Introduction
Stroke is the second most common mortality among
global diseases, with ischemic stroke accounting for 85% of cases
(1-3).
One of the most effective treatments for acute ischemic stroke is
intravenous thrombolytic therapy (4). However, thrombolytic therapy is often
accompanied by side effects or aggravation. Vascular recanalization
induces a series of pathological processes to varying degrees, such
as oxidative stress, inflammation and peripheral immune cell
infiltration, leading to neuronal cell death near the lesion;
clinically known as cerebral ischemia reperfusion injury (CIRI).
Vascular recanalization also triggers transient or permanent
neurological dysfunctional diseases such as vascular dementia
(5-7).
Therefore, to protect brain tissue from ischemia-reperfusion injury
and further improve functional prognosis, the development of
neuroprotective drugs is urgently needed for patients with ischemic
stroke after thrombolytic therapy.
Traditional Chinese medicine has a multi-target,
multi-pathway compound effect and its monomer preparations are
characterized by small side effects and significant therapeutic
effects (8). Such features allow
the treatment of ischemic stroke by traditional Chinese medicine to
have great potential for its application in a wide range of
diseases. Herbs from Yunnan are widely used in the prevention and
treatment of cardiovascular, gastrointestinal and traumatic
diseases and they have been documented in the ‘Southern Yunnan
Materia Medica’ and applied for hundreds of years (9). One of these herbs, Rubia
yunnanensis, is the dried root and rhizome of Rubia
yunnanensis Diels (a plant of the Rubiaceae family) and
is an indigenous medicine endemic to Yunnan, China. Rubia
yunnanensis has pharmacological effects, such as
anti-oxidation, anti-ischemia and anti-thrombosis actions (10). Rubia yunnanensis was commonly
used by some famous Chinese doctors, such as Zhang Chao, a famous
Chinese medicine practitioner in Yunnan Province, to treat certain
cerebral ischemic disorders, such as dizziness and anemia caused by
cerebral ischemia. Some Chinese medicine practitioners also
commonly use Rubia yunnanensis to treat dizziness and
anemia. This application suggests that Rubia yunnanensis may
have the potential to treat ischemic stroke and subsequent
validation in more direct human trials may lead to the gradual
clinical application of Rubia yunnanensis. HT22 cells that
lack the N-methyl-D-aspartic acid receptor are the most commonly
used in vitro cell model in oxidative stress-related
studies. By exploring the potential therapeutic effects and
mechanisms of the Rubia yunnanensis, new ideas and methods
may be provided for the prevention and treatment of ischemic
stroke.
In terms of the innovative aspects of the present
study, it demonstrated the antioxidant protective effect of RY-A in
mouse hippocampal HT22 cells used as an oxygen-glucose
deprivation/reoxygenation (OGD/R) model, investigated the metabolic
alterations between OGD/R and RY-A groups using a metabolomics
approach and explored the involvement of MAPK signaling pathways in
its molecular mechanism. The results suggested that RY-A may be a
potentially effective therapeutic herb for patients with CIRI.
Materials and methods
Preparation of the alcohol extract of
Rubia yunnanensis
Rubia yunnanensis was purchased from Yunnan
Huide Pharmaceutical Co., Ltd. and was identified by Professor Zili
Yin of Yunnan University of Traditional Chinese Medicine (Yunnan,
China). The alcohol extract of Rubia yunnanensis was
obtained by soaking 500 g of powdered Rubia yunnanensis in
95% ethanol for 24 h. Heating and refluxing at 60˚C, then
repeatedly soaking, heating and refluxing for four times to obtain
the alcohol extract of Rubia yunnanensis.
Cell cultivation and preparation of
the OGD/R model
HT22 cells (Shanghai Enzyme Research Biotechnology
Co., Ltd.) were derived from the mouse hippocampal neuronal cell
line. HT22 cells were cultured in high glucose DMEM (cat. no.
2024059) containing 10% fetal bovine serum (cat. no. 04-001-1A) and
1% penicillin-streptomycin (cat. no. 2114092; all from Biological
Industries) and were routinely passaged in an incubator at 37˚C
and, 5% CO2. The cells were cultured in 96-well plates
at a density of 1x105/ml for 24 h during the logarithmic
growth period and then grouped. In vitro cultured HT22 cells
were randomly divided into control group, OGD/R group, OGD/R group,
OGD/R + 100 µmol/l edaravone (Beijing Solarbio Science &
Technology Co., Ltd.; EDA group) and OGD /R + 10, 20, or 40 µg/ml
RY-A (RY-A group). The control group was left untreated and the
OGD/R group was cultured in oxygen/sugar deprivation conditions for
2 h in sugar-free DMEM medium (11)
containing 10 mmol/l Na2S2O4 (cat.
no. 2029548; Biological Industries) and was then cultured in
serum-free high-sugar DMEM for 2 h for reconstitution. Cells were
incubated in DMEM for 2 h for reoxygenation to establish an in
vitro OGD/R model. The EDA and RY-A were added at final
concentrations starting 24 h before oxygen deprivation and until
the end of reoxygenation.
Cell viability assay. HT22 cells were
routinely cultured in 96-well plates at a density of
1x105/ml during the logarithmic growth phase for 24 h.
After treating the cells in each group according to the
aforementioned methods, 20 µl thiazolyl blue tetrazolium bromide
(MTT; 5 mg/ml; Shanghai Suoqiao Biotech Co., Ltd.) was added to
each well and incubated at 37˚C for 4 h. The liquid in the wells
was carefully pipetted off and 150 µl of dimethyl sulfoxide
solution was added. Wells were shaken at low speed for 5 min until
the crystals were completely dissolved. Absorbance was measured at
490 nm on an enzyme labeling instrument (VariosKan Flash; Thermo
Fisher Scientific, Inc.).
Cell mortality assay
The cell culture conditions and grouping were
unchanged and 96-well plates were used. After 2 h of sugar
restoration and reoxygenation, the original medium was replaced by
200 µl/well of serum-free medium and the cell mortality rate was
detected according to the specifications of a lactate dehydrogenase
(LDH) Cytotoxicity Detection Kit (cat. no. C0016, Shanghai
Biyuntian Biotechnology Co., Ltd.).
Detection of intracellular reactive
oxygen species (ROS) levels
HT22 cells were routinely cultured and ROS levels
were detected using a ROS detection kit (cat. no. S0033S; Shanghai
Biyuntian Biotechnology Co., Ltd.). Cells were cultured in 6-well
plates at a density of 6.5x104/ml during the logarithmic
growth phase for 24 h. After treating the cells in each group
according to the aforementioned methods, 20 min before the end of
the treatment, dichlorodihydrofluorescein diacetate was added to
each well at a ratio of 1:1,000 and aspirated well to make complete
contact between the probes and cells. The cells were incubated at
37˚C for 20 min and then washed three times with phosphate-buffered
saline. The fluorescence intensity was detected in an enzyme
labeling instrument (VariosKan Flash; Thermo Fisher Scientific,
Inc.) using 488 nm excitation wavelength and 525 nm emission
wavelength.
Determination of nitric oxide (NO) and
malondialdehyde (MDA) content and superoxide dismutase (SOD) and
glutathione peroxidase (GSH-Px) activities
HT22 cells were routinely cultured in 6-well plates
for 24 h at a density of 6.5x104/ml during the
logarithmic growth phase. After treating the cells in each group,
the cells were analyzed according to the BCA Protein Concentration
Assay Kit (cat. no. P0010), Nitric oxide (NO) Detection Kit (cat.
no. S0021S), Malondialdehyde (MDA) Detection Kit (cat. no. S0131S),
Superoxide dismutase (SOD) Detection Kit (cat. no. S0101S) and
Glutathione peroxidase (GSH-Px) kit (cat. no. S0057S; Shanghai
Biyuntian Biotechnology Co., Ltd.) for the determination of NO,
MDA, SOD and GSH-Px.
Metabolomics assay
HT22 cells were routinely cultured at a density of
6.5x104/ml during the logarithmic growth phase in 6-well
plates for 24 h. Cells were divided into the OGD/R and RY-A (OGD/R
+ 20 µg/ml RY-A) groups, with one sample/two wells and six samples
of each collected for analysis.
The cell sample was pipetted into a 2 ml centrifuge
tube and 100 mg of glass beads added.
Acetonitrile:methanol:H2O (1,000 µl; 2:2:1, v:v:v) was
added and the sample vortexed for 30 sec. The tube was placed in
the 2 ml adapter and frozen in liquid nitrogen for 5 min. The tube
was removed and thawed at room temperature. The tube was then
placed again in the 2 ml adapter and mounted in the tissue grinder
and ground at 60 Hz for 2 min, repeated twice. The tube was removed
and centrifuged at 14,000 x g for 10 min at 4˚C. The supernatant
was concentrated and dried. The sample was reconstituted by adding
exactly 300 µl of 2-chloro-L-phenylalanine (4 ppm) solution in
acetonitrile:0.1% formic acid (1:9; v:v) and the supernatant was
filtered through a 0.22 µm membrane. The filtrate was added to the
assay vial and used for ultra-high performance liquid
chromatography (UHPLC) and mass spectrometry (MS) detection.
UHPLC-MS conditions. UHPLC
conditions
A Thermo Vanquish (Thermo Fisher Scientific, Inc.)
UHPLC system was used with an ACQUITY UPLC HSS T3 (2.1x100 mm, 1.8
µm; Waters Corporation) column. The flow rate was 0.3 ml/min, the
column temperature was 40˚C and the injection volume was 2 µl. The
mobile phases were 0.1% formic acid in acetonitrile (B2) and 0.1%
formic acid with water (A2) in positive ionization mode and the
gradient elution program was as follows: 0-1 min, 8% B2; 1-8 min,
8-98% B2; 8-10 min, 98% B2; 10-10.1 min, 98-8% B2; 10.1-12 min, 8%
B2. In negative ionization mode, the mobile phases were
acetonitrile (B3) and 5 mM ammonium formate in water (A3) and the
gradient elution program was: 0-1 min, 8% B3; 1-8 min, 8-98% B3;
8-10 min, 98% B3; 10~10.1 min, 98-8% B3; and 10.1-12 min, 8%
B3(12).
MS conditions
Data were acquired separately in positive and
negative ion mode on a Thermo Orbitrap Exploris 120 MS detector
(Thermo Fisher Scientific, Inc.) with an electrospray ionization
source. The positive ion spray voltage was 3.50 kV, the negative
ion spray voltage was -2.50 kV, the sheath gas was 40 arb and the
auxiliary gas was 10 arb. The capillary temperature was 325˚C and a
primary full scan was performed at a resolution of 60,000, with a
primary ion scanning range of 100-1,000 m/z. Secondary cleavage was
performed using an HCD with a collision energy of 30% and a
secondary resolution of 15,000. The first four ions of the acquired
signal were fragmented, while dynamic exclusion was used to remove
unnecessary MS/MS information (13).
Metabolomics analysis
Chromatographic data were converted to mzXML file
format by the MSConvert tool in the ProteoWizard software package
(v3.0.8789) (14), then the
retention time correction, peak detection, peak filtering and peak
alignment processes were performed by the R XCMS (v3.12.0) software
package (15). The parameters were
set to bw=2, ppm=15, peakwidth=c(5,30),
mzwid=0.015, mzdiff=0.01 and method=‘centWave’, resulting in a
quantitative list of substances containing the retention times,
mass-to-charge ratios and peak areas in data matrices. Substance
identification was performed using spectral databases, such as the
Human Metabolome Database (16),
Massbank (17), LipidMaps (18), Mzcloud (19), Kyoto Encyclopedia of Genes and
Genomes (20) and the metabolite
database build by Panomix Biomedical Tech Co., Ltd. (http://query.biodeep.cn/), for searching and
comparing, characterization and annotation. The peak area data of
metabolites were imported into the R language Ropls package
(21) for multivariate statistical
analysis, including principal component analysis (PCA), partial
least squares-discriminant analysis (PLS-DA) and orthogonal partial
least squares discriminant analysis (OPLS-DA).
The model was tested for overfitting using the
replacement test. R2X and R2Y denoted the
explanatory rate of the constructed model for the X and Y matrices,
respectively and Q2 labels the predictive power of the
model. Values closer to 1 indicated that the fit of model is
improved and samples in the training set can be more accurately
classified into their original attributions. The P-value was
calculated according to the statistical test and the variable
projection importance (VIP) was calculated by the OPLS-DA
dimensionality reduction method. Fold change (FC) was calculated by
the multiplicity of difference between groups to measure the
strength of the influence of each metabolite content on the
classification discrimination of samples and the explanatory
ability, which assisted in the screening of marker metabolites.
Metabolite molecules were considered statistically significantly
different when P<0.05 and VIP>1. Functional pathway
enrichment and topological analysis of the screened differential
metabolites were performed using the MetaboAnalyst software package
(22).
Western blotting to detect protein
expression levels
HT22 cells were routinely cultured in 6-well plates
at a density of 6.5x104/ml during the logarithmic growth
phase for 24 h. After treatment of the cells in each group, the
cells were lysed on ice for 20 min with RIPA (cat. no. P0013C;
Beyotime Institute of Biotechnology) lysis buffer [PSMF (cat. no.
MFCD00007424; Amresco, LLC):RIPA lysate=1:100]. The supernatant was
centrifuged at 4˚C for 5 min at 12,000 x g. Protein concentration
was determined by the BCA method (Beyotime Institute of
Biotechnology). Total protein (80 µg) was separated by 8% SDS-PAGE
gel electrophoresis and then transferred to a polyvinylidene
difluoride membrane (0.45 µm; cat. no. IPFL85R; MilliporeSigma).
After membrane transfer, the membrane was blocked with 5% skimmed
milk powder at room temperature for 2 h. Subsequently, the cells
were incubated with primary antibodies: IDO (dilution 1:1,000; cat.
no. 51851; Cell Signaling Technology, Inc.), p38 (dilution 1:2,000;
cat. no. 14064-1-AP; Proteintech Group, Inc.), p-p38 (dilution
1:1,000; cat. no. 28796-1-AP; Proteintech Group, Inc.), ERK1+2
antibody (dilution 1:10,000; cat. no. ab184699; Abcam),
phosphorylated (p-)ERK antibody (dilution 1:1,000; cat. no.
ab201015; Abcam), JNK antibody (dilution 1:1,000; cat. no. 9252;
Cell Signaling Technology, Inc.), p-JNK antibody (dilution 1:1,000;
cat. no. 9251; Cell Signaling Technology, Inc.), β-Actin (cat. no.
ab8226; Abcam) overnight at 4˚C, followed by incubation with
secondary antibody [Goat Anti-Rabbit IgG Hirsl (HRP), dilution
1:10,000; cat. no. Ab6721; Rabbit Anti-Mouse IgG hanger L (HRP),
dilution 1:10,000; cat. no. Ab6728; Abcam] for 2 h at room
temperature. Chemiluminescence was visualized and imaged, the bands
were analyzed semi-quantitatively using an automated gel imaging
system (Bio-Rad Laboratories, Inc.) and ImageJ v2 (National
Institute of Health). β-Actin was used as a reference.
Statistical analysis
Data are expressed as mean ± standard deviation
(SD). Data were analyzed using GraphPad Prism 8.0 software
(GraphPad Software, Inc.; Dotmatics). If the data were normally
distributed with homogeneous variance, Bonferroni's multiple
comparison test was performed following one-way analysis of
variance (ANOVA). If the data were normally distributed but the
variance was not homogeneous, Welch's ANOVA test followed by
Dunnett's T3 multiple comparisons test was used. P<0.05 was
considered to indicate a statistically significant difference.
Results
Determination of the dose-dependent
actions of RY-A on HT22 cells
HT22 cells were treated with different
concentrations of RY-A for 24 h and the effect of RY-A on cell
viability was detected. Compared with the control group, cell
viability increased from10 to 20 µg/ml and decreased with
increasing concentrations of RY-A from 40 to 80 µg/ml, but there
was no significant difference. After reaching 160 µg/ml RY-A, high
concentrations of RY-A resulted in a significant dose-dependent
decrease in cell viability and toxic effects (P<0.001), as shown
in Fig. 1A.
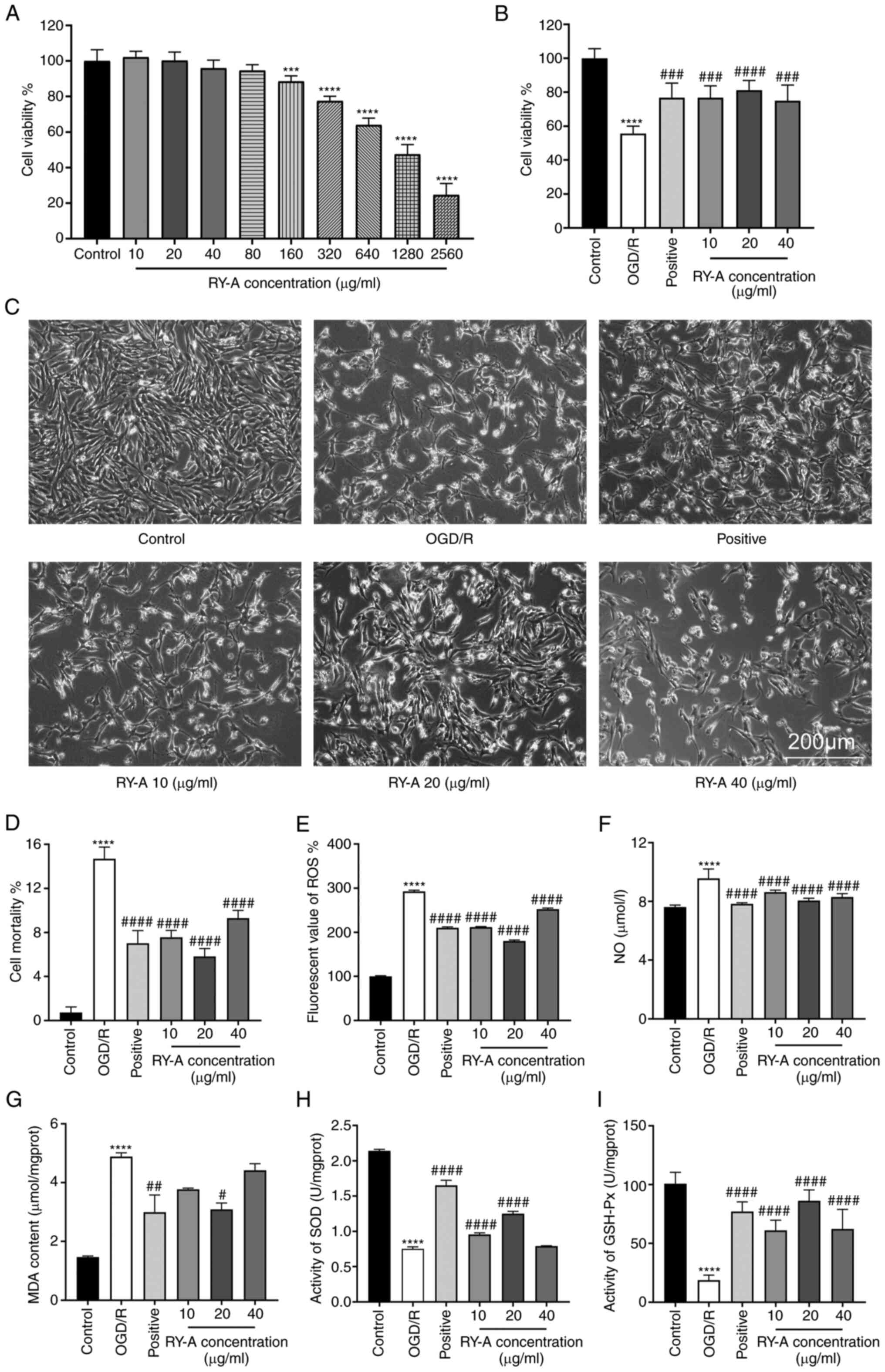 | Figure 1Cell safety of RY-A and effects of
RY-A on cell viability, cell morphology, LDH release and oxidative
stress in OGD/R-induced HT22 cells. (A) Effect of RY-A on the
viability of HT22 cells. (B) Effect of RY-A on the viability of
OGD/R-induced HT22 cells. (C) HT22 cell morphology in each group.
(D) Detection of cellular mortality using the LDH cytotoxicity
assay kit. Assay kits was used to detect (E) ROS and (F) NO. (G)
Lipid oxidation assay kit was used to measure MDA. Assay kits was
used to detect (H) SOD and (I) GSH-Px activity. Positive: EDA
group, OGD/R + 100 µmol/l edaravone. Results were expressed as mean
± SD (n=6). ***P<0.001 and ****P<0.0001
vs. Control group #P<0.05, ##P<0.01,
###P<0.001 and ####P<0.0001 vs. OGD/R
group. RY-A, Rubia yunnanensis alcohol extract; LDH, lactate
dehydrogenase; OGD/R, oxygen-glucose deprivation/reoxygenation;
ROS, reactive oxygen species; NO, nitric oxide; MDA SOD, superoxide
dismutase; GSH-Px, glutathione peroxidase. |
Effect of RY-A on cell viability of
OGD/R treated HT22 cells
The MTT results showed that the cell survival rate
of the OGD/R group decreased significantly and reached half lethal
levels (P<0.0001) compared with the control group. Compared with
the OGD/R group, the cell survival rate was dose-dependently
increased in the range of 10-20 µg/ml RY-A, whereas cell survival
was decreased with 40 µg/ml RY-A, yielding an optimal effective
concentration of 20 µg/ml, as shown in Fig. 1B.
Morphology of HT22 cells
The cells in the control group were morphologically
normal, triangular, with bipolar or multipolar protrusions, large
cytosol, complete and smooth cell membranes and connected into a
network. After 4 h of OGD/R treatment, the number of cells in the
OGD/R group was significantly reduced and most of the cells lost
their protrusions and were rounded. However, the effects of OGD/R
were significantly reversed by RY-A pretreatment, as shown in
Fig. 1C.
Effect of RY-A on LDH release and
oxidation levels in OGD/R treated HT22 cells
LDH release is an important indicator for detecting
cell membrane integrity and is a widely used cell mortality assay.
The results of the LDH assay showed that 20 µg/ml RY-A (mortality
of 5.82±0.65%) significantly inhibited the amount of LDH release
compared with that in the OGD/R group (mortality of 14.69±0.97%),
which resulted in a significant decrease in cell death
(P<0.0001) as shown in Fig. 1D.
The results related to oxidative levels showed that the levels of
NO, ROS and MDA were significantly higher in the OGD/R group
compared with the control group (P<0.0001). RY-A and EDA
significantly reduced the levels of NO, ROS and MDA compared with
the OGD/R group; with a significant difference observed at 20 µg/ml
RY-A (P<0.05), as shown in Fig.
1E-G.
Effect of RY-A on antioxidant levels
in OGD/R treated HT22 cells
The results relating to antioxidant levels showed
that the activities of SOD and GSH-Px were significantly inhibited
in the OGD/R group compared with the control group (P<0.0001).
The EDA group had increased activities of SOD and GSH-Px compared
with the OGD/R group (P<0.0001). Compared with the OGD/R group,
RY-A 10 and 20 µg/ml significantly elevated SOD activity
(P<0.0001), whereas 40 µg/ml RY-A did not significantly increase
SOD activity. The activity of GSH-Px was significantly elevated in
the RY-A group and 20 µg/ml RY-A resulted in the highest GSH-Px
activity (P<0.0001), as shown in Fig. 1H and I.
Metabolomic analysis of OGD/R treated
HT22 cells before and after RY-A treatment
A total of 3,826 metabolites were identified by a
combination of positive and negative ion modes. The cellular
samples were analyzed using the validated UHPLC-MS method and after
calibration and normalization, the data were imported into the R
language Ropls package for PCA. Tight aggregation of quality
control (QC) samples was observed in both positive and negative ion
modes, indicating good stability and reproducibility of the
instrument and method (Fig. 2A and
C). The relative standard deviation
(RSD) results of the QC samples showed that the number of
characteristic peaks with RSD ≤30% in the QC samples accounted for
more than 70% of the total characteristic peaks (Fig. 2B and D), indicating good data suitable for
further analysis (13).
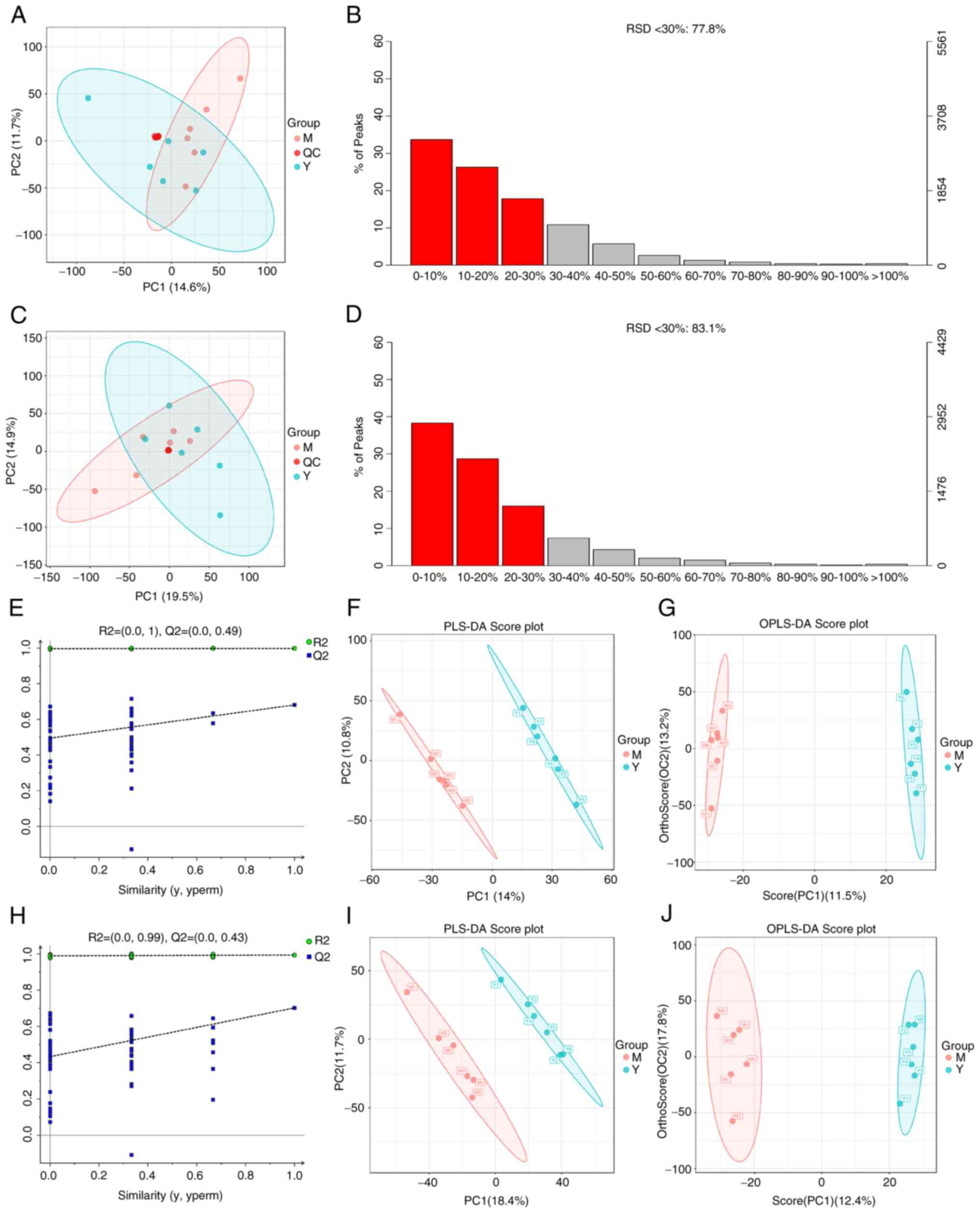 | Figure 2Instrument stability monitoring,
validity analysis of the model and screening of differential
metabolites. (A and C) PCA of cell samples and QC samples, in order
of positive and negative ion PCA. (B and D) RSD analysis of QC
samples, in order of positive and negative ion RSD. (E and H) The
replacement test was used to verify whether the PLS-DA model was
overfitted or not, in order of positive and negative ion
replacement test. (F and I) PLS-DA of metabolic changes between
samples from OGD/R group and RY-A group based on LC-MS/MS system,
in order of positive ion and negative ion PLS-DA.(G and J)
Screening of differential metabolites between OGD/R group and RY-A
group using OPLS-DA, in order of Positive and negative ion OPLS-DA.
PCA, principal component analysis; RSD, Relative Standard
Deviation; QC, quality control; PLS-DA, partial least squares
discriminant analysis; OGD/R, oxygen-glucose
deprivation/reperfusion; RY-A, Rubia yunnanensis alcohol
extract; OPLS-DA, orthogonal partial least squares discriminant
analysis; M, OGD/R group; Y, RY-A group. |
Enrichment analysis of differential
metabolites and their metabolic pathways
The relationship between metabolite expression and
sample category was first analyzed by PLS-DA. To avoid overfitting
of the PLS-DA model during the modeling process, the replacement
test was used to test the model and ensure its validity. The
results showed that its R2X, R2Y and
Q2 values were 0.247, 0.998 and 0.681, respectively, for
the positive ion model (Fig. 2E)
and 0.302, 0.993 and 0.702, respectively, for the negative ion
model (Fig. 2H), indicating that
the constructed model was good without overfitting. As shown by
PLS-DA (Fig. 2F and I), each sample in the OGD/R and RY-A
groups showed a tendency of intra-group aggregation and inter-group
dispersion. The PLS-DA model could distinguish between the two
groups of samples, indicating that the administration of RY-A
affected the metabolites in the OGD/R treated HT22 cells.
Differential metabolites were screened by the OPLS-DA (Fig. 2G and J) to obtain VIP, which is used to
illustrate the degree of contribution of the variables to the
model, measuring the strength of the effect of differences in
accumulation of each metabolite on the categorization and
differentiation of samples in each group and the explanatory power.
Differential metabolites with biological significance were mined.
In the present study, VIP>1 and P<0.05 were used as screening
criteria for significant differences in metabolites. A total of 20
metabolites were significantly different between the RY-A and OGD/R
groups. Of these, five were upregulated: L-tryptophan, xanthine,
uracil 5-carboxylic acid, guanidino-succinic acid and L-dopa. The
other 15 metabolites were downregulated: ornithine,
eicosapentaenoic acid-d5, isosafrole, inosine, adenosine
3'-monophosphate, 5'-methylthioadenosine, glycerol 3-phosphate,
d-mannose, palmitoyl-L-carnitine, L-carnitine, cortexolone,
L-cysteine, L-phenylalanine, shikimic acid, allocholic acid
(Table I).
 | Table IScreening results of differential
metabolites in oxygen-glucose deprivation/reoxygenation group and
Rubia yunnanensis alcohol extract group. |
Table I
Screening results of differential
metabolites in oxygen-glucose deprivation/reoxygenation group and
Rubia yunnanensis alcohol extract group.
| No. | Metabolite | Formula | m/z | RT(s) | Fold change | P-value | Variable projection
importance | Electrospray
ionization mode | Trend |
|---|
| 1 | L-Tryptophan |
C11H12N2O2 | 205.098 | 193.045 | 0.941 |
1.16x10-3 | 2.585 | [M+H]+ | Up |
| 2 | Ornithine |
C5H12N2O2 | 133.097 | 45.265 | 1.792 |
1.82x10-3 | 2.347 | [M+H]+ | Down |
| 3 | Eicosapentaenoic
acid-d5 |
C20H30O2 | 302.221 | 573.912 | 1.257 |
2.52x10-3 | 2.229 | [M-H]- | Down |
| 4 | Isosafrole |
C10H10O2 | 163.076 | 533.436 | 1.112 |
3.85x10-3 | 2.224 | [M+H]+ | Down |
| 5 | Xanthine |
C5H4N4O2 | 152.035 | 382.348 | 0.124 |
4.51x10-3 | 2.292 | [M-H]- | Up |
| 6 | Inosine |
C10H12N4O5 | 267.074 | 67.378 | 2.243 |
1.02x10-2 | 2.240 | [M-H]- | Down |
| 7 | Adenosine
3'-monophosphate |
C10H14N5O7P | 348.070 | 53.845 | 2.194 |
1.79x10-2 | 1.969 | [M+H]+ | Down |
| 8 | Uracil 5-carboxylic
acid |
C5H4N2O4 | 156.966 | 145.502 | 0.367 |
1.94x10-2 | 1.938 | [M+H]+ | Up |
| 9 |
5'-Methylthioadenosine |
C11H15N5O3S | 298.096 | 165.446 | 2.05 |
2.04x10-2 | 1.996 | [M+H]+ | Down |
| 10 | Glycerol
3-phosphate |
C3H9O6P | 171.006 | 46.213 | 1.674 |
2.10x10-2 | 1.956 | [M-H]- | Down |
| 11 | D-Mannose |
C6H12O6 | 179.988 | 686.854 | 1.281 |
2.13x10-2 | 1.936 | [M-H]- | Down |
| 12 | Guanidino-succinic
acid |
C5H9N3O4 | 174.957 | 141.376 | 0.776 |
2.31x10-2 | 1.770 | [M-H]- | Up |
| 13 |
Palmitoyl-L-carnitine |
C23H45NO4 | 400.340 | 519.413 | 1.169 |
2.41x10-2 | 1.920 | [M+H]+ | Down |
| 14 | L-Carnitine |
C7H15NO3 | 162.112 | 51.576 | 1.837 |
2.49x10-2 | 1.846 | [M+H]+ | Down |
| 15 | Cortexolone |
C21H30O4 | 346.331 | 461.884 | 1.54 |
2.88x10-2 | 1.885 | [M+H]+ | Down |
| 16 | L-Cysteine |
C3H7NO2S | 122.019 | 44.167 | 2.181 |
3.20x10-2 | 1.781 | [M+H]+ | Down |
| 17 | L-Dopa |
C9H11NO4 | 196.000 | 59.143 | 0.553 |
3.28x10-2 | 1.844 | [M-H]- | Up |
| 18 |
L-Phenylalanine |
C9H11NO2 | 164.072 | 90.228 | 1.322 |
3.31x10-2 | 1.829 | [M-H]- | Down |
| 19 | Shikimic acid |
C7H10O5 | 173.046 | 147.982 | 1.974 |
3.70x10-2 | 1.845 | [M-H]- | Down |
| 20 | Allocholic
acid |
C24H40O5 | 408.370 | 637.214 | 1.578 |
4.04x10-2 | 1.828 | [M+H]+ | Down |
To more fully and visually analyze the differences
between the OGD/R and RY-A groups, the relationships between the
samples and differences in the accumulation of metabolites in
different samples were demonstrated. Differential metabolites with
FC>1.5 or <0.67 and P<0.05 were visualized as volcano
plots (Fig. 3A). Selected
metabolites were analyzed by clustering, with metabolites in the
same cluster having similar accumulations and potentially similar
functions or participation in the same metabolic processes or
cellular pathways (Fig. 3B).
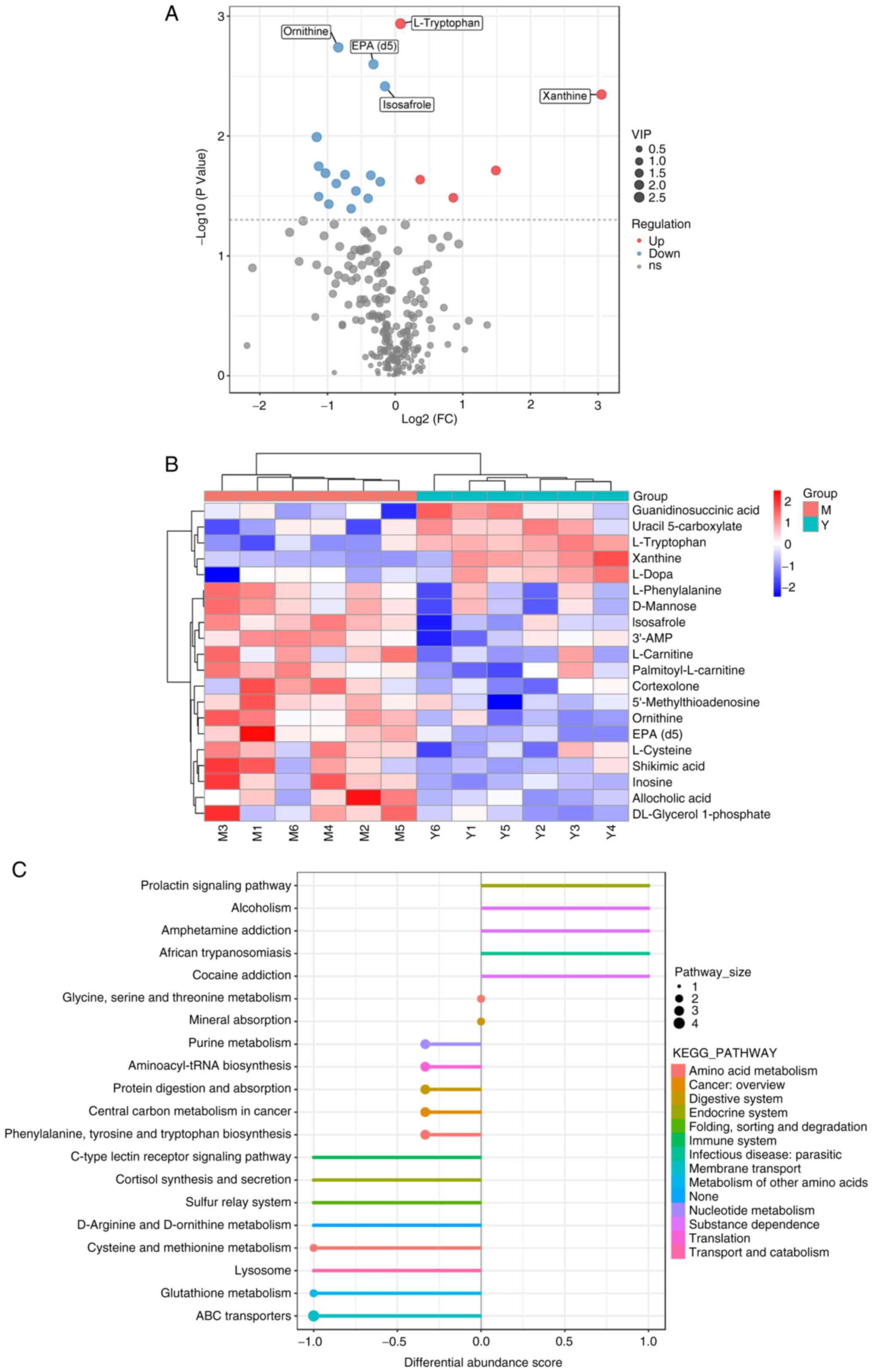 | Figure 3Enrichment analysis of differential
metabolites and their metabolic pathways. (A) Differential
metabolite volcano plot. The larger the absolute value of the
horizontal coordinate, the larger the fold change in expression
between the OGD/R group and the RY-A group. The larger the vertical
coordinate value, the greater the statistical significance of its
expression. Blue dots: downregulated metabolites, red dots:
upregulated metabolites, gray dots: metabolites that did not meet
the conditions of differential screening. The top five metabolite
names with the smallest P-value are displayed by default. (B)
Heatmap analysis of overall clustering of samples. The left
clustering line is the metabolite clustering line and the top is
the sample clustering line, the redder color indicates the higher
expression level. (C) DA score plot of different metabolic
pathways. The closer the score of a metabolic pathway is to 1.0,
the more the overall expression of the pathway tends to be
upregulated and vice versa, downregulated. The larger the dots are,
the higher the number of metabolites contained. The color indicates
the magnitude of the P-value, with redder reflecting a smaller
P-value. OGD/R, oxygen-glucose deprivation/reperfusion; RY-A,
Rubia yunnanensis alcohol extract; DA, differential
abundance; OGD/R, oxygen-glucose deprivation/reperfusion; RY-A,
Rubia yunnanensis alcohol extract; M, OGD/R group; Y, RY-A
group. |
To observe overall alterations in metabolism, the
present study captured the average and overall changes of all
metabolites in pathways based on differential enrichment scores. As
shown in Fig. 3C, the differential
metabolites between the RY-A and OGD/R groups interacted with each
other mainly through different pathways, such as ‘phenylalanine,
tyrosine and tryptophan biosynthesis’, ‘prolactin signaling
pathway’ and ‘amphetamine addiction’ and were collectively
affecting body systems such as ‘amino acid metabolism’, ‘endocrine
system’ and ‘immune system’.
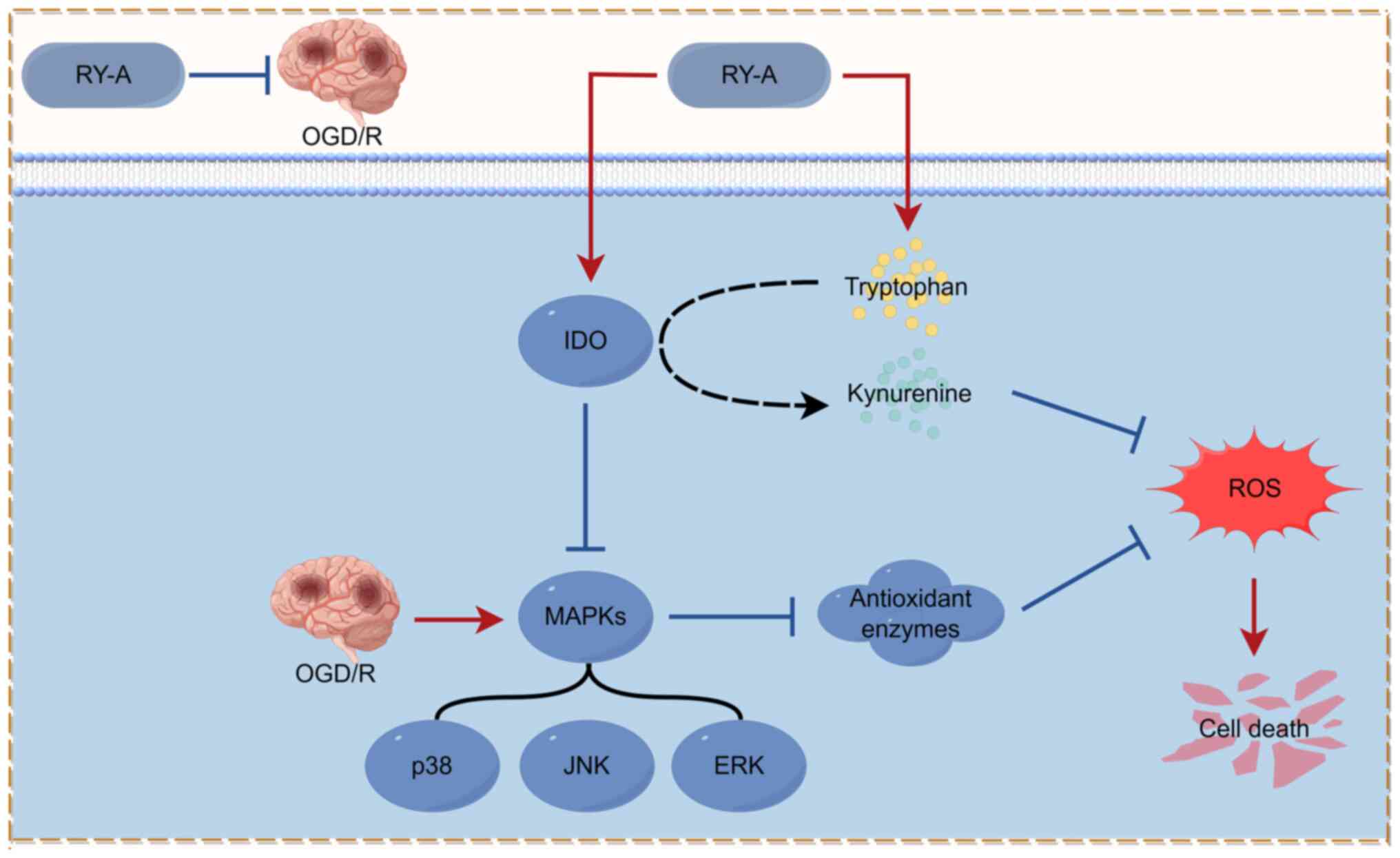
Effect of RY-A treatment on
indoleamine 2,3-dioxygenase (IDO) expression and MAPK signaling
pathways
The metabolite with the most significant difference
following RY-A treatment was tryptophan. Indoleamine
2,3-dioxygenase is one of the major rate-limiting enzymes of the
tryptophan-kynurenine pathway, which catalyzes the production of
kynurenine from the essential amino acid L-tryptophan in mammalian
extrahepatic tissues to exert an antioxidant effect (23). The signaling pathways of MAPKs (p38,
ERK and JNK) have been highly correlated with oxidative
stress-induced cell death (24-26).
Also, an increase in kynurenine to tryptophan ratio after drug
intervention in the metabolomics analysis of the present study
implied elevated IDO activity (27). In the present study, western
blotting was performed at two separate times to detect the
expression levels of IDO proteins and major proteins of MAPK
signaling pathways (p38, p-p38, ERK, p-ERK, JNK, p-JNK). Western
blotting showed that the expression of IDO proteins was
significantly downregulated and the phosphorylation of p-p38, p-ERK
and p-JNK proteins was significantly upregulated in OGD/R treated
HT22 cells compared with controls. In contrast, RY-A treatment
enhanced OGD/R-induced expression of IDO in HT22 cells and
attenuated the effect of OGD/R on the expression of major proteins
of MAPK signaling pathways (Fig.
4A-F). These results suggest that RY-A may ameliorate ischemic
injury in brain tissue by activating IDO protein expression and
inhibiting the phosphorylation of MAPKs.
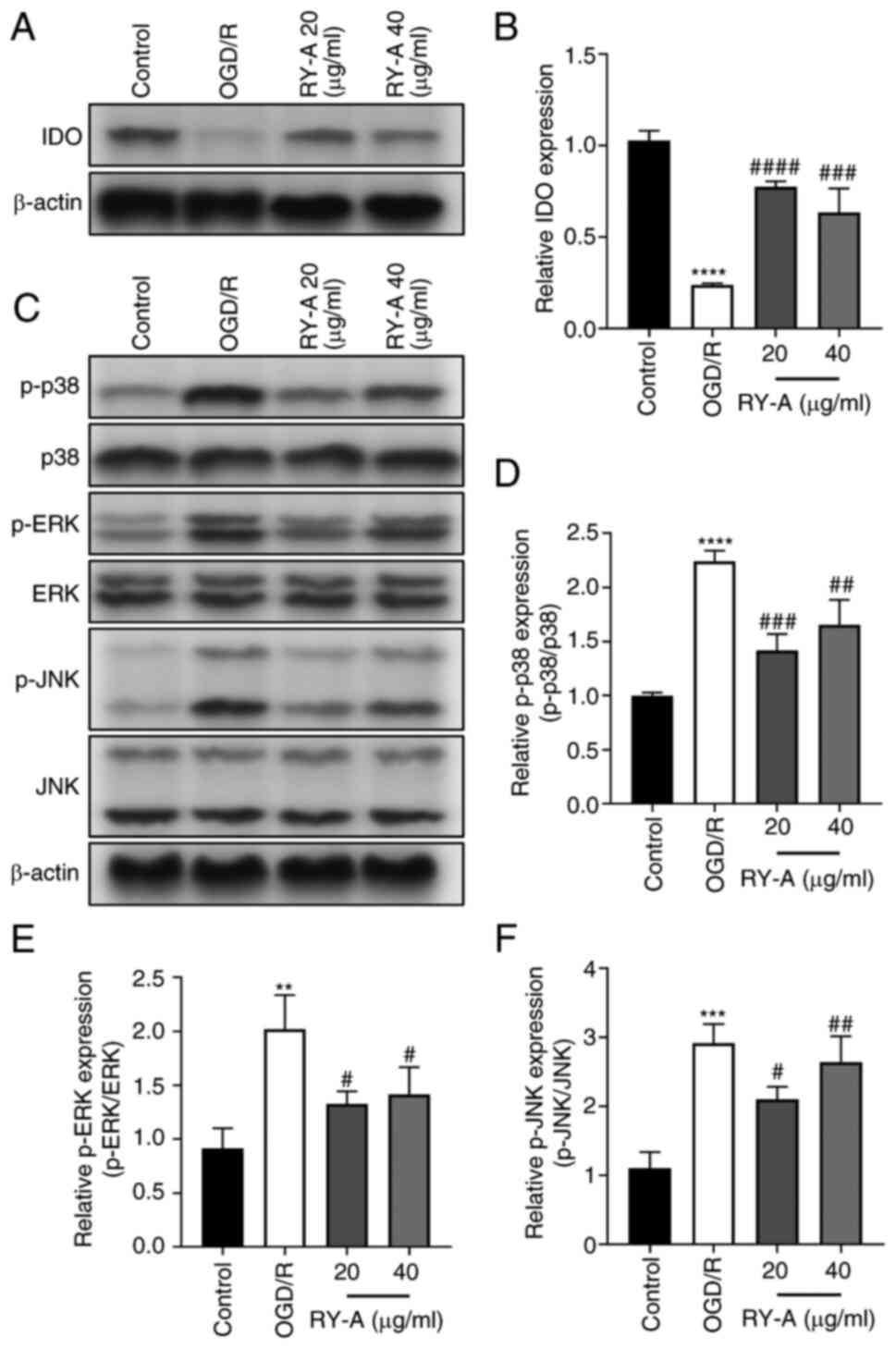 | Figure 4Effect of RY-A on IDO and MAPKs
pathway protein expression in OGD/R-induced HT22 cells. Western
blotting was performed to detect the expression of (A and B) IDO,
(C and D) p-p38, (C and E) p-ERK, (C and F) p-JNK in OGD/R-induced
HT22 cells and actin was used as a reference. Results are expressed
as mean ± SD (n=3). **P<0.01,
***P<0.001 and ****P<0.0001 vs. Control
group, #P<0.05, ##P<0.01,
###P<0.001, ####P<0.0001 vs. OGD/R
group. RY-A, Rubia yunnanensis alcohol extract; IDO,
indoleamine 2,3-dioxygenase; OGD/R, oxygen-glucose
deprivation/reperfusion; p-, phosphorylated. |
Discussion
Our previous research used high-performance liquid
chromatography to analyze the two main components, Rubia
yunnanensis naphthoquinones A and Rubia yunnanensis
quinone B, of the drug RY-A used in the present study (28). This analysis ensured that the
quality of RY-A was up to standard. Mouse hippocampal neuron HT22
cells are widely used in the in vitro study of mechanisms of
neurodegenerative disease (29).
The present study investigated the neuroprotective effects of RY-A
on HT22 cells by simulating an in vitro CIRI model through
OGD/R. Metabolic pathways underlying the effects of RY-A were
examined by employing a metabolomics approach. It was found that
OGD/R induced oxidative stress and reduced viability of HT22 cells
by downregulating IDO enzyme activity and upregulating
phosphorylation levels of MAPKs. The present study showed that
10-80 µg/ml RY-A had no toxic effects on normal HT22 cells and
tended to increase cell viability. Experiments to observe the
effects of RY-A on the viability of OGD/R treated HT22 cells found
that 10-20 µg/ml RY-A dose-dependently increased cell viability,
with 20 µg/ml providing the optimal concentration. Further
metabolomics analyses were performed with 20 µg/ml RY-A.
OGD/R is closely associated with the aggravation of
oxidative stress. The massive production of ROS with oxidative
stress is one of the most critical aspects in the pathomechanisms
of CIRI. Excessive oxidative stress also leads to lipid
peroxidation as well as severe inflammation of neuronal cell
membranes and organelles (30). The
hallmark substances of oxidative stress include MDA, SOD and
GSH-Px. The present study found that RY-A protected HT22 cells from
OGD/R-induced oxidative damage and stress through the
downregulation of ROS, NO and MDA oxidation products and the
upregulation of SOD and GSH-Px antioxidant enzyme activities.
The metabolomics analysis showed that the protective
effect of RY-A against OGD/R-induced cellular damage was mainly
through affecting metabolites such as amino acids and their
derivatives and organic acids. A total of 20 differential
metabolites were obtained by comparative analysis of the RY-A and
OGD/R groups, in which RY-A upregulated five metabolites
(L-tryptophan, xanthine, uracil 5-carboxylic acid,
guanidino-succinic acid and L-dopa) and downregulated 15
metabolites (ornithine, eicosapentaenoic acid-d5, isosafrole,
inosine, adenosine 3'-monophosphate, 5'-methylthioadenosine,
glycerol 3-phosphate, D-mannose, palmitoyl-L-carnitine,
L-carnitine, cortexolone, L-cysteine, L-phenylalanine, shikimic
acid and allocholic acid). Among them, L-tryptophan levels showed
the most significant change. Tryptophan is an essential amino acid
required for protein synthesis and >95% of free tryptophan is
converted to kynurenine mainly by IDO and
tryptophan-2,3-dioxygenase rate-limiting enzymes (31). Tryptophan metabolism has been
associated with a variety of neurodegenerative diseases, including
Alzheimer's disease and a metabolite of tryptophan, kynurenine, has
a recognized neuroprotective effect (32-34).
A review has shown that another metabolite of tryptophan,
5-hydroxytryptamine (serotonin), is involved in the regulation of
neurological functions, such as behavior, mood and cognition
(35). Supplementation of dietary
tryptophan has a positive effect on neurodevelopment and
improvement of sleep (36,37). Wang et al (38) found that oral administration of
tryptophan significantly improved depression and anxiety-like
behaviors via the gut-brain-axis pathway in chronically and mildly
stressed mice. Oğuz et al (39) found that carotid artery infusion of
tryptophan-containing histidine-tryptophan-ketoglutaric acid
solution significantly reduced the degree of ischemia and the
number of apoptotic cells in rabbits with CIRI and effectively
enhanced the resistance of the brain to ischemic conditions.
Xanthine is a purine base commonly used as a mild
stimulant. In vitro and in vivo studies have found
that xanthine promotes the division and proliferation of mammary
stem cells and enhances the activity of stem and progenitor cells
(40,41). Guanidino-succinic acid, a
constituent of normal urine, has been found to inhibit
lipopolysaccharide-induced production of tumor necrosis factor-α by
human monocytes, while the presence of 2-guanidino-succinic acid,
methyl guanidine and guanidinoacetic acid limited the
chemiluminescence produced by phorbol myristate acetate-treated
HL-60 cells (42).
L-dopa is a hydroxylated form of complex amino acids
that forms various catecholamines, such as dopamine, which is
closely related to the modulation of neurological function. L-dopa
pretreatment significantly attenuates dexamethasone-induced
ischemia and promotes angiogenesis (43). L-dopa treatment increases the number
of oligodendrocyte transcription factor 2-positive cells in the
infarcted region of experimental animals and stimulates post-stroke
plasticity (44). In addition,
conversion of L-dopa to dopamine by decarboxylase inhibits
H2O2-induced oxidative stress (45).
Ornithine is a nonprotein amino acid that serves as
a core metabolite in the urea cycle. Zanatta et al (46) showed that ornithine treatment
reduces the survival of astrocytes in the cerebral cortex and
impairs mitochondrial function and antioxidant capacity of cells by
decreasing mitochondrial membrane potential and GSH-Px
concentrations. In addition, Fonteh et al (47) showed that increased ornithine in
urine may serve as one of the biomarkers of Alzheimer's disease.
Isosafrole, a natural product, has been evaluated as a Group III
carcinogen by the International Agency for Research on Cancer
(48). Adenosine 3'-monophosphate
is a nucleotide that is reported to inhibit the proliferation of
pro-glomerular vascular smooth muscle cells and thylakoid cells via
either adenosine or A2B receptors (49). 5'-Methylthioadenosine is a
sulfur-containing adenosine that is considered a tumor metabolite
whose dysregulated metabolism negatively affects the immune
function of a variety of immune cells, including T and NK cells
(50). Glycerol 3-phosphate is an
endogenous metabolite produced by the cytoplasmic glycerol
3-phosphate dehydrogenase pathway. Zhang et al (51) found that
H2O2-induced oxidative stress in glucose
medium increases glycerol 3-phosphate synthesis and stimulates the
expression of glycerol kinase GlcA. D-Mannose is an important sugar
involved in the glycosylation of several cellular molecules. A
recent report (52) found that
serum mannose levels can be used as an early biomarker for ovarian
cancer. Palmitoyl-L-carnitine is a fatty acid metabolite. Recently,
Guan et al (53) conducted
the first clinical study using metabolomics and machine learning
approaches and identified ornithine and palmitoyl-L-carnitine as
potential biomarkers for screening lung cancer. Other research
showed that an increase in palmitoyl-L-carnitine promotes fatty
acid oxidation, which exacerbates oxidative stress and induces
insulin resistance (54). Shikimic
acid, a monomeric compound derived from anise, is reported to
increase the risk of gastric and esophageal cancer (55-57).
Ma and Ning (58) showed that
shikimic acid promotes in vitro cell proliferation through
the miR-300-mediated NF-κB pathway in MCF-7 and T47D cell models of
breast cancer. Allocholic acid is a bile acid found in vertebrates.
It has been found that patients with cirrhosis and brain tumors
contain higher levels of allocholic acid compared with normal
subjects (59,60). There appears to be no
pharmacological studies related to uracil 5-carboxylic acid and
only one chemical study of its enzymatic carboxylation has been
reported (61). In summary, the
underlying mechanisms of RY-A actions in the treatment of cerebral
ischemia may be related to modulation of the aforementioned
metabolites, in terms of anti-oxidant, anti-apoptotic and
anti-inflammatory properties and the promotion of cell
proliferation and angiogenesis.
The current study found that the metabolite with the
most significant change in levels among the aforementioned
metabolites was tryptophan, which plays a key role in cerebral
ischemia. IDO enzymes are the main rate-limiting enzymes for
tryptophan degradation (23). IDO
enzymes consist of two members, IDO1 and IDO2. Studies have shown
that overexpressed IDO proteins inhibits intracellular ROS levels
and DNA damage in a rat model of ischemic injury and prevents
neuronal cell death via free radical scavengers (62,63).
Studies in models of certain cancers and neuronal diseases have
also demonstrated that IDO expression exerts potent antioxidant
functions, thereby significantly inhibiting oxidative
stress-induced cell death (64,65).
In addition, it is well known that the MAPK (p38, ERK and JNK)
signaling pathways are highly associated with oxidative
stress-induced cell death (24-26).
It has been reported that Tat-IDO-1 transduced into HT22 cells
reduces cell death, ROS production, DNA fragmentation and
H2O2-induced MAPK phosphorylation (23). Other studies have also shown that
overexpression of IDO proteins inhibits the activation of MAPK
signaling pathways under conditions of oxidative stress (66,67).
Therefore, the present study investigated the mechanism by which
RY-A increases tryptophan by examining the expression of IDO and
MAPK pathway proteins. The current study showed that RY-A increased
IDO protein expression levels and inhibited the protein
phosphorylation of MAPKs (p38, ERK and JNK) in OGD/R treated HT22
cells, suggesting that RY-A may inhibit cerebral ischemia through
IDO-mediated inhibition of MAPK phosphorylation.
Hashimoto et al (68) compared the differences in
osteoarthritis (OA) development between lectin-like oxidized
low-density lipoprotein receptor-1 (LOX-1) knockout mice and
wild-type mice by replicating a mouse knee OA model of medial
meniscus instability using LOX-1 knockout mice. They analyzed the
expression of LOX-1 in articular cartilage and bone remnants by
immunohistochemistry and double staining techniques and assessed
the expression levels of molecular markers (e.g., Runt-related
transcription factor 2 and type X collagen) associated with LOX-1.
The findings revealed that LOX-1-deficient mice were resistant to
osteoarthritis and also confirmed the involvement of the
LOX-1/low-density lipoprotein (oxLDL) system in cartilage
degeneration and bone capsule formation in the development of OA
(69,70). It was concluded that the binding of
ox-LDL to LOX-1 increases the production of ROS, which subsequently
causes oxidative stress (71). It
is well known that various lifestyle-related diseases such as
hyperlipidemia, hypertension, OA and diabetes involve oxidative
stress and these conditions can lead to atherosclerosis via the
LOX-1/ox-LDL system (68,72-74).
Therefore, ROS will mainly affect those organs that contain a large
amount of blood such as the liver (75), lungs (76), heart and brain (77), as well as tissues and organs that
contain synovial fluid such as the joints, stomach (78), brain (79) and medulla oblongata (80).
In conclusion, the present study demonstrated the
effectiveness of RY-A in treating HT22 cells in an in vitro
OGD/R model and its possible molecular mechanisms. Following
OGD/R-induced injury, RY-A significantly reduced the content of
oxidized products such as ROS, NO and MDA and enhanced the
activities of SOD and GSH-Px antioxidant enzymes, thus ameliorating
damage from oxidative stress and reducing neuronal death. It was
hypothesized that the neuroprotective effect of RY-A may be
associated with regulation of the tryptophan metabolic pathway and
MAPK signaling pathways during OGD/R (Fig. 5). These findings suggested that RY-A
has some potential therapeutic value for patients with CIRI. The
oxygen-glucose deprivation/reoxygenation (OGD/R) model is widely
used for ex vivo studies of cerebral ischemia-reperfusion
injury secondary to ischemic stroke. While neurons in the
hippocampal region of the brain are vulnerable sites of cerebral
ischemia/reperfusion, they are commonly used in studies of cerebral
ischemia/reperfusion injury. The HT22 cell line, an immortalized
mouse hippocampal neuronal cell line, is a subclone of the mouse T4
cell line, which lacks an important receptor of the
N-methyl-D-aspartic acid receptor, there is almost a consensus that
HT22 cells are not excitable and this cell has rightly become one
of the most commonly used in vitro cell models for oxidative
stress-related studies (81,82).
Therefore, the hippocampal neuron HT22 cells were selected as the
main research object in the present study.
There are still some limitations to the present
study, such as no animal-related in vivo experiments. RY-A
is a crude extract and it is not known whether it also has a
protective effect on cortex-associated neuronal cells and the
efficacy of its main monomer constituents needs further in-depth
study. Related studies on the major compounds in Rubia
yunnanensis will be covered in subsequent studies by the group
and follow-up studies may study relevant cells in the cortex.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Xingdian Talent
Support Program-Special for Young Talent (grant no.
XDYC-QNRC-2022-0284), the High-level Talents Projects of Yunnan
University of Chinese Medicine-Fifth Level Talents, the National
Administration of Traditional Chinese Medicine High-level Key
Discipline Construction Project ‘Minority medicine (Dai Medicine)’
(grant no. Zyyzdxk-2023193) and the Scientific Research Foundation
of The Education Department of Yunnan Province (grant no.
2024Y372).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. Metabolomics data have
been deposited to the EMBL-EBI MetaboLights database (DOI:
10.1093/nar/gkad1045, PMID:37971328) with the identifier MTBLS9545
(URL: www.ebi.ac.uk/metabolights/MTBLS9545).
Authors' contributions
JC was the main contributor in writing the
manuscript. JC, LY and XD designed the experiments. JC and GL were
responsible for the statistical and data analysis. JC and XD
drafted and revised the original manuscript. PC and GL confirmed
the authenticity of all the original data. PC and XD interpreted
the results of the study and gave the final approval for the
forthcoming version. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lemmerman LR, Balch MHH, Moore JT,
Alzate-Correa D, Rincon-Benavides MA, Salazar-Puerta A, Gnyawali S,
Harris HN, Lawrence W, Ortega-Pineda L, et al:
Nanotransfection-based vasculogenic cell reprogramming drives
functional recovery in a mouse model of ischemic stroke. Sci Adv.
7(eabd4735)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mahmood A and Muir KW: Tenecteplase or
alteplase: What is the thrombolytic agent of the future? Curr Treat
Options Neurol. 24:503–513. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bayraktutan U: Endothelial progenitor
cells: Potential novel therapeutics for ischaemic stroke. Pharmacol
Res. 144:181–191. 2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mokin M, Ansari SA, McTaggart RA, Bulsara
KR, Goyal M, Chen M and Fraser JF: Society of NeuroInterventional
Surgery. Indications for thrombectomy in acute ischemic stroke from
emergent large vessel occlusion (ELVO): Report of the SNIS
standards and guidelines committee. J Neurointerv Surg. 11:215–220.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Banjara M and Ghosh C: Sterile
neuroinflammation and strategies for therapeutic intervention. Int
J Inflam. 2017(8385961)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gülke E, Gelderblom M and Magnus T: Danger
signals in stroke and their role on microglia activation after
ischemia. Ther Adv Neurol Disord.
11(1756286418774254)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jurcau A and Ardelean IA: Molecular
pathophysiological mechanisms of ischemia/reperfusion injuries
after recanalization therapy for acute ischemic stroke. J Integr
Neurosci. 20:727–744. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu JY, Liu FY, Liu SX, Xie LZ, Li J, Ma YT
and Han FJ: Plant-derived Chinese medicine monomers on ovarian
cancer via the Wnt/β-catenin signaling pathway: Review of
mechanisms and prospects. J Oncol. 2021(6852867)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yi S, Lin Q, Zhang X, Wang J, Miao Y and
Tan N: Selection and validation of appropriate reference genes for
quantitative RT-PCR analysis in Rubia yunnanensis diels
based on transcriptome data. Biomed Res Int.
2020(5824841)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang R, Miao Y, Chen L, Yi S and Tan N:
De novo transcriptome analysis reveals putative genes involved in
anthraquinone biosynthesis in Rubia yunnanensis. Genes
(Basel). 13(521)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Luo L, Lü L, Lu Y, Zhang L, Li B, Guo K,
Chen L, Wang Y, Shao Y and Xu J: Effects of hypoxia on progranulin
expression in HT22 mouse hippocampal cells. Mol Med Rep.
9:1675–1680. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zelena E, Dunn WB, Broadhurst D,
Francis-McIntyre S, Carroll KM, Begley P, O'Hagan S, Knowles JD and
Halsall A: HUSERMET Consortium. Wilson ID and Kell DB: Development
of a robust and repeatable UPLC-MS method for the long-term
metabolomic study of human serum. Anal Chem. 81:1357–1364.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Want EJ, Masson P, Michopoulos F, Wilson
ID, Theodoridis G, Plumb RS, Shockcor J, Loftus N, Holmes E and
Nicholson JK: Global metabolic profiling of animal and human
tissues via UPLC-MS. Nat Protoc. 8:17–32. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rasmussen JA, Villumsen KR, Ernst M,
Hansen M, Forberg T, Gopalakrishnan S, Gilbert MTP, Bojesen AM,
Kristiansen K and Limborg MT: A multi-omics approach unravels
metagenomic and metabolic alterations of a probiotic and synbiotic
additive in rainbow trout (Oncorhynchus mykiss). Microbiome.
10(21)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Navarro-Reig M, Jaumot J, Garcia-Reiriz A
and Tauler R: Evaluation of changes induced in rice metabolome by
Cd and Cu exposure using LC-MS with XCMS and MCR-ALS data analysis
strategies. Anal Bioanal Chem. 407:8835–8847. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wishart DS, Tzur D, Knox C, Eisner R, Guo
AC, Young N, Cheng D, Jewell K, Arndt D, Sawhney S, et al: HMDB:
The human metabolome database. Nucleic Acids Res. 35 (Database
Issue):D521–D526. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Horai H, Arita M, Kanaya S, Nihei Y, Ikeda
T, Suwa K, Ojima Y, Tanaka K, Tanaka S, Aoshima K, et al: MassBank:
A public repository for sharing mass spectral data for life
sciences. J Mass Spectrom. 45:703–714. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sud M, Fahy E, Cotter D, Brown A, Dennis
EA, Glass CK, Merrill AH Jr, Murphy RC, Raetz CR, Russell DW and
Subramaniam S: LMSD: LIPID MAPS structure database. Nucleic Acids
Res. 35 (Database Issue):D527–D532. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Abdelrazig S, Safo L, Rance GA, Fay MW,
Theodosiou E, Topham PD, Kim DH and Fernández-Castané A: Metabolic
characterisation of Magnetospirillum gryphiswaldense MSR-1 using
LC-MS-based metabolite profiling. RSC Adv. 10:32548–32560.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ogata H, Goto S, Sato K, Fujibuchi W, Bono
H and Kanehisa M: KEGG: Kyoto encyclopedia of genes and genomes.
Nucleic Acids Res. 27:29–34. 1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Thévenot EA, Roux A, Xu Y, Ezan E and
Junot C: Analysis of the human adult urinary metabolome variations
with age, body mass index, and gender by implementing a
comprehensive workflow for univariate and OPLS statistical
analyses. J Proteome Res. 14:3322–3335. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xia J and Wishart DS: Web-based inference
of biological patterns, functions and pathways from metabolomic
data using MetaboAnalyst. Nat Protoc. 6:743–760. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Park JH, Kim DW, Shin MJ, Park J, Han KH,
Lee KW, Park JK, Choi YJ, Yeo HJ, Yeo EJ, et al: Tat-indoleamine
2,3-dioxygenase 1 elicits neuroprotective effects on ischemic
injury. BMB Rep. 53:582–587. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jia L, Chen Y, Tian YH and Zhang G: MAPK
pathway mediates the anti-oxidative effect of chicoric acid against
cerebral ischemia-reperfusion injury in vivo. Exp Ther Med.
15:1640–1646. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kwon SH, Hong SI, Kim JA, Jung YH, Kim SY,
Kim HC, Lee SY and Jang CG: The neuroprotective effects of Lonicera
japonica THUNB. Against hydrogen peroxide-induced apoptosis via
phosphorylation of MAPKs and PI3K/Akt in SH-SY5Y cells. Food Chem
Toxicol. 49:1011–1019. 2011.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu N, Cai C, Zhou A, Zhao X, Xiang Y and
Zeng C: Schisandrin B prevents hind limb from
ischemia-reperfusion-induced oxidative stress and inflammation via
MAPK/NF-κB pathways in rats. Biomed Res Int.
2017(4237973)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mallik SB, Mudgal J, Kinra M, Hall S,
Grant GD, Anoopkumar-Dukie S, Nampoothiri M, Zhang Y and Arora D:
Involvement of indoleamine 2,3-dioxygenase (IDO) and brain-derived
neurotrophic factor (BDNF) in the neuroprotective mechanisms of
ferulic acid against depressive-like behaviour. Metab Brain Dis.
38:2243–2254. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Li G, Cheng J, Yang L, Chen P and Duan X:
Ethanol extract of Rubia yunnanensis inhibits carotid
atherosclerosis via the PI3K/AKT signaling pathway. Biomed Rep.
20(19)2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhao J, Liu L, Zhang L, Lv J, Guo X, Li X
and Zhao T: Sodium ferulate attenuates high-glucose-induced
oxidative injury in HT22 hippocampal cells. Exp Ther Med.
18:2015–2020. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li S, Jiang D, Ehlerding EB, Rosenkrans
ZT, Engle JW, Wang Y, Liu H, Ni D and Cai W: Intrathecal
administration of nanoclusters for protecting neurons against
oxidative stress in cerebral ischemia/reperfusion injury. ACS Nano.
13:13382–13389. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pathakoti K, Goodla L, Manubolu M and
Tencomnao T: Metabolic alterations and the protective effect of
punicalagin against glutamate-induced oxidative toxicity in HT22
cells. Neurotox Res. 31:521–531. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kincses ZT, Toldi J and Vécsei L:
Kynurenines, neurodegeneration and Alzheimer's disease. J Cell Mol
Med. 14:2045–2054. 2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Campesan S, Green EW, Breda C,
Sathyasaikumar KV, Muchowski PJ, Schwarcz R, Kyriacou CP and
Giorgini F: The kynurenine pathway modulates neurodegeneration in a
Drosophila model of Huntington's disease. Curr Biol. 21:961–966.
2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Meier-Stephenson FS, Meier-Stephenson VC,
Carter MD, Meek AR, Wang Y, Pan L, Chen Q, Jacobo S, Wu F, Lu E, et
al: Alzheimer's disease as an autoimmune disorder of innate
immunity endogenously modulated by tryptophan metabolites.
Alzheimers Dement (N Y). 8(e12283)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Roth W, Zadeh K, Vekariya R, Ge Y and
Mohamadzadeh M: Tryptophan metabolism and gut-brain homeostasis.
Int J Mol Sci. 22(2973)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Carhart-Harris RL and Nutt DJ: Serotonin
and brain function: A tale of two receptors. J Psychopharmacol.
31:1091–1120. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cowen P and Sherwood AC: The role of
serotonin in cognitive function: Evidence from recent studies and
implications for understanding depression. J Psychopharmacol.
27:575–583. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang D, Wu J, Zhu P, Xie H, Lu L, Bai W,
Pan W, Shi R, Ye J, Xia B, et al: Tryptophan-rich diet ameliorates
chronic unpredictable mild stress induced depression- and
anxiety-like behavior in mice: The potential involvement of
gut-brain axis. Food Res Int. 157(111289)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Oğuz S, Aşgün HF and Büyük B:
Effectiveness of brain protection with
histidine-tryptophan-ketoglutarate solutions. Heart Surg Forum.
23:E510–E516. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Deng WW and Ashihara H: Profiles of purine
metabolism in leaves and roots of Camellia sinensis seedlings.
Plant Cell Physiol. 51:2105–2118. 2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kawasaki H, Shimaoka M, Usuda Y and
Utagawa T: End-product regulation and kinetic mechanism of
guanosine-inosine kinase from Escherichia coli. Biosci Biotechnol
Biochem. 64:972–979. 2000.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Glorieux GL, Dhondt AW, Jacobs P, Van
Langeraert J, Lameire NH, De Deyn PP and Vanholder RC: In vitro
study of the potential role of guanidines in leukocyte functions
related to atherogenesis and infection. Kidney Int. 65:2184–2192.
2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Price CF, Burgess DJ and Kastellorizios M:
l-DOPA as a small molecule surrogate to promote angiogenesis and
prevent dexamethasone-induced ischemia. J Control Release.
235:176–181. 2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Talhada D, Marklund N, Wieloch T, Kuric E
and Ruscher K: Plasticity-enhancing effects of levodopa treatment
after stroke. Int J Mol Sci. 22(10226)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lotsios NS, Arvanitis N, Charonitakis AG,
Mpekoulis G, Frakolaki E, Vassilaki N, Sideris DC and
Vassilacopoulou D: Expression of human L-dopa decarboxylase (DDC)
under conditions of oxidative stress. Curr Issues Mol Biol.
45:10179–10192. 2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zanatta A, Rodrigues MDN, Amaral AU, Souza
DG, Quincozes-Santos A and Wajner M: Ornithine and homocitrulline
impair mitochondrial function, decrease antioxidant defenses and
induce cell death in menadione-stressed rat cortical astrocytes:
Potential mechanisms of neurological dysfunction in HHH syndrome.
Neurochem Res. 41:2190–2198. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Fonteh AN, Harrington RJ, Tsai A, Liao P
and Harrington MG: Free amino acid and dipeptide changes in the
body fluids from Alzheimer's disease subjects. Amino Acids.
32:213–224. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
No authors listed. Overall evaluations of
carcinogenicity: An updating of IARC Monographs volumes 1 to 42.
IARC Monogr Eval Carcinog Risks Hum Suppl. 7:1–440. 1987.PubMed/NCBI
|
|
49
|
Zschocke S, Heidrich V and Kuhlmann E:
Mapping the spontaneous EEG in focal disorders. EEG EMG Z
Elektroenzephalogr Elektromyogr Verwandte Geb. 21:233–242.
1990.PubMed/NCBI(In German).
|
|
50
|
Jacobs B, Schlögl S, Strobl CD, Völkl S,
Stoll A, Mougiakakos D, Malmberg KJ, Mackensen A and Aigner M: The
Oncometabolite 5'-deoxy-5'-methylthioadenosine blocks multiple
signaling pathways of nk cell activation. Front Immunol.
11(2128)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang C, Gu H, Ren Y and Lu L:
GlcA-mediated glycerol-3-phosphate synthesis contributes to the
oxidation resistance of Aspergillus fumigatus via decreasing the
cellular ROS. Fungal Genet Biol. 149(103531)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chen Y, Yao Q, Zhang L and Zeng P: HPLC
for simultaneous quantification of free mannose and glucose
concentrations in serum: Use in detection of ovarian cancer. Front
Chem. 11(1289211)2023.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Guan X, Du Y, Ma R, Teng N, Ou S, Zhao H
and Li X: Construction of the XGBoost model for early lung cancer
prediction based on metabolic indices. BMC Med Inform Decis Mak.
23(107)2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Turnbull PC, Dehghani AC, Theriau CF,
Connor MK and Perry CGR: Synergistic activation of mitochondrial
metabolism and the glutathione redox couple protects HepG2
hepatocarcinoma cells from palmitoylcarnitine-induced stress. Am J
Physiol Cell Physiol. 317:C1324–C1329. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Brown AC: Cancer related to herbs and
dietary supplements: Online table of case reports. Part 5 of 5. J
Diet Suppl. 15:556–581. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Jones RS, Ali M, Ioannides C, Styles JA,
Ashby J, Sulej J and Parke DV: The mutagenic and cell transforming
properties of shikimic acid and some of its bacterial and mammalian
metabolites. Toxicol Lett. 19:43–50. 1983.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Stavric B and Stoltz DR: Shikimic acid.
Food Cosmet Toxicol. 14:141–145. 1976.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ma X and Ning S: Shikimic acid promotes
estrogen receptor(ER)-positive breast cancer cells proliferation
via activation of NF-κB signaling. Toxicol Lett. 312:65–71.
2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Fine JM, Vrieze LA and Sorensen PW:
Evidence that petromyzontid lampreys employ a common migratory
pheromone that is partially comprised of bile acids. J Chem Ecol.
30:2091–2110. 2004.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ohdoi C, Nyhan WL and Kuhara T: Chemical
diagnosis of Lesch-Nyhan syndrome using gas chromatography-mass
spectrometry detection. J Chromatogr B Analyt Technol Biomed Life
Sci. 792:123–130. 2003.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Palmatier RD, McCroskey RP and Abbott MT:
The enzymatic conversion of uracil 5-carboxylic acid to uracil and
carbon dioxide. J Biol Chem. 245:6706–6710. 1970.PubMed/NCBI
|
|
62
|
Liu H, Liu L and Visner GA: Nonviral gene
delivery with indoleamine 2,3-dioxygenase targeting pulmonary
endothelium protects against ischemia-reperfusion injury. Am J
Transplant. 7:2291–2300. 2007.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Taguchi A, Hara A, Saito K, Hoshi M, Niwa
M, Seishima M and Mori H: Localization and spatiotemporal
expression of IDO following transient forebrain ischemia in
gerbils. Brain Res. 1217:78–85. 2008.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Freewan M, Rees MD, Plaza TSS, Glaros E,
Lim YJ, Wang XS, Yeung AW, Witting PK, Terentis AC and Thomas SR:
Human indoleamine 2,3-dioxygenase is a catalyst of physiological
heme peroxidase reactions: Implications for the inhibition of
dioxygenase activity by hydrogen peroxide. J Biol Chem.
288:1548–1567. 2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Taniguchi T, Sono M, Hirata F, Hayaishi O,
Tamura M, Hayashi K, Iizuka T and Ishimura Y: Indoleamine
2,3-dioxygenase. Kinetic studies on the binding of superoxide anion
and molecular oxygen to enzyme. J Biol Chem. 254:3288–3294.
1979.PubMed/NCBI
|
|
66
|
Grant RS, Naif H, Espinosa M and Kapoor V:
IDO induction in IFN-gamma activated astroglia: A role in improving
cell viability during oxidative stress. Redox Rep. 5:101–104.
2000.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Guillemin GJ, Smythe G, Takikawa O and
Brew BJ: Expression of indoleamine 2,3-dioxygenase and production
of quinolinic acid by human microglia, astrocytes, and neurons.
Glia. 49:15–23. 2005.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Hashimoto K, Mori S, Oda Y, Nakano A,
Sawamura T and Akagi M: Lectin-like oxidized low density
lipoprotein receptor 1-deficient mice show resistance to
instability-induced osteoarthritis. Scand J Rheumatol. 45:412–422.
2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Akagi M, Kanata S, Mori S, Itabe H,
Sawamura T and Hamanishi C: Possible involvement of the oxidized
low-density lipoprotein/lectin-like oxidized low-density
lipoprotein receptor-1 system in pathogenesis and progression of
human osteoarthritis. Osteoarthritis Cartilage. 15:281–290.
2007.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Hashimoto K, Oda Y, Nakamura F, Kakinoki R
and Akagi M: Lectin-like, oxidized low-density lipoprotein
receptor-1-deficient mice show resistance to age-related knee
osteoarthritis. Eur J Histochem. 61(2762)2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Kattoor AJ, Pothineni NVK, Palagiri D and
Mehta JL: Oxidative stress in atherosclerosis. Curr Atheroscler
Rep. 19(42)2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Mitra S, Deshmukh A, Sachdeva R, Lu J and
Mehta JL: Oxidized low-density lipoprotein and atherosclerosis
implications in antioxidant therapy. Am J Med Sci. 342:135–142.
2011.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Li X, Tang X, Liu B, Zhang J, Zhang Y, Lv
H, Liu D, Mehta JL and Wang X: LOX-1 deletion attenuates myocardial
fibrosis in the aged mice, particularly those with hypertension.
Front Cardiovasc Med. 8(736215)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Qiu J, Liu J, Tian L, Yu J, Duan Q, Liu Y,
Zhao W, Si H, Lu X and Zhang Q: Knockdown of LOX-1 ameliorates bone
quality and generation of type H blood vessels in diabetic mice.
Arch Biochem Biophys. 752(109870)2024.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Paradies G, Paradies V, Ruggiero FM and
Petrosillo G: Oxidative stress, cardiolipin and mitochondrial
dysfunction in nonalcoholic fatty liver disease. World J
Gastroenterol. 20:14205–14218. 2014.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Valavanidis A, Vlachogianni T, Fiotakis K
and Loridas S: Pulmonary oxidative stress, inflammation and cancer:
Respirable particulate matter, fibrous dusts and ozone as major
causes of lung carcinogenesis through reactive oxygen species
mechanisms. Int J Environ Res Public Health. 10:3886–3907.
2013.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Songbo M, Lang H, Xinyong C, Bin X, Ping Z
and Liang S: Oxidative stress injury in doxorubicin-induced
cardiotoxicity. Toxicol Lett. 307:41–48. 2019.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Chen X, Zhao Y, Luo W, Chen S, Lin F,
Zhang X, Fan S, Shen X, Wang Y and Liang G: Celastrol induces
ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in
gastric cancer cells. Theranostics. 10:10290–10308. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Jimenez-Blasco D, Almeida A and Bolaños
JP: Brightness and shadows of mitochondrial ROS in the brain.
Neurobiol Dis. 184(106199)2023.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Braga VA, Colombari E and Jovita MG:
Angiotensin II-derived reactive oxygen species underpinning the
processing of the cardiovascular reflexes in the medulla oblongata.
Neurosci Bull. 27:269–274. 2011.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Majrashi M, Altukri M, Ramesh S,
Govindarajulu M, Schwartz J, Almaghrabi M, Smith F, Thomas T,
Suppiramaniam V, Moore T, et al: β-hydroxybutyric acid attenuates
oxidative stress and improves markers of mitochondrial function in
the HT-22 hippocampal cell line. J Integr Neurosci. 20:321–329.
2021.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Yan W, Guo T, Liu N, Cui X, Wei X, Sun Y,
Hu H and Chen L: Erythropoietin ameliorates cognitive deficits by
improving hippocampal and synaptic damage in streptozotocin-induced
diabetic mice. Cell Signal. 106(110614)2023.PubMed/NCBI View Article : Google Scholar
|