Introduction
Bladder outlet obstruction (BOO) developing
secondary to benign prostatic hyperplasia (BPH) is observed at
varying degrees in ∼80% of male patients over the age of 50 years
(1). Increased wall thickness
induced by BOO augments the bladder injury by resulting in cyclic
ischemia/reperfusion (I/R) injury during each urination (2). I/R injury in the bladder leads to the
generation of reactive oxygen species which may be a pathogenic
factor in the inflammation of the dysfunctional detrusor and thus
antioxidants may be beneficial for treating bladder dysfunction
secondary to BPH/BOO (3). Major
cytokines stimulating apoptosis include tumor necrosis factor
(TNF)-α, interleukin (IL)-6 and inducible nitric oxide synthase
(iNOS) which have been observed to be present at increased levels
in the inflammation of rat bladder (4,5).
Similarly, the permanence of membrane injury may
also prepare the ground for partial BOO-associated progressive
bladder dysfunction (2). Increased
bladder outlet resistance results in decreased compliance and
increased pressure, as well as a loss of bladder viscoelasticity
due to fibroproliferative development in the mucosa and collagen
accumulation. Collagen production is of critical significance in
this process. Although type III collagen is elastic in character,
collagen that is first produced by fibroblasts and then by muscle
cells destroys the viscoelastic character of the bladder, leading
to poor compliance (6).
Studies have been conducted on the efficacy of
αlipoic acid (ALA), an ideal, unique and universal antioxidant, on
bladder contractility in animal models of partial BOO. However, to
the best of our knowledge, no studies had been performed on the
effects of ALA on type I and III collagen ratios and iNOS mRNA gene
expression, as well as on cytokines such as TNF-α and IL-6 which
are involved in apoptosis and oxidative injury in the bladder. It
was proposed that these parameters should be investigated to
observe the I/R injury occurring in BOO and to evaluate the
efficacy of antioxidant therapy. Silymarin has anticarcinogenic,
antiapoptotic and antioxidant characteristics. It also favors cell
proliferation, while offering protection against neurotoxins and
cardiotoxins, with estrogenic and antiestrogenic effects (7,8).
Despite the protective character of silymarin in various tissues
and organs, no studies have been conducted with regard to its
effects on the bladder to the best of our knowledge.
We proposed that the use of antioxidants may avoid
the release of free oxygen radicals during reperfusion. The
decision was made to use ALA and silymarin as antioxidant agents in
the present study to provide protection against reperfusion injury,
the underlying cause of bladder injury.
Materials and methods
Animals
A total of 32 6-month-old female Sprague Dawley rats
weighing 200–250 g were used in the study. Approval was obtained
(dated 13.05.2009, no. 19/111) from the ESOGÜ Local Ethics
Committee on Animal Experiments for all procedures that were to be
performed on animals.
The rats were divided into four groups, each
consisting of eight rats: group I (sham), group II (BOO), group II
(control), group III (ALA, 100mg/kg, intraperitoneally) and group
IV (silymarin, 25mg/kg, intraperitoneally). ALA and silymarin were
dissolved in dimethyl sulfoxide. The BOO model was same as that of
Hashimoto et al(9). In the
sham group, placebo surgery with a median laparotomy was performed.
For the control group, a urethral catheter (1.2 mm) was inserted
into the bladder. Following the median laparotomy the bladder and
urethra were exposed. A 3-0 suture was placed in the urethrovesical
junction. Experimental BOO was completed by removing the urethral
catheter. The same procedure was performed for groups III and IV.
Medications were then administered intraperitoneally (IP) for one
month.
Experimental protocol
Once the experimental protocol had been completed,
the rats were administered general anesthesia with ketamine (75
mg/kg, IP) and xylazine (15 mg/kg, IP) following 12 h of fasting.
After reaching the bladder through a suprapubic incision with a
median laparotomy, the bladder tissue was resected proximal to the
sutured area from the ureterovesical junction. Intracardiac blood
samples were collected using a syringe. The rats were then
sacrificed by drawing excessive amounts of blood. The bladder
weight of each rat was recorded and the bladder was dissected into
three equal sections, from the bladder dome to bladder neck. One of
the sections was set aside for the histological investigation of
the changes in I/R, apoptosis and collagen-detrusor muscle ratio
parameters. The samples collected at this stage were evaluated
using various techniques such as direct microscopy, immune staining
and TdT-mediated biotin nick end-labeling (TUNEL) staining. Blood
samples and one-third of the bladder tissue were set aside for
investigating the biochemical parameters. I/R parameters, including
malondialdehyde (MDA), IL-6 and TNF-α levels, were measured in the
bladder tissue and the remaining one-third of the bladder tissue
was evaluated in terms of iNOS gene expression using real-time PCR
(RT-PCR).
Histological evaluation
The tissue samples obtained were immediately
immersion fixed in neutral-buffered formalin for 24 h prior to
processing and embedding in paraffin wax. Sections (5 μm) were cut
using a microtome and stained with Masson's trichrome to examine
the smooth muscle/collagen ratio. Slides were examined using an
Olympus BX51 light microscope and photographed with an Olympus DP70
camera. The slides were analyzed using a ocular micrometer with a
BAB Bs 200 ProP image analysis system.
Immunohistochemical evaluation of
collogen types I and III
The primary antibodies were rabbit polyclonal
collagen type I (Abcam, Cambridge, MA, USA; ab292) vs. mouse
monoclonal collagen type III (Abcam; ab6310). The collagen type I
and collagen type III analysis was performed by two examiners using
the modified scoring system introduced by Cör et al(10). A minimal staining reaction was
scored as (+), a medium staining reaction as (++) and a markedly
positive staining reaction as (+++). The data were analyzed and
compared in relation to the collagen type I and collagen type III
content in both groups.
TUNEL staining
The DNA fragmentation characteristic of apoptosis
was detected with a TUNEL assay and using an Apoptag Plus
Peroxidase In Situ Apoptosis Detection kit (S7101, Chemicon
International, Temecula, CA, USA). TUNEL evaluation was performed
by two blinded investigators. The evaluations were performed on 25
randomly selected sections at x40 magnification in at least 20
areas. TUNEL (+) cells were counted to establish the apoptotic
index. Apoptotic cells in the bladder sections of the experimental
groups were regarded as TUNEL (+) cells.
Bladder tissue and serum MDA levels
Lipid peroxidation was assayed by measuring MDA
levels on the basis of MDA reacted with thiobarbituric acid (TBA)
in tissue homogenates and serum samples, according to Ohkawa et
al(11). Serum and tissue MDA
levels were expressed in nmol/ml and nmol/mg protein, respectively.
Tissue protein levels were determined according to the Biuret
method.
IL-6 and TNF-α concentrations were measured using
enzyme-linked immunosorbent assays (Bender MedSystems, GmbH,
Vienna, Austria) according to the manufacturer's instructions using
a Victor/X3 microplate reader (Perkin Elmer, Waltham, MA, USA).
RNA extraction and RT-PCR
The mRNA level of iNOS relative to the housekeeping
gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was
determined using RT-PCR with a Taqman probe. Total RNA was
extracted from the renal tissue using the RNA stabilization reagent
(Qiagen, Hilden, Germany), according to the manufacturer's
instructions and quantified by measuring the absorbance at 260 nm
(Nanodrop1000; Thermo, Wilmington, DE, USA). Aliquots (20 μl) of
RNA from each group were used for the production of comple mentary
DNA (cDNA). The newly synthesized cDNA, stored at −20°C, was used
for the mRNA assay of the iNOS isoform with RT-PCR. cDNA (5 μl)
from each group was amplified in 20 μl reaction mixture. RT-PCR was
performed by monitoring in real time the increase in the amount of
Taqman probe using a Rotor-Gene 6000 RT-PCR system (Qiagen). The
oligonucleotide sequences of the cDNA primers were designed at Gene
Research Laboratories UK (Southampton, UK). The forward primer for
rat iNOS was 5′-CACCACCCTCCTTGTTCAAC-3′ and the reverse primer was
5′-CAATCCACAACTCGCTCCAA-3′. Sobajima et al also used GAPDH
(housekeeping gene) to normalize iNOS (target gene) data using
RT-PCR (12).
The RT-PCR thermal cycling conditions were as
follows: 15 min at 42°C and 10 min at 4°C for cDNA synthesis, 10
min at 95°C and 20 sec at 95°C, 30 sec at 55°C and 20 sec at 72°C
for 50 cycles. RT-PCR data were collected using a Rotor-Gene 6000
detec tion system. Cycle threshold (CT) values were determined
using automated threshold analysis. Primer quality (lack of
primer-dimer amplification) was confirmed using a melting curve.
Relative quantification of the gene expression was performed using
the standard curve method, constructed with serial dilutions of
control mRNA or RT-PCR amplicons. All experiments were performed in
triplicate. iNOS levels were standardized with GAPDH (ratio,
iNOS:GAPDH) to account for loading differences. Gene expression
levels (mRNA) were reported using the median as a point estimator
and the range of values.
Statistical analysis
SPSS 17.0 and SigmaStat 3.1 software were used for
the analyses of all data in the study. Continuous quantitative data
were expressed as number, mean and standard deviation, while
qualitative data were expressed as numbers and percentages.
Continuous data with normal distributions obtained from independent
measurements were analyzed using one-way ANOVA, while data with
non-normal distributions obtained from independent measurements
were analyzed with the Kruskal-Wallis test. Correlation analysis
was used to establish the correlation between variables. P<0.05
was considered to indicate statistically significant
differences.
Results
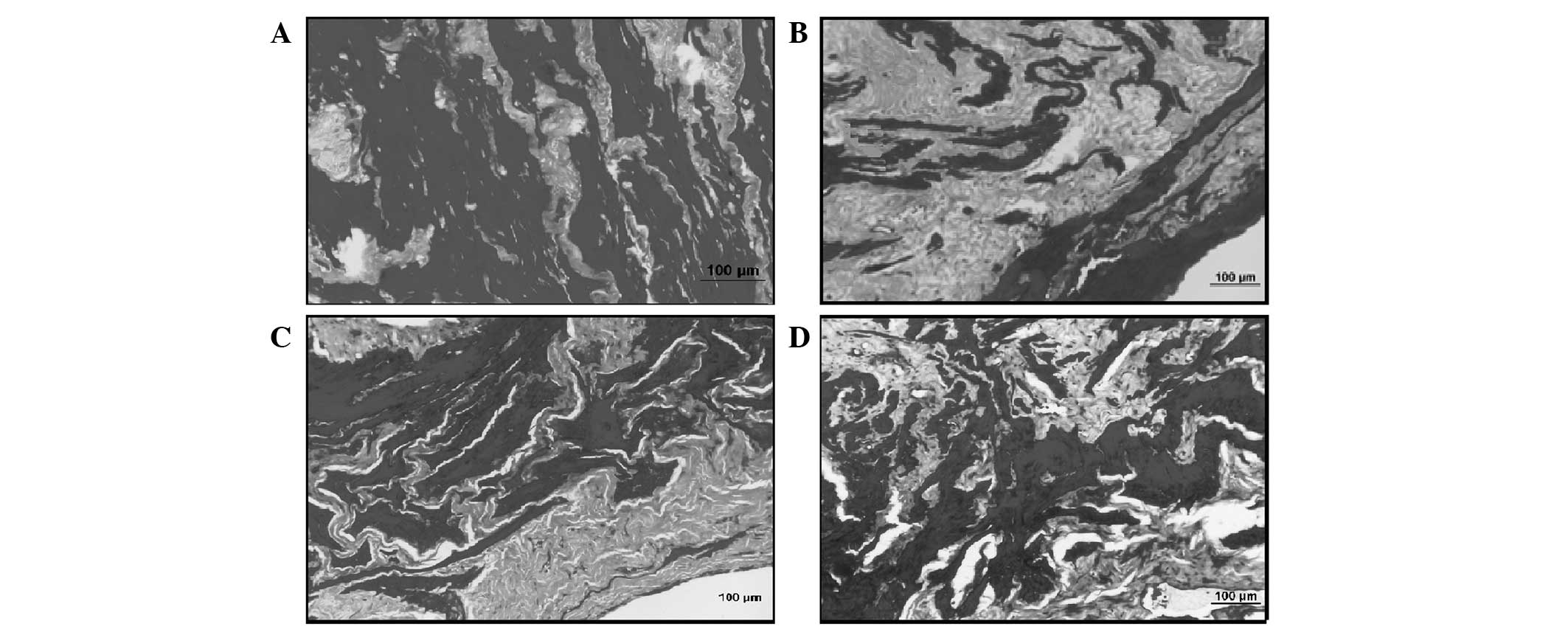
Histological results
Bladder sections of the control group revealed a
multi-layered transitional epithelium and lamina propria with a
loose connective tissue layer underneath forming the tunica mucosa.
In the tunica muscularis layer, smooth muscle bundles were
interrupted by connective tissue fibers. It was noted that the
percentages of smooth muscle bundles (stained black) and collagen
fibers (stained grey) were equal in the sections stained with
Masson's trichrome stain (Fig.
1A). A thicker bladder wall was observed in the sections of the
BOO-induced sham group, when compared with the controls. Mild
edema, separations and irregularities in the epithelium were noted.
The increase in connective tissue among the smooth muscle bundles
and in all layers was notable. However, there was a decrease in the
smooth muscle quantity. Furthermore, fibroblasts were observed to
have increased as well (Fig. 1B).
The results observed in the BOO + silymarin group were similar to
those noted in the control group. Dense collagen fibers were
observed among the smooth muscle bundles (Fig. 1C). By contrast, the BOO + ALA group
had relatively equal percentages of collagen and smooth muscle
bundles, similar to the sham group. However, edema and
irregularities were noted in the epithelium (Fig. 1D).
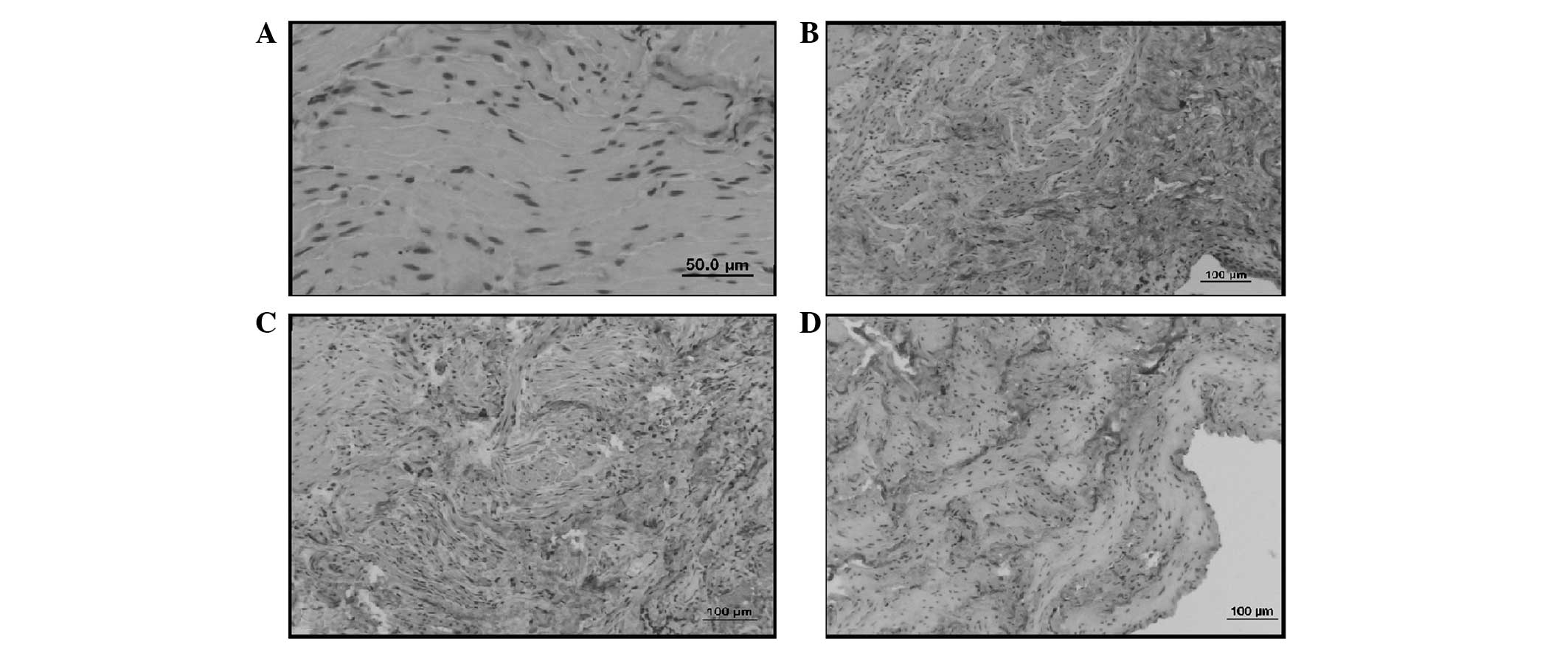
Immunohistochemical examination of types
I and III collagen
In the sham group, type I and type III collagen
staining was noted to be generally localized in the lamina propria
layer (Figs. 2A and 3A).
In the control group, type III collagen staining was
observed to be more marked than in the sham group. Type III
collagen staining was also more marked than type I collagen
staining in the sham group (Figs.
2B and 3B).
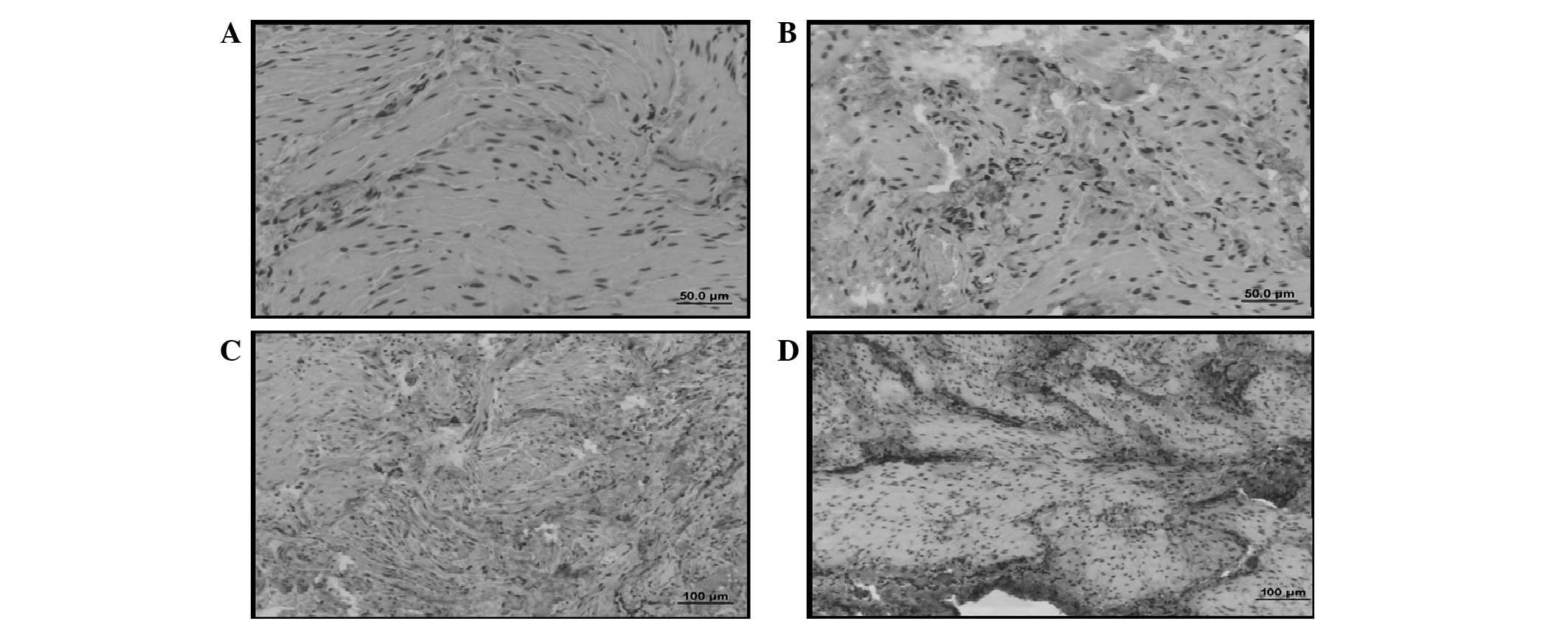
The type III collagen distribution in the BOO +
silymarin group was similar to that observed in the control group
(Figs. 2C and 3C). Silymarin was observed to be
ineffective at reducing the type III collagen levels.
BOO + ALA group was observed as having less intense
type I and type III collagen staining when compared with the
control group. The type III collagen staining was notably less
intense (Figs. 2D and 3D). Histological examination revealed
that ALA prevented I/R injury in BOO and reduced the amount of type
III collagen, maintaining bladder functions.
Evaluation of bladder weight
It was observed that the increase in bladder weight
in the control group was significantly higher compared with the
sham (P<0.001). The bladder weight was similar to that of the
sham group in the ALA and silymarin groups and the differences with
the control group were statistically significant (P<0.001;
Table I).
 | Table I.Comparison of experimental groups
according to bladder weights, TUNEL and iNOS levels. |
Table I.
Comparison of experimental groups
according to bladder weights, TUNEL and iNOS levels.
| Variable | Sham | Control | Silymarin | ALA |
|---|
| Bladder weights
(g) | 0.13±0.05 | 0.78±0.32c | 0.32±0.19f | 0.15±0.05f |
| TUNEL | 15.87±4.79 | 33.12±23.02c | 22.37±15.30 | 5.37± 2.92f |
| iNOS mRNA levels | 0.117±0.02 | 6.95±2.43c | 0.676±0.29 | 0.246±0.223f |
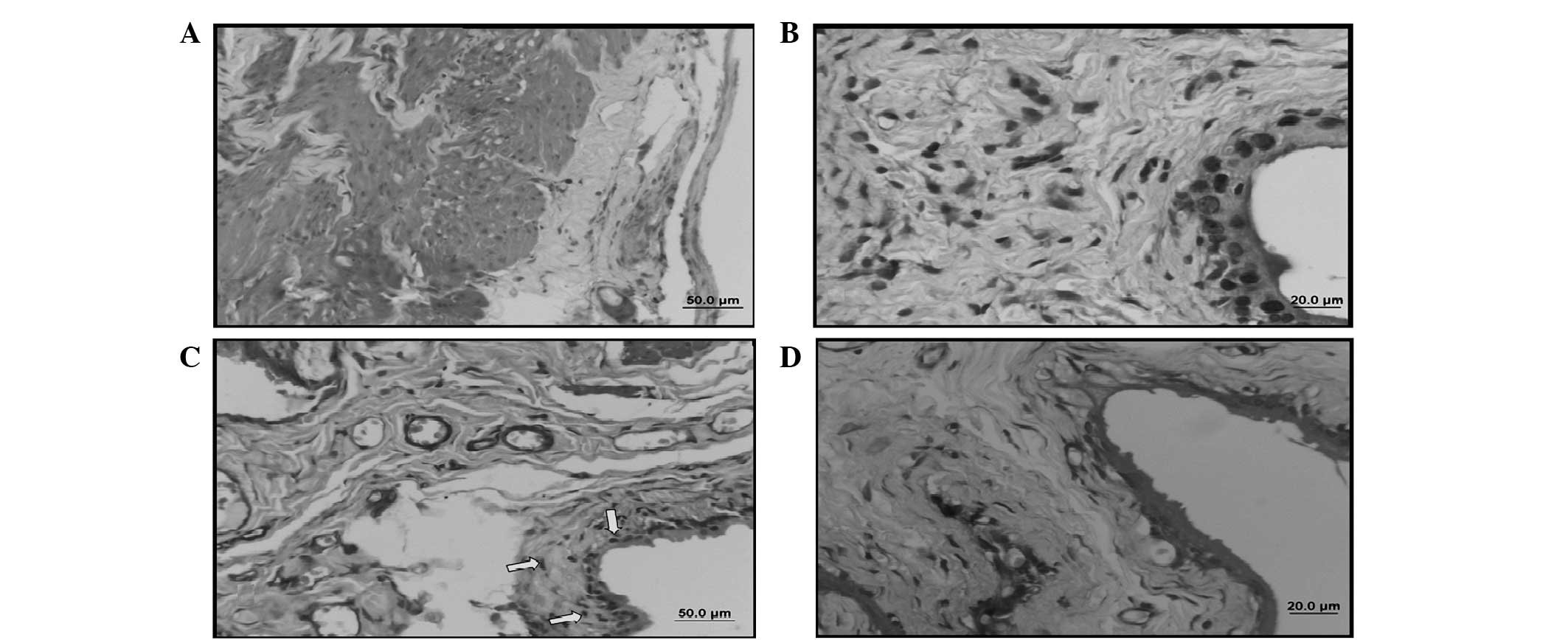
TUNEL staining
TUNEL was evaluated blindly by two examiners. In the
sham group, a limited number of TUNEL (+) cells was observed in the
epithelium and connective tissue. However, a large number of TUNEL
(+) cells was observed in the smooth muscle and epithelium in the
control group. The number of TUNEL (+) cells in the BOO + silymarin
group was similar in terms of quantity and localization to that of
the control group. By contrast, a low number of TUNEL (+) cells was
observed in the BOO + ALA group similar to that of the sham group.
The number of TUNEL (+) cells in the control group was higher than
that observed in the sham group and this increase was statistically
significant (P<0.001). Although there was a decrease in the
number of TUNEL (+) cells in the ALA- and silymarin-treated groups,
the decrease in the silymarin group was not statistically
significant. However, the decrease in the number of TUNEL (+) cells
was statistically significant in the ALA group (P<0.001). ALA
was revealed to be effective at decreasing apoptosis (Table II, Fig. 4).
 | Table II.Comparison of experimental groups
according to serum and tissue levels of MDA and TNF. |
Table II.
Comparison of experimental groups
according to serum and tissue levels of MDA and TNF.
| Variable | Sham | Control | Silymarin | ALA |
|---|
| Serum MDA | 3.1±0.59 | 4.08±0.89a | 1.96±0.47f | 1.36±0.77f |
| Tissue MDA | 5.57±1.63 | 7.53±1.00a | 2.60±1.22f | 3.21±1.21f |
| Serum TNF-α | 7.17±5.50 | 14.17±6.88b | 7.05±3.31e | 5.31±2.39e |
| Tissue TNF-α | 80.27±40.23 |
127.62±44.80a | 74.02±19.69a | 62.31±37.41a |
Expression of iNOS mRNA in the urinary
bladder
Due to the abnormal distribution of the parameters,
the Kruskal Wallis test was used and a significant difference in
the gene expression levels of iNOS was observed between the groups
(P<0.001). To identify the significance of the differences
between the groups, the Tukey HSD test was used and iNOS levels in
the ALA group were observed to be slightly decreased compared with
the control group (P<0.001). However, in the silymarin group
this decrease was not significant (P>0.05; Table I).
Tissue and serum MDA and TNF-α
levels
In the ALA and silymarin groups, the decreases in
the MDA and TNF-α levels were significant compared with the control
group (P<0.001 and P<0.01, respectively). Serum TNF-α levels
were observed to be significantly decreased in the ALA and
silymarin groups (P<0.05; Table
II).
Discussion
The most significant factor that initiates
I/R-associated tissue injury is the presence of free oxygen
radicals. The destructive effects of radicals cause cell death as a
result of apoptosis and necrosis (3). Similarly, ongoing membrane injury may
also be the basis of progressive bladder dysfunction associated
with BOO (2). Therefore, while
designing the present study the use of antioxidant agents was
preferred to protect against free oxygen radicals that are produced
during reperfusion and are the main cause of bladder injury.
The increase in bladder wall thickness leads to
cyclic I/R injury during each urination and uninhibited
contraction. The decrease in blood flow and tissue oxygenation
becomes increasingly larger during urination and uninhibited
continuous contractions (13). In
the present study, a macroscopic increase in the bladder volume was
observed in the control group when compared with the sham group
following BOO. Studies demonstrated that certain therapeutic agents
maintained only the contractile functions of the bladder but
offered no protection against the increase in bladder wall
thickness and weight. According to current data, the contractile
functions of the bladder are maintained in a limited manner prior
to the decompensating phase, but as the increase in bladder wall
thickness prolongs the I/R injury, the bladder enters into the
decompensating stage. ALA and silymarin were observed to be
efficacious in terms of providing protection against I/R-induced
increases in bladder wall thickness and weight in BOO.
Studies conducted so far have reported no data with
regard to the role of TNF-α in bladder I/R injury, to the best of
our knowledge. TNF-α released from leukocytes during I/R injury may
be a critical parameter for evaluating the injury and assessing the
treatment efficacy.
Silymarin has protective properties against hepatic
and biliary conditions, with anticarcinogenic, antiapoptotic and
antioxidant effects. It promotes cell proliferation and has
neuroprotective, cardioprotective, estrogenic and antiestrogenic
effects (14,15). Juan et al conducted a study
in 2008 with rabbits and reported increased bladder weights after
four and seven weeks of BOO. The authors demonstrated that the
groups undergoing αlipoic acid and coenzyme Q10 treatment had
significantly reduced bladder weights when compared with those of
the 7-week obstruction group (16).
Saito and Miyagawa measured MDA levels to evaluate
lipid peroxidation in I/R injury resulting from acute urinary
retention in rats and observed elevated MDA levels following
reperfusion and demonstrated that free radicals released during
reperfusion led to bladder dysfunction (17). The present study revealed that ALA
and silymarin prevented lipid peroxidation and therefore, cell
injury resulting from I/R in BOO.
The decrease in the levels of proinflammatory
mediators responsible for organ injury, as well as in MDA which is
the main cause of oxidative injury, indicated that ALA was
effective at providing protection against I/R injury (18).
Several studies investigated TNF-α levels as an
indicator of I/R injury in a number of organs, including the
kidneys, small intestine, stomach and liver (19–21).
However, no studies had been performed to investigate TNF-α and
IL-6 levels in I/R injury of the bladder to the best of our
knowledge
Elevated levels of TNF-α were observed in the
bladder tissue and serum of rats in the control group as a result
of I/R injury resulting from BOO, compared with those of the sham
group. The results of the present study were consistent with the
results reported in studies performed on I/R injury in various
organs. The present study demonstrated that antioxidants protect
the bladder by inhibiting the release of proinflammatory
mediators.
In the present study, it was observed that although
ALA had an anti-apoptotic effect in the bladder, silymarin did not.
An increase in the number of TUNEL (+) cells in the control group
was revealed when compared with the sham group and the increase was
statistically significant (P<0.001). The number of TUNEL (+)
cells was observed to decrease in the ALA- and silymarin-treated
groups, although the decrease in the silymarin group was not
significant. By contrast, the decrease in the number of TUNEL (+)
cells in the ALA group was significant (P<0.001), indicating the
antiapoptotic properties of ALA.
Data obtained previously has revealed that ischemic
damage is involved with iNOS, while most iNOS is associated with
inflammatory causes (22).
However, the role of NO in BOO remains controversial. Several
studies have suggested that NO induces cellular cytotoxicity and
tissue injury via lipid peroxidation, as well as DNA damage and
pro-apoptotic effects in I/R injury (23–25).
However, there are studies which demonstrate that the increased
activity of NOS is associated with reduced BOO induced injury
(26). According to Bae et
al, the protective effect of ALA treatment in the kidney
following I/R was dependent on the reduction of iNOS levels
(27). In the present study,
quantitative iNOS expression increased in the I/R group and no
significant difference was observed between the I/R and silymarin
treatment groups, while ALA had significant decreasing effect.
In conclusion, ALA not only has antioxidant and
anti-apoptotic activities but also an inhibitory effect on iNOS,
collagen formation, lipid peroxidation and TNF-α levels in bladder
tissue protection during I/R injury in rats. Therefore, ALA may be
used to protect bladder tissue in I/R injury which develops
following BOO or during surgical procedures.
Abbreviations:
|
BOO
|
bladder outlet obstruction
|
|
ALA
|
αlipoic acid
|
|
MDA
|
malondialdehyde
|
|
PCR
|
polymerase chain reaction
|
|
iNOS
|
inducible nitric oxid synthase
|
|
I/R
|
ischemia-reperfusion
|
Acknowledgements
This study was funded by the
Scientific Research Projects section of Osmangazi University.
References
|
1.
|
Zderic SA, Levin RM and Wein AJ: Voiding
function and dysfunction: a relevant anatomy, physiology, and
pharmacology, and molecular biology. Adult and Pediatric Urology.
Gillenwater JY, Grayhack JT, Howards SS and Duckett JW: 3rd
edition. Mosby Year Book Medical Publishers; Chicago: pp.
1159–1219. 1996
|
|
2.
|
Parekh MH, Lobel R, O'Connor LJ, Leggett
RE and Levin RM: Protective effect of vitamin E on the response of
the rabbit bladder to partial outlet obstruction. J Urol.
166:341–346. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sahna E, Deniz E and Aksulu HE: Myocardial
ischemia-reper-fusion injury and melatonin. Anadolu Kardiyol Derg.
6:163–168. 2006.(In Turkish).
|
|
4.
|
Bonvissuto G, Minutoli L, Morgia G, Bitto
A, Polito F, Irrera N, Marini H, Squadrito F and Altavilla D:
Effect of Serenoa repens, lycopene, and selenium on proinflammatory
phenotype activation: an in vitro and in vivo comparison study.
Urology. 77:2482011. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Chen LM, Wang C, Chen M, Marcello MR, Chao
J, Chao L and Chai KX: Prostasin attenuates inducible nitric oxide
synthase expression in lipopolysaccharide-induced urinary bladder
inflammation. Am J Physiol Renal Physiol. 291:F567–F577. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sjuve R, Haase H, Morano, Uvelius B and
Arner A: Contraction kinetics and myosin isoform composition in
smooth muscle from hypertrophied rat urinary bladder. J Cell
Biochem. 63:86–93. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Moini H, Packer L and Saris NE:
Antioxidant and prooxidant activities of alpha-lipoic acid and
dihydrolipoic acid. Toxicology and Applied Pharmacology. 182:84–90.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Packer L and Tritschler HJ: Alpha-lipoic
acid: the metabolic antioxidant. Free Radic Biol Med. 20:625–626.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hashimoto T, Nagabukuro H and Doi T:
Effects of the selective acetylcholinesterase inhibitor TAK-802 on
the voiding behavior and bladder mass increase in rats with partial
bladder outlet obstruction. J Urol. 174:1137–1141. 2005. View Article : Google Scholar
|
|
10.
|
Cör A, Barbic M and Kralj B: Differences
in the quantity of elastic fibres and collagen type I and type III
in endopelvic fascia between women with stress urinary incontinence
and controls. Urol Res. 31:61–65. 2003.PubMed/NCBI
|
|
11.
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Sobajima S, Shimer AL, Chadderdon RC,
Kompel JF, Kim JS, Gilbertson LG and Kang JD: Quantitative analysis
of gene expression in a rabbit model of intervertebral disc
degeneration by real-time polymerase chain reaction. Spine J.
5:14–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Greenland JE and Brading AF: Urinary
bladder blood flow changes during the micturition cycle in a
conscious pig model. J Urol. 156:1858–1861. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Fraschini F, Demartini G and Esposti D:
Pharmacology of Silymarin. Clin Drug Investig. 22:51–56. 2002.
View Article : Google Scholar
|
|
15.
|
Kren V and Walterová D: Silybin and
silymarin - new effects and applications. Biomed Pap Med Fac Univ
Palacky Olomouc Czech Repub. 149:29–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Juan YS, Levin RM, Chuang SM, Hydery T, Li
S, Kogan B, Schuler C, Huang CH and Mannikarottu A: The beneficial
effect of coenzyme Q10 and lipoic acid on obstructive bladder
dysfunction in the rabbit. J Urol. 180:2234–2240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Saito M and Miyagawa I: Bladder
dysfunction after acute urinary retention in rats. J Urol.
165:1745–1747. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Sehirli O, Sener E, Cetinel S, Yüksel M,
Gedik N and Sener G: Alpha-lipoic acid protects against renal
ischaemia-reperfusion injury in rats. Clin Exp Pharmacol Physiol.
35:249–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hacioglu A, Algin C, Pasaoglu O, Pasaoglu
E and Kanbak G: Protective effect of leptin against
ischemia-reperfusion injury in the rat small intestine. BMC
Gastroenterol. 5:372005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Erkasap N, Uzuner K, Serteser M, Köken T
and Aydin Y: Gastroprotective effect of leptin on gastric mucosal
injury induced by ischemia-reperfusion is related to gastric
histamine content in rats. Peptides. 24:1181–1187. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Colletti LM, Remick DG, Burtch GD, Kunkel
SL, Strieter RM and Campbell DA Jr: Role of tumor necrosis
factor-alpha in the pathophysiologic alterations after hepatic
ischemia/reperfusion injury in the rat. J Clin Invest.
85:1936–1943. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kinaci MK, Erkasap N, Kucuk A, Koken T and
Tosun M: Effects of quercetin on apoptosis, NF-κB and NOS gene
expression in renal ischemia/reperfusion injury. Exp Ther Med.
3:249–254. 2012.
|
|
23.
|
López-Neblina F, Paez AJ, Toledo AH and
Toledo-Pereyra LH: Role of nitric oxide in ischemia/reperfusion of
the rat kidney. Circ Shock. 44:91–95. 1994.
|
|
24.
|
Wan LL, Xia J, Ye D, Liu J, Chen J and
Wang G: Effects of quercetin on gene and protein expression on NOX
and NOS after myocardial ischemia and reperfusion in rabbit.
Cardiovasc Ther. 27:28–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Martínez-Flórez S, Gutiérrez-Fernández B,
Sánchez-Campos S, González-Gallego J and Tuñón MJ: Quercetin
attenuates nitric oxide production and nuclear factor kappa B
activation in inter-leukin-1 beta activated rat hepatocytes. J
Nutr. 135:1359–1365. 2005.PubMed/NCBI
|
|
26.
|
Chen H, Xing B, Liu X, Zhan B, Zhou J, Zhu
H and Chen Z: Ozone oxidative preconditioning protects the rat
kidney from reperfusion injury: the role of nitric oxide. J Surg
Res. 149:287–295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Bae EH, Lee KS, Lee J, Ma SK, Kim NH, Choi
KC, Frøkiaer J, Nielsen S, Kim SY, Kim SZ, Kim SH and Kim SW:
Effects of alpha-lipoic acid on ischemia-reperfusion-induced renal
dysfunction in rats. Am J Physiol Renal Physiol. 294:F272–F280.
2008. View Article : Google Scholar : PubMed/NCBI
|