Introduction
It is common for immunosuppressed patients, such as
kidney (1) and hematopoietic stem
cell transplantation recipients (2),
to exhibit dyslipidemia. Such patients are likely to develop
lipoprotein modifications that have an atherogenic profile. It has
previously been indicated that mortality rates from cardiovascular
disease are up to 10-fold greater among immunosuppressed patients
than among the normal population (1). Guidelines for the management of
dyslipidemia recommend a low-fat diet, exercise and body weight
reduction, as well as reductions in alcohol consumption and smoking
(3). Dyslipidemia is also commonly
found in patients with malignancies. Total cholesterol and
low-density lipoprotein (LDL) levels among patients with breast
cancer have been shown to be significantly elevated as compared
with those in controls (4).
Dyslipidemia has also been observed in patients with esophageal
carcinoma (5), chronic myeloid
leukemia (CML) (6) or acute
myeloblastic leukemia (AML) (7). It
has been proposed that lipoproteins in the blood influence
carcinogenesis, as a result of their fundamental role in the
maintenance of cell integrity (8).
The exact mechanisms through which lipoproteins may contribute to
carcinogenesis remain unidentified.
A previous study indicated that levels of
oxidatively modified LDLs (oxLDLs) are elevated in the plasma of
patients with acute myocardial infarction as compared with those in
healthy individuals (9). The
initiation of atherosclerosis is mainly triggered by endothelial
dysfunction or activation elicited by oxLDL (10). oxLDL exerts numerous biological
activities that are crucial for atherogenesis, including inducing
the migration, proliferation and transformation of cells by
impairing the production of nitric oxide, activating T lymphocytes
and inducing endothelial-leukocyte adhesion molecules, smooth
muscle growth factors and proatherogenic genes (11,12).
oxLDL-induced activated monocytes upregulate transforming growth
factor (TGF-β) expression and increase disease progression
(13). In addition, the expression
of lectin-like oxidized LDL receptor 1 in endothelial cells and
monocytes has a critical function in plaque formation, progression
and thrombosis (14). These
aforementioned effects lead to fibrosis of the intimal vessels.
To date, the majority of studies on hyperlipidemia
have been performed in immunosuppressed patients who have undergone
a transplant. Although previous reports have revealed a close
association between oxLDL and the malignancy status, there is a
missing link between variations in the oxLDL and cytokine/chemokine
expression levels for different hematological diseases. We
hypothesized that the plasma levels of oxLDL in patients with
hematological malignancies are different from those in patients
with hematological diseases. We additionally hypothesized that
differences in oxLDL levels influence the expression of cytokines,
growth factors and inflammatory molecules, which play an important
role in inflammatory responses. To confirm the above hypothesis,
the aim of the present study was to evaluate the association
between the plasma oxLDL levels and the expression of cytokines,
chemokines and growth factors in 39 patients and 9 healthy
individuals. The patients were stratified into groups according to
the type of hematological disorder, namely CML, AML,
myelodysplastic syndrome (MDS) or iron deficiency anemia (IDA).
Materials and methods
Patients
The present study included 39 adult patients with
hematological disorders or malignancies. Patients who were
considered to have a hematological disorder included those patients
with MDS (n=8) or IDA (n=11), whereas patients who were considered
to have a hematological malignancy included those patients with AML
(n=9) or CML (n=11). All patients received the first diagnosis
based on clinical and laboratory features. Blood smears of their
bone marrow were extracted in the Department of Hematology of
Sichuan Provincial People's Hospital (Chengdu, China) between 2009
and 2012.
Nine normal healthy controls were selected from a
community screening examination in Chengdu, China. Informed consent
was obtained from all subjects. The study protocol was approved by
the local Ethics Review Committee.
Enzyme-linked immunosorbent assay
(ELISA) for oxLDL
The plasma of the patients and normal controls was
prepared through centrifugation (600 × g, 5 min) of the fresh blood
samples. An ELISA for oxLDL was performed with an oxLDL kit
(Diagnostic Systems Laboratories, Inc., Webster, TX, USA). Plasma
was diluted 1:1 and incubated in 96-well oxLDL-coated
microtitration strips for 30 min at room temperature, in accordance
with the manufacturer's instructions. The plates were then washed
three times and incubated with horseradish peroxidase for 30 min at
room temperature. Once the unbound conjugates had been removed by
washing the samples three times, tetramethylbenzidine was added to
the wells as a chromogenic substrate. The mixture was incubated for
10 min at room temperature in the dark. A stop solution was used to
terminate color development and the absorbance was measured at 450
nm within 30 min. A standard curve was constructed, using the
standards included in the kits, for the purpose of calculating the
oxLDL titer. The oxLDL concentration in the samples was quantified
in biomedical units, as defined by the manufacturer.
Quantitative polymerase chain reaction
(qPCR) analysis
Total RNA was extracted using RNeasy Mini kit
(Qiagen, Düsseldorf, Germany). cDNA was synthesized using ReverTra
Ace® qPCR RT kit (TOYOBO, Kagoshima, Japan), and the reverse
transcription conditions were 65°C for 5 min, followed by 37°C for
15 min and 98°C for 5 min. The qPCR was conducted using RealMaster
Mix (SYBR Green) [cat. no. FP202-01; Tiangen Biotech (Beijing) Co.,
Ltd., Beijing, China] in a Bio-Rad iCycler iQ™ Optical Module
(584BR; Bio-Rad, Hercules, CA, USA) with the following conditions:
One cycle of 95°C for 30 sec; 40 cycles of 95°C for 30 sec, 58°C
for 30 sec and 72°C for 30 sec; followed by 80 repeats of 55°C for
10 sec to collect melting curve data. The primers used for the PCR
assays are listed in Table I. GAPDH
was used as an internal control. Bio-Rad iQ5 software was used for
the analysis of the qPCR data, and GraphPad Prism 5 software
(GraphPad Software, Inc., San Diego, CA, USA) was used for the
construction of the figures.
 | Table I.Oligonucleotides used for the
quantitative polymerase chain reaction. |
Table I.
Oligonucleotides used for the
quantitative polymerase chain reaction.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | GenBank number |
|---|
| IL-5 |
AGCTGCCTACGTGTATGCCA |
GTGCCAAGGTCTCTTTCACCA | J03478 |
| IL-6 |
GACCCAACCACAAATGCCA |
GTCATGTCCTGCAGCCACTG | M14584 |
| IL-10 |
GGTGATGCCCCAAGCTGA |
TCCCCCAGGGAGTTCACA | U16720 |
| IL-11 |
CGAGCGGACCTACTGTCCTA |
GCCCAGTCAAGTGTCAGGTG | NM_000641 |
| IL-12 |
CGGTCATCTGCCGCAAA |
CAAGATGAGCTATAGTAGCGGTCCT | M65272 |
| IL-15 |
GACCCCACCAAAGCTGGAC |
TCACAGTGCTGCTGTCTGCTG | M90391 |
| IFN-α |
GGTGCTCAGCTGCAAGTCAA |
GCTACCCAGGCTGTGGGTT | J00207 |
| IFN-β |
CAGCAATTTTCAGTGTCAGAAGCT |
TCATCCTGTCCTTGAGGCAGT | M28622 |
| IFN-γ |
CCAACGCAAAGCAATACATGA |
CGCTTCCCTGTTTTAGCTGC | J00219 |
| TGF-β |
TATCGACATGGAGCTGGTGAAG |
CAGCTTGGACAGGATCTGGC | X02812 |
| TNF-α |
GGTGCTTGTTCCTCAGCCTC |
CAGGCAGAAGAGCGTGGTG | M10988 |
| iNOS |
CCAACAATGGCAACATCAGG |
TCGTGCTTGCCATCACTCC | L09210 |
| IP-10 |
TGAAATTATTCCTGCAAGCCAA |
CAGACATCTCTTCTCACCCTTCTTT | NM_001565 |
| MIP-1α |
AGCTGACTACTTTGAGACGAGCAG |
CGGCTTCGCTTGGTTAGGA | NM_002983 |
| MIP-1β |
CTGCTCTCCAGCGCTCTCA |
GTAAGAAAAGCAGCAGGCGG | NM_002984 |
| RANTES |
GACACCACACCCTGCTGCT |
TACTCCTTGATGTGGGCACG | NM_002985 |
| MCP-2 |
GTTTCTGCAGCGCTTCTGTG |
TGGCTGAGCAAGTCCCTGA | Y10802 |
| MCP-3 |
AGCAGAGGCTGGAGAGCTACA |
GGGTCAGCACAGATCTCCTTGT | NM_006273 |
| p53 |
CTTGCATTCTGGGACAGCCAAG |
CACGCAAATTTCCTTCCACTCGG | DQ892492 |
| cdc2 |
CAGGTTATATCTCATCTTTGAG |
GTTGAGTAACGAGCTGACCCC | AM393287 |
| ITBG9 |
GCAGTGACCGCTGGCCACTT |
GCGCACAAGGAGGAGCCGAA | NM_002207.2 |
| ICAM-1 |
CGAGCTTGGCTGTGGCCTCC |
TCTCCGCCATCCCAGCCTCC | NR_002947.1 |
| VCAM-1 |
AGGTGACGAATGAGGGGACCACA |
CCAGCCTCCAGAGGGCCACT | NM_001078.3 |
| CCL21 |
TGGTTCTGGCCTTTGGCA |
AGGCAACAGTCCTGAGCCC | NM_002989 |
| CCL24 |
AGCCTTCTGTTCCTTGGTGTCT |
GGGAGAGGGTATGACCACAGAG | NM_002991 |
| CCL25 |
CAGGAAGGTGTGTGGGAACC |
CGAGCATCCAGGAGCTTCA | NM_005624 |
| CCL27 |
GAGCCCAGACCCTACAGCAG |
GGCTTTCGGTAGAGCTGAGTACA | NM_006664 |
| GAPDH |
GAAGGTGAAGGTCGGAGTC |
GAAGATGGTGATGGGATTTC | J04038 |
Statistical analysis
All statistical analyses were performed using SPSS
16.0 (SPSS Inc., Chicago, IL, USA). The characteristics of the
patients are presented as the mean ± standard deviation. The t-test
was used to perform quantitative data analysis. P≤0.05 was
considered to indicate a statistically significant difference. The
data obtained from healthy volunteers were used as the control
data.
Results
Patient characteristics
Table II shows the
clinical and biochemical characteristics of the patients that took
part in the study. No significant differences were found in the
systolic blood pressure or the red blood cell, urea, total protein,
albumin, alanine aminotransferase, mean corpuscular volume and mean
corpuscular hemoglobin levels of the patients; however, the gender
distribution, age, diastolic blood pressure and white blood cell,
neutrophil, lymphocyte, hemoglobin, platelet, creatine, aspartate
aminotransferase and γ-glutamyl transpeptidase levels were
significantly different among the five groups.
 | Table II.Characteristics of the patients and
normal controls in the present study. |
Table II.
Characteristics of the patients and
normal controls in the present study.
|
Characteristics | AML, n=9 | CML, n=11 | MDS, n=8 | IDA, n=11 | NL, n=9 |
|---|
| Gender
(male/female) | 3/6 | 4/7 | 7/1 | 2/9 | 5/4 |
| Age
(years)a |
53.6±16.0 |
46.3±20.2 |
47.8±21.2 |
47.3±19.7 |
38.4±10.2 |
| SBP
(mmHg)a |
123.2±28.9 |
108±10.6 |
131.6±23.6 |
113.0±12.3 |
112.4±9.6 |
| DBP
(mmHg)a |
69.5±16.6 |
62.6±8.8 |
81.4±16.3 |
65.9±9.9 |
78.3±6.9 |
| Hypertension
(Y/N) | 1/8 | 0/11 | 2/8 | 1/11 | 0/9 |
| WBC
(109/l)a |
33.4±11.6 |
25.5±4.8 |
14.8±1.3 |
7.4±5.6 |
7.5±2.3 |
| Neutrophil
(%)a |
49.5±29.8 |
57.9±28.2 |
67.9±20.9 |
76.1±15.9 |
65.8±6.7 |
| Lymphocyte
(%)a |
47.9±22.1 |
26.2±11.2 |
12.6±6.7 |
15.8±5.8 |
11.4±2.3 |
| RBC
(1012/l)a |
2.3±0.9 |
3.1±0.8 |
2.1±0.3 |
3.1±1.8 |
2.6±1.1 |
| Hb
(g/l)a |
65.6±29.1 |
79.2±29.3 |
53.4±4.4 |
67.9±25.2 |
68.2±6.7 |
| PLT
(109/l)a |
50.6±6.6 |
77.9±58.8 |
48.1±57.5 |
203.6±22.1 |
69.3±12.2 |
| Urea
(mM)a |
4.9±0.8 |
5.2±1.9 |
5.8±3.0 |
5.3±4.4 |
4.6±0.8 |
| Creatine
(µM)a |
70.1±10.0 |
53.3±23.6 |
83.8±33.1 |
64.5±12.4 |
65.3±22.3 |
| TP
(g/l)a |
56.5±4.8 |
61.7±11.6 |
65.9±14.7 |
62.7±7.5 |
61.8±5.7 |
| ALB
(g/l)a |
38.2±3.4 |
37.7±7.3 |
38.7±14.2 |
37.1±6.2 |
36.2±5.2 |
| AST
(U/l)a |
23.5±10.4 |
62.1±7.8 |
28.0±16.6 |
15.8±7.8 |
19.4±2.3 |
| ALT
(U/l)a |
25.0±10.9 |
29.9±17.1 |
25.5±16.7 |
25.8±7.8 |
25.1±5.7 |
| GGT
(U/l)a |
93.7±11.4 |
50.3±8.3 |
40.0±30.1 |
14.5±7.1 |
22.5±5.6 |
| MCV
(fl)a |
97.0±4.4 |
87.3±7.9 |
93.4±8.6 |
84.9±21.5 |
87.2±15.3 |
| MCH
(pg)a |
32.7±2.2 |
27.8±3.7 |
31.0±3.5 |
32.5±2.5 |
31.6±8.3 |
Comparison of the serum oxLDL levels
among the five groups
The ELISA results showed that the plasma oxLDL
levels differed among the groups, with significant elevations in
the AML and CML groups compared with the control group
(P<0.001). The average plasma oxLDL levels in the AML and CML
groups were 350 and 260 µg, respectively, while in the MDS and IDA
groups the average levels were 120 and 130 µg, respectively, which
were comparable with the levels in the normal controls (Fig. 1).
Expression of cancer-related genes and
chemokines in patients with hematological disorders
In order to determine the association between the
oxLDL level and gene expression variation, qPCR was conducted. The
qPCR results indicated changes in the expression of genes
associated with the following biological processes: i) Cell cycle,
ii) immune and inflammatory responses, iii) defense response to
viruses and other pathogens, iv) regulation of cell proliferation,
v) regulation of cell migration, vi) leukocyte chemotaxis and vii)
cell adhesion.
p53 is a well-known anticancer gene. Suppressed
expression of p53 was found in all patients, as compared with the
normal controls (P<0.001) (Fig.
2). By contrast, the expression of the cell cycle gene
cyclin-dependent kinase 1 (cdc2) was significantly higher in
patients with hematological disorders than that in the normal
controls (P<0.001). These results indicated increased cell
proliferation in those patients (Fig.
2).
Notably, the detection of chemokine expression
showed the overexpression of chemokine (C-C motif) ligand-21
(CCL-21), CCL-24, CCL-25 and CCL-27 in patients with MDS or IDA
(P<0.001), while only a slight increase was observed in patients
with AML or CML (P<0.05) (Fig.
2). These results suggest that high levels of oxLDL suppress
the proinflammatory response in disease states.
Variation in the gene expression of
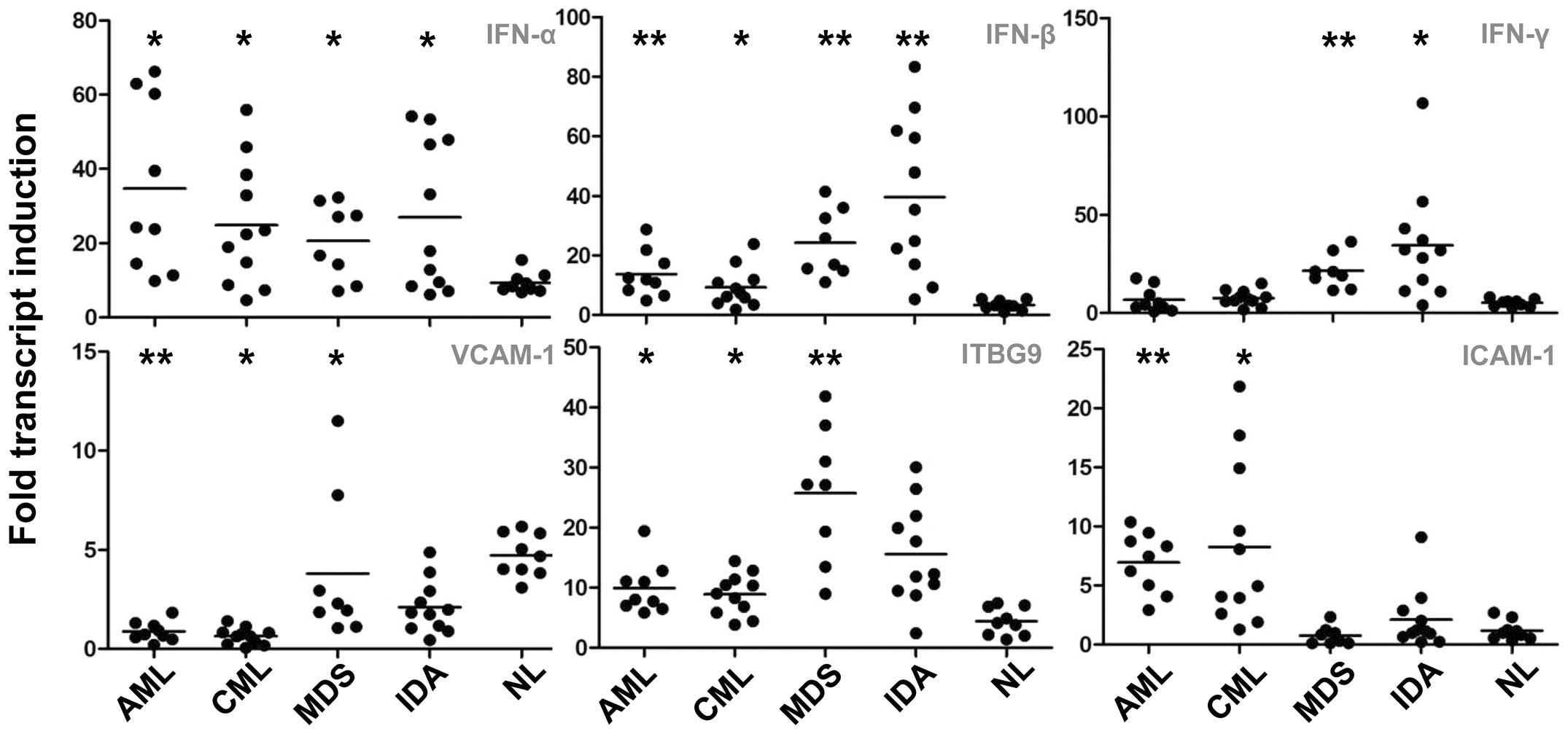
interferons (IFNs) and adhesion molecules
IFNs play an important role in the host defense
against viruses and other pathogens. Upon analysis of the three
major members of this family, IFN-α, -β and -γ, higher levels of
IFN-α and -β were found in the patients with hematological
disorders as compared with the normal controls (P<0.05)
(Fig. 3). The level of IFN-γ was
observed to be increased only in the MDS and IDA groups (P<0.05)
(Fig. 3).
With regard to the cell adhesion molecules, the
expression of the three most important families [intercellular
adhesion molecules (ICAMs), vascular cell adhesion molecules
(VCAMs) and integrin β (ITGB) 9] was assessed. When compared with
the expression in healthy volunteers, the expression of VCAM-1 was
downregulated in patients with tumors (P<0.05), while ITGB9 and
ICAM-1 expression was increased in patients with AML or CML
(Fig. 3).
Gene expression of interleukins (ILs)
is increased in patients with non-malignant disorders
ILs play a key role in inflammatory responses,
immunomodulation and cell proliferation. It was observed that,
while IL-10 and IL-15 expression in patients with AML or CML
remained the same as that in the normal controls, the expression of
IL-11 was reduced (P<0.05) (Fig.
4). The expression levels of IL-6, IL-12 and IL-5 were slightly
increased in patients with AML or CML (P<0.05) (Fig. 4) compared with levels in the
controls. Based on this observation, we hypothesized that the
activation of IL-related immune responses is suppressed in patients
with hematological carcinomas.
Gene expression of growth factors and
macrophage chemotaxis proteins
Compared with the control expression levels, the
expression of tumor necrosis factor-α (TNF-α) was found to be
significantly upregulated in the MDS and IDA groups (P<0.001),
while it was slightly increased in the AML and CML groups
(P<0.05). The TGF-β expression in the AML (P<0.05) and CML
(P<0.001) groups was greater than that in the controls, while in
the MDS and IDA groups it remained at a normal level. The
expression levels of monocyte chemotactic protein-2 (MCP-2),
macrophage inflammatory protein-1β (MIP-1β) and inducible nitric
oxide synthase (iNOS) were increased markedly in patients without
tumors (P<0.001), while a minor upregulation was observed in
those with malignancies (P<0.05). The MCP-3 expression levels
were only increased in the MDS (P<0.05) and IDA (P<0.001)
groups. MIP-1α, 10 kDa IFN-γ-induced protein and regulated on
activation normal T-cell expressed and secreted expression was
similarly upregulated in all the patient groups (Fig. 5).
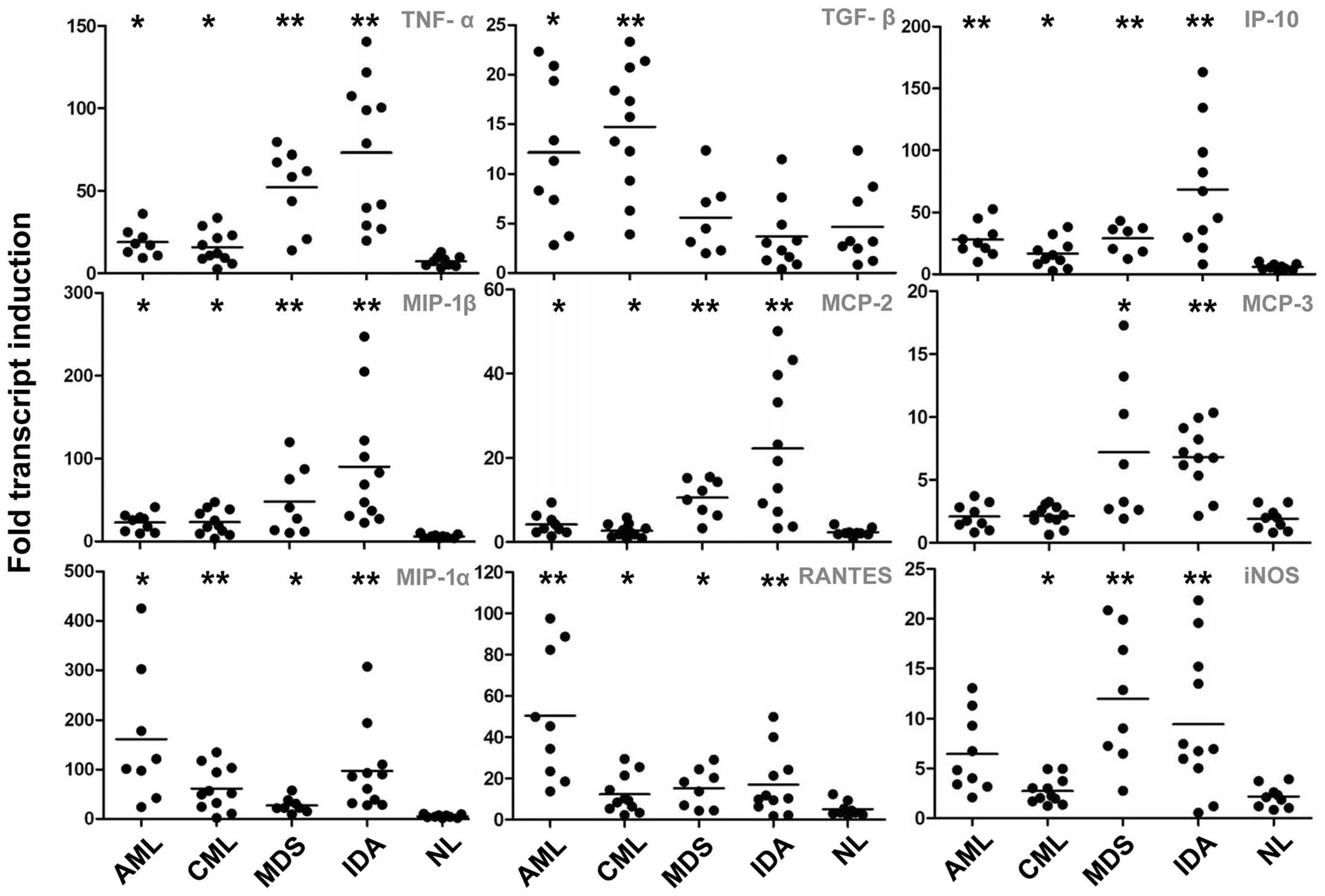 | Figure 5.Gene expression variation IV: Growth
factors and macrophage chemotactic proteins. *P<0.05 and
**P<0.001 versus the control group. AML, acute myeloblastic
leukemia; CML, chronic myeloid leukemia; MDS, myelodysplastic
syndrome; IDA, iron deficiency anemia; NL, normal control; TNF,
tumor necrosis factor; TGF, transforming growth factor; IP-10, 10
kDa interferon γ-induced protein; MIP, macrophage inflammatory
protein; MCP monocyte chemotactic protein; RANTES, regulated on
activation normal T-cell expressed and secreted; iNOS, inducible
nitric oxide synthase. |
Discussion
Hyperlipidemia is a common and serious problem among
adult renal and bone marrow stem cell transplantation recipients
and patients with malignancies, and it has been demonstrated that
the condition contributes to an increase in the risk of
cardiovascular diseases and elevated morbidity and mortality within
these populations (15). Previous
research has demonstrated an association between plasma/serum
lipoproteins and different types of cancers, such as breast,
prostate and gynecological cancer and acute lymphoblastic leukemia
(8). The main question is whether
dyslipidemia predisposes one to cancer or whether it is an effect
of the malignancy.
Previous studies have found that oxLDL plays an
important role in various types of malignancies (16,17).
oxLDL expression has been shown to decrease during the progression
of esophageal cancer (16), while
Petridou et al (17) have
reported an association between adiponectin expression and
childhood myeloblastic leukemia. A study using an animal model has
suggested that a high oxLDL level is closely associated with
carcinogenesis (18). Although
conflicting data have been reported regarding the link between
oxLDL level and the risk of cancer-related mortality (19,20),
accumulating evidence suggests that oxLDL induces the production of
leukocyte adhesion molecules and chemokines and decreases the
release of nitric oxide, which is associated with endothelial
dysfunction (11). It has also been
shown that oxLDL may be involved in atherogenesis and
atherothrombosis by monocyte activation and the accumulation of
foam cells (21).
Cytokines and chemokines, as well as numerous other
growth factors and adhesion molecules, play an important role in
carcinoma progression and inflammatory responses. The progression
of carcinomas is closely associated with the unusual expression of
several cytokines and growth factors; however, there are no reports
regarding the link between plasma oxLDL levels and
cytokine/chemokine expression in patients with hematological
disorders. In the present study, patients were divided into four
groups: Two groups with malignancies (AML and CML) and two groups
with nonmalignant disorders (MDS and IDA). The findings of the
study demonstrated that the plasma oxLDL levels were significantly
higher in the AML and CML groups as compared with those in the
non-carcinoma groups (MDS and IDA) and the normal controls. This
result is consistent with the results of a previous study, in which
the oxLDL serum levels in patients with hepatocellular carcinoma
and squamous cell carcinoma of the esophagus were lower than those
in the normal controls (22).
Under pathological conditions, a number of immune
response-related factors are upregulated and secreted in response
to the hostile environment. In the qPCR analysis that was performed
in the present study, the upregulation of p53 and the
downregulation of cdc2 in patients with hematological disorders
suggested that these patients had a decreased antitumor effect and
increased cell proliferation. The most noteworthy result of this
study was that the activation of numerous proinflammatory genes,
including CCL-21, CCL-24, CCL-27, IFN-β, IFN-γ, IL-5, IL-6, IL-10,
IL-15, TNF-α, MCP-2, MCP-3 and iNOS, was inhibited in the patients
with cancer (the AML and CML groups). Among these factors, IFN-β
and IFN-γ are known to mediate antitumor effects, and TNF-α is
known to inhibit tumor progression by inducing the apoptosis of
cancer cells (23). It remains to be
determined whether these differences were due to the dyslipidemic
condition.
There is considerable evidence suggesting that lipid
peroxidation products, including oxLDL and anti-oxLDL
autoantibodies, play a key role in cancer development (24,25).
High serum levels of oxLDL could lead to uncontrolled cell
proliferation and reduced apoptosis, contributing to carcinogenesis
induction. oxLDL increases the activity of protein kinase-C, which
is a serine-threonine-kinase that is involved in the cytoplasmic
transduction of the mytogenic signaling pathway (26). oxLDL is also able to induce the
production and delivery of other growth factors, such as
platelet-derived growth factor, possibly through IL-1 and TNF-α,
thereby regulating tumor growth (27).
A number of contradictory results, such as the
downregulation of VCAM-1 and upregulation of ICAM 1, were found in
the present study. The same was also found for IL-5, IL-11 and
IL-12, in that the results were not consistent in the patients with
cancer. It is possible, however that these contradictions were due
to the small number of patients in each study group. Another
limitation was that only gene expression detection was used. It is
possible that protein expression and secretion studies would
enhance the results. The results of the current study suggest a
close association between high oxLDL levels and the status of
patients with hematological disorders. Further research is required
to delineate the detailed mechanisms of the oxLDL-mediated
regulation of disease.
Acknowledgements
This study was supported by the National Basic
Research Program of China (grant no. 2009CB522401). The authors
would like to thank the nursing staff for their support in this
clinical study.
References
|
1
|
Chadban S, Chan M, Fry K, et al: CARI: The
CARI guidelines. Nutritional management of dyslipidaemia in adult
kidney transplant recipients. Nephrology (Carlton). 15:Suppl 1.
S62–S67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Griffith ML, Savani BN and Boord JB:
Dyslipidemia after allogeneic hematopoietic stem cell
transplantation: Evaluation and management. Blood. 116:1197–1204.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tonkin A, Barter P, Best J, et al:
National Heart Foundation of Australia; Cardiac Society of
Australia and New Zealand: National Heart Foundation of Australia
and the Cardiac Society of Australia and New Zealand: Position
statement on lipid management - 2005. Heart Lung Circ. 14:275–291.
2005.PubMed/NCBI
|
|
4
|
Ray G and Husain SA: Role of lipids,
lipoproteins and vitamins in women with breast cancer. Clin
Biochem. 34:71–76. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diao Y, Li H, Li H, et al: Association of
serum levels of lipid and its novel constituents with the different
stages of esophageal carcinoma. Lipids Health Dis. 8:482009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gottardi M, Manzato E and Gherlinzoni F:
Imatinib and hyperlipidemia. N Engl J Med. 353:2722–2723. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Diao YT, Li HQ, et al: The
association between serum levels of oxLDL-IgG and oxLDL-IgM
autoantibody with adult acute myeloblastic leukaemia. Lipids Health
Dis. 9:112010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bielecka-Dąbrowa A, Hannam S, Rysz J and
Banach M: Malignancy-associated dyslipidemia. Open Cardiovasc Med
J. 5:35–40. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tatsuguchi M, Furutani M, Hinagata J, et
al: Oxidized LDL receptor gene (OLR1) is associated with the risk
of myocardial infarction. Biochem Biophys Res Commun. 303:247–250.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen M, Masaki T and Sawamura T: LOX-1,
the receptor for oxidized low-density lipoprotein identified from
endothelial cells: Implications in endothelial dysfunction and
atherosclerosis. Pharmacol Ther. 95:89–100. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Castelló IB: Hyperlipidemia: A risk factor
for chronic allograft dysfunction. Kidney Int Suppl. 73–77. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Drüeke TB, Abdulmassih Z, Lacour B, Bader
C, Chevalier A and Kreis H: Atherosclerosis and lipid disorders
after renal transplantation. Kidney Int Suppl. 31:S24–S28.
1991.PubMed/NCBI
|
|
14
|
Mehta JL, Chen J, Hermonat PL, Romeo F and
Novelli G: Lectin-like, oxidized low-density lipoprotein receptor-1
(LOX-1): A critical player in the development of atherosclerosis
and related disorders. Cardiovasc Res. 69:36–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Silverstein DM, Palmer J, Polinsky MS,
Braas C, Conley SB and Baluarte HJ: Risk factors for hyperlipidemia
in long-term pediatric renal transplant recipients. Pediatr
Nephrol. 14:105–110. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Li H, Diao Y, et al: Relationship
between oxidized LDL antibodies and different stages of esophageal
carcinoma. Arch Med Res. 39:760–767. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petridou E, Mantzoros CS, Dessypris N,
Dikalioti SK and Trichopoulos D: Adiponectin in relation to
childhood myeloblastic leukaemia. Br J Cancer. 94:156–160. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chung FL, Nath RG, Ocando J, Nishikawa A
and Zhang L: Deoxyguanosine adducts of t-4-hydroxy-2-nonenal are
endogenous DNA lesions in rodents and humans: Detection and
potential sources. Cancer Res. 60:1507–1511. 2000.PubMed/NCBI
|
|
19
|
Eichholzer M, Stähelin HB, Gutzwiller F,
Lüdin E and Bernasconi F: Association of low plasma cholesterol
with mortality for cancer at various sites in men: 17-y follow-up
of the prospective Basel study. Am J Clin Nutr. 71:569–574.
2000.PubMed/NCBI
|
|
20
|
Iribarren C, Reed DM, Burchfiel CM and
Dwyer JH: Serum total cholesterol and mortality. Confounding
factors and risk modification in Japanese-American men. JAMA.
273:1926–1932. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Puccetti L, Pasqui AL, Bruni F, et al:
Lectin-like oxidized-LDL receptor-1 (LOX-1) polymorphisms influence
cardiovascular events rate during statin treatment. Int J Cardiol.
119:41–47. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uchida K: 4-Hydroxy-2-nonenal: A product
and mediator of oxidative stress. Prog Lipid Res. 42:318–343. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vicari AP and Caux C: Chemokines in
cancer. Cytokine Growth Factor Rev. 13:143–154. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guyton KZ and Kensler TW: Oxidative
mechanisms in carcinogenesis. Br Med Bull. 49:523–544.
1993.PubMed/NCBI
|
|
25
|
Wiseman H and Halliwell B: Damage to DNA
by reactive oxygen and nitrogen species: Role in inflammatory
disease and progression to cancer. Biochem J. 313:17–29.
1996.PubMed/NCBI
|
|
26
|
Kelly K, Cochran BH, Stiles CD and Leder
P: Cell specific regulation in normal and neoplastic cells. Adv
Cancer Res. 56:1–48. 1990.
|
|
27
|
Oberhammer F, Bursch W, Parzefall W, et
al: Effect of transforming growth factor beta on cell death of
cultured rat hepatocytes. Cancer Res. 51:2478–2485. 1991.PubMed/NCBI
|