1. CeO2-x and CeO2-x
nanoparticles
Nanotechnology has become a promising ally for
research and treatment of diseases. In addition to a wide range of
established applications in multiple areas of research (1), cerium has captured the interest of
researchers due to its redox properties. Cerium, a rare earth
metal, can exist in both +3 and +4 states. Thus, cerium oxide
(CeO2-x) can occur in two different forms:
CeO2 and Ce2O3 in the bulk state,
due to the coexistence of the element cerium (Ce) in two different
oxidation states: Ce3+ [(Xe) 4f1] and
Ce4+ [(Xe)]. The
CeO2-Ce2O3 phase transition
depends on the oxygen pressure and system temperature, as well as
the reduction transition (2,3). Among
the compounds with Ce4+, the CeO2 phase
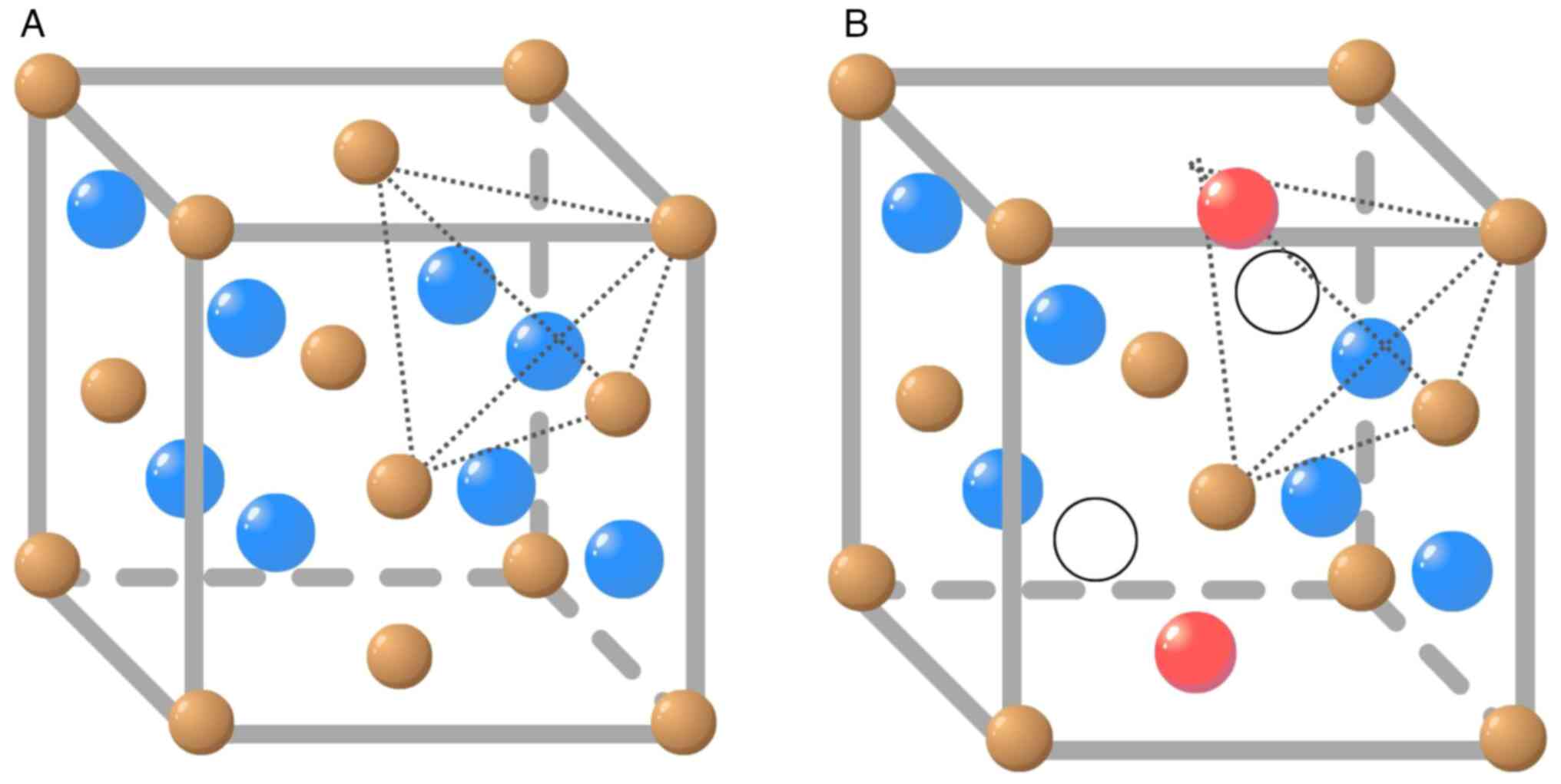
presents the most stable structure: A face-centred cubic
crystalline network (FCC) of the fluorite type (Fm3m)
(4,5). Each tetravalent cerium cation
(Ce4+) coordinates with eight oxygen anions
(O-2) (Fig. 1A), further
providing greater stability compared with the hexagonal structure
of Ce2O3 (6).
The CeO2 structure could present intrinsic and extrinsic
defects in the atom arrangement. Intrinsic defects are related to
the thermal disorder of the material or the result of atmosphere
surrounding the material, the redox process (7). At the nanoscale, however, the
formation of non-stoichiometric oxides is observed in the redox
process in CeO2-x, which creates pure CeO2-x
nanoparticle structures where 0 <x <0.5(8). In this process, electrons are
transferred from the oxygen anion (O2-) to the cerium
cation (Ce4+), which generates an oxygen vacancy from
the reduction of Ce4+ to Ce3+ (Fig. 1B; Eq. 1).
(Eq. 1: ø represents the empty position due to the
atom displacement in the structure after electron transfer).
An oxygen vacancy is a structural disarrangement
caused by the increase or decrease of oxygen concentration inside
the particle and cerium ion radius. It is important to note that
the concentration of oxygen defects increase with the reduction in
particle size, which confers better redox properties of
CeO2-x nanoparticles compared with their oxide (9,10). In
chemical catalysis, vacancy is defined as the ability of an oxide
to store and release oxygen. The description of oxygen vacancies in
transition oxides and rare earth oxides is an unexplored challenge
for modern calculations of electronic structure (11). Due to the increase in the
surface-to-volume ratio, CeO2-x nanoparticles have
higher concentrations of Ce3+, compared with
CeO2-x particles. Thus, there is greater mobility of
oxygen in the structure for the rapid generation of surface
vacancies. This property provides easy switching between
Ce3+ and Ce4+ oxidation states, generating
numerous active points for redox reactions to occur on the surface
of nanoparticles. Due to their oxygen buffering capacity,
CeO2-x nanoparticles can also self-regenerate to the
initial state of Ce4+ with no side reactions (12).
Nanoparticle structural
defects-vacancies
Vacancies are structural defects in a particle,
which can be formed through electronic relocation in the structure
of the material, generating redox hotspots. Vacancies are not
restricted to the oxide surface only. For CeO2-x,
studies demonstrate that the electrons resulting from the formation
of oxygen vacancies on the surface and subsurface in the
crystallographic plane (111; Miller index) may not go to the atoms
directly linked to the electron donator atom, but to more distant
atoms instead (11,13). Due to the increase in the surface
area, CeO2-x nanoparticles have more Ce3+
ions on their surface and their vacancies can occur by a quantum
ionization/displacement process of cerium 4f electrons, as proposed
by Skorodumova et al (14).
Electrons in the f subshell create redox hotspots by the
reduction of Ce4+ to Ce3+ (11). In addition, the migration of
electrons from 2p orbital oxygen states in the valence to the
conduction band is only possible if the energy between them (band
gap) is relatively small (14,15).
In CeO2-x nanoparticles, vacancies are dynamic and can
change spontaneously or in response to physical parameters, such as
temperature, partial pressure of oxygen, doping with other ions and
application of an electric field or surface stress (16,17).
Another phenomenon responsible for causing vacancies is entropic
stabilization, which will appear on surfaces with numerous empty
spaces. Beyond particle size, some other factors may influence the
redox activity of the nanoparticles, such as suspension medium and
formation of agglomerates. Reducible oxide surfaces, such as
CeO2-x nanoparticles, are highly disordered at the
nanoscale, causing a greater formation of empty spaces,
facilitating the formation of vacancies, and presenting even
greater redox activity (18). Due
to their high redox activity, CeO2-x nanoparticles have
started to gain attention in the biomedical field, particularly
with regard to diseases in which a redox imbalance is observed. It
is important to note that a redox imbalance can be found in a wide
range of diseases, including cancer (19). Therefore, some studies have
addressed the oxidative stress-associated cytotoxic effects of
CeO2-x nanoparticles in biological models, particularly
focusing on the modulation of the apoptotic pathway, the most
studied regulated cell death modality (Tables I and II). This is relevant, considering the
increasing body of evidence characterizing genetically-regulated
distinct mechanisms of cell death (20). Due to the wide diversity of cell
death subroutines, the present study focuses only on the effects of
nanoparticles on apoptotic cell death.
 | Table IEffects of cerium oxide nanoparticles
in non-cancer disease models. |
Table I
Effects of cerium oxide nanoparticles
in non-cancer disease models.
| Disease models | Redox
activity/Apoptosis regulation | Nanoparticle
characteristics | (Refs.) |
|---|
| Alveolar epithelial
cells |
Antioxidant/Oxidant | Not described | Lord et al
(21) |
| Progressive
cochlear and retinal degeneration in Tubby mice |
Antioxidant/Apoptosis inhibition | Medium, saline | Kong et al
(40) |
| Hepatic
fibrosis |
Antioxidant/Apoptosis inhibition | Synthesis, chemical
precipitation; Size, 4-20 nm; medium, aqueous solution of
TMAOH | Oró et al
(41) |
| Type 1
diabetes |
Antioxidant/Apoptosis inhibition | Purchased from
Sigma-Aldrich; size, 90 nm; shape, cubes | Khurana et
al (42) |
| Activated and
non-activated human monocytic cells |
Antioxidant/Oxidant | Synthesis, flame
spray pyrolysis; size: 3-94 nm | Schwotzer et
al (43) |
 | Table IIEffects of cerium oxide nanoparticles
in cancer cellular models. |
Table II
Effects of cerium oxide nanoparticles
in cancer cellular models.
| Cancer model | Redox
activity/Apoptosis regulation | Nanoparticle
characteristics | (Refs.) |
|---|
| Breast cancer and
healthy breast cells | No effect in breast
cancer cells and antioxidant in healthy breast cells | Synthesis,
microemulsion process; size, 3-5 nm | Tarnuzzer et
al (50) |
| Lung carcinoma,
melanoma and colorectal adenocarcinoma and healthy (origin)
cells | Oxidant in
tumors/No apoptosis induction | Synthesis, SPRT;
shape, cubic; size: 4 nm | Pešić et al
(62) |
| Alveolar
adenocarcinoma, hepatoma, colorectal cancer, cervical cancer and
healthy (origin) cells | Antioxidant | Purchased from
Sigma-Aldrich; size, <25 nm; medium, DMEM | Rubio et al
(57) |
| Hepatoma | Oxidant | Shape, hexahedral;
size, 20-30 nm | Cheng et al
(58) |
| Hepatoma | Unchanged/Apoptosis
inhibition | Not described | Cheng et al
(61) |
| Hepatoma | Apoptosis
induction | Synthesis, chemical
precipitation; size, 4-5 nm; medium, aqueous solution of TMAOH | Fernández-Varo
et al (60) |
| Melanoma | Oxidant/Apoptosis
induction | Purchased from
Sigma- Aldrich; size, 20-40 nm | Ali et al
(53) |
| Melanoma | Oxidant/Apoptosis
induction | Purchased from
Sciventions; Medium, water | Aplak et al
(63) |
| Colorectal
carcinoma | Oxidant/Apoptosis
induction | Synthesis, chemical
precipitation; size, 30-40 nm | Datta et al
(64) |
2. CeO2-x nanoparticles mimic
antioxidant enzymes
Due to their physical and chemical redox properties,
CeO2-x nanoparticles have begun to be used in the
biomedical field, with the aim of restoring normal tissue
homeostasis. CeO2-x nanoparticles exhibit controversial
pro-oxidant and antioxidant activity, which enable them to react
with chemical elements such as oxygen, nitrogen, sulphur and
chloride (21). It has previously
been described that CeO2-x nanoparticles have the
ability to mimic some enzymes, such as catalase (CAT) and
superoxide dismutase (SOD), and neutralise reactive oxygen species
(ROS) (21).
Korsvik et al (22) have described CeO2-x
nanoparticles exhibiting SOD-like activity, hypothesizing that they
confer cellular protection. These findings revealed that the
surface oxidation state of CeO2-x nanoparticles plays an
essential role in the SOD mimetic activity, found to be dependent
on the concentration of the +3 oxidation state (22). It suggests a positive association
between the trivalent oxidation state of CeO2-x
nanoparticles and the mimetic activity of SOD (22,23). A
single nanoparticle has been shown to be more efficient as a SOD
catalyst than the natural SOD enzyme, with a catalytic rate of
3.6x109 M-1sec-1 compared with 1.3
and 2.8x109 M-1sec-1 of a natural
SOD enzyme (22). In contrast to
results attributing a SOD mimetic activity for CeO2-x
nanoparticles, experiments conducted by Pirmohamed et al
(24) showed CeO2-x
nanoparticles acting as CAT mimetics in a redox state-dependent
manner. Their findings demonstrated that only CeO2-x
nanoparticles with fewer surface cerium atoms in the +3 state
exhibited any significant CAT mimetic activity. These findings are
especially noteworthy, since CeO2-x nanoparticles with
lower +3/+4 ratios were revealed to be less efficient in their SOD
mimetic activity, and thus there appears to be an inverse
realtionship between catalysis and the cerium oxidation state of
the nanoparticle (23).
It should be noted that the effects of SOD and CAT
mimetics can be enhanced or suppressed by CeO2-x
nanoparticle surface modifications. Yadav and Singh (23) demonstrated that CeO2-x
nanoparticles coated with phosphotungstic acid (PTA) exhibited an
enhanced SOD and CAT mimetic activity independent of the majority
of nanoparticle surface charges (+3 or +4). When covering the
nanoparticle with phosphomolybdic acid (PMA), SOD activity was
suppressed in CeO2-x nanoparticles with its +3 surface
charge, however no effect was observed on CAT activity. In
addition, PMA covering exerted no effect on SOD while CAT acitvity
was enhanced in CeO2-x nanoparticles with a +4 surface
charge. PTA and PMA are both electron-dense molecules and display
quick and reversible multielectron redox reactions, a probable
explanation for the surface charge-dependent effects on SOD/CAT
(23).
Notably, a molecule exhibiting the same
characteristics as PTA and PMA was demonstrated to exert the
opposite effects from those aforementioned. Triethyl phosphite
(TEP) altered the SOD and CAT mimetic activities of
CeO2-x nanoparticles. A higher
Ce4+/Ce3+ surface oxidation state exerted a
decrease in CAT mimetic activity while SOD activity was increased.
In addition, in this study a correlation between TEP concentration
and the formation of surface oxygen vacancies was reported
(25).
Of note, PMA, PTA and TEP are molecules that contain
phosphorus. For example, Karakoti et al (26) described that CeO2-x
nanoparticles coated with the polymer polyethylene glycol (PEG),
which does not hold phosphorus in its composition, did not affect
the SOD mimicking activity of the nanoparticle. In view of the
results herein mentioned, CeO2-x nanoparticles exhibit
SOD and CAT mimetic activity, and their effects may be enhanced or
suppressed according to which type of molecules cover the the
surfaces of the nanoparticles. Further studies analysing the
influence of different chemical functional groups on SOD and CAT
mimetic activities on the surface of nanoparticles are
required.
3. Biological effects of CeO2-x
nanoparticles in disease models with dysregulated apoptosis and
redox imbalance
Apoptosis is the most studied cell death modality
and can be triggered by a wide range of stimuli, such as DNA
damage, nutrient deprivation, hypoxia, viral infections, growth
factor and hormone signalling. In addition, apoptosis involves the
activation of two main biochemical pathways (intrinsic and
extrinsic) and leads to various events such as cell retraction,
bleb formation, protein cleavage, DNA degradation, and phagocyte
recognition (20). Apoptosis
dysregulation is involved in the pathogenesis of numerous diseases,
including progressive cochlear and retinal degeneration (27), liver fibrosis (28), hypoxia in brain cells (29), type 1 diabetes (30), neurodegenerative diseases (31) and cancer (32). For some of these conditions,
apoptosis is defective, and thus cells are capable of surviving
even upon cell death stimuli. For others, apoptosis is aberrant,
and is associated with organ degeneration and failure. Restoring
apoptosis is essential for tissue homeostasis and great effort has
been devoted to inducing or inhibiting this form of cell death,
depending on the cellular and disease context. Although the scope
of this manuscript is apoptosis, the increasingly significant role
of other regulated cell death modalities directly modified by
cellular oxidative stress, such as ferroptosis or paraptosis,
cannot be excluded (20).
Furthermore, the accumulation of ROS has been
described to be associated with the development of pathologies such
as diabetes, atherosclerosis, stroke, arthrosis, amyotrophic
lateral sclerosis, neurodegenerative disorders including
Parkinson's and Alzheimer's diseases and cancer (10). ROS play a central role in cell
signaling as well as regulation of the main pathways of apoptosis
mediated by mitochondria, death receptors and the endoplasmic
reticulum (ER) (33-35).
Notably, several cytotoxic agents have been shown to induce
apoptosis by ROS production (36-39).
Nevertheless, low levels of ROS play an important role in the
signal transduction process inside the cell, acting as second
messengers in the physiological environment. Therefore, it is
important to finely tune the balance between cellular oxidative
stressors and the antioxidative defence to maintain homeostasis and
impair pathological states (10).
In this context, with the increasing interest in the
redox characteristics of CeO2-x nanoparticles, numerous
authors have dedicated studies to their role in modulating
apoptotic signalling pathways in a wide range of disease
models.
Effects of CeO2-x
nanoparticles in disease models exhibiting excessive apoptosis
Some studies have shown CeO2-x
nanoparticles as negatively regulating apoptosis pathways in animal
models of diseases that exhibit aberrant apoptosis (Table I; Fig.
2). For example, in an in vivo model of cochlear and
retinal degeneration, CeO2-x nanoparticles were shown to
upregulate, at the protein level, the basic fibroblast growth
factor (bFGF)/receptor tyrosine kinase (RTK)/Ras/extracellular
signal-regulated kinase (ERK) pathway, indicated as essential for
cell proliferation and survival (40). An increase in the protein expression
of thioredoxin (Trx), nuclear factor 2 (Nrf-2), and nuclear Nrf-2
antioxidant proteins and a decrease in the ROS concentration and in
the mRNA levels of caspase 8 and BCL-1-antagonist-killer (Bak-1)
was also revealed in this study. In addition, a decrease in the
catalytic activity of caspases 3 and 9 and improved release of
cytochrome c from the mitochondria were reported (40). These findings clearly attribute an
antioxidant activity to CeO2-x nanoparticles, at least
in the model in context.
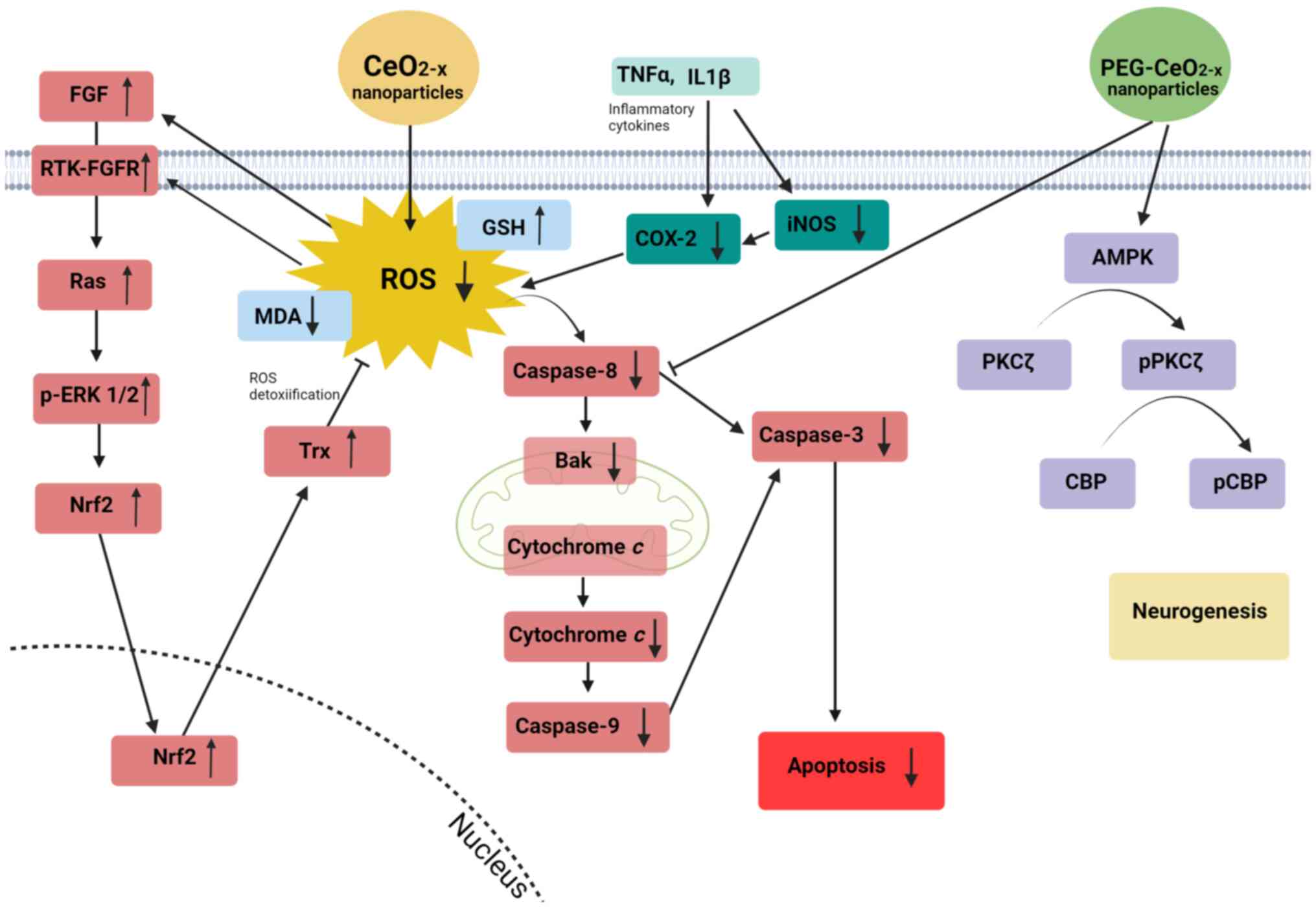 | Figure 2Cellular signalling pathways affected
by CeO2-x nanoparticles in disease models with excessive
apoptosis. In an in vivo model of cochlear and retinal
degeneration (40).
CeO2-x nanoparticles stimulate the bFGF/RTK/Ras/ERK
pathway by reducing ROS. Under this stimulus, the Ras-p-ERK cascade
activates Nrf2, which then increases the expression of Trx, further
inhibiting the production of ROS. Reduced ROS levels lead to a
decrease in the activity of caspases 3, 8 and 9, Bak-1 expression
and release of cytochrome c from the mitochondria,
inhibiting the apoptosis process. In liver fibrosis,
CeO2-x nanoparticles reduce the levels of TNFα, IL1β,
COX-2 and iNOS pro-inflammatory cytokines (41). In type 1 diabetes, CeO2-x
nanoparticles exhibit a significant decrease in the levels of MDA
and an increase in GSH, which may suggest lower ROS levels after
treatment with CeO2-x nanoparticles (42). In hypoxic brain cells,
CeO2-x nanoparticles coated with polyetilenoglycol
(PEG-CeO2-x) act on the AMPK/PKCζ/p-PKCζ/CBP/p-CBP
pathway and inhibit caspase-8, leading to neurogenesis and
inhibition of the apoptotic process (29). CeO2-x, cerium oxide; FGF,
fibroblast growth factor; RTK, receptor tyrosine kinase; ERK,
extracellular signal-regulated kinase; ROS, reactive oxygen
species; p-, phosphorylated; Nrf2, nuclear factor 2; Trx,
thioredoxin; Bak-1, BCL-1-antagonist-killer; TNFα, tumor necrosis
factor α; IL1β, interleukin-1β; COX-2, cyclooxygenase-2; iNOS,
inducible nitric oxide synthase; MDA, malondialdehyde; GSH,
glutathione; AMPK, 5' AMP-activated protein kinase; PKCζ, protein
kinase Cζ; CBP, CREB-binding protein. |
For liver fibrosis, a chronic liver disease
characterized by excessive apoptosis (28), Oró et al (41) demonstrated that CeO2-x
nanoparticles reduced steatosis (accumulation of fat in the liver),
portal hypertension (abnormal increase in blood pressure in the
portal vein that transposes blood from the intestine to the liver)
and the levels of hepatic pro-inflammatory cytokines in rats,
thereby attenuating the inflammatory response. Additionally, a
marked reduction in the mRNA expression of inflammatory cytokines
[tumor necrosis factor α (TNFα), interleukin-1β (IL1β),
cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase
(iNOS)], endothelin 1 (ET-1) and messengers related to the
oxidative stress signaling pathway [eosinophil peroxidase precursor
(Epx), neutrophil cytosolic factor 1 and 2 (Ncf1 and Ncf2)] or
endoplasmic reticulum [cyclic AMP-dependent transcription factor
(Atf3) and heat shock protein family A (Hsp70) member 5 (Hspa5)]
was observed. This was associated with reduced macrophage
infiltration and reduced abundance of active caspase-3 protein,
immunostaining of α-smooth muscle actin (α-SMA) and both protein
and gene expression of inflammatory cytokines.
An in vivo study conducted by Khurana et
al (42) for type 1 diabetes
induced in Swiss mice, revealed a significant decrease in
malondialdehyde (MDA), a ROS marker, and nitric oxide levels and an
increase in glutathione (GSH; part of antioxidant defence) levels
and insulin production following CeO2-x nanoparticle
treatment. In CeO2-x nanoparticle-treated mice, the
levels of intracellular SOD increased while the expression of
caspase-3 and DNA damage decreased. Collectively, these findings
indicate that CeO2-x nanoparticles act as antioxidant
agents and modulate signalling pathways (Fig. 2), particularly those resulting in
apoptosis inhibition, in the context of diseases with excessive
apoptotic levels.
Conversely, some studies have shown
CeO2-x nanoparticles acting as an oxidant agent
generating inflammatory and oxidative stress in models exhibiting
defective apoptosis, further inducing positively the apoptotic
pathway. As an example, a previous study performed by Schwotzer
et al (43), revealed
CeO2-x nanoparticles acting as inflammatory and
oxidative stress agents in in vitro tests in alveolar
epithelial cells, verified through the release of chemokines and
characterized by the release of increased monocyte activation with
subsequent neutrophil and lymphocyte infiltrations. These findings,
although in contrast to the previous ones aforementioned, show the
duality of CeO2-x nanoparticles and a possible role in
the inflammatory system, which remains to be investigated. In
cancer, in which cell death is defective (44) mainly due to dysregulation of Bcl-2
family members (45) and inhibitor
of apoptosis proteins (46),
CeO2-x nanoparticles can also act as an oxidative agent.
In the present study, an overview of the effects of
CeO2-x nanoparticles in cancer models, from the first
published studies to the analysis of how CeO2-x
nanoparticles modulate the apoptotic pathway is presented.
Effects of CeO2-x
nanoparticles in models exhibiting defective apoptosis:
CeO2-x nanoparticles and cancer
It is well known that the development and
progression of cancer are associated with adaptation to oxidative
stress and dysregulation of the expression of antioxidant enzymes
(47,48). In addition, ROS can act as
messengers in signal transduction and induce DNA damage, further
leading to carcinogenic lesions (49). As described in the previous section,
CeO2-x nanoparticles have been shown to be potent
scavengers of certain free radicals and may then be potentially
effective in diseases exhibiting high levels of ROS production.
However, CeO2-x nanoparticles have also been
demonstrated to be either protective or induce oxidative stress in
cancer, indicating the diversity of the biological effects of
CeO2-x nanoparticles in this scenario.
Radioprotective and sensitizing effects of
CeO2-x nanoparticles. The first study
described in literature assessing the effects of CeO2-x
nanoparticles on tumour lines was carried out by Tarnuzzer et
al (50), who treated breast
carcinoma tumour cells (MCF-7) and breast epithelial cells
(CRL8798) with CeO2-x nanoparticles and analysed their
response to radiation. While healthy cells were protected by
nanoparticles (showing no cytotoxicity), breast tumour cells were
more sensitive to radiation. The radioprotective capacity of
CeO2-x nanoparticles in healthy epithelial cells could
be possibly explained by their antioxidant activity attributed to
the chemical characteristic of its self-regenerating redox
activity. Conversely, most solid tumours have an acidic
microenvironment due to the glycolytic metabolic pathway and
exacerbated lactate production (51). Tumour acidosis may then disable the
antioxidant activity of the nanoparticle, potentiating their
oxidizing activity and, consequently, sensitizing the tumour to
radiation therapy (52).
Effect of pH on CeO2-x
nanoparticle activity. Variations in pH are important
factors affecting CeO2-x nanoparticle activity. In
general, neutral pH promotes cytoprotective effects, while acidic
pH leads to cytotoxic effects (52). Due to its redox behaviour,
CeO2-x nanoparticles can mimic some enzymes such as as
SOD, CAT and oxidases (50). When
CeO2-x nanoparticles mimic SOD, the dismutation of
O2•- and generation of
H2O2 and then, O2 is observed. In
this case, a higher Ce3+ surface concentration leads the
nanoparticles to exert the same mechanism of action as SOD. On the
flip side, a higher surface concentration of Ce4+ leads
the nanoparticles to mimic CATs, degrading
H2O2. In an acidic pH, CAT properties
decrease significantly, but SOD properties remain the same. A
single CeO2-x nanoparticle has been revealed to be more
efficient as a SOD catalyst than the natural enzyme (22,53).
Some studies have shown that the CAT-like scavenger activity of
CeO2-x nanoparticles is inhibited in an acidic pH
environment. Notably, while the rate of superoxide conversion to
peroxide is not affected by pH variations, CeO2-x
nanoparticles cannot detoxify the hydrogen peroxide at the same
rate in an acidic pH. Therefore, CeO2-x nanoparticles
could be harmful in a low pH environment (54-56).
A study by Rubio et al (57) identified antioxidant activity of
CeO2-x nanoparticles in both human tumoral cell lines
and mouse embryonic fibroblast (MEF) non-neoplastic cells. In this
study, the influence of pH on the action of CeO2-x
nanoparticles in the A549 human lung alveolar adenocarcinoma cell
line was assessed, but it was found that the antioxidant properties
of CeO2-x nanoparticles were not influenced.
Modulation of the apoptotic pathway by
CeO2-x nanoparticles in cancer. A study
conducted by Cheng et al (58) revealed that CeO2-x
nanoparticles were effective in inducing apoptosis in the SMMC-7721
human hepatoma cell line. In this study, CeO2-x
nanoparticles had a non-spherical hexahedral shape between 20-30
nm. After treatment with CeO2-x nanoparticles, increased
levels of ROS and MDA marker, and decreased levels of antioxidant
SOD, GSH-px and CAT were found (Fig.
3). In addition, there was an increase in the expression of
phosphorylated (p)-p38, p-c-Jun N-terminal kinase (JNK) and
p-ERK1/2, followed by improved apoptosis rates. According to a
study by Xia et al (59),
CeO2-x nanoparticles can induce spontaneous ROS
production inducing a protective response. This is likely since
cellular responses such as inflammation or mitochondrial damage
also act as injury response pathways to stressors other than
oxidative stress. Upon adaptation, cells can activate the SOD, GSH
and CAT antioxidant enzymes.
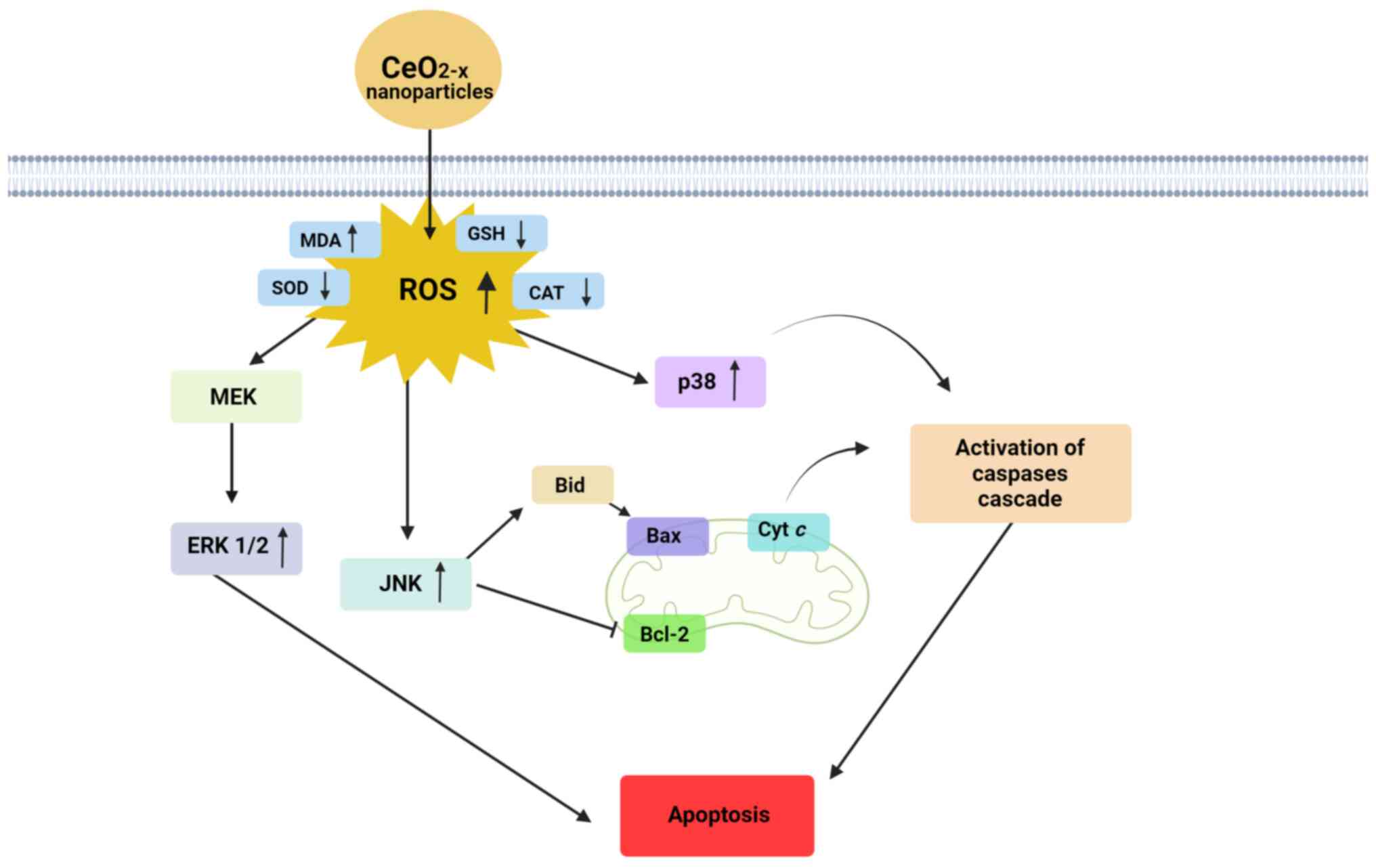 | Figure 3Cellular signalling pathways affected
by CeO2-x nanoparticles in cancer. For human hepatomas,
CeO2-x nanoparticles decreased ROS concentration, as
shown by the increase in the levels of MDA and decrease in the
levels of GSH (58). Under ROS
stimuli, expression of MEK/ERK, JNK, p38 and caspase-3 was also
increased, leading to activation of an apoptotic cascade. JNK acts
on the regulation of Bid and on the inhibition of Bcl-2, affecting
the mitochondrial membrane potential and leading to the activation
of the apoptotic pathway via caspases. In human melanoma models,
CeO2-x nanoparticle treatment promoted an increase in
ROS and MDA, but a decrease in GSH, SOD and CAT levels (61). For colorectal carcinoma models, the
induction of apoptosis through activation of caspase-3 and
caspase-9, release of cytochrome c in the cytoplasm, high
ROS levels and disrupted membrane potential (64) is observed. CeO2-x, cerium
oxide; ROS, reactive oxygen species; MDA, malondialdehyde; GSH,
glutathione; MEK, mitogen-activated protein kinase; ERK,
extracellular signal-regulated kinase; JNK, c-Jun N-terminal
kinase; SOD, superoxide dismutase; CAT, catalase. |
Accordingly, a previous study by Fernández-Varo
et al (60) demonstrated
that hepatoma cells treated with CeO2-x nanoparticles
had an activation of caspase-3, increased expression of ERK1/2 and
a decrease in the expression of p-ERK-1/2. Collectively, the
results from Cheng et al (58) and Fernández-Varo et al
(60) indicate that
CeO2-x nanoparticles may activate distinct signalling
pathways when triggering apoptosis cell death in hepatoma in
vitro models. Conversely, PEG-coated CeO2-x
nanoparticles conjugated with alendronate (specific bone resorption
inhibitory drug) were found to have protective effects on hepatoma
cells, marked by an increased in the ratio of Bcl-2/Bax and
p-protein kinase B (p-AKT) and p-ERK, with no change in the levels
of ROS (61). In addition, tumour
size in vivo increased after CeO2-x nanoparticle
treatment, further confirming that CeO2-x
nanoparticle-PEG-alendronate promotes cell proliferation and should
not be considered for potential therapeutic anticancer
purposes.
In human melanoma models, CeO2-x
nanoparticles were revealed to have impaired cell viability in a
dose-dependent-manner, decreased levels of GSH and increased levels
of caspase-3, ROS, MDA and SOD. In addition, there was an increase
in double strand DNA breaks following ROS generation, suggesting
that CeO2-x may promote oxidative stress-mediated
apoptosis and DNA damage (63).
Furthermore, in melanoma, Aplak et al (63) found that CeO2-x
nanoparticles could induce the levels of mitochondrial ROS,
accompanied by an increase in the oxidation of mitochondrial thiol
and promotion of hydrogen peroxide-linked mitochondrial
dysfunctions. The cytotoxic effects of CeO2-x
nanoparticles have also been demonstrated in colorectal carcinoma
models, in which a dose-dependent induction of apoptosis was found.
This was associated with activation of caspase-3 and caspase-9,
release of cytochrome c in the cytoplasm, high ROS levels
and disrupted membrane potential (64). Taken together, most preclinical
studies indicate that CeO2-x nanoparticles exhibit
cytotoxic activity in experimental models of cancers from different
origins, which may encourage future work with animal models.
4. Concluding remarks and future
perspectives
With the increasing interest in the dual
characteristics of CeO2-x nanoparticles, as either an
oxidant or antioxidant, numerous authors have begun to investigate
their participation in apoptosis signalling pathways. In some cases
(as for example, in cancer), its induction would be favourable to
help control the disease. In other cases (such as in liver
fibrosis), its inhibition would be ideal to interrupt the
disease-associated degenerative features.
Apoptosis can be triggered by different factors and
several pathways can be activated to promote cell termination.
Notably, CeO2-x nanoparticles can modulate some of these
pathways, which could be particularly useful for future cancer
treatments (44). Although this
review highlights the effects of CeO2-x nanoparticles in
modulating apoptotic cell death, other forms of cell death have
also been shown to be triggered by these nanoparticles (65-67).
The cytotoxic effects of CeO2-x nanoparticles in
non-neoplastic cells are minor, some even cytoprotective against
cytotoxic stimuli.
Considering the dual behaviour of CeO2-x
nanoparticles as either antioxidant or pro-oxidant (oxidation no.
change between +3 → +4 → +3), it is important to mention that
nanoparticle action may not only be influenced by tumour features,
but also by its structural and chemical characteristics. It is most
likely that the effects of CeO2-x nanoparticles are
dependent upon the tumour origin and oxidative state, the
subcellular compartment where it is located within cells and how
acidic the tumour microenvironment is.
Last but not least, it should be noted that
numerous factors such as particle shape, size and structure as well
as the solvents used to prepare them for experimental cellular
assays may all affect the properties of CeO2-x
nanoparticles. Unfortunately, this piece of information is most
often overlooked by studies, which lack discussion on the subject.
In general, studies have been carried out with nanoparticles of
different sizes, synthesis routes and procedures used to prepare
their suspension for biological assays. For a better understanding
of the effects of CeO2-x nanoparticles in a biological
environment, a more detailed description of the chemical structure
and properties of nanoparticles must be elucidated (43,54).
Combining information from both chemical and biological models is
key to address the potential future role of CeO2-x
nanoparticles and other nanoparticles in therapeutics and further
biomedical applications.
Acknowledgements
Not applicable.
Funding
Funding: The present study was financed in part by the
Coordination of Superior Level Staff Improvement-Brazil-(CAPES
Finance Code 001), the Carlos Chagas Filho Foundation for Research
Support in the State of Rio de Janeiro (FAPERJ nos.
SEI-26003/004812/2021 and SEI-260003/001554/2022), and the Military
Institute of Engineering (IME) and Scholarship for Women in Science
L'Oréal-UNESCO-ABC.
Availability of data and materials
Not applicable.
Authors' contributions
All authors (MBDSA, GNDM and KRDS) designed and
conceived the study, performed the research, and wrote and edited
the manuscript. Data authentication is not applicable. All authors
have read and agreed to the published version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Montini T, Melchionna M, Monai M and
Fornasiero P: Fundamentals and Catalytic Applications of
CeO2 -Based Materials. Chem Rev. 116:5987–6041.
2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Choudhury B and Choudhury A: Ce3+ and
oxygen vacancy mediated tuning of structural and optical properties
of CeO2 nanoparticles. Mater Chem Phys. 131:666–671.
2012.
|
|
3
|
Gangopadhyay S, Frolov DD, Masunov AE and
Seal S: Structure and properties of cerium oxides in bulk and
nanoparticulate forms. J Alloys Compd. 584:199–208. 2014.
|
|
4
|
Guo W, Zhang M, Lou Z, Zhou M, Wang P and
Wei H: Engineering nanoceria for enhanced peroxidase mimics: A
solid solution strategy. ChemCatChem. 11:737–743. 2019.
|
|
5
|
Sisubalan N, Ramkumar VS, Pugazhendhi A,
Karthikeyan C, Indira K, Gopinath K, Hameed ASH and Basha MHG:
ROS-Mediated Cytotoxic Activity of ZnO and CeO2
Nanoparticles Synthesized Using the Rubia Cordifolia L. Leaf
Extract on MG-63 Human Osteosarcoma Cell Lines. Environ Sci Pollut
Res. 25:10482–10492. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Da Silva JLF: Stability of the
Ce2O3 Phases: A DFT + U Investigation. Phys
Rev B. 76(193108)2007.
|
|
7
|
Trovarelli Alessandro Catalysis by Ceria
and Related Materials; 2002; Vol. 2; ISBN 978-1-78326-131-4.
|
|
8
|
Gangavarapu RR and Mishra B: Structural,
Redox and Catalytic Chemistry of Ceria Based Materials. Bull Catal
Soc India. 2:122–134. 2003.
|
|
9
|
Deshpande S, Patil S, Kuchibhatla SV and
Seal S: Size dependency variation in lattice parameter and valency
states in nanocrystalline cerium oxide. Appl Phys Lett.
87(133113)2005.
|
|
10
|
Das S, Dowding JM, Klump KE, McGinnis JF,
Self W and Seal S: Cerium Oxide Nanoparticles: Applications and
prospects in nanomedicine. Nanomedicine (Lond). 8:1483–1508.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ganduglia-Pirovano MV, Da Silva JL and
Sauer J: Density-Functional calculations of the structure of
near-surface oxygen vacancies and electron localization on
CeO2 (111). Phys Rev Lett. 102(026101)2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen BH and Stephen Inbaraj B: Various
physicochemical and surface properties controlling the bioactivity
of cerium oxide nanoparticles. Crit Rev Biotechnol. 38:1003–1024.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jerratsch JF, Shao X, Nilius N, Freund HJ,
Popa C, Ganduglia-Pirovano MV, Burow AM and Sauer J: Electron
Localization in Defective Ceria Films: A study with
scanning-tunneling microscopy and density-functional theory. Phys
Rev Lett. 106(246801)2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Skorodumova NV, Simak SI, Lundqvist BI,
Abrikosov IA and Johansson B: Quantum origin of the oxygen storage
capability of ceria. Phys Rev Lett. 89(166601)2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pinto FM, Suzuki VY, Silva RC and La Porta
FA: Oxygen defects and surface chemistry of reducible oxides. Front
Mater. 6(260)2019.
|
|
16
|
Sun C, Li H and Chen L: Nanostructured
ceria-based materials: Synthesis, properties, and applications.
Energy Environ Sci. 5:8475–8505. 2012.
|
|
17
|
Calvache-Muñoz J, Prado FA and
Rodríguez-Páez JE: Cerium oxide nanoparticles: Synthesis,
characterization and tentative mechanism of particle formation.
Colloids Surf Physicochem. Eng Asp. 529:146–159. 2017.
|
|
18
|
Capdevila-Cortada M and López N: Entropic
contributions enhance polarity compensation for
CeO2(100) Surfaces. Nat Mater. 16:328–334.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hussain SP, Hofseth LJ and Harris CC:
Radical Causes of Cancer. Nat Rev Cancer. 3:276–285.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the Nomenclature Committee on Cell Death 2018. Cell Death Differ.
25:486–541. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lord MS, Berret JF, Singh S, Vinu A and
Karakoti AS: Redox active cerium oxide nanoparticles: Current
status and burning issues. Small. 17(e2102342)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Korsvik C, Patil S, Seal S and Self WT:
superoxide dismutase mimetic properties exhibited by vacancy
engineered ceria nanoparticles. Chem Commun (Camb).
1056-1058:2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yadav N and Singh S:
Polyoxometalate-Mediated vacancy-engineered cerium oxide
nanoparticles exhibiting controlled biological enzyme-mimicking
activities. Inorg Chem. 60:7475–7489. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pirmohamed T, Dowding JM, Singh S,
Wasserman B, Heckert E, Karakoti AS, King JES, Seal S and Self WT:
Nanoceria exhibit redox state-dependent catalase mimetic activity.
Chem Commun. 46(2736)2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yadav N, Patel V, McCourt L, Ruppert M,
Miller M, Inerbaev T, Mahasivam S, Bansal V, Vinu A, Singh S and
Karakoti A: Tuning the enzyme-like activities of cerium oxide
nanoparticles using a triethyl phosphite ligand. Biomater Sci.
10:3245–3258. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Karakoti AS, Singh S, Kumar A, Malinska M,
Kuchibhatla SV, Wozniak K, Self WT and Seal S: PEGylated Nanoceria
as Radical Scavenger with Tunable Redox Chemistry. J Am Chem Soc.
131:14144–14145. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ikeda A, Nishina PM and Naggert JK: The
tubby-like proteins, a family with roles in neuronal development
and function. J Cell Sci. 115(Pt 1):9–14. 2002.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schwabe RF and Luedde T: Apoptosis and
necroptosis in the liver: A matter of life and death. Nat Rev
Gastroenterol Hepatol. 15:738–752. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jiang L, Xie Y, Sun X and Yin Z:
Relationship between apoptosis of brain cells and free radical
scavenging ability in rats during acute intermittent hypoxia. Space
Med Med Eng (Beijing). 11:83–86. 1998.PubMed/NCBI
|
|
30
|
Eizirik DL, Colli ML and Ortis F: The role
of inflammation in insulitis and beta-cell loss in type 1 diabetes.
Nat Rev Endocrinol. 5:219–226. 2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li W, Khor TO, Xu C, Shen G, Jeong WS, Yu
S and Kong AN: Activation of Nrf2-antioxidant signaling Attenuates
NFkappaB-Inflammatory response and elicits apoptosis. Biochem
Pharmacol. 76:1485–1489. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Carneiro BA and El-Deiry WS: Targeting
Apoptosis in Cancer Therapy. Nat Rev Clin Oncol. 17:395–417.
2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Davis W Jr, Ronai Z and Tew KD: Cellular
thiols and reactive oxygen species in drug-induced apoptosis. J
Pharmacol Exp Ther. 296:1–6. 2001.PubMed/NCBI
|
|
34
|
Fleury C, Mignotte B and Vayssière JL:
Mitochondrial reactive oxygen species in cell death signaling.
Biochimie. 84:131–141. 2002.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Redza-Dutordoir M and Averill-Bates DA:
Activation of apoptosis signalling pathways by reactive oxygen
species. Biochim Biophys Acta. 1863:2977–2992. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hockenbery DM, Giedt CD, O'Neill JW,
Manion MK and Banker DE: Mitochondria and apoptosis: New
therapeutic targets. Adv Cancer Res. 85:203–242. 2002.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Henry-Mowatt J, Dive C, Martinou JC and
James D: Role of mitochondrial membrane permeabilization in
apoptosis and cancer. Oncogene. 23:2850–2860. 2004.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Aroui S, Brahim S, De Waard M, Bréard J
and Kenani A: Efficient induction of apoptosis by doxorubicin
coupled to cell-penetrating peptides compared to unconjugated
doxorubicin in the human breast cancer cell line MDA-MB 231. Cancer
Lett. 285:28–38. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fulda S, Galluzzi L and Kroemer G:
Targeting mitochondria for cancer therapy. Nat Rev Drug Discov.
9:447–464. 2010.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kong L, Cai X, Zhou X, Wong LL, Karakoti
AS, Seal S and McGinnis JF: Nanoceria extend photoreceptor cell
lifespan in tubby mice by modulation of apoptosis/survival
signaling pathways. Neurobiol Dis. 42:514–523. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Oró D, Yudina T, Fernández-Varo G, Casals
E, Reichenbach V, Casals G, González De La Presa B, Sandalinas S,
Carvajal S, Puntes V and Jiménez W: Cerium oxide nanoparticles
reduce steatosis, portal hypertension and display antiinflammatory
properties in rats with liver fibrosis. J Hepatol. 64:691–698.
2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Khurana A, Tekula S and Godugu C:
Nanoceria suppresses multiple low doses of streptozotocin-induced
type 1 diabetes by inhibition of Nrf2/NF-κB pathway and reduction
of apoptosis. Nanomedicine (Lond). 13:1905–1922. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Schwotzer D, Niehof M, Schaudien D, Kock
H, Hansen T, Dasenbrock C and Creutzenberg O: cerium oxide and
barium sulfate nanoparticle inhalation affects gene expression in
alveolar epithelial cells type II. J Nanobiotechnology.
16(16)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hanahan D: Hallmarks of cancer: New
dimensions. Cancer Discov. 12:31–46. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Delbridge ARD, Grabow S, Strasser A and
Vaux DL: Thirty Years of BCL-2: Translating cell death discoveries
into novel cancer therapies. Nat Rev Cancer. 16:99–109.
2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mohamed MS, Bishr MK, Almutairi FM and Ali
AG: Inhibitors of apoptosis: Clinical implications in cancer.
Apoptosis. 22:1487–1509. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Arfin S, Jha NK, Jha SK, Kesari KK,
Ruokolainen J, Roychoudhury S, Rathi B and Kumar D: Oxidative
stress in cancer cell metabolism. Antioxidants (Basel).
10(642)2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Waris G and Ahsan H: Reactive oxygen
species: Role in the development of cancer and various chronic
conditions. J Carcinog. 5(14)2006.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Perillo B, Di Donato M, Pezone A, Di Zazzo
E, Giovannelli P, Galasso G, Castoria G and Migliaccio A: ROS in
cancer therapy: The bright side of the moon. Exp Mol Med.
52:192–203. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Tarnuzzer RW, Colon J, Patil S and Seal S:
Vacancy engineered ceria nanostructures for protection from
radiation-induced cellular damage. Nano Lett. 5:2573–2577.
2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Robertson-Tessi M, Gillies RJ, Gatenby RA
and Anderson ARA: Impact of metabolic heterogeneity on tumor
growth, invasion, and treatment outcomes. Cancer Res. 75:1567–1579.
2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Asati A, Santra S, Kaittanis C and Perez
JM: Surface-Charge-Dependent cell localization and cytotoxicity of
cerium oxide nanoparticles. ACS Nano. 4:5321–5331. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Ali D, Alarifi S, Alkahtani S, AlKahtane
AA and Almalik A: Cerium oxide nanoparticles induce oxidative
stress and genotoxicity in human skin melanoma cells. Cell Biochem.
Biophys. 71:1643–1651. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Xu C and Qu X: Cerium oxide nanoparticle:
A remarkably versatile rare earth nanomaterial for biological
applications. NPG Asia Mater. 6(e90)2014.
|
|
55
|
Alili L, Sack M, Karakoti AS, Teuber S,
Puschmann K, Hirst SM, Reilly CM, Zanger K, Stahl W, Das S, et al:
Combined cytotoxic and anti-invasive properties of redox-active
nanoparticles in tumor-stroma interactions. Biomaterials.
32:2918–2929. 2011.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wason MS, Colon J, Das S, Seal S, Turkson
J, Zhao J and Baker CH: Sensitization of pancreatic cancer cells to
radiation by cerium oxide nanoparticle-induced ROS production.
Nanomedicine. 9:558–569. 2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Rubio L, Marcos R and Hernández A:
Nanoceria acts as antioxidant in tumoral and transformed cells.
Chem Biol Interact. 291:7–15. 2018.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Cheng G, Guo W, Han L, Chen E, Kong L,
Wang L, Ai W, Song N, Li H and Chen H: Cerium oxide nanoparticles
induce cytotoxicity in human hepatoma SMMC-7721 cells via oxidative
stress and the activation of MAPK signaling pathways. Toxicol In
Vitro. 27:1082–1088. 2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Xia T, Kovochich M, Liong M, Mädler L,
Gilbert B, Shi H, Yeh JI, Zink JI and Nel AE: Comparison of the
mechanism of toxicity of zinc oxide and cerium oxide nanoparticles
based on dissolution and oxidative stress properties. ACS Nano.
2:2121–2134. 2008.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Fernández-Varo G, Perramón M, Carvajal S,
Oró D, Casals E, Boix L, Oller L, Macías-Muñoz L, Marfà S, Casals
G, et al: Bespoken Nanoceria: An effective treatment in
experimental hepatocellular carcinoma. Hepatology. 72:1267–1282.
2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Cheng H, Liao ZL, Ning LH, Chen HY, Wei
SS, Yang XC and Guo H: Alendronate-Anchored PEGylation of ceria
nanoparticles promotes human hepatoma cell proliferation via
AKT/ERK signaling pathways. Cancer Med. 6:374–381. 2017.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Pešić M, Podolski-Renić A, Stojković S,
Matović B, Zmejkoski D, Kojić V, Bogdanović G, Pavićević A, Mojović
M, Savić A, et al: Anti-Cancer effects of cerium oxide
nanoparticles and its intracellular redox activity. Chem Biol
Interact. 232:85–93. 2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Aplak E, Von Montfort C, Haasler L, Stucki
D, Steckel B, Reichert AS, Stahl W and Brenneisen P: CNP mediated
selective toxicity on melanoma cells is accompanied by
mitochondrial dysfunction. PLoS One. 15(e0227926)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Datta A, Mishra S, Manna K, Saha KD,
Mukherjee S and Roy S: Pro-Oxidant therapeutic activities of cerium
oxide nanoparticles in colorectal carcinoma cells. ACS Omega.
5:9714–9723. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Hirst SM, Karakoti AS, Tyler RD,
Sriranganathan N, Seal S and Reilly CM: Anti-Inflammatory
Properties of Cerium Oxide Nanoparticles. Small. 5:2848–2856.
2009.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zal Z, Ghasemi A, Azizi S, Asgarian-Omran
H, Montazeri A and Hosseinimehr SJ: Radioprotective effect of
cerium oxide nanoparticles against genotoxicity induced by ionizing
radiation on human lymphocytes. Curr Radiopharm. 11:109–115.
2018.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Cai R, Xiao L, Qiu J, Zhao L, Li Z, Ju H,
Sun M, Zhu W, Wang Z and Du F: Fabrication of cerium doped carbon
dots with highly radical scavenging activity alleviates
ferroptosis-induced oxidative damage. Nanotechnology.
32:2021.PubMed/NCBI View Article : Google Scholar
|