Introduction
The prevention and treatment of osteoporosis (OP) is
a global health problem due to the high morbidity and mortality
associated with this condition. Glucocorticoid-induced OP (GIO) may
be divided into primary, secondary and idiopathic GIO. Among the
types of secondary OP, GIO is the most common. Long-term use of
glucocorticoids may impair kidney function, which results in bone
damage and loss of myeloid cells. Thus, the pathogenesis of GIO
includes kidney asthenia and marrow deficiency (1). Invigorating kidney and nourishing
essence Chinese medicine-containing serum has been reported to
promote osteogenic differentiation in bone marrow stromal cells
(BMSCs) (2,3). Experimental studies have shown that
beyond the physiological dose, glucocorticoids promote the
differentiation of BMSCs into fat cells and inhibit their
differentiation into bone cells (4–6),
thereby causing an imbalance in osteoblast-osteoclast coupling,
which results in bone loss and GIO. BMSC-derived osteoblasts and
adipocytes exhibit intra-plasticity and, under certain conditions,
are capable of differentiating into each other. Differentiation
among these cells has become a topic of much interest to
cytological research (7,8).
The present study aimed to investigate the effects
of kidney-reinforcing and marrow-beneficial traditional Chinese
medicine (TCM)-intervened (KRMBTI)-serum on rat BMSC proliferation
and differentiation in vitro. In particular, the effect of
KRMBTI-serum on the balance of BMSC osteoblast and adipocyte
differentiation and the underlying mechanisms was analyzed.
Materials and methods
Animals
A total of 75 pathogen-free, eight-month-old,
Sprague-Dawley rats were obtained from the Experimental Animal
Center of Liaoning Medical University (Jinzhou, China), 50% of
which were male and 50% of which were female. All experimental
procedures were performed in accordance with the Guide for the Care
and Use of Laboratory Animals of the National Institutes of Health
(8th edition, 2011). The animal protocol was reviewed and approved
by the Institutional Animal Care and Use Committee of Liaoning
Medical University.
Preparation of reagents
KRMBT was prepared as a suspension containing 10 g
lyophilized powder of fresh antler, 5 g oyster powder and 15 g
Epimedium brevicornum decoction, according to the daily dose
required for a 60 kg adult. The osteogenic differentiation-inducing
medium was generated by mixing 10–8 mol/l dexamethasone,
50 μmol/l vitamin C, 10 mmol/l β-glycerophosphate and 10% fetal
bovine serum (FBS) and was stored at 4°C.
Drug administration
From the group of 75 rats, 45 rats were randomly
selected and divided into high-dose (HD), middle-dose (MD) and
low-dose (LD) groups (n=15 per group). The rats in each group were
randomly divided into five subgroups, with three rats in each
subgroup according to the time at which blood was collected. The
KRMBT dose was calculated based on the dose/kg body weight of the
animal compared with that of humans. The KRMBT doses in the
different groups were as follows: LD group, 3.125 g/kg body
weight/day; MD group, three-fold that in the LD group; and HD
group, nine-fold that in the LD group. KRMBT administration was
performed concurrently in all groups for 10 weeks. Thereafter, 5–8
ml abdominal aortic blood was collected at 0.5, 1.0, 1.5, 2.0 and
2.5 h after the last administration at 10 weeks. The blood was then
centrifuged at 600 × g for 20 min and the serum was preserved at
−70°C.
Isolation, cultivation and subcultivation
of BMSCs
A further 20 rats were dipped in 75% ethanol for 20
min, then anesthetized using an injection of chloral hydrate. Under
aseptic conditions, the bilateral lower limb femurs of the rats
were separated and the attached fatty, connective tissue and
periosteum were removed. Femurs were placed in a small aseptic
beaker and transferred to a ultraclean bench. The femurs were then
placed in a sterile culture dish. Subsequent to washing with
phosphate-buffered saline (PBS), the bilateral ends in the femurs
were resected. A total of 5 ml high glucose Dulbecco’s modified
Eagle’s medium (DMEM) containing 10% FBS was mixed with 0.5 ml
heparin and used to wash the marrow cavity of the femur three or
four times. The flushing fluid was fully mixed, followed by cell
resuspension with DMEM containing 10% FBS. The cells
(1×106 cells/ml) were inoculated in a 25-cm2
culture bottle and were incubated at 37°C with 5% CO2
and saturated humidity. The medium was changed every three days.
After the adherent cells had reached 80–90% confluency, the culture
medium was removed and the cells were washed three times with PBS.
Preheated (37°C) digestion liquid containing 0.25% trypsin and
0.02% EDTA was used at room temperature to passage the cells at a
ratio of 1:2.
MTT assay
The effects of the different blood sampling
time-points, doses of KRMBT and concentrations of KRMBTI-serum on
the proliferation of BMSCs were determined by MTT assay. The
P3-generation BMSCs were seeded at 1×104 cells/well, and
RMBTI-serum was added after 24 h. Five blood sampling time-points
(0.5, 1, 1.5, 2, 2.5 h) and 10 KRMBTI-serum addition concentrations
(5, 10, 15, 20, 25, 30, 40, 50, 60 and 80%) were set. Each
concentration had three repeated wells, and one blank control well
was prepared. The final liquid volume of each well was 200 μl.
After 72 h, 20 μl 5 mg/ml MTT (Shanghai Sangon Biological
Engineering Technology & Services Co., Ltd., Shanghai, China)
was added to each well and the plate was incubated for 4 h. Finally
the optical density (OD) value at 450 nm of each well was measured
using a Synergy NEO HTS plate reader (BioTek Instruments, VT,
USA).
Detection of ALP expression
The P3-generation BMSCs were seeded at a density of
1×105 cells/well, and the serum-free medium was replaced
following 24 h of cultivation. After 24 h following the medium
replacement, the drug-containing sera at concentrations of 10, 20
and 30% were added for the induction of the BMSCs. Optimum blood
sampling time-point, optimum dose and optimum concentration
subgroups were established, on the basis of optimum values
determined by the MTT assay. The final volume of each well was 1
ml, and a blank control well was also prepared. The supernatant,
following induction for 3, 7, 10, 14 and 15 days, was collected.
ALP expression was measured by an ELISA method using an ALP kit
(R&D Systems Inc., Minneapolis, MN, USA) according to
manufacturer’s instructions.
Detection of TGF-β1 expression
The P3-generation BMSCs with good growth were
divided into four groups. These were: the control group, in which
the BMSCs were cultured only with culture medium; the osteoblast
group, in which BMSCs were cultured with culture medium and
osteogenic differentiation-inducing medium; the serum group, in
which BMSCs were cultured with culture medium and serum with the
optimum concentration for bone differentiation; and the
comprehensive induction group, in which BMSCs were cultured with
culture medium, osteogenic differentiation-inducing medium and
serum with the optimum concentration for bone differentiation. In
each group, all the culture medium was replaced every three days.
The TGF-β1 concentration in the cell culture supernatant was
detected by an ELISA method using a TGF-β1 kit (R&D Systems
Inc.) according to manufacturer’s instructions.
Reverse transcription polymerase chain
reaction (RT-PCR) analysis
The remaining 10 rats were randomly divided into
control and HD KRMBT groups. In the HD KRMBT group, 3 days after
the grouping, KRMBT was intragastrically administered once daily at
10:00 a.m. for 10 consecutive weeks. Experimental samples were
taken on the fifth and tenth weeks. The rats were weighed weekly
and the dose was adjusted based on changes in body weight. The rats
in the control group were administered equal volumes of saline.
Bilateral livers were taken 1 h after the final administration,
under sterile conditions.
Hepcidin mRNA expression in the liver was determined
using an RNA PCR kit (AMV) Ver. 3.0 (Takara Bio Inc., Dalian,
China) and a 600 bp DNA ladder marker (Mianyang Gaoxin Tianze
Genetic Engineering Co. Ltd., Sichuan, China). Primer Premier 5.0
software (Premier Biosoft, Palo Alto, CA, USA) was used to design
PCR primer sequences for β-actin and hepcidin, based on the rat
β-actin and hepcidin gene sequences registered in GenBank.
Approximately 100 mg fresh rat liver tissue was homogenized in
liquid nitrogen. Total RNA extraction was performed using TRIzol
reagent (Takara Bio Inc.) according to the manufacturer’s
instructions. An RT-PCR kit (Takara Bio Inc.) was used to
synthesize the first strand of cDNA according to the manufacturer’s
instructions. The RT-PCR reaction conditions were as follows: 42°C
for 30 min, 99°C for 5 min, 5°C for 5 min followed by preservation
at 4°C in a ABI PCR machine (Applied Biosystems@2720 Thermal
Cycler, Grand Island, NY, USA). PCR products were electrophoresed
using 3 μl DNA ladder marker with molecular weight standards (100
bp) as the reference. Electrophoresis was performed at 90 V for 1
h.
Statistical analysis
SPSS software, version 11.0 (SPSS, Inc., Chicago,
IL, USA) was used for the statistical analysis of the experimental
data. Data are presented as mean ± standard deviation. The
electrophoresis results were determined using FluorChem V2.0
software (Model: Gene Genus; Alpha Innotech Corporation, San
Leandro, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
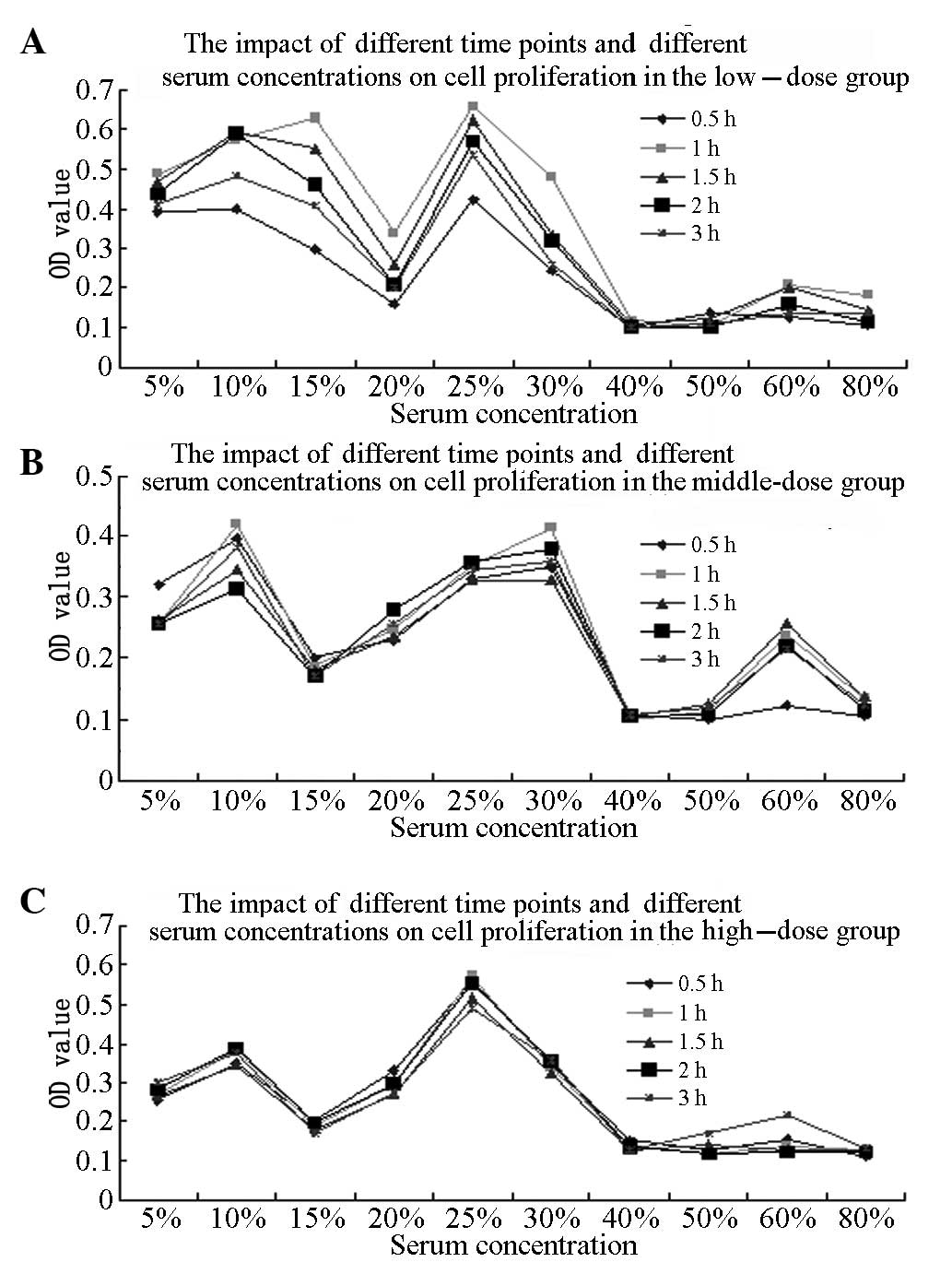
Optimum BMSC proliferation-promoting
conditions
The randomized block-designed analysis of variance
(ANOVA) results for the comparison of different blood sampling
time-points showed that the OD value of the BMSCs treated with the
KRMBTI-serum collected at 1 h was higher than that for the BMSCs
treated with KRMBTI-serum collected at the other four time points
(P<0.05). Furthermore, the OD value with a 25% concentration of
KRMBTI-serum was observed to be significantly higher than that with
the other concentrations (P<0.01). Analysis of the different
doses using concentrations as covariants showed that P<0.05,
indicating that the KRMBT dose exhibited a linear regression
correlation with the concentration of KRMBTI-serum added to the
BMSCs and the blood-sampling time points (P<0.01; Table I).
 | Table IEffect of dose, serum sampling
time-point and concentration of kidney-reinforcing and
marrow-beneficial traditional Chinese medicine-intervened-serum on
bone marrow stromal cell proliferation as determined by MTT
assay. |
Table I
Effect of dose, serum sampling
time-point and concentration of kidney-reinforcing and
marrow-beneficial traditional Chinese medicine-intervened-serum on
bone marrow stromal cell proliferation as determined by MTT
assay.
| Optical density |
|---|
|
|
|---|
| Parameters | 0.5 h | 1.0 h | 1.5 h | 2.0 h | 2.5 h |
|---|
| Low-dose group
(%) |
| 0 | 0.2354±0.0436 | 0.2424±0.0357 | 0.2652±0.0463 | 0.2571±0.0642 | 0.2523±0.0384 |
| 5 | 0.3932±0.0876 | 0.4869±0.1540 | 0.4668±0.0378 | 0.4370±0.0768 | 0.4094±0.0154 |
| 10 | 0.4099±0.0557 | 0.5732±0.0162 | 0.5954±0.0852 | 0.5902±0.0159 | 0.4849±0.0198 |
| 15 | 0.2954±0.0286 | 0.6281±0.0318 | 0.5523±0.0428 | 0.4589±0.0078 | 0.4071±0.0077 |
| 20 | 0.1609±0.0390 | 0.3391±0.0396 | 0.2698±0.0843 | 0.2104±0.0193 | 0.2024±0.0542 |
| 25 | 0.4209±0.0874 | 0.6583±0.1231a | 0.6256±0.1678 | 0.5714±0.1072 | 0.5363±0.1347 |
| 30 | 0.2423±0.0546 | 0.4813±0.0398 | 0.3349±0.0430 | 0.3182±0.0251 | 0.2569±0.0279 |
| 40 | 0.1034±0.0152 | 0.1189±0.0295 | 0.1123±0.0239 | 0.1023±0.0218 | 0.1029±0.0364 |
| 50 | 0.1379±0.0542 | 0.1073±0.0145 | 0.1209±0.0146 | 0.1017±0.0255 | 0.1089±0.0137 |
| 60 | 0.1251±0.0126 | 0.2079±0.0518 | 0.2023±0.0198 | 0.1609±0.0355 | 0.1384±0.0148 |
| 80 | 0.1079±0.0281 | 0.1807±0.0325 | 0.1459±0.0145 | 0.1149±0.0335 | 0.1384±0.0289 |
| Middle-dose group
(%) |
| 5 | 0.3209±0.0257 | 0.2574±0.0385 | 0.2609±0.0529 | 0.2573±0.0392 | 0.2582±0.1389 |
| 10 | 0.3970±0.0333 | 0.4189±0.0546 | 0.3459±0.0534 | 0.3125±0.0535 | 0.3809±0.0169 |
| 15 | 0.2009±0.0187 | 0.1849±0.0236 | 0.1814±0.0436 | 0.1608±0.0171 | 0.1703±0.0122 |
| 20 | 0.2310±0.0354 | 0.2459±0.0123 | 0.2351±0.0252 | 0.2799±0.0255 | 0.2580±0.0353 |
| 25 | 0.3289±0.0678 | 0.3522±0.0117 | 0.3279±0.0557 | 0.3583±0.0254 | 0.3462±0.0327 |
| 30 | 0.3489±0.0242 | 0.4129±0.0574 | 0.3284±0.0336 | 0.3783±0.0534 | 0.3592±0.0247 |
| 40 | 0.1007±0.0152 | 0.1013±0.0067 | 0.1049±0.0365 | 0.1059±0.0384 | 0.1068±0.0188 |
| 50 | 0.1012±0.0033 | 0.1119±0.0143 | 0.1231±0.0130 | 0.1082±0.0165 | 0.1209±0.0274 |
| 60 | 0.1223±0.0160 | 0.2379±0.0825 | 0.2569±0.1686 | 0.02219±0.0401 | 0.2164±0.0209 |
| 80 | 0.1050±0.0022 | 0.1348±0.0294 | 0.1359±0.0203 | 0.1161±0.0567 | 0.1253±0.0152 |
| High-dose group
(%) |
| 5 | 0.2542±0.0487 | 0.2709±0.0182 | 0.2682±0.0388 | 0.2802±0.0394 | 0.3019±0.2661 |
| 10 | 0.3501±0.0356 | 0.3831±0.0534 | 0.3479±0.0561 | 0.3892±0.0716 | 0.3775±0.0239 |
| 15 | 0.2029±0.0126 | 0.1902±0.0336 | 0.1839±0.0465 | 0.1969±0.0151 | 0.1713±0.0157 |
| 20 | 0.3314±0.0264 | 0.2969±0.0178 | 0.2704±0.0357 | 0.2981±0.0479 | 0.2729±0.0345 |
| 25 | 0.5634±0.0678 |
0.5723±0.0234a | 0.5164±0.0688 | 0.5569±0.0769 | 0.4912±0.0634 |
| 30 | 0.3413±0.0377 | 0.3369±0.0568 | 0.3235±0.0367 | 0.3524±0.0367 | 0.3583±0.0257 |
| 40 | 0.1507±0.0147 | 0.1359±0.0137 | 0.1309±0.0344 | 0.1359±0.0474 | 0.1284±0.0342 |
| 50 | 0.1249±0.0130 | 0.1189±0.0243 | 0.1479±0.0468 | 0.1192±0.0321 | 0.1709±0.0321 |
| 60 | 0.1573±0.0278 | 0.1372±0.0587 | 0.1289±0.2334 | 0.1224±0.1335 | 0.2159±0.0178 |
| 80 | 0.1109±0.0121 | 0.1289±0.0322 | 0.1274±0.0243 | 0.1208±0.0467 | 0.1321±0.0243 |
ANOVA was performed on the OD values for the HD, MD
and LD subgroups at the l-h time-point, with an added concentration
of 25%, as well as in the control group. The OD values for the HD
and LD subgroups were found to be significantly higher than those
for the control and MD groups (P<0.01), but no statistically
significant difference was found between the HD and LD groups
(P>0.05; Fig. 1).
Optimum KRMBTI-serum concentration
Analysis of covariance using KRMBTI-serum
concentrations as fixed factors revealed that the ALP values in
each group at 14 and 15 days were significantly higher than those
at 3, 7 and 10 days (P<0.05); however, no significant difference
was identified between the HD and LD groups (P>0.05). When
induction times were set as fixed factors, ALP values at a
concentration of 20% for the LD group and 30% for the HD group were
found to be significantly higher than those of other groups
(P<0.01), with no significant difference identified between any
other two groups (P>0.05). When KRMBTI-serum concentrations and
induction times were set as the covariants, the analysis showed
that P<0.05, which indicates that the KRMBT dose exhibited a
linear regression correlation with the KRMBTI-serum concentration
and treatment duration (Table
II).
 | Table IIEffect of dose, induction time and
concentration of kidney-reinforcing and marrow-beneficial
traditional Chinese medicine-intervened-serum on bone marrow
stromal cell differentiation. |
Table II
Effect of dose, induction time and
concentration of kidney-reinforcing and marrow-beneficial
traditional Chinese medicine-intervened-serum on bone marrow
stromal cell differentiation.
| Alkaline
phosphatase (U/l) |
|---|
|
|
|---|
| Parameters | 3 days | 7 days | 10 days | 14 days | 15 days |
|---|
| Low-dose group
(%) |
| 0 |
4.8975±0.0586a,b |
4.7546±0.0346a,b |
4.4281±0.3449a,b |
4.6847±0.0487a,b |
4.1469±0.0254a,b |
| 10 |
2.1063±1.2771a,b |
14.1718±1.2270a,b |
17.6483±1.5439a,b |
26.6462±3.3789a,b |
24.6012±1.2270a,b |
| 20 | 5.7873±0.9371 | 20.7154±1.4168 | 28.4867±2.3227 | 32.7812±2.7664 | 35.0307±1.6232 |
| 30 |
1.0839±1.2771a,b |
10.4061±1.2032a,b |
15.9518±0.5355a,b |
23.5787±2.1545a,b |
25.2147±1.2270a,b |
| High-dose group
(%) |
| 10 |
1.2883±0.6135a,b |
16.8302±2.1545a,b |
25.0102±2.3227a,b |
31.1452±2.1545a,b |
28.8957±2.4540a,b |
| 20 |
3.0538±0.9371a,b |
17.4438±1.8743a,b |
19.4888±1.8657a,b |
27.8732±1.7986a,b |
21.7381±1.8513a,b |
| 30 | 4.3558±1.2270 | 21.7659±0.9375 | 29.7136±2.1546 | 35.2352±0.9482 | 34.0082±0.9482 |
As shown in Fig. 2,
ALP activity in the HD group at the different concentrations and
time-points was higher than in the LD group at the corresponding
induction time-points. The ALP value peaked between 10 and 14 days
when the added concentrations were 20 or 30%, which would
significantly promote the osteogenic differentiation of BMSCs.
TGF-β1 expression
Univariate ANOVA analysis revealed that the TGF-β1
expression levels in the osteoblast, serum and comprehensive
induction groups at 14 days were significantly higher than those in
the control group (P<0.01). The TGF-β1 expression level in the
comprehensive induction group was higher than that in the other
groups, with a significant difference among the groups (P<0.01).
Furthermore, the TGF-β1 expression level in the osteoblast group
was significantly higher than that in the serum group (P<0.05;
Table III).
 | Table IIITGF-β1 expression in bone marrow
stromal cells in the different treatment groups. |
Table III
TGF-β1 expression in bone marrow
stromal cells in the different treatment groups.
| Group | TGF-β1 (pg/ml) |
|---|
| Control | 15.3992±3.1623 |
| Osteoblast |
41.7784±10.8950a,c |
| Serum |
31.7868±2.0429a |
| Comprehensive
induction |
69.8832±4.3906a,b,d |
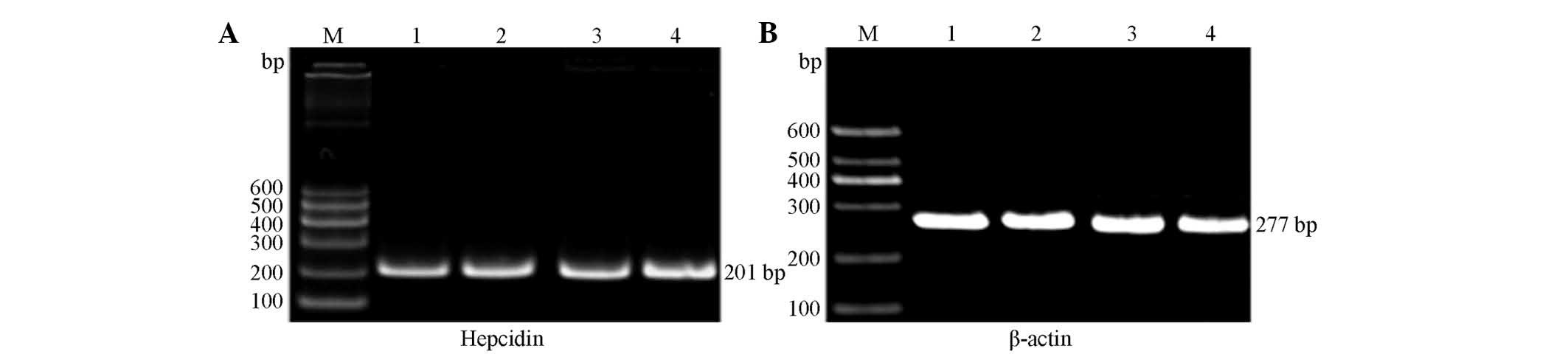
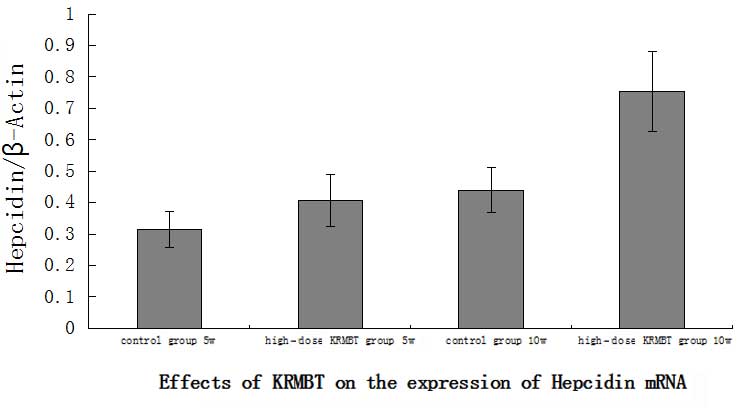
Hepcidin mRNA expression
The primer internal reference gene and target genes
of each group were subjected to RT-PCR amplification, which was
performed using rat liver tissue. RT-PCR analysis revealed two
bands at ~201 and 277 bp, as shown in Fig. 3. The image analysis software
indicated that the expression of hepcidin in the HD KRMBT group was
significantly higher than that in the control group. On the fifth
week, hepcidin expression in the HD group was ~1.3-fold higher than
that in the control group and on the tenth week it was ~1.8-fold
higher (Fig. 4).
Discussion
A number of scholars believe that supplementing
kidney tongbi and strengthening the body to eliminate pathogenic
factors are the basic principles in the treatment of bone
osteoporosis (9).
Oysters are naturally salty and are high in natural
vitamin D-like substances, iron and zinc, all of which enter the
kidneys (10,11). The CHCl3 extract of deer
antler has been reported to inhibit the activity of differentiated
osteoclasts and to regulate bone resorption (12). The aqueous extract, deer antler
aqua-acupuncture (DAA), is rich in antioxidant polyphenols, which
have been shown to regulate bone circulation and treat arthritis
(13). Furthermore, E.
brevicornum has been found to promote the activity of
osteoblasts and to inhibit the differentiation of osteoclasts, as
well as to promote the osteoblast differentiation of BMSCs
(14,15). Qian et al (12) reported that E. brevicornum
regulates core binding factor α1 expression. Icariside I and II and
epimedin B and C, which may be extracted from Epimedii Brevicornus,
have also been reported to promote in vitro osteoblast
proliferation and mineralization (15,16).
In the present study, various KRMBT groups,
including three dose groups and five blood sampling time-points
with final concentrations between 0 and 80% were investigated. A
dose-effect relationship was observed and the optimum BMSC
proliferation-promoting effect was found for serum sampled 1 h
after the administration of KRMBT, while the optimum KRMBTI-serum
concentration was observed to be 25%. These findings are consistent
with those of a previous study (17). The optimum BMSC osteogenic
differentiation-promoting serum concentration was between 20 and
30% in the HD group.
TGF-β is one of the most abundant cell growth
factors present in bone tissues. This factor not only promotes
osteoblast proliferation and differentiation, but also stimulates
bone formation, supports osteoclast formation and stimulates bone
resorption. TGF-β is an important coupling factor in the regulation
of bone formation and resorption (18,19).
In addition, TGF-β has an important function in inducing bone and
cartilage formation and calcification in different periods of
fracture healing (20). Shin et
al (21) and Ozmen et
al (22) demonstrated that Erk
channels are involved in the osteogenic differentiation of BMSCs,
and that TGF-β1 activates such channels. In the present study,
KRMBT was found to promote TGF-β1 expression.
Houtkooper et al (23) reported that lactoferrin promotes
the proliferation of osteoblasts and inhibits the activity of
osteoclasts in vivo. Moreover, when the calcium intake of
postmenopausal women is sufficient, iron intake levels have been
found to be positively correlated with bone mineral density
(23). Hepcidin, an ion regulator
isolated from human urine and named by Park et al (24) in 2001, has been reported to
regulate the absorption, utilization, storage and re-use of the
iron alternative pathway. Pigeon et al (25) confirmed that increased dietary iron
content or increased hepatic iron may increase liver hepcidin
expression. In the present study, hepcidin expression in the HD
KRMBT group was observed to be significantly higher than that in
the control group and was significantly higher on the tenth week
compared with the fifth week, indicating that KRMBT increased
hepcidin expression. This mechanism may be the underlying process
by which KRMBT treats osteoporosis.
References
|
1
|
Liu Z, Wang YF and Liu ZH: Research
progress in the association of glucocorticoid and osteoporosis.
Zhongguo Gu Zhi Shu Song Za Zhi. 16:713–718. 2010.(In Chinese).
|
|
2
|
Liu GW, Gao J, Guo HJ, et al: Effect of
invigorating kidney and nourishing essence Chinese medicine
containing serum on Wnt/β-catenin osteogenic differentiation signal
pathway in BMSCs in ovariectomized mice. Zhongguo Gu Zhi Shu Song
Za Zhi. 19:324–329. 2013.(In Chinese).
|
|
3
|
Zheng JC, Fan YG, Liu JR, et al:
Experimental study of directional differentiation of bone
mesenchymal stem cells (BMSCs) to osteoblasts guided by serum
containing Cistanche deserticola. Zhongguo Gu Shang. 23:606–608.
2010.(In Chinese).
|
|
4
|
Kerachian MA, Séguin C and Harvey EJ:
Glucocorticoids in osteonecrosis of the femoral head: a new
understanding of the mechanisms of action. J Steroid Biochem Mol
Biol. 114:121–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inoue S, Horii M, Asano T, et al: Risk
factors for nontraumatic osteonecrosis of the femoral head after
renal transplantation. J Orthop Sci. 8:751–756. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cui QJ, Wang GJ, Su CC and Balian G; The
Otto Aufranc Award. Lovastatin prevents steroid induced
adipogenesis and osteonecrosis. Clin Orthop Relat Res. 344:8–19.
1997. View Article : Google Scholar
|
|
7
|
Schilling T, Nöth U, Klein-Hitpass L, et
al: Plasticity in adipgenesis and osteogenesis of human mesenchymal
stem cells. Mol Cell Endocrinol. 271:1–17. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kazama T, Fujie M, Endo T and Kano K:
Mature adipocyte-derived dedifferentiated fat cells can
transdifferentiate into skeletal myocytes in vitro. Biochem Biophys
Res Commun. 377:780–785. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang XX, Zhang YL and Huang QF: Discussion
on the main pathogenesis in traditional Chinese medicine and
etiology about primary osteoporosis. Zhong Xi Yi Jie He Xue Bao.
8:1119–1123. 2010.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujita T, Ohue T, Fujii Y, Miyauchi A and
Takagi Y: Heated oyster shell-seaweed calcium (AAA Ca) on
osteoporosis. Calcif Tissue Int. 58:226–230. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Anić B and Grazio S: Current osteoporosis
treatment: reasons for adding vitamin D to alendronate. Reumatizam.
53:63–65. 2006.(In Croatian).
|
|
12
|
Ji JX, Qian J, Huang FJ, et al: Study on
the active principles and pharmacological actions of hairy antler.
Zhongguo Sheng Hua Yao Wu Za Zhi. 30:141–143. 2009.(In
Chinese).
|
|
13
|
Kim KH, Kim KS, Choi BJ, et al: Anti-bone
resorption activity of deer antler aqua-acupunture, the pilose
antler of Cervus korean TEMMINCK var. mantchuricus Swinhoe
(Nokyong) in adjuvant-induced arthritic rats. J Ethnopharmacol.
96:497–506. 2005. View Article : Google Scholar
|
|
14
|
Qian G, Zhang X, Lu L, Wu X, Li S and Meng
J: Regulation of Cbfa1 expression by total flavonoids of Herba
epimedii. Endocr J. 53:87–94. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen KM, Ge BF, Ma HP, et al: Effects of
icariin on the osteogenic differentiation of bone marrow
mysenchymal stem cells in vitro. Zhongguo Gu Zhi Shu Song Za Zhi.
14:642–645. 2008.(In Chinese).
|
|
16
|
Meng FH, Li YB, Xiong ZL, Jiang ZM and Li
FM: Osteoblastic proliferative activity of Epimedium brevicornum
Maxim. Phytomedicine. 12:189–193. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo X, Song M and Liu Y: Development of
the experimental research of the effects of the drug serum in herba
epimendii on osteoblast proliferation and differentiation. Zhongguo
Gu Zhi Shu Song Za Zhi. 14:818–822. 2008.(In Chinese).
|
|
18
|
Grainger DJ, Percival J, Chiano M and
Spector TD: The role of serum TGF-beta isoforms as potential
markers of osteoporosis. Osteoporos Int. 9:398–404. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nesti LJ, Caterson EJ, Li WJ, Chang R,
McCann TD, Hoek JB and Tuan RS: TGF-beta1 calcium signaling in
osteoblasts. J Cell Biochem. 101:348–359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arnott JA, Zhang X, Sanjay A, et al:
Molecular requirements for induction of CTGF expression by
TGF-beta1 in primary osteoblasts. Bone. 42:871–885. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shin MK, Kim MK, Bae YS, et al: A novel
collagen-binding peptide promotes ostegenic differentiation via
Ca2+/calmodulin-dependent protein kinase II/ERK/AP-1
signaling pathway in human bone marrow-derived mesenchymal stem
cells. Cell Singal. 20:613–624. 2008.
|
|
22
|
Ozmen B, Kirmaz C, Aydin K, Kafesciler SO,
Guclu F and Hekimsoy Z: Influence of the selective oestrogen
receptor modulator (raloxifene hydrochloride) on IL-6, TNF-alpha,
TGF-beta1 and bone turnover markers in the treatment of
postmenopausal osteoporosis. Eur Cytokine Netw. 18:148–153.
2007.PubMed/NCBI
|
|
23
|
Houtkooper LB, Stanford VA, Metcalfe LL,
Lohman TG and Going SB: Preventing osteoporosis the bone estrogen
strength training way. ACSMS Health Fit J. 11:21–27. 2007.
View Article : Google Scholar
|
|
24
|
Park CH, Valore EV, Waring AJ and Ganz T:
Hepcidin, a urinary antimicrobial peptide synthesized in the liver.
J Biol Chem. 276:7806–7810. 2001. View Article : Google Scholar
|
|
25
|
Pigeon C, Ilyin G, Courselaud B, Leroyer
P, Turlin B, Brissot P and Loréal O: A new mouse liver-specific
gene, encoding a protein homologous to human antimicrobial peptide
hepcidin, is overexpressed during iron overload. J Biol Chem.
276:7811–7819. 2001. View Article : Google Scholar
|