Introduction
One of the most commonly affected sites in a number
of diseases is the neuromuscular synapse. The neuromuscular
junction is involved in various conditions, such as autoimmune
diseases, genetic polymorphisms and toxic disorders. The most
prominent symptom in all such conditions is muscular weakness,
which is caused by the interference of acetylcholine-mediated
transmission of signals from the presynaptic nerve to the skeletal
muscle, which subsequently impairs muscle contraction (1). Autoimmune, genetic and toxic conditions
are known to affect this transmission either presynaptically or
postsynaptically (2).
Lambert-Eaton myasthenic syndrome (LEMS) is one such
autoimmune disease. This syndrome, also termed myasthenic syndrome,
affects acetylcholine release at the presynaptic membrane of the
neuromuscular junction (3). LEMS can
be divided into tumorous LEMS (T-LEMS) and non-tumorous LEMS
(NT-LEMS) forms (4). The condition
is typically recognized as a paraneoplastic syndrome associated
with small cell lung carcinoma (SCLC); the association of LEMS with
other neuroendocrine lung tumors, including carcinoids or large
cell lung carcinoma, is highly unusual (5). T-LEMS is more often observed in a
clinical setting compared with NT-LEMS, and SCLC is the most
commonly associated type of tumor in patients with T-LEMS. However,
the clinical observation of mediastinal small cell cancer in
association with LEMS is extremely rare. A number of patients are
commonly misdiagnosed, resulting in delayed treatment (6). The aim of the present case report was
to provide further understanding with regard to the diagnosis and
treatment of T-LEMS. Thus, we herein describe a rare case of LEMS
in association with an atypical mediastinal carcinoid tumor, which
exhibited transient clinical and electrophysiological remission
following surgical resection, chemotherapy and radiotherapy
(7).
Case report
A 53-year-old man was admitted to the Bethune First
Hospital (Changchun, China) with a 3-month history of weakness in
the lower extremities that had become aggravated during the
previous 20 days. Three months earlier, the patient had begun to
feel weakness in the lower limbs, extending from the inguinal
region downward to the knees, without a known muscle weakness. The
patient reported aggravation of this weakness during the previous
20 days, most notably a lack of strength in the two lower limbs,
although the patient was able to walk unaided. Weakness of the
upper limbs was also experienced, although the patient was able to
hold things independently. The patient also reported the symptom of
a dry mouth. No breathing difficulties or drooping eyelids were
observed. The patient had a >20-year smoking history involving
an average of 20 cigarettes per day, as well as a >20-year
history of occasional alcohol use.
A physical examination revealed no abnormalities of
consciousness, language fluency, memory, orientation or calculation
ability. Bilateral pupil dilation was present, and the pupils were
round, of the same size, and sensitive to light. Both eyeballs
moved freely without nystagmus. The bilateral nasolabial grooves
were deep, and the patient's tongue was oriented along the midline
when protruded. The muscle tension in the limbs was normal. More
specifically, the muscle strength in the upper limbs was normal,
and the muscle strength in the lower limbs was graded as 4+,
indicating that the patient was able to resist gravity and perform
a resistance movement with 75% of normal muscle strength. The
patient exhibited no marked ataxia, and the pathological reflex was
negative for the lower limbs. No evident abnormalities were
identified during the whole-body examination.
An auxiliary examination revealed no abnormalities
in the thyroid function test, serum electrolyte levels or tumor
marker levels. In addition, no marked findings were observed with
the electrocardiography or magnetic resonance imaging of the head,
cervical vertebrae and thoracic vertebrae. The neostigmine test
result was negative. With regard to the electromyography (EMG),
low-frequency repetitive nerve stimulation (RNS) resulted in a
>15% reduction in the compound muscle action potential (CMAP)
amplitude, and high-frequency RNS resulted in a >100% increase
in the CMAP amplitude. The EMG examination demonstrated a typical
presynaptic membrane neuromuscular transmission obstacle, which was
in accordance with LEMS. A chest computed tomography (CT) scan
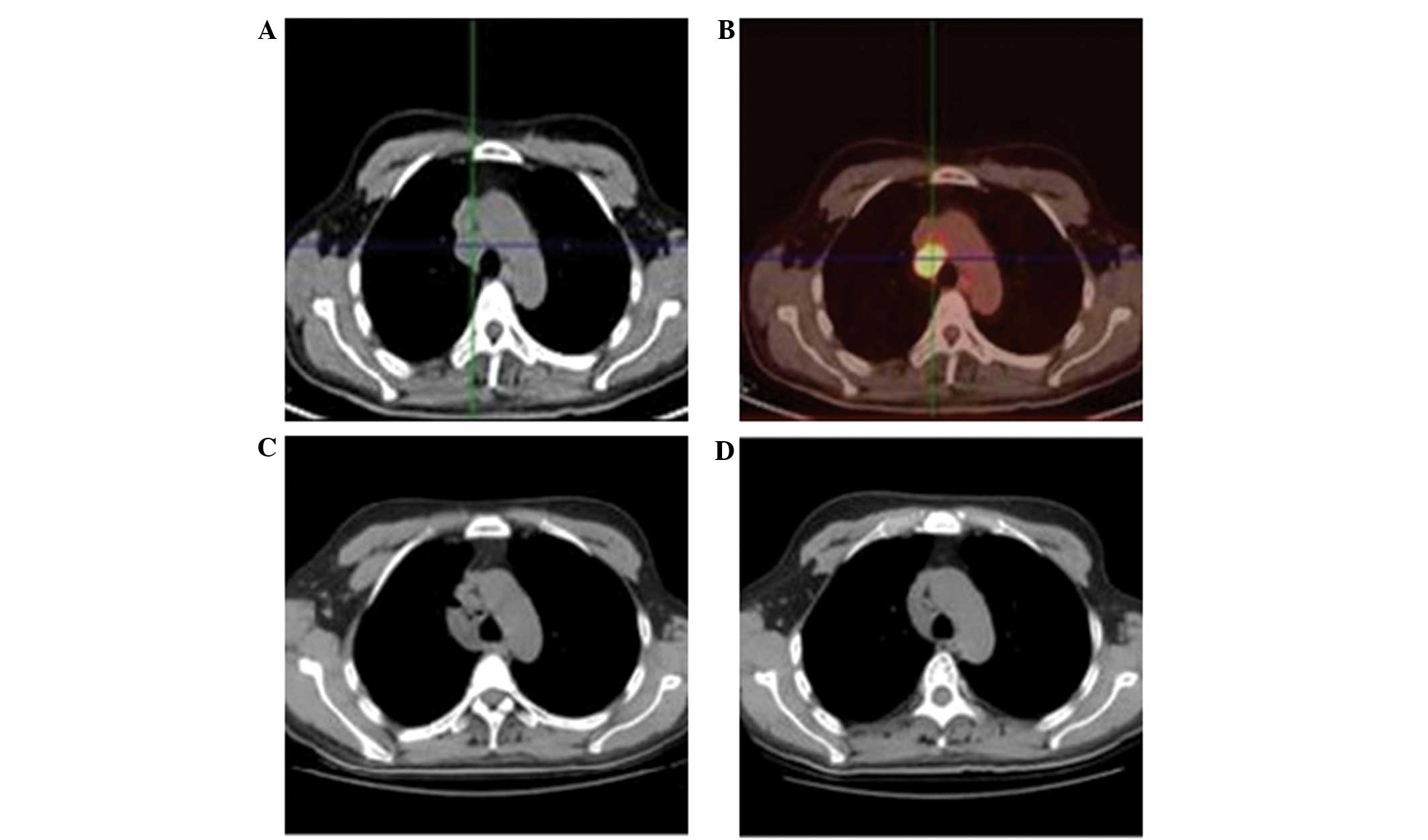
revealed no abnormalities. However, positron emission tomography
(PET)-CT (Fig. 1A and B) revealed
enlargement and hypermetabolic activity of multiple lymph nodes
between the vena cava and trachea, which indicated the presence of
inflammation. No other abnormalities were identified from the
scans. Therefore, the patient was diagnosed with LEMS. Following
the hospitalization period, a mediastinal lymph node biopsy under
thoracoscopic guidance was performed due to the absence of
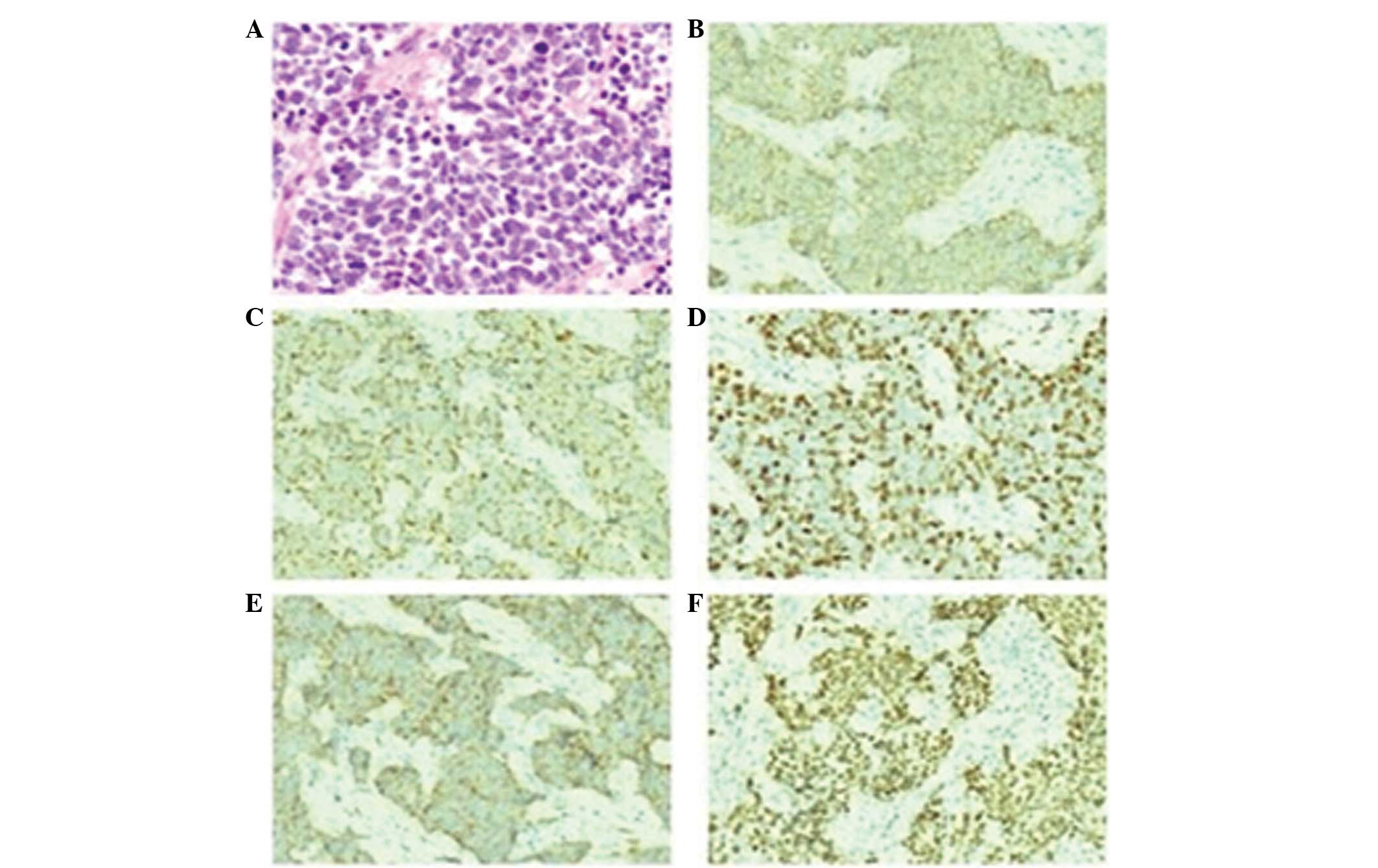
histological pathology. The pathology report indicated a high
prevalence of small cell neuroendocrine carcinoma (Fig. 2A). Hematoxylin and eosin staining
revealed oat cells and short spindle cells, nuclear
hyperchromatism, and indistinct nucleoli. The immunohistochemistry
results were as follows: Cluster of differentiation (CD)56 (+;
Fig. 2B), cytokeratin (CK; +;
Fig. 2C), Ki-67 (+50, meaning that
50% of the cells exhibited Ki-67 expression; Fig. 2D), synaptophysin (+; Fig. 2E), thyroid transcriptional factor-1
(+; Fig. 2F), epithelial membrane
antigen (+), neuron-specific enolase (+), P63 (weakly +), leukocyte
common antigen (–), CD99 (–), vimentin (–) and CK5/6 (–).
Immunohistological figures for epithelial membrane antigen,
neuron-specific enolase, P63, leukocyte common antigen, CD99,
vimentin and CK5/6 not shown due to poor image quality.
Based on the aforementioned observations, the
patient was clinically diagnosed with LEMS accompanied with
mediastinal small cell carcinoma. The patient underwent one course
of chemotherapy, consisting of 80 mg/m2 cisplatin on day
1 and 100 mg/m2 etoposide on days 1–3; however, the
patient failed to respond. Subsequently, the patient underwent a
different chemotherapy regimen, which significantly improved the
symptoms. This regimen consisted of 80 mg/m2 cisplatin
on day 1, 100 mg/m2 etoposide on days 1–3 and 1.2
g/m2 ifosfamide on days 1–3. This second regimen was
administered four times, with each course lasting four days and a
21-day interval between each course. Written informed consent was
obtained from the patient. The primary lesion was not identified on
the lung CT images following the treatment. Additionally, the
mediastinal lymph nodes were clearly diminished (Fig. 1C and D). Subsequently, the patient
underwent two courses of radiotherapy (40 Gy/22f/6W).
Discussion
LEMS is an autoimmune disease involving the
dysregulation of acetylcholine release at the presynaptic membrane
of the neuromuscular junction (3).
The symptoms of the condition resemble those of myasthenia gravis.
LEMS is often associated with tumors, and 50–60% of patients with
LEMS have an underlying tumor (T-LEMS). SCLC has been observed in
90% of T-LEMS cases. By contrast, NT-LEMS is often associated with
the human leukocyte antigen-B8-DR3 haplotype and a variety of other
autoimmune diseases, such as rheumatoid arthritis, pernicious
anemia, thyroid disorders and Sjögren's syndrome (7).
In the majority of cases, LEMS involves muscle
fatigue and weakness of the limbs and trunk. Lower limb weakness is
more common compared with upper limb weakness; similarly, the
proximal aspects of the limbs are more commonly affected than the
distal aspects. Dominant cranial nerve muscles are not generally
involved. A small number of patients have symptoms of drooping
eyelids, double vision, difficulty swallowing and dysarthria.
Muscle weakness is felt in the resting state, and strength
increases after a few seconds due to the vigorous contracting of
muscles. Evidence suggests that half of patients with LEMS also
exhibit disruption of the autonomic nervous system. These
individuals may experience a dry mouth, impotence and impaired tear
secretion and sweating (8).
Furthermore, in a small number of patients, an impaired performance
of certain regions of the central nervous system, such as the
cerebellum, can be observed (4).
In the majority of patients with LEMS, muscle
weakness appears prior to symptoms of cancer. There have only been
a few cases where tumors have been diagnosed earlier than LEMS
(9). Therefore, careful screening to
detect any possible associated cancers at an early stage is a
crucial step in optimal disease management (7).
A timely diagnosis of LEMS is crucial not only to
ensure proper treatment of the neurological disease, but also to
detect any underlying tumors early in the course of the disease.
However, early diagnosis is often hampered by the rarity of LEMS
and its fluctuating symptoms (4).
Even when the patient's symptoms have been properly associated with
a neuromuscular transmission disorder, LEMS is often misdiagnosed
as myasthenia gravis. Clinical suspicion of LEMS should be
confirmed with an EMG. Although weakness is more evident in the
proximal limbs, electrophysiological abnormalities are more easily
detected in the distal muscles. Therefore, the diagnosis of LEMS is
based on the presence of the typical triad, comprising a low CMAP
amplitude at rest (0.1–6.0 mV), a further decrease in the CMAP
amplitude during low-frequency RNS (2–5 Hz), and an increase in the
CMAP amplitude during high-frequency RNS (20–50 Hz) or immediately
after a brief maximal voluntary contraction (15–20 sec). The latter
(an increase in the CMAP amplitude during high-frequency RNS or
immediately after a brief maximal voluntary contraction) is the
technique of choice due to the higher level of tolerance (7). A ≥100% increase in the CMAP
amplitude/area (post-tetanic/postexercise facilitation) in at least
two tested muscles is fairly typical of LEMS, with a sensitivity
ranging between 84 and 96% (4).
Low-frequency RNS during maximal contraction may increase the
sensitivity of the EMG. In addition, single-fiber EMG may be more
sensitive compared with RNS; however, the technique does not
distinguish between myasthenia gravis and LEMS (5). In the present case, the EMG results
revealed that the CMAP amplitude with low-frequency RNS diminished
by >15%, while that with high-frequency RNS increased by
>100%.
Antibodies targeting the P/Q-type voltage-gated
calcium channel (VGCC) are present in 75–90% of patients with LEMS.
In patients with T-LEMS, this rate can further increase to 100%,
and the condition is generally associated with high antibody titers
(4). Research has indicated that
monitoring the anti-P/Q-type VGCC serum antibody titer may be
important for evaluating the efficacy of chemotherapy for LEMS
associated with SCLC (10). Based on
the two aforementioned positive findings, the patient in the
current study was definitively diagnosed with LEMS with mediastinal
small cell cancer.
Treatment of T-LEMS should initially target the
malignant tumor. Thus, surgical resection should be performed to
remove the primary tumor, followed by chemotherapy and radiation
therapy. The symptoms of LEMS, including muscle weakness, have been
reported to improve following this treatment regimen (4).
In patients with T-LEMS, treatment of the associated
tumor generally induces a significant improvement in the
neurological symptoms. Severely affected patients requiring a rapid
therapeutic response may benefit from high-dose prednisone in
combination with plasmapheresis or intravenous immunoglobulin
(11). However, the use of
immunosuppressants other than steroids in patients with T-LEMS is
controversial; the general trend is to avoid such agents as much as
possible, although there is no definite contraindication to
azathioprine treatment (7).
In the present case report, the patient underwent a
chemotherapy regimen described in an earlier study (4). Briefly, 80 mg/m2 cisplatin
was administered on day 1 and 100 mg/m2 etoposide was
administered on days 1–3. However, the patient failed to respond.
Subsequently, the patient underwent a different chemotherapy
regimen, consisting of 80 mg/m2 cisplatin on day 1, 100
mg/m2 etoposide on days 1–3 and 1.2 g/m2
ifosfamide on days 1–3, which significantly improved the symptoms
of myasthenia. Furthermore, the mediastinal lymph nodes were found
to have clearly diminished. The patient completed four courses of
chemotherapy and two courses of radiotherapy (40 Gy/22f/6W).
Following treatment, the muscle strength returned to normal, and
the patient was able to freely move his limbs during normal
activities. The patient is continuing to undergo follow-up.
The present case report serves as a reminder that in
patients with T-LEMS, muscle weakness occurs prior to the
presentation of tumor-related symptoms. Once a patient is diagnosed
with LEMS, the selection of the most appropriate treatment is
dependent upon the correct identification of the underlying cause,
which may be a tumor or an additional autoimmune disease.
In conclusion, routine examinations, including chest
CT, PET-CT, fiber bronchoscopy and pathological examination, can
lead to a definitive diagnosis of T-LEMS. In cases of successful
post-treatment tumor regression, the patient should undergo regular
follow-up examinations over a rational period of time to ensure the
early detection of tumor recurrence.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81272607).
Glossary
Abbreviations
Abbreviations:
|
LEMS
|
Lambert-Eaton myasthenic syndrome
|
|
T-LEMS
|
tumorous Lambert-Eaton myasthenic
syndrome
|
|
NT-LEMS
|
non-tumorous Lambert-Eaton myasthenic
syndrome
|
|
SCLC
|
small cell lung carcinoma
|
|
EMG
|
electromyography
|
|
RNS
|
repetitive nerve stimulation
|
|
CMAP
|
compound muscle action potential
|
|
CT
|
computed tomography
|
|
PET-CT
|
positron emission tomography-computed
tomography
|
|
CK
|
cytokeratin
|
|
CD
|
cluster of differentiation
|
|
VGCC
|
voltage-gated calcium channel
|
References
|
1
|
Lee JH, Shin JH, Kim DS, et al: A case of
Lambert-Eaton myasthenic syndrome associated with atypical
bronchopulmonary carcinoid tumor. J Korean Med Sci. 19:753–755.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilhus NE: Myasthenia and the
neuromuscular junction. Curr Opin Neurol. 25:523–529. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tomiyasu K, Ito H, Kanazawa N, Saito T and
Kowa H: Anti-Hu antibody in a patient with Lambert-Eaton myasthenic
syndrome and early detection of small cell lung cancer. Intern Med.
34:1082–1085. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
LoMonaco M, Milone M, Padua L and Tonali
P: Combined low-rate nerve stimulation and maximal voluntary
contraction in the detection of compound muscle action potential
facilitation in Lambert-Eaton myasthenic syndrome. Muscle Nerve.
20:1207–1208. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Titulaer MJ, Lang B and Verschuuren JJ:
Lambert-Eaton myasthenic syndrome: from clinical characteristics to
therapeutic strategies. Lancet Neurol. 10:1098–1107. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martín-García E, Mannara F,
Gutiérrez-Cuesta J, et al: Intrathecal injection of P/Q type
voltage-gated calcium channel antibodies from paraneoplastic
cerebellar degeneration cause ataxia in mice. J Neuroimmunol.
261:53–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu X, Wang J, Liu Y and Liu K: Clinical
presentation and differential diagnosis of Lambert-Eaton myasthenic
syndrome. Neurosciences (Riyadh). 18:169–172. 2013.PubMed/NCBI
|
|
8
|
Nakamur S, Kawagishi Y, Kato S, et al:
Long-term survival case of small-cell lung cancer with
Lambert-Eaton myasthenic syndrome without anticancer therapy. Nihon
Kokyuki Gakkai Zasshi. 48:918–922. 2010.(In Japanese). PubMed/NCBI
|
|
9
|
Baggi F, Ubiali F, Nava S, et al: Effect
of IgG immunoadsorption on serum cytokines in MG and LEMS patients.
J Neuroimmunol. 201–202:104–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsukiji J, Kaneko T, Saito H, et al: A
case of cT0N2M0 small cell lung cancer with Lambert-Eaton
myasthenic syndrome. Nihon Kokyuki Gakkai Zasshi. 42:820–824.
2004.(In Japanese). PubMed/NCBI
|
|
11
|
Oh SJ, Kurokawa K, Claussen GC and Ryan HF
Jr: Electrophysiological diagnostic criteria of Lambert-Eaton
myasthenic syndrome. Muscle Nerve. 32:515–520. 2005. View Article : Google Scholar : PubMed/NCBI
|