Introduction
Diabetes causes various cardiovascular
complications, for example diabetic cardiomyopathy, which has
become the major cause of morbidity and mortality among patients
with diabetes. Previous studies have suggested that cardiomyocyte
apoptosis has a key role in diabetic cardiac damage in animals and
humans (1–3). Hyperglycemia is known to cause
apoptosis in cardiomyocytes, which leads to diabetic cardiomyopathy
(1,4,5).
Attenuation of hyperglycemia-induced cardiomyocyte cell death has
been shown to prevent the progression of cardiac complications
associated with diabetes (1,6).
The multi-ligand receptor for advanced glycation end
products (RAGE), which was first identified as receptor for the
advanced glycation end products (AGEs), is a signal transduction
receptor belonging to the immunoglobulin superfamily (7). RAGE is expressed on multiple cell
types, including vascular cells, inflammatory cells, neurons
(central and peripheral nervous systems) and glomerular epithelial
cells (8), and hyperglycemia has
been shown to directly induce RAGE expression in endothelial cells
(9) and retinal Müller glia
(10). However, RAGE interacts
with ligands other than AGEs; non-AGE ligands of RAGE include high
mobility group box 1 (HMGB1), members of the S100/calgranulin
family, amyloid-β peptide and Mac-1 (8). Diabetic RAGE-null mice are
significantly protected from the adverse effects of ischemia and
reperfusion injury of the heart. In addition, important markers of
apoptosis, specifically, caspase-3 activity and cytochrome c
release, have been demonstrated to be decreased in the hearts of
diabetic RAGE-null mice compared with those in wild-type diabetic
littermates during myocardial ischemia and reperfusion (11). Therefore, this suggests that
hyperglycemia-induced RAGE expression may have an important role in
diabetic cardiac damage.
Glucagon-like peptide-1 (GLP-1), a gut hormone
secreted in a nutrient-dependent manner, stimulates insulin
secretion and inhibits glucagon secretion and gastric emptying
(12). Therefore, GLP-1 has been
proposed to be a potential therapeutic target for the treatment of
patients with type 2 diabetes mellitus. Clinical studies have shown
that GLP-1 improves endothelial function in patients with type 2
diabetes (13), and transient
GLP-1 administration is able to improve cardiovascular outcomes in
patients with myocardial infarction (MI) (14) or congestive heart failure (CHF)
(15,16). Furthermore, previous studies have
suggested that exendin-4 (EX-4), a GLP-1 receptor agonist, may
protect against myocardial ischemia and reperfusion injury and
reduce rates of oxidative phosphorylation in the adult rat heart
(17,18), as well as prevent cardiac
remodeling in the hearts of rats with type 1 diabetes (19). However, the mechanism by which EX-4
protects against myocardial injury associated with diabetes remains
unclear. Therefore, the present study investigated whether EX-4
inhibits hyperglycemia-induced apoptosis in myocardial cells by
suppressing RAGE expression.
Materials and methods
Cell culture and treatment
Neonatal rat ventricular myocytes were prepared from
the hearts of Sprague-Dawley rats (aged between 1 and 3 days) by
enzymatic dissociation, as previously described (20). Briefly, the rats were euthanized
and their hearts excised. Following homogenization using a scalpel,
the heart tissue was treated with 0.1% (w/v) collagenase for 20 min
at 37°C, and then incubated with 0.25% (w/v) trypsin overnight at
4°C. Experimental protocols were conformed to the Guide for the
Care and Use of Laboratory Animals published by the National
Institutes of Health, and were approved by Wuhan University (Wuhan,
China) Cardiomyocytes were enriched by Percoll gradient
centrifugation (Amersham Pharmacia Biotech, Piscataway, NJ, USA)
and plated at a density of 5×105/ml in Dulbecco’s
modified Eagle medium supplemented with 15% (v/v) fetal calf serum
at 37°C and 5% (v/v) CO2. Following incubation in serum
for 24 h, the cells were washed and cultured in serum-free medium
for 24 h, and the cultures were then subjected to different
treatments.
To determine the effect of glucose on the expression
of RAGE, cells were exposed to high levels of glucose (HG;
Sigma-Aldrich, St. Louis, MO, USA) for different time periods (0,
6, 12, 24 and 48 h). A total of 25 mmol/l D-glucose was used for
the HG treatments, compared with 5 mmol/l D-glucose used as the
normal control (NG), as previously described (4,5). To
exclude a hyperosmolar effect, the same concentration of mannitol
(25 mmol/l; Sigma-Aldrich) was used in control cultures. For the
purpose of testing the cardioprotective effect of GLP-1, cells were
treated with 25 mmol/l D-glucose either with or without EX-4
(Sigma-Aldrich). The concentrations of EX-4 tested were 0.1, 1 and
10 nM.
3-(4,5-Dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT)
assay for the determination of cell viability
The cultured cells were seeded at a density of
1×105/ml per well. Subsequently, MTT (Sigma-Aldrich) was
added (final concentration, 5 mg/ml) to each well. The cells were
incubated for 4 h, and then, following the addition of 100 μl 10%
sodium dodecyl sulfate (SDS) and 0.01 N HCl to dissolve the
crystals, the cells were incubated for a further 16 h. The
absorbance was determined using an automatic microplate reader at a
wavelength of 570 nm. The relative cell viability was expressed as
a percentage of the cell viability of the control group.
Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) staining for detecting cardiac myocyte
apoptosis
Apoptosis was detected using an annexin V-FITC/PI
kit (BD Pharmingen, San Diego, CA, USA) in accordance with the
manufacturer’s instructions, as previously described (20). Briefly, cells were harvested after
24 h and washed with phosphate-buffered saline, prior to being
pelleted by centrifugation at 1500 × g for 5 min. Cells were
resuspended in 300 μl binding buffer (1×105 cells/ml)
followed by staining with 5 μl annexin V-FITC and 5 μl PI for 15
min at room temperature in the dark. The percentage of cell
apoptosis was then determined using flow cytometry with a BD
FACSCalibur platform (BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
Cardiomyocytes were lysed using a Protein Complete
Lysis kit (Beyotime Institute of Biotechnology, Haimen, China). The
protein concentration in each sample was determined using a Protein
Assay kit (Beyotime Institute of Biotechnology) using bovine serum
albumin as a standard. For the immunoblot analysis, proteins were
separated using SDS-PAGE, and then transferred onto a
polyvinylidene fluoride (PVDF) membrane as previously described
(5,20). The membranes were probed using
antibodies against RAGE and caspase-3 (Cell Signaling Technology,
Inc., Danvers, MA, USA), followed by horseradish
peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). The membranes were
visualized using an enhanced chemiluminescence system (Beyotime
Institute of Biotechnology). The levels of protein expression were
normalized against glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
expression.
Statistical analysis
Statistical analysis was performed using SPSS
software, version 13.0 (SPSS, Inc., Chicago, IL, USA). All values
are expressed as the mean ± standard deviation. The Student’s
t-test was used for between-group comparisons. Welch’s analysis of
variance was used for comparisons among groups, and the
Student-Neuman-Keuls or Dunnett’s T3 test was used for post-hoc
multiple comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
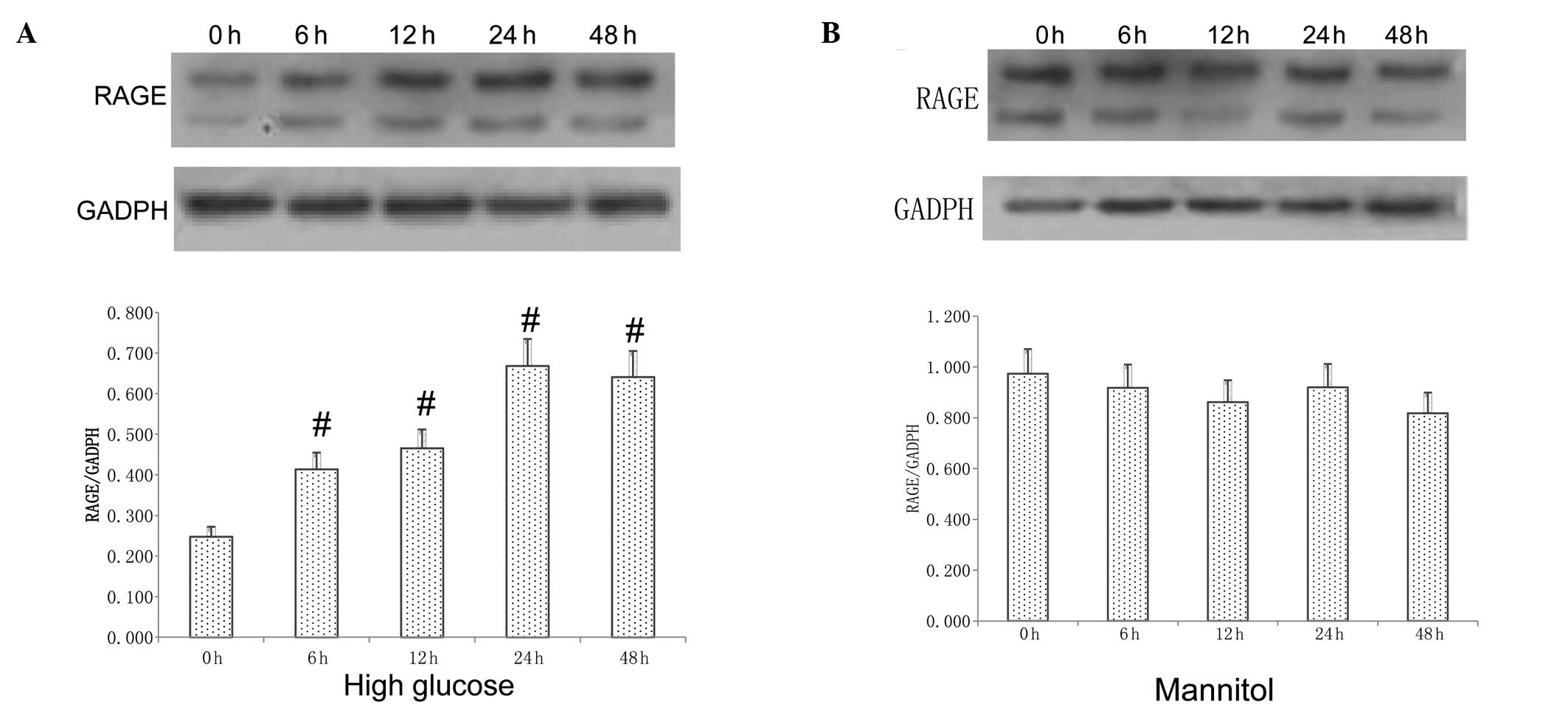
Effect of HG on RAGE expression
Incubation of myocytes with HG led to a
time-dependent activation of RAGE expression compared with that
observed in a low-glucose environment, and the protein expression
of RAGE was increased at 6 h and peaked at 24 h (P<0.05;
Fig. 1A). However, the protein
levels of RAGE in myocytes were not changed following treatment
with 25 mmol/l mannitol (Fig.
1B).
Cell viability
As shown in Fig. 2,
the treatment of myocytes with HG for 24 h resulted in
significantly decreased cell viability compared with that in the NG
group (P<0.05), as shown by an MTT assay. In the myocardial
cells cultured with HG and various concentrations of EX-4, the cell
viability was increased by EX-4 in a dose-dependent manner. At a
concentration of 10 nM EX-4, the cell viability of the myocardial
cells was significantly improved compared with that in the HG group
(P<0.05).
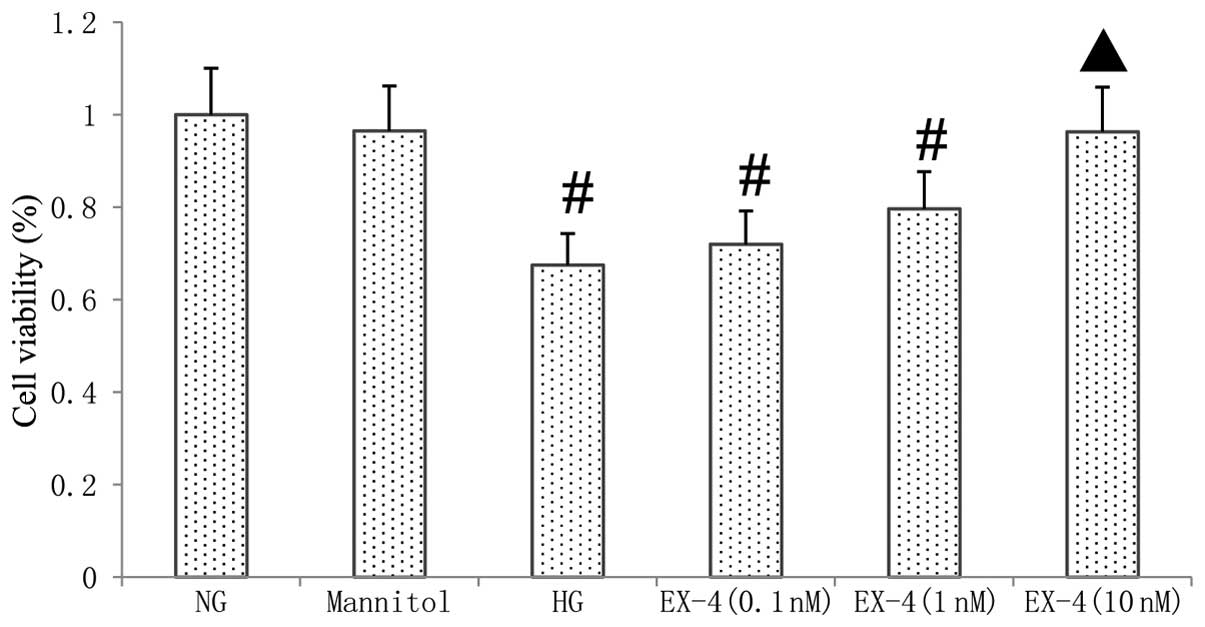 | Figure 2Effect of HG and EX-4 on cell
viability. Cardiomyocytes were treated with HG or mannitol
with/without EX-4, a GLP-1 receptor agonist. After 24 h, cell
viability was analyzed using the MTT assay. #P<0.05
vs. NG group, ▲P<0.05 vs. HG group. NG, 5 mmol/l
glucose; mannitol, 25 mmol/l mannitol; HG, high glucose
concentration of 25 mmol/l; EX-4 (0.1 nM), 0.1 nM EX-4 and 25
mmol/l glucose; EX-4 (1 nM), 1 nM EX-4 and 25 mmol/l glucose; EX-4
(10 nM), 10 nM EX-4 and 25 mmol/l glucose. NG, normal glucose;
EX-4, exendin-4; HG, high glucose; MTT,
3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide. |
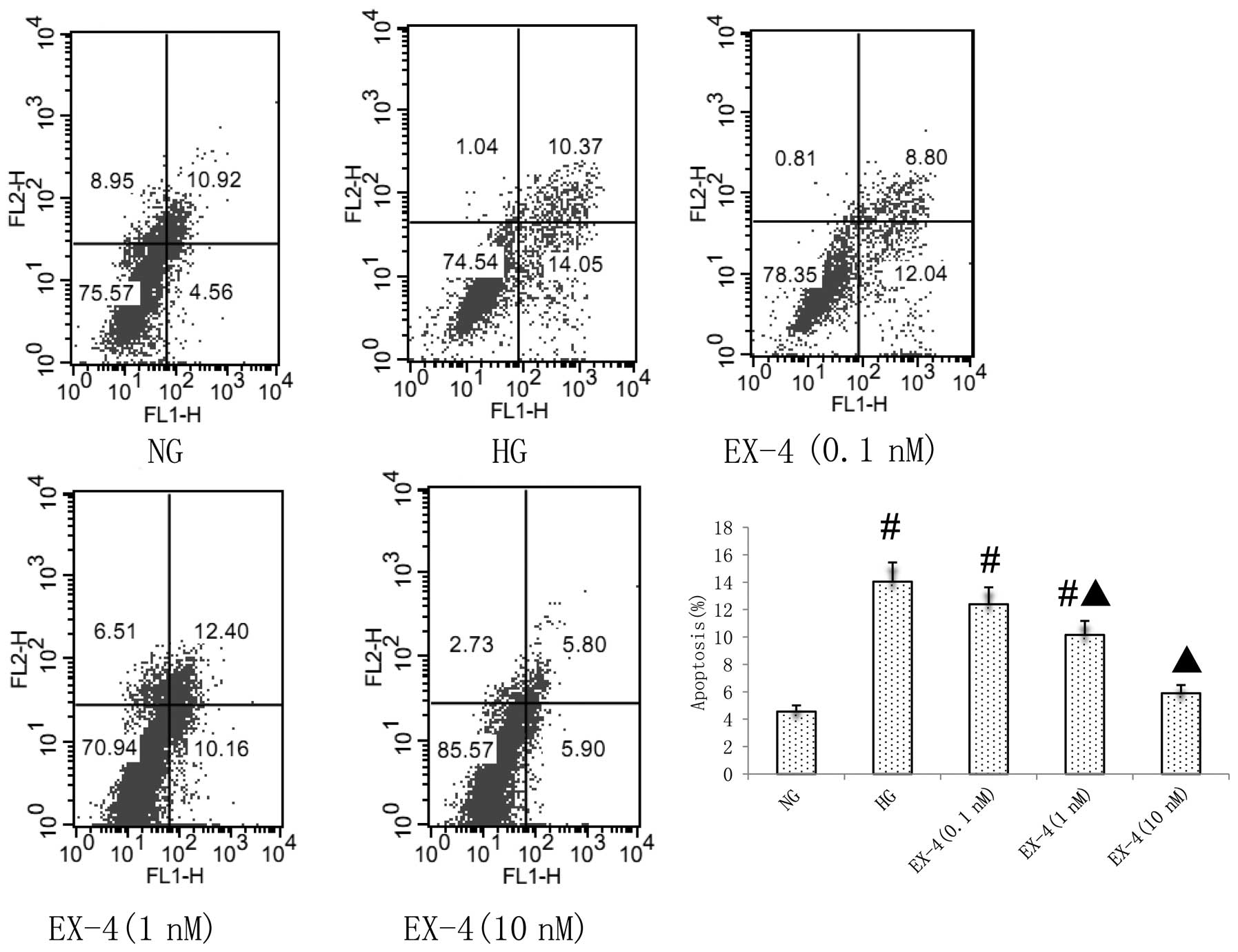
Effect of EX-4 on HG-induced myocyte
apoptosis
According to the results from the MTT assay, the
effects of different concentrations of EX-4 (0.1–10 nM) on
myocardial cell apoptosis were determined. Flow cytometric analysis
demonstrated that EX-4 inhibited the apoptosis of neonatal myocytes
induced by HG in a dose-dependent manner (P<0.05; Fig. 3).
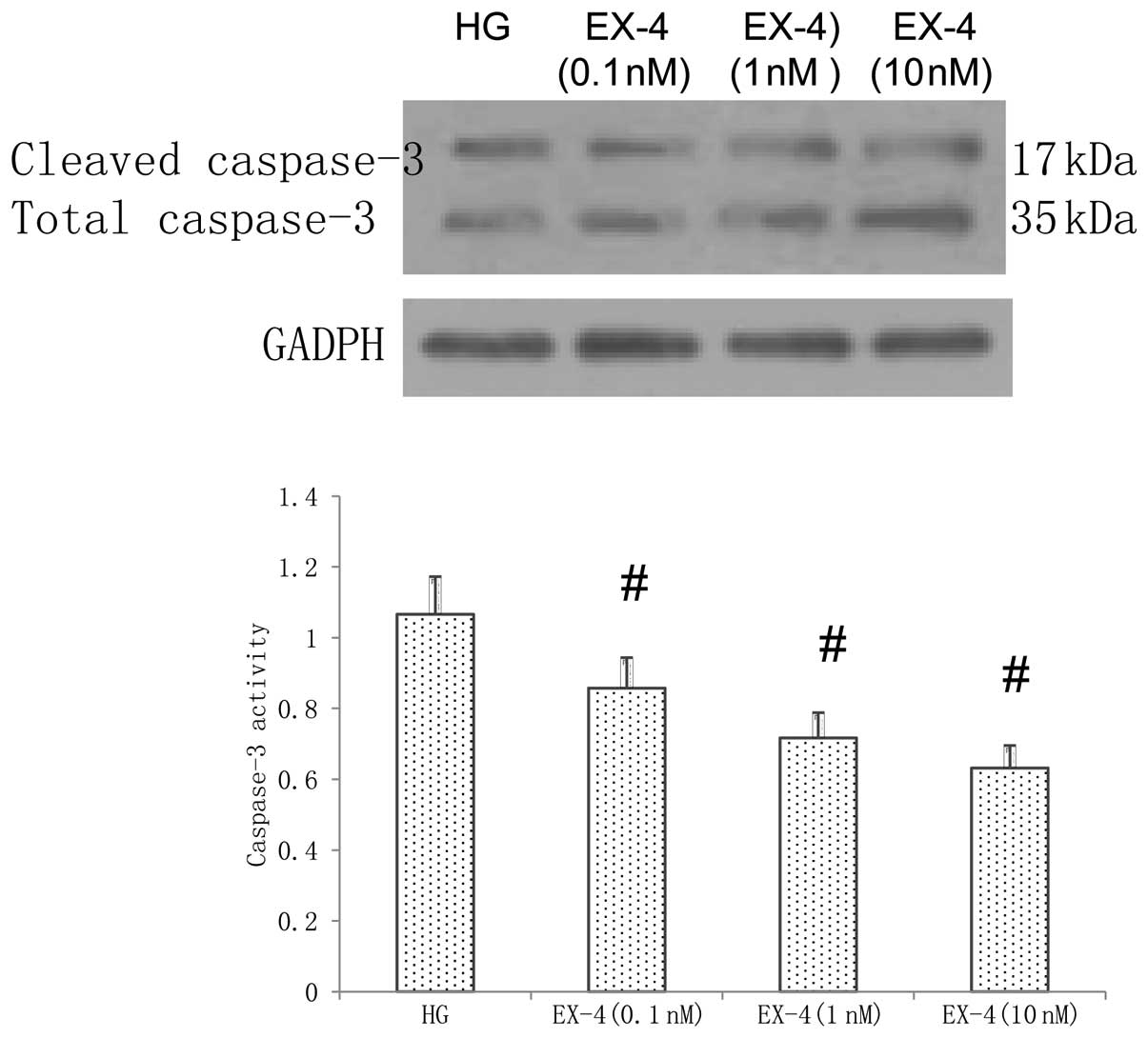
Cleaved caspase-3 is a key executor in the apoptotic
process, which is induced by HG. Therefore, in the present study,
the expression of caspase-3 was detected using western blot
analysis. As shown in Fig. 4, the
HG-induced activity of caspase-3 was inhibited in the cells treated
with EX-4 in a dose-dependent manner compared with that in the
cells in the HG group (P<0.05).
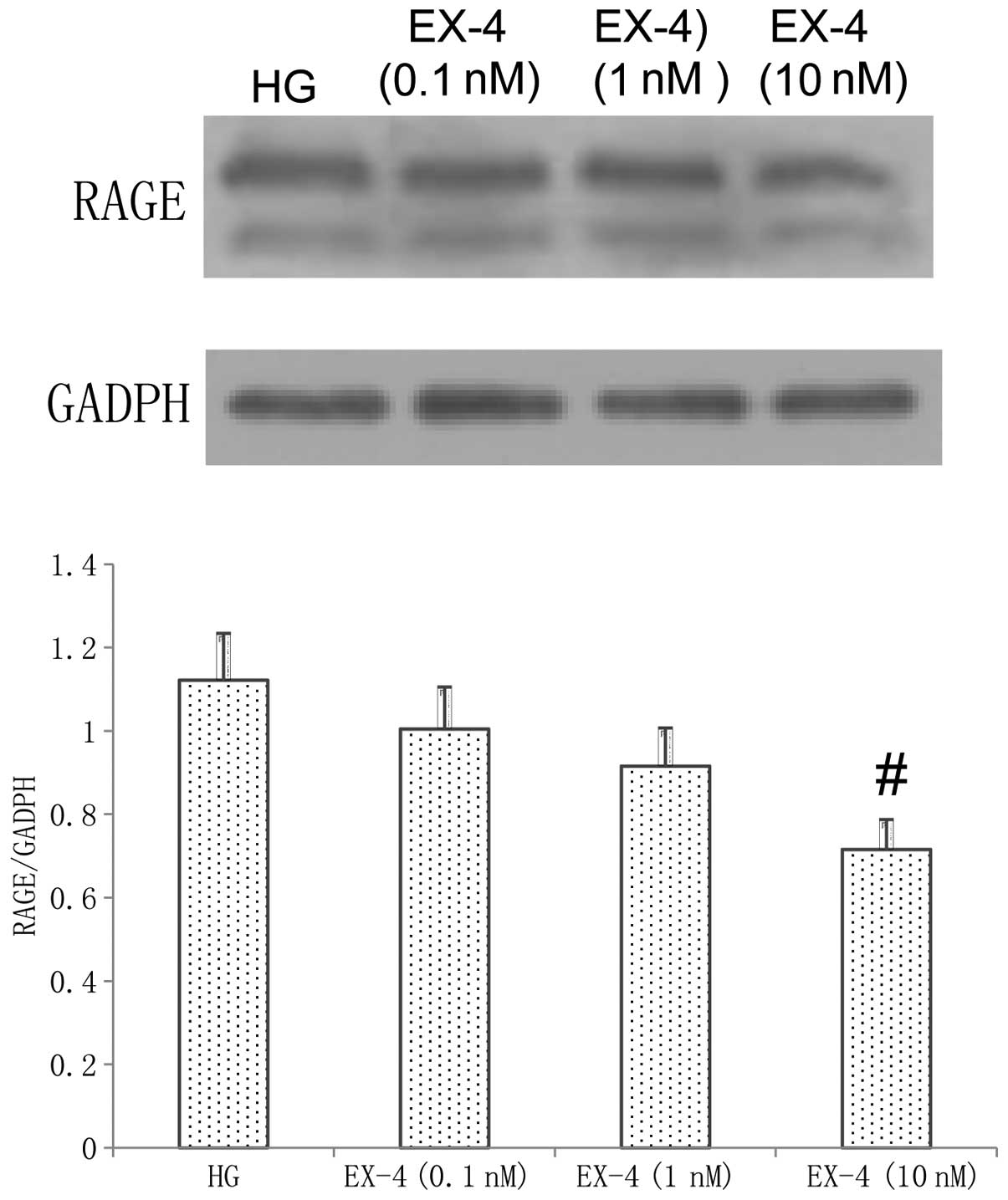
Effect of EX-4 on RAGE expression
As shown in Fig. 5,
following incubation with HG and EX-4 for 24 h, EX-4 inhibited the
RAGE expression induced by HG. At a concentration of 10 nM EX-4,
the expression of RAGE was significantly improved compared with
that in the HG group (P<0.05).
Discussion
In the present study, it was demonstrated that
hyperglycemia increases the expression of RAGE and induces
apoptosis in myocytes. In addition, EX-4, a GLP-1 receptor agonist,
was shown to protect against myocardial apoptosis in a
dose-dependent manner. The inhibitory effect of EX-4 on apoptosis
may be via a reduction in caspase-3 activity and the inhibition of
RAGE expression. These results indicate that the cardioprotective
effect induced by EX-4 during diabetic cardiomyopathy may be
associated with the inhibition of RAGE expression.
Hyperglycemia, which directly causes abnormalities
at the cardiac myocyte level, including apoptosis, is the main
pathogenetic factor of diabetic cardiomyopathy. Anderson et
al (21) demonstrated that the
myocardium in patients with diabetes has a greater overall
propensity for mitochondrial-dependent cell death, possibly as a
result of metabolic stress-imposed changes that have occurred
within the mitochondria. In addition, a previous study demonstrated
that hyperglycemia directly induces apoptosis in the myocardium,
and it is mediated by activation of the cytochrome
c-activated caspase-3 pathway (1). The results from the present study are
in accordance with these observations.
Furthermore, in a previous study we demonstrated
that HMGB1 promotes the apoptosis of neonatal myocytes in a
dose-dependent manner (20). Since
HMGB1 is a ligand of RAGE, the role of the RAGE axis in
hyperglycemia-induced apoptosis in myocytes was investigated in the
present study. The results demonstrated that hyperglycemia
increases RAGE expression in myocardial cells, and the protein
expression of RAGE was increased at 6 h and peaked at 24 h. Yao and
Brownlee (9) previously found that
HG increase RAGE and HMGB1 expression, and that this effect is
mediated by reactive oxygen species-induced methylglyoxal, the
major substrate of glyoxalase 1. Furthermore, the interaction
between HMGB1 and RAGE has been shown to be important in neuronal
cell apoptosis (22), and the RAGE
axis is also involved in the apoptosis of a number of different
cell types, including pancreatic β cells (23), esophageal squamous cell carcinoma
(24) and neuronal cells (22). In addition, RAGE-null mice with
diabetes have been previously demonstrated to be significantly
protected against the adverse impact of ischemia/reperfusion (I/R)
injury of the heart, and key markers of apoptosis, including
caspase-3 activity and cytochrome c release, were found to
be decreased in the hearts of RAGE-null mice with diabetes compared
with those in wild-type mice with diabetes during myocardial I/R
(11). In combination, these
results suggest that hyperglycemia-induced RAGE expression has an
important role in diabetic cardiac damage and the RAGE axis may be
a therapeutic target for diabetic cardiovascular complications.
As a novel hypoglycemic agent, GLP-1 acts through a
distinct heptahelical G-protein-coupled receptor (GLP-1R). Since
this receptor is abundantly expressed in β cells and throughout the
gut, heart, vascular smooth muscle cells, endothelial cells,
kidney, lung and peripheral nervous system (25), GLP-1 appears to modulate a wide
variety of physiologic effects. The cardioprotective effect induced
by GLP-1 and GLP-1 receptor agonists has been demonstrated in
previous studies (13–19); however, the mechanism has yet to be
elucidated. Ravassa et al (26) demonstrated that GLP-1 (100 nM)
inhibits staurosporine-induced apoptosis in murine HL-1
cardiomyocytes, and GLP-1 also inhibits palmitate- and
ceramide-induced DNA fragmentation, which is an integral part of
apoptosis. However, Chen et al (27) demonstrated that treatment with EX-4
had no effect on LPS-induced apoptosis in H9c2 cardiomyoblast
cells. The reasons for the different results may be due to the
different cell types, the different GLP-1 doses used and the
different apoptosis models. In the present study the effects of
various EX-4 doses (0.1, 1 and 10 nM) on hyperglycemia-induced
apoptosis in neonatal rat ventricular myocytes were investigated.
The results indicated that EX-4 protects against myocardial
hyperglycemia-induced apoptosis in a dose-dependent manner via a
downregulation of caspase-3 cleavage; this effect was most
significant at a 10-nM concentration of EX-4.
Hyperglycemia may activate the RAGE axis, which
contributes to myocyte apoptosis; therefore, in the present study,
it was investigated whether EX-4 was able to suppress RAGE
expression in myocardial cells. The results showed that the
expression of RAGE in myocytes was significantly decreased by EX-4,
and this inhibitory effect was greatest in the cells treated with
10 nM EX-4. Ishibashi et al (28,29)
previously demonstrated that GLP-1 may directly act on mesangial
cells and human umbilical vein endothelial cells via GLP-1R and it
may work as an anti-inflammatory agent against AGEs by reducing
RAGE expression. The results from the present study also indicated
that EX-4 is able to inhibit the protein expression of RAGE that is
induced by hyperglycemia in myocytes.
In conclusion, the results from the present study
suggest that the cardioprotective effect induced by a GLP-1
receptor agonist (EX-4) during diabetic cardiomyopathy may be
associated with the inhibition of RAGE expression. However, there
were certain limitations in the present study, as the effects of
EX-4 on RAGE expression and apoptosis were only observed following
their induction by hyperglycemia. Therefore, the precise mechanisms
underlying the observations require further investigation.
Acknowledgements
The present study was partially supported by a grant
from National Natural Science foundation of China (no. 81100146), a
grant from the Fundamental Research Funds for the Central
Universities (no. 111023) and the Specialized Research Fund for the
Doctoral Program of Higher Education of China (no.
20110141120060).
References
|
1
|
Cai L, Li W, Wang G, Guo L, Jiang Y and
Kang YJ: Hyperglycemia-induced apoptosis in mouse myocardium:
mitochondrial cytochrome c-mediated caspase-3 activation
pathway. Diabetes. 51:1938–1948. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Z, Zhang T, Dai H, et al: Involvement
of endoplasmic reticulum stress in myocardial apoptosis of
streptozocin-induced diabetic rats. J Clin Biochem Nutr. 41:58–67.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuethe F, Sigusch HH, Bornstein SR, Hilbig
K, Kamvissi V and Figulla HR: Apoptosis in patients with dilated
cardiomyopathy and diabetes: a feature of diabetic cardiomyopathy?
Horm Metab Res. 39:672–676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu XY, Song YH, Geng YJ, et al: Glucose
induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1.
Biochem Biophys Res Commun. 376:548–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang JL, Xiao DZ, Liu XY, et al: High
glucose induces apoptosis in AC16 human cardiomyocytes via
macrophage migration inhibitory factor and c-Jun N-terminal kinase.
Clin Exp Pharmacol Physiol. 37:969–973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fiordaliso F, Bianchi R, Staszewsky L, et
al: Antioxidant treatment attenuates hyperglycemia-induced
cardiomyocyte death in rats. J Mol Cell Cardiol. 37:959–968. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Neeper M, Schmidt AM, Brett J, et al:
Cloning and expression of a cell surface receptor for advanced
glycosylation end products of proteins. J Biol Chem.
267:14998–15004. 1992.PubMed/NCBI
|
|
8
|
Yan SF, Ramasamy R and Schmidt AM:
Receptor for AGE (RAGE) and its ligands - cast into leading roles
in diabetes and the inflammatory response. J Mol Med (Berl).
87:235–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao D and Brownlee M:
Hyperglycemia-induced reactive oxygen species increase expression
of the receptor for advanced glycation end products (RAGE) and RAGE
ligands. Diabetes. 59:249–255. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zong H, Ward M, Madden A, et al:
Hyperglycaemia-induced pro-inflammatory responses by retinal Müller
glia are regulated by the receptor for advanced glycation
end-products (RAGE). Diabetologia. 53:2656–2666. 2010.PubMed/NCBI
|
|
11
|
Bucciarelli LG, Ananthakrishnan R, Hwang
YC, et al: RAGE and modulation of ischemic injury in the diabetic
myocardium. Diabetes. 57:1941–1951. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mannucci E and Rotella CM: Future
perspectives on glucagon-like peptide-1, diabetes and
cardiovascular risk. Nutr Metab Cardiovasc Dis. 18:639–645. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hiki M, Shimada K, Kiyanagi T, et al:
Single administration of alpha-glucosidase inhibitors on
endothelial function and incretin secretion in diabetic patients
with coronary artery disease - Juntendo University trial: effects
of miglitol on endothelial vascular reactivity in type 2 diabetic
patients with coronary heart disease (J-MACH). Circ J.
74:1471–1478. 2010.
|
|
14
|
Nikolaidis LA, Mankad S, Sokos GG, et al:
Effects of glucagon-like peptide-1 in patients with acute
myocardial infarction and left ventricular dysfunction after
successful reperfusion. Circulation. 109:962–965. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sokos GG, Nikolaidis LA, Mankad S, Elahi D
and Shannon RP: Glucagon-like peptide-1 infusion improves left
ventricular ejection fraction and functional status in patients
with chronic heart failure. J Card Fail. 12:694–699. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Halbirk M, Nørrelund H, Møller N, et al:
Cardiovascular and metabolic effects of 48-h glucagon-like
peptide-1 infusion in compensated chronic patients with heart
failure. Am J Physiol Heart Circ Physiol. 298:H1096–H1102. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brown SB, Libonati JR, Selak MA, Shannon
RP and Simmons RA: Neonatal exendin-4 leads to protection from
reperfusion injury and reduced rates of oxidative phosphorylation
in the adult rat heart. Cardiovasc Drugs Ther. 24:197–205. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sonne DP, Engstrøm T and Treiman M:
Protective effects of GLP-1 analogues exendin-4 and GLP-1(9–36)
amide against ischemia-reperfusion injury in rat heart. Regul Pept.
146:243–249. 2008.PubMed/NCBI
|
|
19
|
Barakat GM, Nuwayri-Salti N, Kadi LN,
Bitar KM, Al-Jaroudi WA and Bikhazi AB: Role of glucagon-like
peptide-1 and its agonists on early prevention of cardiac
remodeling in type 1 diabetic rat hearts. Gen Physiol Biophys.
30:34–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu X, Zhou X, He B, et al: Minocycline
protects against myocardial ischemia and reperfusion injury by
inhibiting high mobility group box 1 protein in rats. Eur J
Pharmacol. 638:84–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anderson EJ, Rodriguez E, Anderson CA,
Thayne K, Chitwood WR and Kypson AP: Increased propensity for cell
death in diabetic human heart is mediated by
mitochondrial-dependent pathways. Am J Physiol Heart Circ Physiol.
300:H118–H124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SW, Lim CM, Kim JB, et al:
Extracellular HMGB1 released by NMDA treatment confers neuronal
apoptosis via RAGE-p38 MAPK/ERK signaling pathway. Neurotox Res.
20:159–169. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu Y, Shu T, Lin Y, et al: Inhibition of
the receptor for advanced glycation endproducts (RAGE) protects
pancreatic β-cells. Biochem Biophys Res Commun. 404:159–165.
2011.PubMed/NCBI
|
|
24
|
Jin Q, Chen H, Luo A, Ding F and Liu Z:
S100A14 stimulates cell proliferation and induces cell apoptosis at
different concentrations via receptor for advanced glycation end
products (RAGE). PLoS One. 6:e193752011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davidson MH: Cardiovascular effects of
glucagonlike peptide-1 agonists. Am J Cardiol. 108:33B–41B. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ravassa S, Zudaire A, Carr RD and Díez J:
Antiapoptotic effects of GLP-1 in murine HL-1 cardiomyocytes. Am J
Physiol Heart Circ Physiol. 300:H1361–H1372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen TH, Wo HT, Wu CC, et al: Exendin-4
attenuates lipopolysaccharides induced inflammatory response but
does not protects H9c2 cells from apoptosis. Immunopharmacol
Immunotoxicol. 34:484–490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishibashi Y, Nishino Y, Matsui T, Takeuchi
M and Yamagishi S: Glucagon-like peptide-1 suppresses advanced
glycation end product-induced monocyte chemoattractant protein-1
expression in mesangial cells by reducing advanced glycation end
product receptor level. Metabolism. 60:1271–1277. 2011. View Article : Google Scholar
|
|
29
|
Ishibashi Y, Matsui T, Takeuchi M and
Yamagishi S: Glucagon-like peptide-1 (GLP-1) inhibits advanced
glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA
levels in endothelial cells by suppressing AGE receptor (RAGE)
expression. Biochem Biophys Res Commun. 391:1405–1408. 2010.
View Article : Google Scholar : PubMed/NCBI
|