Introduction
In International Commission on Radiological
Protection (ICRP) Publication 118, the threshold radiation dose for
cataract development in the lens of the eye was revised, and the
threshold radiation dose (0.5 Gy) for cardiovascular and
cerebrovascular diseases was newly introduced (1). The threshold dose may be exceeded
during procedures involving radiation such as interventional
radiology and radiation therapy (2). Therefore, in addition to regulating
radiation dose, it is necessary to develop strategies to prevent
and mitigate development of cardiovascular diseases.
Cellular senescence is the irreversible arrest of
cell proliferation and includes replicative and premature
senescence (3). Replicative
senescence is caused by telomere shortening, while various stimuli,
such as oncogenic stress and DNA-damaging agents, including
ionizing radiation, induce premature senescence. Senescent cells
exhibit morphological changes such as flattening and hypertrophy,
activation of senescence-associated β-galactosidase (SA-β-gal),
induction of cell cycle-associated factors such as p16 and p21 and
resistance to apoptosis (4-7).
Additionally, senescent cells secrete factors such as inflammatory
cytokines and proteases (8); this
phenomenon is termed SA secretory phenotype (SASP) and affects
surrounding cells or tissue (9).
As proliferation of cells with DNA damage may lead
to the development of cancer cells, cellular senescence may be a
tumor-suppressing mechanism (10).
However, evidence has shown that senescent cells are also involved
in age-associated diseases (11).
Senescent cells cause chronic inflammation, neurodegenerative
disease and rheumatoid arthritis (12-14).
Furthermore, senescence of vascular endothelial cells (VECs)
increases risk of not only vascular dysfunction but also
development of diseases such as hypertension and atherosclerosis
(15). Therefore, regulating
senescence of VECs may prevent and mitigate cardiovascular disease
in individuals exposed to radiation.
The p53 pathway, which serves an important role in
tumor suppression, is involved in induction of premature cellular
senescence (16). Following DNA
damage, p53 is activated and transcriptionally upregulates p21,
which is a cyclin-dependent kinase (CDK) inhibitor that binds to
the cyclin-CDK dimer to form inactive trimeric complexes, resulting
in G1 arrest that leads to cellular senescence (16,17).
In addition to cellular senescence, p53 can also induce apoptosis,
thereby contributing to maintenance of genome integrity by
eliminating cells with unrepairable DNA damage (18). These facts suggest that although p53
may be a potential target to regulate cellular senescence, p53
inhibition may disrupt genome integrity. Therefore, it is key to
identify factors that regulate senescence of VECs without causing
undesirable effects such as inhibiting apoptosis and downregulating
EC function.
Retinoic acid-inducible gene-I (RIG-I)-like
receptors (RLRs) serve an important role to induce anti-viral
response (19,20). RLRs are located in the cytoplasm and
recognize virus-derived RNA. RLRs include RIG-I, melanoma
differentiation-associated gene 5 (MDA5) and laboratory of genetics
and physiology 2 (LGP2). RIG-I and MDA5 contain an N-terminal
domain consisting of tandem caspase activation and recruitment
domains (CARDs), a central DExD/H box RNA helicase domain and a
C-terminal regulatory domain (21).
Although RIG-I and MDA5 are structurally and functionally similar,
they recognize different types of RNA virus (21). They independently and
synergistically induce anti-viral responses (22), although it is suggested that RIG-I
and MDA5 do not directly interact with each other (23). By contrast, LGP2 can interact with
RIG-I and MDA5(24), but lacks
CARDs, which are responsible for initiating downstream signaling
pathways leading to production of type I interferon (IFN) (19). Although it is well-known that RLRs
serve important roles in anti-viral responses (19,20),
it remains unknown whether they are involved in radiation-indued
cellular senescence.
To identify factors involved in radiation-induced
senescence of VECs, the present study aimed to perform in
silico analysis using the NCBI Gene Expression Omnibus (GEO,
ncbi.nlm.nih.gov/geo/) to search for
candidate factors. Since in silico analysis suggested that
RLRs may be candidate factors for radiation-induced senescence of
VECs, the present study further investigated the involvement of
RLRs in radiation-induced senescence of human umbilical (HU)
VECs.
Materials and methods
Reagents
PBS(-) (Ca2+, Mg2+-free
Dulbecco's) was purchased from Wako Pure Chemical Industries.
Rabbit anti-human MDA5 (cat. no. #5321), RIG-I (cat. no. #4200),
β-actin (cat. no. #4967) and horseradish peroxidase-conjugated
anti-rabbit IgG (cat. no. #7074) and Senescence β-Galactosidase
Activity Assay kit (cat. no. #35302) were purchased from Cell
Signaling Technology, Inc. Silencer® Select pre-designed
RIG-I (#1, cat. no. s223615; #2, cat. no. s24144) and MDA5 small
interfering (si)RNA (#1, cat. no. s34498; #2, vat. no. s34499) and
Negative Control #1 (cat. no. 4390843) were purchased from Thermo
Fisher Scientific, Inc. PI and FBS were purchased from
Sigma-Aldrich (Merck KGaA). FITC-annexin V and annexin V binding
buffer were purchased from BioLegend, Inc.
Cell culture and treatment
HUVECs (cat. no. 200-05n, population doubling level,
15) were purchased from Cell Applications, Inc. After 3-5 passages
(about 1-2 weeks) at 37˚C in a humidified atmosphere of 5%
CO2, cells were used for experiments. HUVECs were seeded
onto collagen I-coated dishes (35 mm; Iwaki Science Products Dept.;
AGC Techno Glass Co., Ltd.) at a density of 3.0x104
cells/dish and cultured with Endothelial Cell Growth Medium kit
(cat. no. C-22110; Takara Bio, Inc.) at 37˚C in a humidified
atmosphere of 5% CO2/95% air. After 6 h incubation and
once cells adhered to the dish, irradiation was performed. Cells
were irradiated with an X-ray generator (cat. no. MBR-1520R-3;
Hitachi, Ltd.) at 450 mm from the focus and at a dose rate of
0.99-1.03 Gy/min (150 kVp; 20 mA; 0.5-mm Al filter and 0.3-mm Cu
filter). Cells were harvested 5 or 10 days after irradiation and
used for subsequent experiments. For cells cultured for 10 days,
non-irradiated cells were harvested on day 5 and reseeded onto new
35-mm dishes at a density of 3.0x104 cells/dish to avoid
overproliferation. The irradiated cells were washed with PBS(-) and
the medium was replaced on day 5.
siRNA transfection
Knockdown of RIG-I and MDA5 was achieved using
Silencer® Select Pre-designed siRNA and RNAiMAX
(Invivogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The sense sequences for RIG-I #1 and #2
were 5'-GAAGCAGUAUUUAGGGAAATT-3' (antisense:
5'-UUUCCCUAAAUACUGCUUCGT-3') and 5'-CCAGAAUUAUCCCAACCGATT-3'
(antisense: 5'-UCGGUUGGGAUAAUUCUGGTT-3'), respectively. The sense
sequences for MDA5 #1 and #2 were 5'-GUAACAUUGUUAUCCGUUATT-3'
(antisense: 5'-UAACGGAUAACAAUGUUACAT-3') and
5'-GGUGUAAGAGAGCUACUAATT-3' (antisense:
5'-UUAGUAGCUCUCUUACACCTG-3'), respectively. Silencer®
Select Negative Control #1 (sequence not available) was used as the
negative control. The final concentration of all siRNAs was 10 nM.
After 48 h transfection at 37˚C in a humidified atmosphere of 5%
CO2/95% air, cells were harvested. The cells were
immediately seeded onto collagen I-coated dishes for subsequent
experiments.
Western blotting
SDS-PAGE and western blot analysis were performed as
previously reported (28). The
following primary antibodies diluted in Can Get Signal®
Immunoreaction Enhancer Solution 1 (Toyobo Life Science) were used:
Anti-RIG-I (1:3,000), anti-MDA5 (1:3,000) and anti-β-actin
(1:4,000). Following overnight dilution at 4˚C, the membrane was
reacted with secondary antibody (1:10,000) diluted in Can Get
Signal® Immunoreaction Enhancer Solution 2 (Toyobo Life
Science) for 1 h at room temperature and detected using Clarity
Western ECL Substrate (Bio-Rad Laboratories, Inc.). Images were
captured by cool saver AE-6955 (ATTO Corporation) or iBright 1500
Image system (Thermo Fisher Scientific, Inc.).
Analysis of SA-β-gal activity
SA-β-gal activity was analyzed using the Senescence
β-Galactosidase Activity Assay kit according to the manufacturer's
instructions. For analysis of SA-β-gal activity by flow cytometry,
after culture of irradiated cells, the culture medium was replaced
with Endothelial Cell Growth Medium containing bafilomycin A1 (100
nM). Following incubation for 1 h at 37˚C, SA-β-Gal substrate
solution (33 µM) was added to the cell culture and incubated for 2
h at 37˚C. After washing three times with PBS(-), cells were
harvested, washed with PBS(-) and suspended in cold PBS(-)
containing 2% FBS. Fluorescence intensity of SA-β-Gal substrate was
analyzed using a flow cytometer (CytoFLEX with CytExpert software
version 2.4.0.28; Beckman-Coulter, Inc., https://www.beckman.jp/).
For analysis of SA-β-gal activity by a confocal
microscope, HUVECs seeded onto collagen coated-glass bottom dish
(cat. no. D11134H; Matsunami Glass Ind., Ltd.) were irradiated with
10 Gy and cultured for 5 days, as aforementioned. Culture medium
was replaced with Endothelial Cell Growth Medium containing
bafilomycin A1 (100 nM). Following incubation for 1 h at 37˚C,
SA-β-Gal substrate solution (33 µM) was added to the cell culture
and incubated for 2 h at 37˚C. After washing three times with
PBS(-), cold PBS(-) containing 2% FBS was added to the dish. After
that, cells were treated with 5 µg/ml Hoechst 33342 (Thermo Fisher,
Scientific, Inc.) for 5 min at room temperature in the dark.
Following washing with PBS(-), images were captured by a confocal
laser microscope (LSM710; Carl Zeiss AG).
Analysis of apoptosis
Apoptosis was analyzed using FITC-annexin V and PI
staining according to the manufacturer's instructions. Briefly,
irradiated cells were harvested, washed twice with PBS(-),
centrifuged at 200 x g for 5 min at room temperature and suspended
in 100 µl annexin V binding buffer. Five µl of FITC-annexin V (90
µg/ml) and PI (1 mg/ml) were added to cell suspension and cells
were incubated for 15 min at room temperature in the dark. After
adding annexin V binding buffer, samples were analyzed by flow
cytometer as aforementioned (CytoFLEX, Beckman-Coulter).
Statistical analysis
Data are presented as the mean ± SD of three
independent experiments. Comparison of multiple groups was
performed using one-way analysis of variance followed by
Tukey-Kramer post hoc test. Statistical analysis was performed
using Excel 2016 (Microsoft Corporation) with the Statcel 4 (OMS
Publishing) add-in. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of genes commonly
upregulated in radiation-induced senescent cells
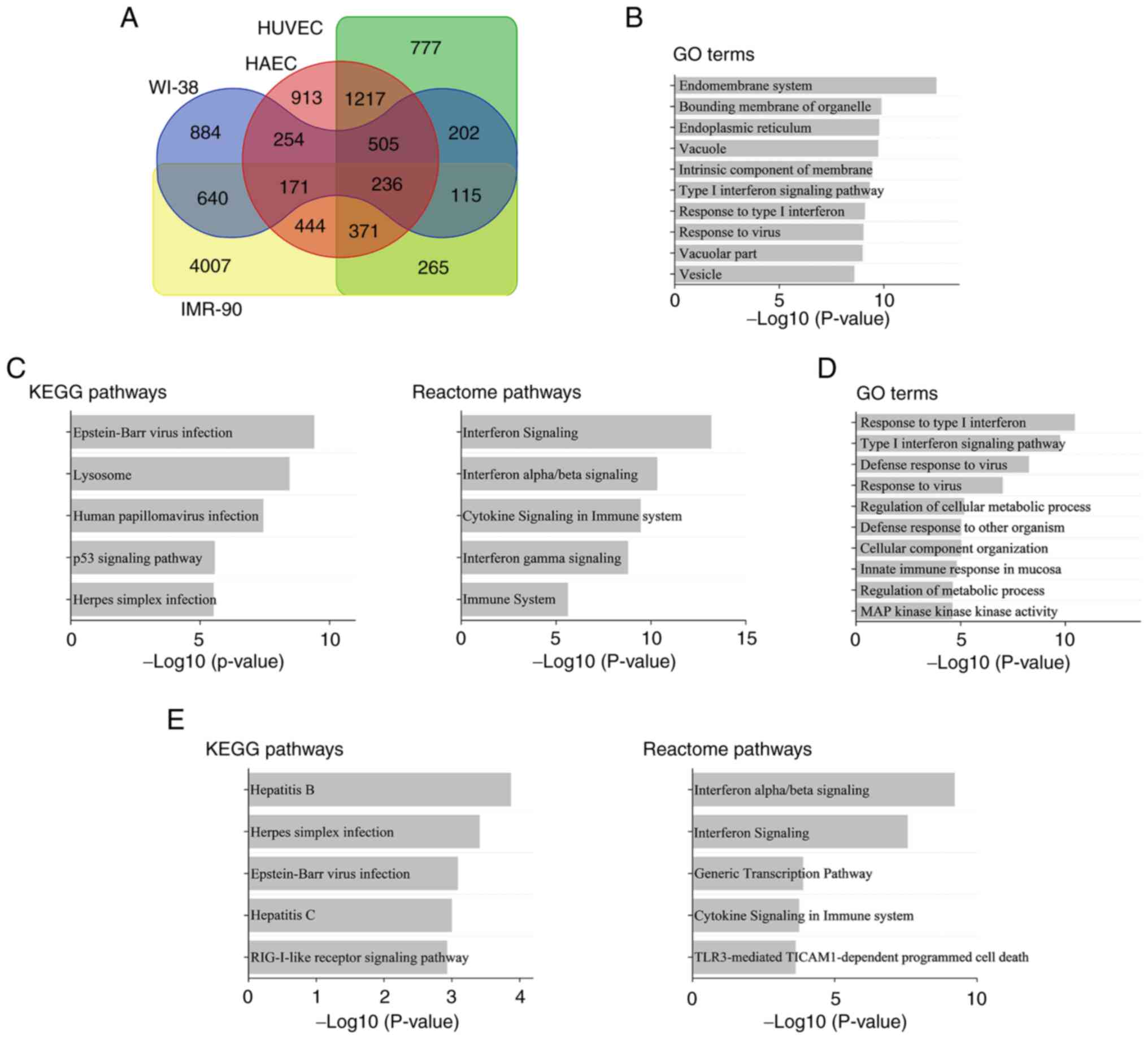
Gene expression in HUVECs, HAECs, WI-38 and IMR-90
cells, which underwent irradiation to induce senescence (25), was re-analyzed to determine the
commonly upregulated genes in senescent cells, revealing 236 genes
(Fig. 1A). To investigate the
characteristics of these genes, GO terms and signaling pathways
were analyzed (Fig. 1B and C). As shown in Fig. 1B, commonly upregulated genes in GO
terms were associated with viral infections (‘Response to virus’)
and inflammation (‘Type I interferon signaling pathway’ and
‘Response to type I interferon’; Fig.
1B). Similarly, pathway analysis (Fig. 1C) showed that commonly upregulated
genes were associated with viral infections (‘Epstein-Bar virus
infection’ and ‘Human papillomavirus infection’) and inflammation
(‘Interferon Signaling’, ‘Interferon alpha/beta signaling’,
‘Cytokine Signaling in Immune system’, ‘Interferon gamma
Signaling’, ‘Immune System’).
Characteristics of genes upregulated
in HUVECs following irradiation
The present study re-analyzed the microarray data of
HUVECs 24 h after irradiation (26). Similar to those observed in
senescent cells, genes associated with viruses and inflammation
were upregulated in irradiated HUVECs (Fig. 1D). RIG-I-like receptor (RLR)
signaling pathway, which plays an important role in the anti-viral
response (29), was altered
(Fig. 1E).
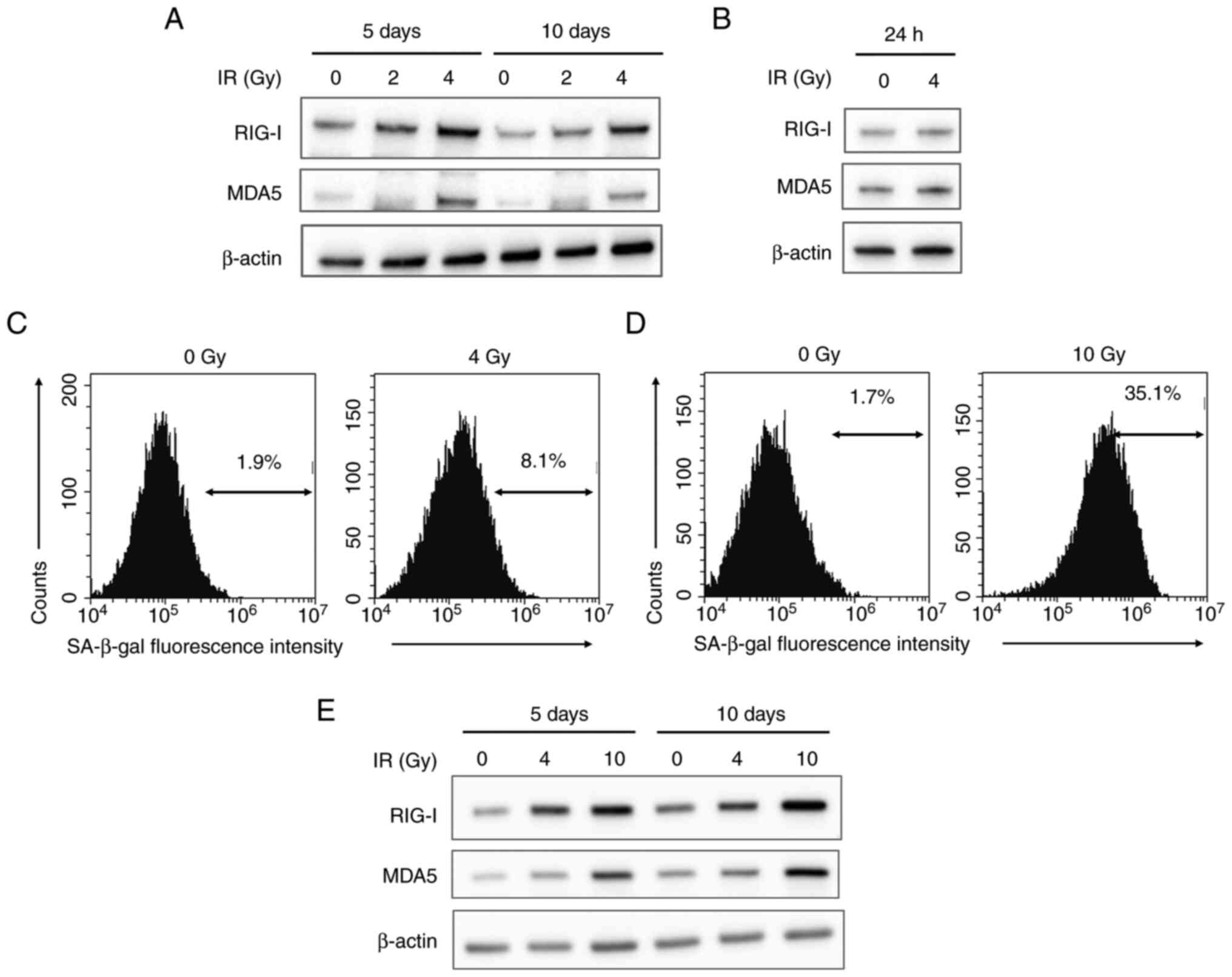
Expression of RLRs and SA-β-gal
activity in irradiated HUVECs
The association between RLRs and senescence in
HUVECs was analyzed in vitro. The present study focused on
RIG-I and MDA5 because they activate downstream signaling pathways
(23). Irradiation (4 Gy) increased
RIG-I and MDA5 expression on post-irradiation day 5 (Fig. 2). Additionally, RIG-I and MDA5
expression remained high 10 days post-irradiation. However, RIG-I
and MDA5 expression 24 h post-irradiation was not notably changed
from that at baseline (Fig.
2B).
To confirm cellular senescence, SA-β-gal activity of
irradiated HUVECs was analyzed (30). At 10 days after 4 Gy-irradiation,
RIG-I and MDA5 expression as well as the proportion of HUVECs with
high SA-β-gal activity were higher than those in non-irradiated
cells (Fig. 2C). Additionally, 10
Gy irradiation effectively increased not only the expression of
RIG-I and MDA5 but also the proportion of cells with high SA-β-gal
activity at 5 days post-irradiation (Fig. 2D and E). These results showed that irradiation
induced senescence of HUVECs accompanied by upregulation of RIG-I
and MDA5 expression.
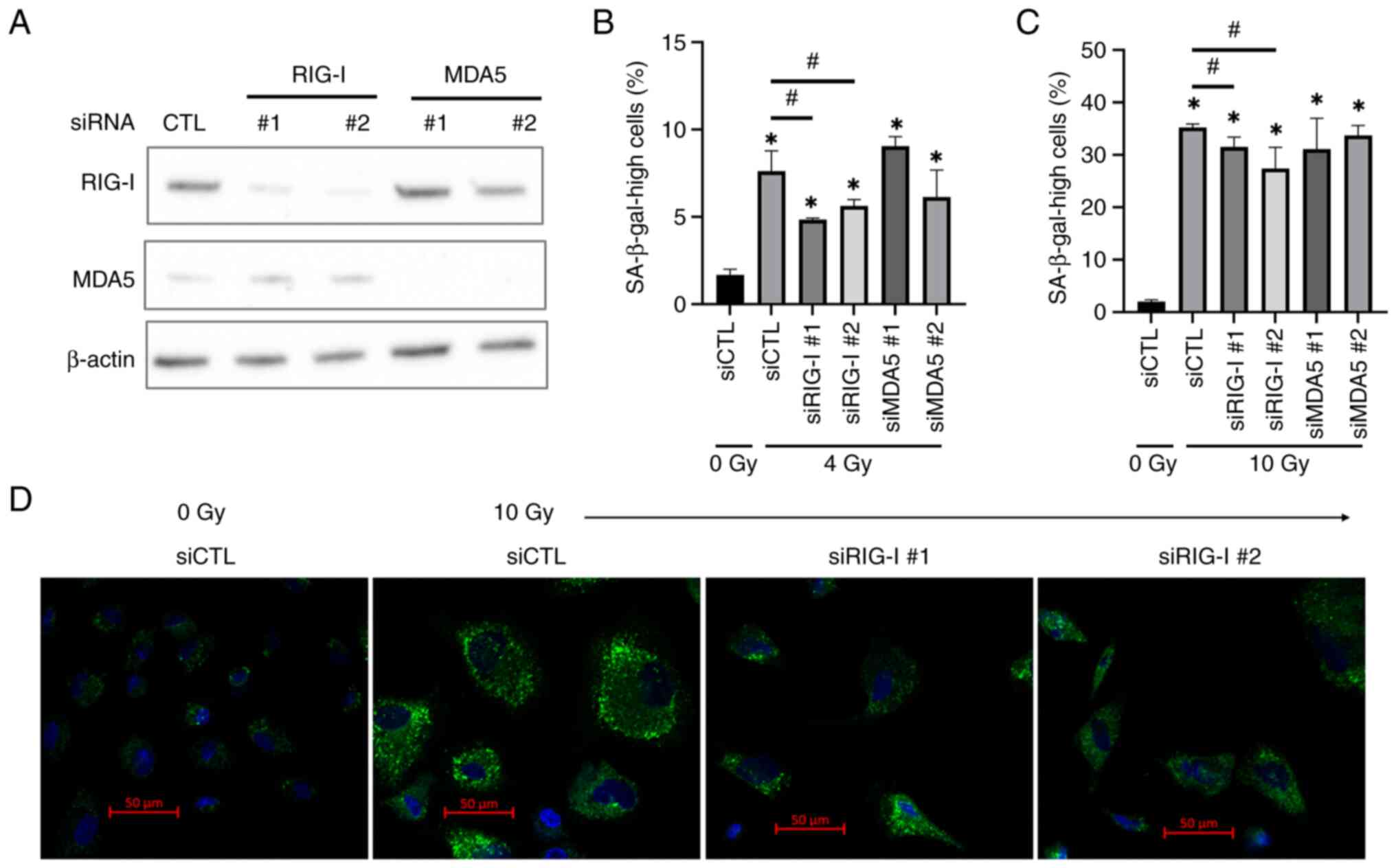
Association between radiation-induced
senescence and RIG-I or MDA5 expression in HUVECs
To investigate involvement of RIG-I and MDA5 in
radiation-induced senescence in HUVECs, RIG-I- or MDA5-knockdown
HUVECs were constructed. Transfection of siRNA targeting RIG-I or
MDA5 decreased their expression in HUVECs (Fig. 3A). Proportion of cells with high
SA-β-gal activity at 10 days following 4 Gy irradiation was
significantly lower in the RIG-I knockdown group than in the
control group; this was not observed in the MDA5 knockdown group
(Fig. 3B). Similar effects by RIG-I
knockdown were observed at 5 days after 10 Gy irradiation (Fig. 3C). Furthermore, 10 Gy-irradiated
HUVECs showed morphological changes such as enlargement and high
fluorescence intensity for SA-β-gal substrate compared with
non-irradiated cells (Fig. 3D). In
addition, in line with the results of flow cytometric analysis, the
number of cells with high fluorescence intensity was low in RIG-I
knockdown group compared with 10 Gy-irradiated cells transfected
with control siRNA (Fig. 3D).
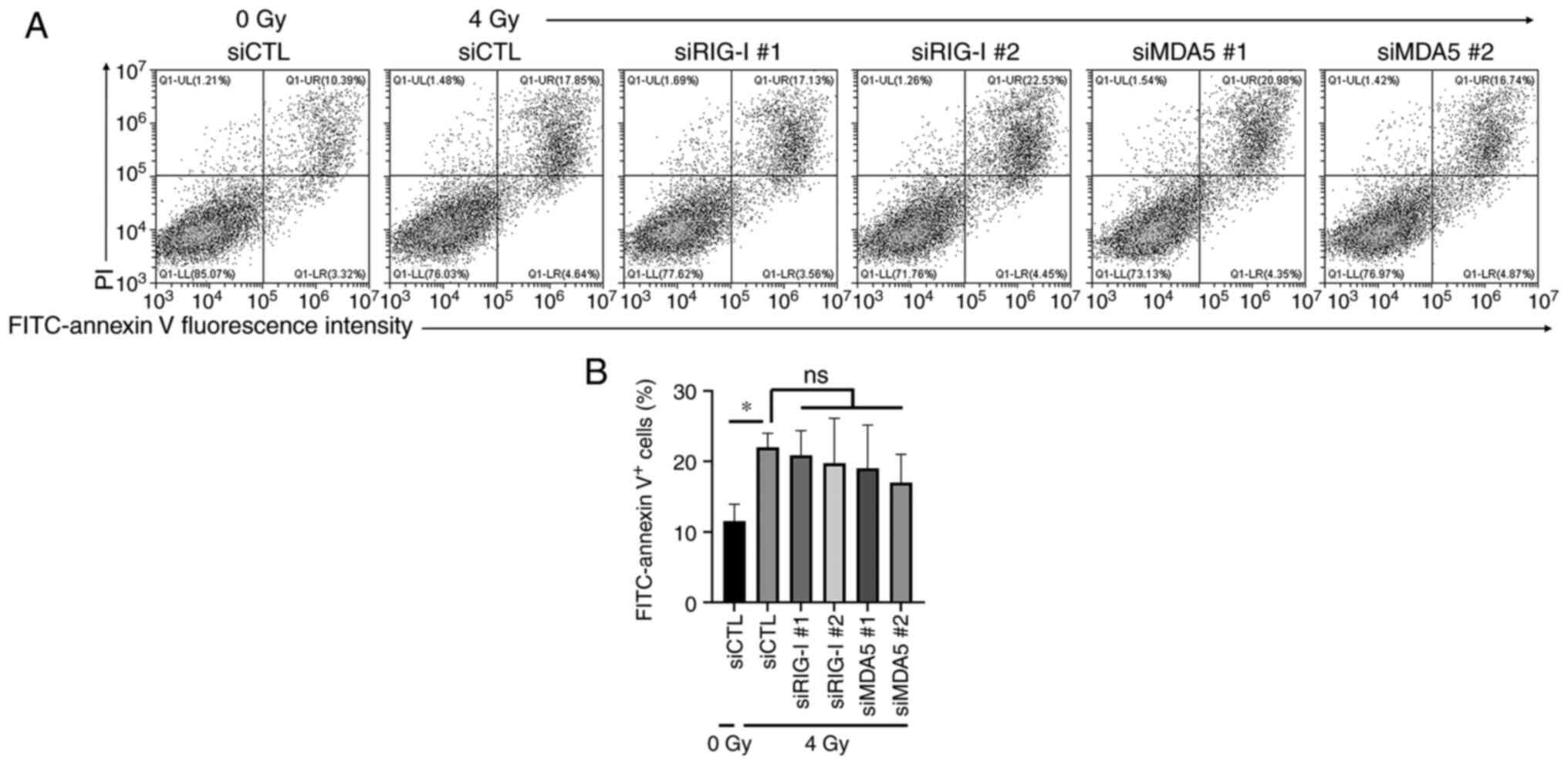
Association between RLRs and
radiation-induced apoptosis in HUVECs
The present study investigated the effects of RIG-I
or MDA5 knockdown on radiation-induced apoptosis in HUVECs 10 days
after 4 Gy-irradiation. Although 4 Gy irradiation statistically
increased the proportion of annexin V-positive apoptotic cells
(Fig. 4A and B), no significant difference in the
proportion of annexin V-positive apoptotic cells in 4 Gy-irradiated
cells was observed between control group and RIG-I or MDA5
knockdown group (Fig. 4B).
Discussion
Although radiotherapy is one of the primary
treatments for cancer, it has adverse effects (31). Exposure of the heart to radiation
during radiotherapy for breast cancer increases risk of heart
disease (32). As senescence of
VECs is associated with vascular diseases (15), regulating cell senescence may
prevent development of vascular diseases due to radiation exposure.
The present study aimed to identify factors that regulate
senescence of irradiated HUVECs. In silico analysis
suggested that RLRs may be involved in radiation-induced senescence
of HUVECs. Additionally, in vitro analysis showed that
radiation induced not only senescence of HUVECs but also
upregulated RIG-I and MDA5 expression and that RIG-I knockdown, but
not MDA5 knockdown, inhibited senescence of irradiated HUVECs.
These results suggested that the role of RIG-I in responses of
HUVECs to radiation was different from that of MDA5, and that RIG-I
was involved in radiation-induced senescence of HUVECs.
Although it is well-known that RLRs serve important
roles in anti-viral responses (23,24),
there is a little information about their involvement in cellular
senescence. RIG-I is induced in replicative senescent cells via the
ataxia telangiectasia mutated-interferon regulatory factor 1 axis
(33). In addition, Zeng et
al (34) reported that in the
senescence-accelerated mouse prone-8 mouse model of premature
aging, the RIG-I/NF-κB signaling pathway is activated. In line with
the aforementioned reports, the present study also showed
upregulation of RIG-I expression in irradiated HUVECs, accompanied
by increased SA-β-gal activity. These data suggested that the
upregulation of RIG-I expression is a feature, and may be a useful
marker, of senescent cells.
Here, RIG-I knockdown attenuated radiation-induced
increase in SA-β-gal activity in cells, suggesting that RIG-I
promoted radiation-induced senescence in HUVECs. To the best of our
knowledge, the present study is the first to show the involvement
of RIG-I in radiation-induced cellular senescence. By contrast,
Zhao et al (35) reported
the anti-aging effects of RIG-I. In the aforementioned study,
RIG-I-/- mice showed age-related features such as
alopecia and shortened survival. Additionally, continuous passage
of RIG-I-/- mouse embryonic fibroblast results in
premature replicative senescence (35). Although the reasons for the
difference in the roles of RIG-I between the present results and
those of the aforementioned study remain unclear, the difference in
types of cells or senescence (replicative or premature senescence)
may be involved due to the effects of senolytic drugs that
eliminates senescent cells depending on cell or type of
senescence-induced stimuli (36,37).
Upon activation, RLRs induce production of
anti-viral cytokines such as type I IFN-β (38), which promotes cellular senescence
(39). Ranoa et al (40) reported that ionizing radiation
induces IFN-β production in both mouse fetal fibroblasts and the
human glioma cell line D54 via the endogenous RNA/RIG-I pathway.
Therefore, it is possible that RIG-I is involved in
radiation-induced senescence via regulation of IFN-β. Additionally,
as IFN-β can induce RLR expression (41), it may also upregulate RLR expression
in irradiated HUVECs. To confirm this, further studies regarding
the role of IFN-β in irradiated HUVECs are needed.
MDA5 knockdown failed to attenuate radiation-induced
senescence in HUVECs, although its activation induces production of
type I IFN (42). The type of RNA
detected by MDA5 is different from that by RIG-I. Briefly, RIG-I
primarily recognizes short double-stranded 5'-triphosphate RNA,
while MDA5 recognizes long double-stranded RNA (22). Ranoa et al (40) reported that MDA5 is not involved in
radiation-induced IFN-β and that radiation enriches endogenous
small RNA molecules in RIG-I complexes. Thus, it is likely that
through endogenous small RNA molecules, radiation activates the
RIG-I pathway, leading to cell senescence.
Although the tumor suppressor gene TP53 may be an
effective target to control cellular senescence induced by DNA
damage, it is also involved in apoptosis regulation (43). As proliferation of cells with DNA
damage may increase the risk of genomic instability (44), suppression of p53 may be
undesirable. Here, RIG-I knockdown did not affect radiation-induced
apoptosis. Therefore, RIG-I may be a potential target for the
regulation of senescence while maintaining genomic stability
following radiation exposure.
In conclusion, the present study revealed a novel
role of RIG-I in regulation of radiation-induced senescence in
HUVECs. As RIG-I mediates SASP (33), regulating RIG-I may prevent and
relieve SASP-mediated adverse effects as well as senescence
induction. Although it is necessary to elucidate the mechanisms of
RIG-I-mediated radiation-induced cellular senescence and the role
of RIG-I in VEC functioning, RIG-I could be a potential target for
prevention and mitigation of vascular damage after radiation
exposure.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Takeda Science
Foundation, JSPS KAKENHI (grant number: JP21K07691) and
Interdisciplinary Collaborative Research Grant for Young
Scientists, Hirosaki University.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HY conceived the study, designed the methodology,
analyzed data and wrote the manuscript. FS, AK, ET and KS analyzed
data. FS wrote the manuscript. ET interpreted data and edited the
manuscript. All authors have read and approved the final
manuscript. FS and HY confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Authors on behalf of ICRP. Stewart FA,
Akleyev AV, Hauer-Jensen M, Hendry JH, Kleiman NJ, Macvittie TJ,
Aleman BM, Edgar AB, Mabuchi K, Muirhead CR, et al: ICRP
publication 118: ICRP statement on tissue reactions and early and
late effects of radiation in normal tissues and organs-Threshold
doses for tissue reactions in a radiation protection context. Ann
ICRP. 41:1–322. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sanchez RM, Siiskonen T and Vano E:
Current status of diagnostic reference levels in interventional
cardiology. J Radiol Prot. 42(041002)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Toutfaire M, Dumortier E, Fattaccioli A,
Van Steenbrugge M, Proby CM and Debacq-Chainiaux F: Unraveling the
interplay between senescent dermal fibroblasts and cutaneous
squamous cell carcinoma cell lines at different stages of
tumorigenesis. Int J Biochem Cell Biol. 98:113–126. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Frediani E, Scavone F, Laurenzana A,
Chillà A, Tortora K, Cimmino I, Leri M, Bucciantini M, Mangoni M,
Fibbi G, et al: Olive phenols preserve lamin B1 expression reducing
cGAS/STING/NFκB-mediated SASP in ionizing radiation-induced
senescence. J Cell Mol Med. 26:2337–2350. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang AS and Dreesen O: Biomarkers of
cellular senescence and skin aging. Front Genet.
9(247)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Salama R, Sadaie M, Hoare M and Narita M:
Cellular senescence and its effector programs. Genes Dev.
28:99–114. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mohamad Kamal NS, Safuan S, Shamsuddin S
and Foroozandeh P: Aging of the cells: Insight into cellular
senescence and detection methods. Eur J Cell Biol.
99(151108)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kaarniranta K, Blasiak J, Liton P, Boulton
M, Klionsky DJ and Sinha D: Autophagy in age-related macular
degeneration. Autophagy. 19:388–400. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Takasugi M, Yoshida Y and Ohtani N:
Cellular senescence and the tumour microenvironment. Mol Oncol.
16:3333–3351. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Campisi J: Cellular senescence: Putting
the paradoxes in perspective. Curr Opin Genet Dev. 21:107–112.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Calcinotto A, Kohli J, Zagato E,
Pellegrini L, Demaria M and Alimonti A: Cellular senescence: Aging,
cancer, and injury. Physiol Rev. 99:1047–1078. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kirkland JL and Tchkonia T: Cellular
senescence: A translational perspective. EBiomedicine. 21:21–28.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Chinta SJ, Woods G, Rane A, Demaria M,
Campisi J and Andersen JK: Cellular senescence and the aging brain.
Exp Gerontol. 68:3–7. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chalan P, van den Berg A, Kroesen BJ,
Brouwer L and Boots A: Rheumatoid arthritis, immunosenescence and
the hallmarks of aging. Curr Aging Sci. 8:131–146. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hwang HJ, Kim N, Herman AB, Gorospe M and
Lee JS: Factors and pathways modulating endothelial cell senescence
in vascular aging. Int J Mol Sci. 23(10135)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Campisi J: Aging, cellular senescence, and
cancer. Annu Rev Physiol. 75:685–705. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Besson A, Dowdy SF and Roberts JM: CDK
inhibitors: Cell cycle regulators and beyond. Dev Cell. 14:159–169.
2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Marei HE, Althani A, Afifi N, Hasan A,
Caceci T, Pozzoli G, Morrione A, Giordano A and Cenciarelli C: p53
signaling in cancer progression and therapy. Cancer Cell Int.
21(703)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kawai T and Akira S: Toll-like receptor
and RIG-I-like receptor signaling. Ann N Y Acad Sci. 1143:1–20.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Carty M, Guy C and Bowie AG: Detection of
viral infections by innate immunity. Biochem Pharmacol.
183(114316)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kato H, Takeuchi O, Mikamo-Satoh E, Hirai
R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T and Akira
S: Length-dependent recognition of double-stranded ribonucleic
acids by retinoic acid-inducible gene-I and melanoma
differentiation-associated gene 5. J Exp Med. 205:1601–1610.
2008.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Brisse M and Ly H: Comparative structure
and function analysis of the RIG-I-Like Receptors: RIG-I and MDA5.
Front Immunol. 10(1586)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Diao F, Li S, Tian Y, Zhang M, Xu LG,
Zhang Y, Wang RP, Chen D, Zhai Z, Zhong B, et al: Negative
regulation of MDA5- but not RIG-I-mediated innate antiviral
signaling by the dihydroxyacetone kinase. Proc Natl Acad Sci USA.
104:11706–11711. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Takahashi T, Nakano Y, Onomoto K, Yoneyama
M and Ui-Tei K: Virus sensor RIG-I represses RNA interference by
interacting with TRBP through LGP2 in mammalian cells. Genes
(Basel). 9(511)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Casella G, Munk R, Kim KM, Piao Y, De S,
Abdelmohsen K and Gorospe M: Transcriptome signature of cellular
senescence. Nucleic Acids Res. 47:7294–7305. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Furusawa Y, Zhao QL, Hattori Y, Tabuchi Y,
Iwasaki T, Nomura T and Kondo T: Comprehensive and computational
analysis of genes in human umbilical vein endothelial cells
responsive to X-irradiation. Genomics Data. 8:126–130.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Gene Ontology Consortium. The Gene
Ontology project in 2008. Nucleic Acids Res. 36 (Database
issue):D440–D444. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yoshino H, Kumai Y and Kashiwakura I:
Effects of endoplasmic reticulum stress on apoptosis induction in
radioresistant macrophages. Mol Med Rep. 15:2867–2872.
2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Loo YM and Gale M Jr: Immune signaling by
RIG-I-like receptors. Immunity. 34:680–692. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee BY, Han JA, Im JS, Morrone A, Johung
K, Goodwin EC, Kleijer WJ, DiMaio D and Hwang ES:
Senescence-associated beta-galactosidase is lysosomal
beta-galactosidase. Aging Cell. 5:187–195. 2006.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Barazzuol L, Coppes RP and van Luijk P:
Prevention and treatment of radiotherapy-induced side effects. Mol
Oncol. 14:1538–1554. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Darby SC, Ewertz M and Hall P: Ischemic
heart disease after breast cancer radiotherapy. N Engl J Med.
368(2527)2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liu F, Wu S, Ren H and Gu J: Klotho
suppresses RIG-I-mediated senescence-associated inflammation. Nat
Cell Biol. 13:254–262. 2011.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Zeng Y, Wang PH, Zhang M and Du JR:
Aging-related renal injury and inflammation are associated with
downregulation of klotho and induction of RIG-I/NF-κB signaling
pathway in senescence-accelerated mice. Aging Clin Exp Res.
28:69–76. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Zhao J, Jiang X, Yan L, Lin J, Guo H, Yu
S, Ye B, Zhu J and Zhang W: Retinoic acid inducible gene-I slows
down cellular senescence through negatively regulating the integrin
β3/p38 MAPK pathway. Cell Cycle. 18:3378–3392. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H,
Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO,
Giles CB, et al: Identification of a novel senolytic agent,
navitoclax, targeting the Bcl-2 family of anti-apoptotic factors.
Aging Cell. 15:428–435. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Malaquin N, Vancayseele A, Gilbert S,
Antenor-Habazac L, Olivier MA, Ait Ali Brahem Z, Saad F, Delouya G
and Rodier F: DNA damage- but not enzalutamide-induced senescence
in prostate cancer promotes senolytic Bcl-xL inhibitor sensitivity.
Cells. 9(1593)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Rehwinkel J and Gack MU: RIG-I-like
receptors: Their regulation and roles in RNA sensing. Nat Rev
Immunol. 20:537–551. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yu Q, Katlinskaya YV, Carbone CJ, Zhao B,
Katlinski KV, Zheng H, Guha M, Li N, Chen Q, Yang T, et al:
DNA-damage-induced type I interferon promotes senescence and
inhibits stem cell function. Cell Rep. 11:785–797. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ranoa DR, Parekh AD, Pitroda SP, Huang X,
Darga T, Wong AC, Huang L, Andrade J, Staley JP, Satoh T, et al:
Cancer therapies activate RIG-I-like receptor pathway through
endogenous non-coding RNAs. Oncotarget. 7:26496–26515.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Berghäll H, Sirén J, Sarkar D, Julkunen I,
Fisher PB, Vainionpää R and Matikainen S: The interferon-inducible
RNA helicase, mda-5, is involved in measles virus-induced
expression of antiviral cytokines. Microbes Infect. 8:2138–2144.
2006.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Takeuchi O and Akira S: MDA5/RIG-I and
virus recognition. Curr Opin Immunol. 20:17–22. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rufini A, Tucci P, Celardo I and Melino G:
Senescence and aging: The critical roles of p53. Oncogene.
32:5129–5143. 2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhang Y, Yan W and Chen X: Mutant p53
cooperates with knockdown of endogenous wild-type p53 to disrupt
tubulogenesis in Madin-Darby canine kidney cells. PLoS One.
8(e85624)2013.PubMed/NCBI View Article : Google Scholar
|