, Lombardi L, Gastaldo E,
Stacchini M and Pastore A: Preoperative and postoperative
electroneurographic facial nerve monitoring in patients with
parotid tumors. Arch Otolaryngol Head Neck Surg. 129:940–943.
2003.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Gupta S, Mends F, Hagiwara M, Fatterpekar
G and Roehm PC: Imaging the facial nerve: A contemporary review.
Radiol Res Pract. 2013(248039)2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Wegscheider H, Volk GF, Guntinas-Lichius O
and Moriggl B: High-resolution ultrasonography of the normal
extratemporal facial nerve. Eur Arch Otorhinolaryngol. 275:293–299.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Guntinas-Lichius O, Silver CE, Thielker J,
Bernal-Sprekelsen M, Bradford CR, De Bree R, Kowalski LP, Olsen KD,
Quer M, Rinaldo A, et al: Management of the facial nerve in parotid
cancer: Preservation or resection and reconstruction. Eur Arch
Otorhinolaryngol. 275:2615–2626. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Guenette JP, Ben-Shlomo N, Jayender J,
Seethamraju RT, Kimbrell V, Tran NA, Huang RY, Kim CJ, Kass JI,
Corrales CE and Lee TC: MR imaging of the extracranial facial nerve
with the CISS sequence. AJNR Am J Neuroradiol. 40:1954–1959.
2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Takahashi N, Okamoto K, Ohkubo M and
Kawana M: High-resolution magnetic resonance of the extracranial
facial nerve and parotid duct: Demonstration of the branches of the
intraparotid facial nerve and its relation to parotid tumours by
MRI with a surface coil. Clin Radiol. 60:349–354. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Coulson SE, O'dwyer NJ, Adams RD and
Croxson GR: Expression of emotion and quality of life after facial
nerve paralysis. Otol Neurotol. 25:1014–1019. 2004.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Walker DT, Hallam MJ, Ni Mhurchadha S,
McCabe P and Nduka C: The psychosocial impact of facial palsy: Our
experience in one hundred and twenty six patients. Clin
Otolaryngol. 37:474–477. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ekman P: Basic emotions. Handb Cogn Emot.
98(16)1999.
|
|
49
|
Guntinas-Lichius O, Straesser A and
Streppel M: Quality of life after facial nerve repair.
Laryngoscope. 117:421–426. 2007.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Park H, Kim DJ, Chung JH, Yoon ES and Park
SH: Quantitative analysis of facial symmetry and animation
following intraoral orthodromic temporalis transfer in facial
paralysis. J Craniomaxillofac Surg. 51:272–279. 2023.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Pinkiewicz M, Dorobisz K and Zatoński T: A
comprehensive approach to facial reanimation: A systematic review.
J Clin Med. 11(2890)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Subramaniam N, Luu E, Asher R, Oates J,
Clark JR and Low TH: The impact of radical parotidectomy with
immediate facial nerve reconstruction: A quality-of-life measure. J
Laryngol Otol. 135:804–809. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Owusu JA, Truong L and Kim JC: Facial
nerve reconstruction with concurrent masseteric nerve transfer and
cable grafting. JAMA Facial Plast Surg. 18:335–339. 2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lin CH, Wallace C and Liao CT: Functioning
free gracilis myocutaneous flap transfer provides a reliable
single-stage facial reconstruction and reanimation following tumor
ablation. Plast Reconstr Surg. 128:687–696. 2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wax MK and Kaylie DM: Does a positive
neural margin affect outcome in facial nerve grafting? Head Neck.
29:546–549. 2007.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Volk GF, Pantel M, Streppel M and
Guntinas-Lichius O: Reconstruction of complex peripheral facial
nerve defects by a combined approach using facial nerve
interpositional graft and hypoglossal-facial jump nerve suture.
Laryngoscope. 121:2402–2405. 2011.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Osinga R, Buncke HJ, Buncke GM and
Meuli-Simmen C: Subdivision of the sural nerve: Step towards
individual facial reanimation. J Plast Surg Hand Surg. 45:3–7.
2011.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ozmen OA, Falcioni M, Lauda L and Sanna M:
Outcomes of facial nerve grafting in 155 cases: Predictive value of
history and preoperative function. Otol Neurotol. 32:1341–1346.
2011.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Humphrey CD and Kriet JD: Nerve repair and
cable grafting for facial paralysis. Facial Plast Surg. 24:170–176.
2008.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Tomita K, Hosokawa K and Yano K:
Reanimation of reversible facial paralysis by the double
innervation technique using an intraneural-dissected sural nerve
graft. J Plast Reconstr Aesthet Surg. 63:e535–e539. 2010.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Bianchi B, Ferri A and Sesenna E: Facial
reanimation after nerve sacrifice in the treatment of head and neck
cancer. Curr Opin Otolaryngol Head Neck Surg. 20:114–119.
2012.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Biglioli F, Frigerio A, Colombo V,
Colletti G, Rabbiosi D, Mortini P, Dalla Toffola E, Lozza A and
Brusati R: Masseteric-facial nerve anastomosis for early facial
reanimation. J Craniomaxillofac Surg. 40:149–155. 2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Vincent AG, Bevans SE, Robitschek JM, Wind
GG and Hohman MH: Masseteric-to-facial nerve transfer and selective
neurectomy for rehabilitation of the synkinetic smile. JAMA Facial
Plast Surg. 21:504–510. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Biglioli F, Allevi F, Rabbiosi D, Cupello
S, Battista VMA, Saibene AM and Colletti G: Triple innervation for
re-animation of recent facial paralysis. J Craniomaxillofac Surg.
46:851–857. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Urken ML, Weinberg H, Vickery C and Biller
HF: The neurofasciocutaneous radial forearm flap in head and neck
reconstruction: A preliminary report. Laryngoscope. 100:161–173.
1990.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Teknos TN, Nussenbaum B, Bradford CR,
Prince ME, El-Kashlan H and Chepeha DB: Reconstruction of complex
parotidectomy defects using the lateral arm free tissue transfer.
Otolaryngol Head Neck Surg. 129:183–191. 2003.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Revenaugh PC, Knott PD, Scharpf J and
Fritz MA: Simultaneous anterolateral thigh flap and temporalis
tendon transfer to optimize facial form and function after radical
parotidectomy. Arch Facial Plast Surg. 14:104–109. 2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Ch'ng S, Ashford BG, Gao K, McGuinness J
and Clark JR: Reconstruction of post-radical parotidectomy defects.
Plast Reconstr Surg. 129:275e–287e. 2012.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Jandali D and Revenaugh PC: Facial
reanimation: An update on nerve transfers in facial paralysis. Curr
Opin Otolaryngol Head Neck Surg. 27:231–236. 2019.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Biglioli F, D'Orto O, Bozzetti A and
Brusati R: Function of the great auricular nerve following surgery
for benign parotid disorders. J Craniomaxillofac Surg. 30:308–317.
2002.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Biglioli F, Colombo V, Pedrazzoli M,
Frigerio A, Tarabbia F, Autelitano L and Rabbiosi D: Thoracodorsal
nerve graft for reconstruction of facial nerve branching. J
Craniomaxillofac Surg. 42:e8–e14. 2014.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Lee MC, Kim DH, Jeon YR, Rah DK, Lew DH,
Choi EC and Lee WJ: Functional outcomes of multiple sural nerve
grafts for facial nerve defects after tumor-ablative surgery. Arch
Plast Surg. 42:461–468. 2015.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Haller JR and Shelton C: Medial
antebrachial cutaneous nerve: A new donor graft for repair of
facial nerve defects at the skull base. Laryngoscope.
107:1048–1052. 1997.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Li H, Zhu W, Wu S, Wei Z and Yang S:
Anatomical analysis of antebrachial cutaneous nerve distribution
pattern and its clinical implications for sensory reconstruction.
PLoS One. 14(e0222335)2019.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Vaughan ED and Richardson D: Facial nerve
reconstruction following ablative parotid surgery. Br J Oral
Maxillofac Surg. 31:274–280. 1993.PubMed/NCBI View Article : Google Scholar
|
|
76
|
White WM, McKenna MJ and Deschler DG: Use
of the thoracodorsal nerve for facial nerve grafting in the setting
of pedicled latissimus dorsi reconstruction. Otolaryngol Head Neck
Surg. 135:962–964. 2006.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Biglioli F, Frigerio A, Rabbiosi D and
Brusati R: Single-stage facial reanimation in the surgical
treatment of unilateral established facial paralysis. Plast
Reconstr Surg. 124:124–133. 2009.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Klebuc M and Shenaq SM: Donor nerve
selection in facial reanimation surgery. Semin Plast Surg.
18:53–60. 2004.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Spira M: Anastomosis of masseteric nerve
to lower division of facial nerve for correction of lower facial
paralysis. Preliminary report. Plast Reconstr Surg. 61:330–334.
1978.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Sakthivel P, Singh CA, Thakar A, Thirumeni
G, Raveendran S and Sharma SC: Masseteric-facial nerve anastomosis:
Surgical techniques and outcomes-A pilot Indian study. Indian J
Otolaryngol Head Neck Surg. 72:92–97. 2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Coombs CJ, Ek EW, Wu T, Cleland H and
Leung MK: Masseteric-facial nerve coaptation-an alternative
technique for facial nerve reinnervation. J Plast Reconstr Aesthet
Surg. 62:1580–1588. 2009.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Borschel GH, Kawamura DH, Kasukurthi R,
Hunter DA, Zuker RM and Woo AS: The motor nerve to the masseter
muscle: An anatomic and histomorphometric study to facilitate its
use in facial reanimation. J Plast Reconstr Aesthet Surg.
65:363–366. 2012.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Manktelow RT, Tomat LR, Zuker RM and Chang
M: Smile reconstruction in adults with free muscle transfer
innervated by the masseter motor nerve: Effectiveness and cerebral
adaptation. Plast Reconstr Surg. 118:885–899. 2006.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Klebuc MJA: Facial reanimation using the
masseter-to-facial nerve transfer. Plast Reconstr Surg.
127:1909–1915. 2011.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Sarhane KA, Shappell H, Slavin B, von
Guinneau N, Hanwright P, Borschel GH and Tuffaha SH: Abstract QS19:
Elucidating the effects of delayed nerve repair on motor vs sensory
functional recovery: A systematic review and meta-analysis. Plast
Reconstr Surg Glob Open. 7 (4 Suppl):S114–S115. 2019.
|
|
86
|
Murphey AW, Clinkscales WB and Oyer SL:
Masseteric nerve transfer for facial nerve paralysis: A systematic
review and meta-analysis. JAMA Facial Plast Surg. 20:104–110.
2018.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Wang W, Yang C, Li Q, Li W, Yang X and
Zhang YX: Masseter-to-facial nerve transfer: A highly effective
technique for facial reanimation after acoustic neuroma resection.
Ann Plast Surg. 73 (Suppl 1):S63–S69. 2014.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Tiago RSL, de Faria FP, de Lima Pontes PA
and do Brasil O de OC: Morphometric aspects of the human
hypoglossal nerve in adults and the elderly. Braz J
Otorhinolaryngol. 71:554–558. 2005.PubMed/NCBI View Article : Google Scholar
|
|
89
|
May M, Sobol SM and Mester SJ:
Hypoglossal-facial nerve interpositional-jump graft for facial
reanimation without tongue atrophy. Otolaryngol Head Neck Surg.
104:818–825. 1991.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Mackinnon SE and Dellon AL: Fascicular
patterns of the hypoglossal nerve. J Reconstr Microsurg.
11:195–198. 1995.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Chuang DCC, Lu JCY, Chang TNJ and Laurence
VG: Comparison of functional results after cross-face nerve graft-,
spinal accessory nerve-, and masseter nerve-innervated gracilis for
facial paralysis reconstruction: The chang gung experience. Ann
Plast Surg. 81 (6S Suppl 1):S21–S29. 2018.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Galli SKD, Valauri F and Komisar A: Facial
reanimation by cross-facial nerve grafting: Report of five cases.
Ear Nose Throat J. 81:25–29. 2002.PubMed/NCBI
|
|
93
|
Kim IA, Maxim T and Echanique K: Modern
cross-facial nerve grafting in facial paralysis. Oper Tech
Otolaryngol Neck Surg. 33:20–28. 2022.
|
|
94
|
Harii K, Ohmori K and Torii S: Free
gracilis muscle transplantation, with microneurovascular
anastomoses for the treatment of facial paralysis. A preliminary
report. Plast Reconstr Surg. 57:133–143. 1976.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Hontanilla B, Marre D and Cabello Á:
Facial reanimation with gracilis muscle transfer neurotized to
cross-facial nerve graft versus masseteric nerve: A comparative
study using the FACIAL CLIMA evaluating system. Plast Reconstr
Surg. 131:1241–1252. 2013.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Rodríguez-Lorenzo A and Tzou CHJ:
Principles of facial nerve reconstruction. In: Facial Palsy:
Techniques for Reanimation of the Paralyzed Face. Tzou CHJ and
Rodríguez-Lorenzo A (eds.) Springer International Publishing, Cham,
pp55-69, 2021.
|
|
97
|
Terzis JK and Tzafetta K: The ‘babysitter’
procedure: Minihypoglossal to facial nerve transfer and
cross-facial nerve grafting. Plast Reconstr Surg. 123:865–876.
2009.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Biglioli F, Colombo V, Tarabbia F,
Pedrazzoli M, Battista V, Giovanditto F, Dalla Toffola E, Lozza A
and Frigerio A: Double innervation in free-flap surgery for
long-standing facial paralysis. J Plast Reconstr Aesthet Surg.
65:1343–1349. 2012.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Vathana T, Larsen M, de Ruiter GCW, Bishop
AT, Spinner RJ and Shin AY: An anatomic study of the spinal
accessory nerve: Extended harvest permits direct nerve transfer to
distal plexus targets. Clin Anat. 20:899–904. 2007.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Thulin CA, Petersen I and Granholm L:
Follow-up study of spinal accessory-facial nerve anastomosis with
special reference to the electromyographic findings. J Neurol
Neurosurg Psychiatry. 27:502–506. 1964.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Wang C, Zhang Y, Nicholas T, Wu G, Shi S,
Bo Y, Wang X, Zhou X and Yuan W: Neurotization of the phrenic nerve
with accessory nerve for high cervical spinal cord injury with
respiratory distress: An anatomic study. Turk Neurosurg.
24:478–483. 2014.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Xu YB, Liu J, Li P, Donelan MB, Parrett BM
and Winograd JM: The phrenic nerve as a motor nerve donor for
facial reanimation with the free latissimus dorsi muscle. J
Reconstr Microsurg. 25:457–463. 2009.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Perret G: Results of phrenicofacial nerve
anastomosis for facial paralysis. Arch Surg. 94:505–508.
1967.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Gaudet AD, Popovich PG and Ramer MS:
Wallerian degeneration: Gaining perspective on inflammatory events
after peripheral nerve injury. J Neuroinflammation.
8(110)2011.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Zhang S, Hembd A, Ching CW, Tolley P and
Rozen SM: Early masseter to facial nerve transfer may improve smile
excursion in facial paralysis. Plast Reconstr Surg Glob Open.
6(e2023)2018.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Falagas ME, Pitsouni EI, Malietzis GA and
Pappas G: Comparison of PubMed, scopus, web of science, and google
scholar: Strengths and weaknesses. FASEB J. 22:338–342.
2008.PubMed/NCBI View Article : Google Scholar
|