Introduction
Overweight and obesity have become serious global
problems in recent years, with a recent report estimating that 1.5
billion adults worldwide are overweight, among whom over 200
million men and almost 300 million women are obese (1). Overweight and obesity were once
considered problems associated with high-income nations, but their
incidence is now increasing in low and middle-income countries, a
trend that is attributed to chronic alcoholism, food
overconsumption and sedentary lifestyle. Overweight and obese
individuals face the risk of numerous chronic diseases, including
heart disease, diabetes and some types of cancer, as well as
psychological and social problems.
Overweight and obesity, as well as the
noncommunicable diseases associated with their development, are
largely preventable. Several steps can be taken not only at the
individual and societal levels but also at the molecular level to
prevent and treat overweight and obesity. At the individual level,
individuals are able to limit their consumption of fat and sugar,
increase their consumption of fruits and vegetables, and engage in
regular physical activity. At the societal level, governments and
the food industry can promote healthy diets. At the molecular
level, researchers identify and/or develop agents that prevent or
treat obesity. A recent research focus has been the development of
anti-obesity agents derived from natural products, a process that
has several safety and economic advantages over the development of
pharmaceuticals via years of investment in drug development. Such
research has been bolstered by recent reports that traditional
herbal extracts have been found effective in reducing body weight
in vivo (2,3).
Among these extracts, the dried aromatic flower buds
of Syzygium aromaticum (L.) Merr. & Perry (family
Myrtaceae), commonly known as cloves, have been reported to be
useful in treating dental pain, headache and respiratory disorders
in Asian countries (4).
Phytochemical studies indicate that eugenol, a member of the
phenylpropanoid class of chemical compounds, and its derivatives
are responsible for the unique aroma of S. aromaticum, and
it has been found to have antimicrobial, insecticidal, antioxidant,
antitumor, anti-inflammatory and anaesthetic properties (5–10).
In spite of these findings and reports of its utility in several
medicinal applications, S. aromaticum has not been
investigated for its potential as an anti-obesity agent or food
additive. Therefore, the present study investigated the effect of
S. aromaticum ethanol extract (SAE) on a mouse model in
which obesity had been induced by administering a high-fat diet
(HFD).
Materials and methods
Materials
Anti-PPARγ (sc-7273), SREBP-1 (sc-366) and CD36
(sc-13572) were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA). Antibodies against FAS (#3180), and β-actin (#4967)
were purchased from Cell Signaling (Danvers, MA, USA). Insulin
(I5500), isobutylmethylxanthine (I7018), dexamethasone (D4902) and
Oil Red O (O0625) were purchased from Sigma-Aldrich (St. Louis, MO,
USA). Serum and tissue triglyceride (TG), total cholesterol (TC)
and high-density lipoprotein-cholesterol (HDL-C) kits were
purchased from Wako Chemicals (Osaka, Japan). An insulin ELISA kit
was obtained from Shibayagi Co. (Gunma, Tokyo, Japan). A leptin
ELISA kit was purchased from R&D Systems (Minneapolis, MN,
USA).
Preparation of extract
Dried flower buds of Syzygium aromaticum were
purchased from the Omni Herb Company (Seoul, South Korea) and were
extracted three times with 70% ethanol at room temperature
overnight. The extracts were combined and concentrated using a
rotary evaporator, and freeze-dried under a vacuum and dissolved in
dimethyl sulfoxide (DMSO).
Cell culture and differentiation
The 3T3-L1 cells were cultured in Dulbecco’s
modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA) with
1% penicillin-streptomycin and 10% bovine calf serum at 37°C in 5%
CO2. The cells were seeded at a density of
4×105 cells/well into a 6-well plate. At 2 days
post-confluence (day 0), the cells were exposed to an MDI solution
containing 0.5 mM of 3-IBMX, 1 μM of dexamethasone and 1
μg/ml of insulin for 2 days in DMEM with 10% fetal bovine
serum (FBS). Following induction, cells were switched to a solution
containing DMEM medium supplemented with 1%
penicillin-streptomycin, 10% FBS and 167 nm insulin for 2 days. The
cells were maintained in a postdifferentiation medium (DMEM
containing 1% penicillin-streptomycin and 10% FBS) and changed
every 2 days. To examine the effects of SAE on the differentiation
of preadipocytes into adipocytes, the cells were treated with
various concentrations of SAE at 2 days post confluence (day
0).
Oil Red O staining
For quantification, cells were fixed with 10%
neutral formalin for 1 h at room temperature, washed with
phosphate-buffered saline (PBS) and then stained for 1 h with 0.5%
Oil Red O in 60% isopropanol. After washing with distilled water,
the stained cells were observed under a microscope. The stained
lipid droplets were then extracted with isopropanol for
quantification by measuring its absorbance at 490 nm.
Animal models
Male C57BL/6J mice aged 4 weeks were obtained from
Orient Bio Inc. (Seoul, South Korea) and acclimatized to laboratory
conditions consisting of a 12:12-h light-dark cycle, 24°C and 55%
humidity for 1 week. At 5 weeks old the mice were then randomly
divided into three groups; a group fed the American Institute of
Nutrition AIN-76A diet (normal group), a group fed a HFD (HFD
group) and a group fed a HFD supplemented with 0.5% (w/w) SAE (HFD
+ SAE group) for 9 weeks. The experimental diets were prepared by
supplementing the basic AIN-76 diet with 20% fat and 0.5%
cholesterol (w/w). Body weight and average daily food intake were
measured weekly. The care and use of the animals followed our
institutional and national guidelines and all experimental
procedures were approved by the Korea Food Research Institute
Animal Care and Use Committee (KFRI-IACUC, #2010-0011).
Sample preparation and procedures
At nine weeks after consuming the experimental
diets, the mice were subjected to fasting for 12 h and then
sacrificed. Blood samples were collected from the abdominal aorta,
centrifuged at 1,000 × g for 15 min and stored at −80°C. The
measurement of serum TG, TC and HDL-C levels was performed with the
appropriate kit. The measurement of serum insulin and leptin
concentrations was performed according to the manufacturer’s
instructions of the mouse insulin and leptin ELISA kits.
Epididymal, perirenal and liver tissues were excised, weighed and
stored at −80°C until use. Liver and epididymal fat pads were
either fixed in 4% formalin solution and processed for histological
examination or snap frozen in liquid nitrogen and stored at −80°C
until protein and RNA extraction.
To determine total lipid, TG and TC levels in the
liver, the livers of each group were homogenized in 5 ml NaCl.
After 20 ml of Folch solution (chloroform:methanol = 2:1) was
subsequently added to each homogenate, the resulting solution was
incubated at 4°C for 12 h. The samples were centrifuged for 10 min
at 1,000 × g before the organic layer was removed and dried using a
Speed Vac. The resulting pellet was dissolved in ethyl alcohol
containing 25% Triton X-100 and then assayed for TG, TC and HDL-C
levels using indicated commercial kits.
Histological analysis
The liver and epididymal fat pads were fixed in 4%
neutral-buffered formalin, embedded in paraffin, sliced at a
thickness of 5 μm and stained with hematoxylin and eosin
(H&E) stain. The pathological changes were assessed and
photographed under an Olympus BX-51 microscope (Olympus, Tokyo,
Japan).
Western blotting
The isolated liver, adipose tissue and cell extracts
were prepared in PRO-PREP protein extraction solution (Intron
Biotechnology, Gyeonggi, South Korea) using an MP 40 system (MD
Biosciences, St. Paul, MN, USA). The extracts were centrifuged at
15,000 rpm for 20 min at 4°C. The supernatants were boiled with a
sodium dodecyl sulfate (SDS)-loading buffer, loaded onto
Tris-glycine gels, transferred to polyvinylidene fluoride (PVDF)
membranes and visualized with a chemiluminescence reagent (Amersham
Bioscience, Piscataway, NJ, USA).
RNA extraction and real-time PCR
Total RNA was isolated from the liver using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. After cDNA was prepared from isolated
RNA using a Maxime RT premix (oligo dT primer), the obtained cDNA
was used as a template for real-time PCR with the Light Cycler 480
system (Roche, Basel, Switzerland). The specific primers were as
follows: Fas sense AGAGATCCCGAGACGCTTCT, antisense
GCCTGGTAGGCATTCTGTAGT; Srebp sense GCAGCC ACCATCTAGCCTG, antisense
CAGCAGTGAGTCTGCCTT GAT; Pparg sense TCGCTGATGCACTGCCTATG, antisense
GAGAGGTCCACAGAGCTGATT; Ppara sense AACATC GAGTGTCGAATATGTGG,
antisense AGCCGAATAGTT CGCCGAAAG; and β-actin sense AATACCCCAGCCATG
TGTGT, antisense ATGGGCACTGTGTGTGACC. The level of mRNA was
expressed as the ratio of signal intensity for each gene relative
to β-actin.
Statistical analysis
Results are expressed as the mean ± SE, and the
differences among the three groups were calculated using an
analysis of multiple ranges and a one-way variance test using
GraphPad Prism4 software (San Diego, CA, USA).
Results
SAE inhibits cell differentiation of
3T3-L1 preadipocytes
The effect of SAE treatment on lipid metabolism
in vitro was examined by investigating its effect on the
adipocyte differentiation on 3T3-L1 cells, a well-established
preadipocyte cell line used for studying the conversion of
preadipocytes into adipocytes. After differentiation with or
without SAE, the resulting mass of accumulated intracellular lipid
was stained with Oil Red O dye and quantified. As shown in Fig. 1, a dose-dependent reduction in
lipid accumulation was observed in the cells treated with SAE,
suggesting that SAE inhibits adipocyte transcription factors during
adipocyte differentiation.
SAE inhibits obesity in HFD-fed mice
On the basis of an in vitro study that
revealed the inhibition of adipocyte differentiation by SAE
treatment, it was hypothesized that SAE may exert anti-obesity
effects in vivo. To test this hypothesis, the body weights
of the three groups of mice were compared after 9 weeks of feeding.
Although no significant difference was observed between the HFD and
HFD + SAE groups with respect to daily food intake, the overall
body weight, as well as the liver, epididymal and retrorenal
fat-pad weights, of the HFD + SAE group were found to have
increased to a significantly lesser extent than that of the HFD
group (P<0.05; Table I and
Fig. 2), suggesting that SAE
supplementation had effectively reduced fat accumulation in HFD-fed
obese mice.
 | Table IEffect of SAE on body weight, liver
and WAT in mice fed a high-fat diet for 9 weeks. |
Table I
Effect of SAE on body weight, liver
and WAT in mice fed a high-fat diet for 9 weeks.
| Growth
parameters | N | HFD | HFD + SAE |
|---|
| Body weight gain
(g) | 8.57±0.75b | 16.60±1.72a | 9.15±0.97b |
| Liver (g/100 bw) | 3.61±0.20c | 4.94±0.15a | 4.08±0.07b |
| Epididymal fat pad
(g/100 g bw) | 1.97±0.04b | 4.56±0.20a | 2.03±0.15b |
| Perirenal fat pad
(g/100 g bw) | 0.93±0.07b | 2.14±0.10a | 1.05±0.14b |
| Food intake
(g/day) | 2.91±0.06 | 3.05±0.06 | 2.95±0.04 |
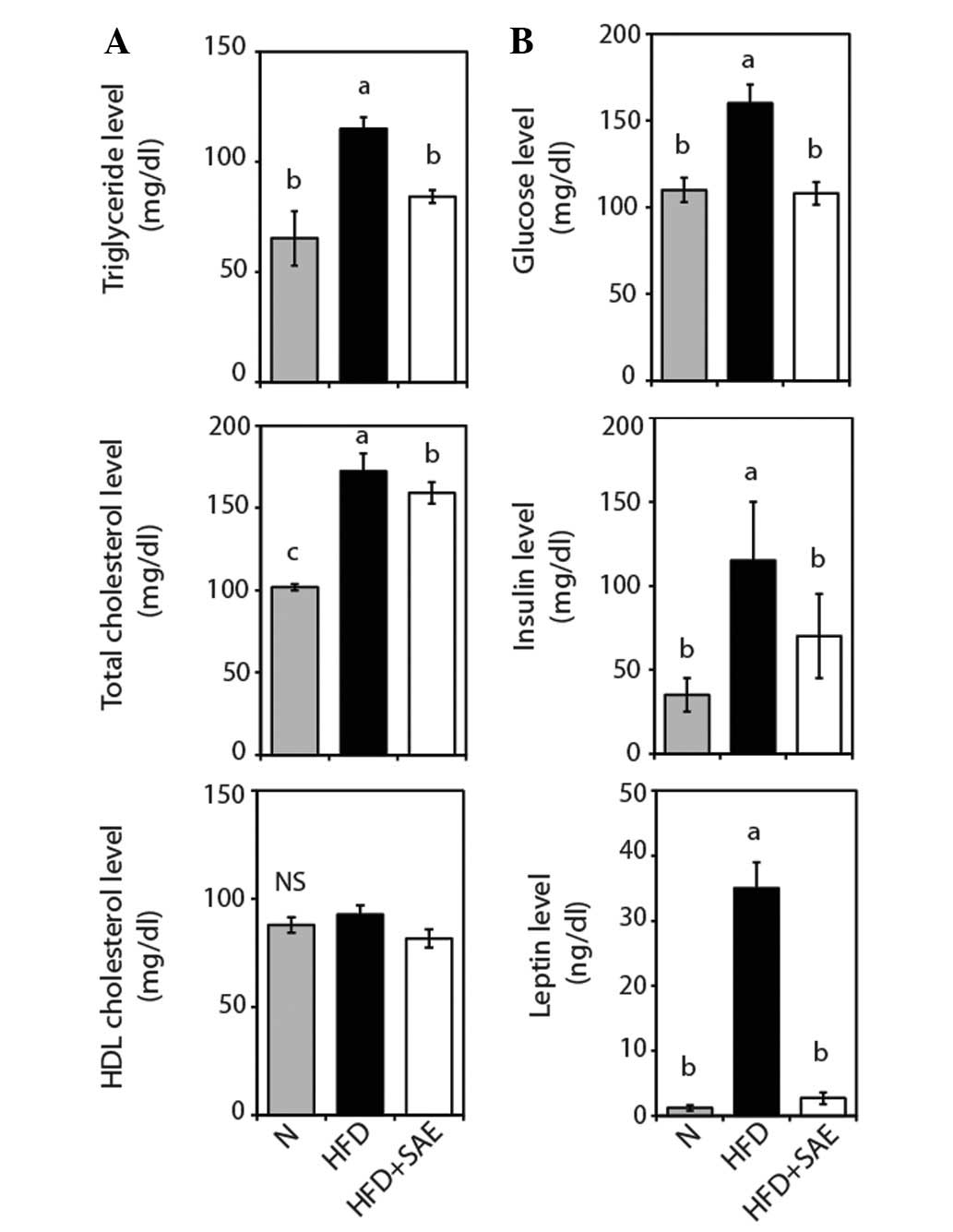
SAE decreases serum TG and TC levels in
HFD-fed mice
After 9 weeks of treatment, the TG and TC levels of
the HFD + SAE group were found to be significantly lower, 33.4 and
8.8% lower, respectively, than those of the HFD group (Fig. 3A), suggesting that SAE exerts a
hypolipidemic effect in HFD-fed mice.
SAE reduces serum glucose, insulin and
leptin levels
The levels of not only serum glucose and insulin but
also of leptin, an adipocyte-derived hormone, of the HFD group were
found to be significantly higher than those of the normal group
(Fig. 3B) and the HFD + SAE group,
suggesting that the increased levels due to HFD feeding had been
significantly decreased by SAE supplementation. These findings
indicate that lower serum glucose and insulin levels may be due to
lower insulin resistance.
SAE suppresses adipogenesis in HFD-fed
mice
SAE supplementation was found to have reduced the
size of the epididymal fat pad in HFD-fed mice (Fig. 4A). Investigation of the manner in
which SAE supplementation reduces the epididymal fat pad weight
revealed that it suppresses the expression of several proteins
related to adipogenesis genes, including SREBP-1, PPARγ and FAS
(Fig. 4B), indicating that it
exerts an anti-obesity effect by inhibiting adipogenesis.
SAE attenuates fatty liver in HFD-fed
mice
Comparison of the effect of SAE supplementation on
the extent of lipid accumulation in the livers of the HFD + SAE and
HFD groups via H&E staining indicated reduced white coloring
and fewer lipid droplets and uncolored circles in the livers of the
HFD + SAE group compared with the HFD group (Fig. 5A). The total lipid, TC and TG
levels in the HFD + SAE group were found to have decreased by 45.2,
37.5 and 39.5%, respectively (Fig.
5B). These results suggest that SAE supplementation had
attenuated the increase in hepatic TG and TC levels due to HFD
feeding.
To further explore the effect of SAE on lipogenesis,
the expression of proteins and mRNA involved in lipogenesis,
including that of SREBP-1 and its primary lipogenic target enzymes
(e.g. FAS), as well that of PPARγ and CD36, was analyzed. The
results indicated that HFD feeding of the experimental groups had
resulted in significant increases in the hepatic levels of
transcriptional factors integral to lipogenesis, including PPARγ,
SREBP-1, FAS and CD36 (Fig. 5C).
Comparison of these factors in the HFD and HFD + SAE groups
indicated that SAE supplementation had reduced their levels in the
HFD + SAE group, providing further evidence of the anti-lipogenic
effects of SAE. Consistent with the inhibition of PPARγ by SAE
supplementation, the supplementation appeared to have also
downregulated the mRNA levels of Pparg (Fig. 5D), as well as to have significantly
activated Ppara, a regulator of fatty acid β-oxidation.
Collectively, these findings indicate that SAE supplementation
inhibits the development of HFD-induced fatty liver through
regulation of lipogenic genes.
Discussion
Numerous herbs and spices have been found to reduce
blood glucose levels and body weight. Among these herbs, S.
aromaticum (family Myrtaceae) is used not only as a culinary
supplement in several global cuisines but also as a traditional
medicinal aid in the treatment of dental pain, headache and
respiratory disorders in several Asian countries. Several studies
have reported that S. aromaticum exerts a variety of
pharmacological actions, including antioxidant, hypoglycemic and
anti-inflammatory activities (7,10,11).
However, to the best of our knowledge, no study has examined its
action on preventing obesity. Thus, this study and its primary
finding that the addition of SAE to the diet reduces body weight in
HFD-fed mice, thereby indicating its potential as a natural
anti-obesity supplement, are significant contributions to the
research and literature in this area.
Adipocyte differentiation of 3T3-L1 preadipocytes
has been found to depend on genes involved in the adipogenic
pathway during the differentiation process (12). In cultured adipocyte models, SAE
has been found to inhibit adipocyte differentiation in 3T3-L1 cells
in a dose-dependent manner. Although analysis of the results of the
in vitro assay performed in this study did not indicate that
SAE supplementation had affected the expression of
adipogenesis-related genes, analysis of the western blot results of
the in vivo assay revealed that it had strongly suppressed
an increase in WAT mass in HFD-fed mice via reduction of PPARγ and
SREBP-1 expression. Analysis of these results indicates that SAE
supplementation reduces WAT mass via a reduction in adipogenesis,
leading to lipid loss, and thus strongly suggests that SAE
supplementation prevents HFD-induced lipid accumulation in
epididymal adipose tissue through regulation of adipogenesis.
HFD feeding elevates serum leptin and insulin
levels, which are associated with energy expenditure, glucose
metabolism and fatty acid oxidation (13). SAE supplementation was found to
have significantly reduced serum leptin and insulin levels in
HFD-fed mice, indicating that SAE improves these HFD-induced
metabolic abnormalities. SAE supplementation was also found to have
decreased HFD-induced increases in liver weight and hepatic lipid,
and TC and TG levels, a finding supported by the results of H&E
staining, which indicated that SAE supplementation had reduced
hepatic lipid accumulation. Investigation of hepatic expression of
the genes regulating lipid metabolism revealed that SAE
supplementation had significantly prevented reduction of Ppara and
had suppressed the expression of both the lipogenic transcription
factor Pparg and lipogenic regulatory proteins, including CD36,
SREBP-1, and PPARγ, thereby regulating the expression of genes
promoting fatty acid oxidation. These findings suggest that SAE
supplementation improves hepatic metabolic parameters via
regulation of lipogenesis-related genes in the liver.
While these findings provide evidence that SAE
supplementation significantly inhibits obesity in HFD-fed mice,
further investigation of the active compounds responsible for
anti-obesity effects is required. Kuroda et al recently
reported that eugenol derivatives, including dehydrodieugenol and
dehydrodieugenol B exert potent hypoglycemic effects via PPARγ
activation in diabetic models (11). Contrary to the results of the in
vitro assay in this study, Kuroda et al found that these
constituents stimulate 3T3-L1 preadipocyte differentiation through
PPARγ activation, indicating that unknown compounds in SAE besides
dehydrodieugenol and dehydrodieugenol B may inhibit PPARγ
activation and exert anti-obesity effects through regulation of
adipogenic-related genes.
Collectively, the results of this study provide
solid evidence that SAE supplementation exerts an anti-obesity
effect on HFD-induced obese mice via regulation of genes related to
lipid metabolism in the liver and WAT in a manner that results in
the reduction of lipid accumulation. These results strongly support
the potential of SAE as an anti-obesity supplement in the
regulation of body weight.
Acknowledgements
This study was supported by the
National Platform Technology Project of the Ministry of Knowledge
Economy in Korea and the Korea Food Research Institute.
References
|
1.
|
World Health Organization: Fact sheet No.
311: obesity and overweight. http://www.who.int/mediacentre/factsheets/fs311/en/index.htmluri.
May 2012.
|
|
2.
|
Hasani-Ranjbar S, Nayebi N, Larijani B and
Abdollahi M: A systematic review of the efficacy and safety of
herbal medicines used in the treatment of obesity. World J
Gastroenterol. 15:3073–3085. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Yin J, Zhang H and Ye J: Traditional
Chinese medicine in treatment of metabolic syndrome. Endocr Metab
Immune Disord Drug Targets. 8:99–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Gurib-Fakim A: Medicinal plants:
traditions of yesterday and drugs of tomorrow. Mol Aspects Med.
27:1–93. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Chaieb K, Hajlaoui H, Zmantar T,
Kahla-Nakbi AB, Rouabhia M, Mahdouani K and Bakhrouf A: The
chemical composition and biological activity of clove essential
oil, Eugenia caryophyllata (Syzigium aromaticum L.
Myrtaceae): a short review. Phytother Res. 21:501–506. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Das S: Anticancer potential of flavouring
agents and their active principles – garlic, saffron, clove. Int J
Cancer Prev. 1:89–97. 2004.
|
|
7.
|
Abdel-Wahhab MA and Aly SE: Antioxidant
property of Nigella sativa (black cumin) and Syzygium
aromaticum (clove) in rats during aflatoxicosis. J Appl
Toxicol. 25:218–223. 2005.
|
|
8.
|
Fu Y, Zu Y, Chen L, Shi X, Wang Z, Sun S
and Efferth T: Antimicrobial activity of clove and rosemary
essential oils alone and in combination. Phytother Res. 21:989–994.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Park MJ, Gwak KS, Yang I, Choi WS, Jo HJ,
Chang JW, Jeung EB and Choi IG: Antifungal activities of the
essential oils in Syzygium aromaticum (L.) Merr. Et Perry
and Leptospermum petersonii Bailey and their constituents
against various dermatophytes. J Microbiol. 45:460–465.
2007.PubMed/NCBI
|
|
10.
|
Tanko Y, Mohammed A, Okasha MA, Umar AH
and Magaji RA: Anti-nociceptive and anti-inflammatory activities of
ethanol extract of Syzygium aromaticum flower bud in Wistar
rats and mice. Afr J Tradit Complement Altern Med. 5:209–212.
2008.PubMed/NCBI
|
|
11.
|
Kuroda M, Mimaki Y, Ohtomo T, Yamada J,
Nishiyama T, Mae T, Kishida H and Kawada T: Hypoglycemic effects of
clove (Syzygium aromaticum flower buds) on genetically
diabetic KK-Ay mice and identification of the active ingredients. J
Nat Med. 66:394–399. 2012.
|
|
12.
|
Madsen L, Petersen RK, Sørensen MB,
Jørgensen C, Hallenborg P, Pridal L, Fleckner J, Amri EZ, Krieg P,
Furstenberger G, et al: Adipocyte differentiation of 3T3-L1
preadipocytes is dependent on lipoxygenase activity during the
initial stages of the differentiation process. Biochem J.
375:539–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Dube MG, Beretta E, Dhillon H, Ueno N,
Kalra PS and Kalra SP: Central leptin gene therapy blocks high-fat
diet-induced weight gain, hyperleptinemia, and hyperinsulinemia:
increase in serum ghrelin levels. Diabetes. 51:1729–1736. 2002.
View Article : Google Scholar : PubMed/NCBI
|