Introduction
As an effective treatment for invasive bladder
tumors, bladder reconstruction, which predominantly comprises ileal
neobladder and ileal-colon neobladder in situ, has a serious
side effect in that the intestinal mucus secretion function remains
(1). Although intestinal villi and
microvilli gradually shrink and the number of goblet cells reduce
with time (2,3), complications associated with the
orthotopic neobladder in the early postoperative period, caused by
a cork of mucus secretion, remain a challenging problem in clinical
practice.
Previously, the effects of several antitumor agents
on ileum mucus secretion in rats was studied, and the results
revealed that mitomycin-C (MMC) inhibited the mucus secretion of
goblet cells in vitro (4). In
the present study, the administration of different MMC dosages into
rat bladder substitution models was investigated with the aim of
determining a suitable MMC dosage for local application.
Materials and methods
Establishment of the rat bladder
substitution models
A total of 50 healthy Sprague Dawley (SD) rats
(weight, 250±20 g) were purchased from the Experimental Animal
Center of Dalian Medical University (Dalian, China). The SD rats
were randomly separated into five groups, which included the
control or sham operation group (Sham, n=10), the normal saline
(NS) group (n=10), the high-dose MMC (HMMC) group (0.6 mg/ml,
n=10), the low-dose MMC (LMMC) group (0.4 mg/ml, n=10) and the
dehydrated alcohol (DA) group (n=10). The animal experiments were
approved by the Animal Research Ethics Committee of Dalian Medical
University.
The rats received an injection of 5% glucose and
sodium chloride (GNS; Pfizer, Inc., Dalian, China) solution
preoperatively at 24 h. In addition, 100,000 units sodium
penicillin was injected intramuscularly at 30 min prior to surgery,
after which intraperitoneal anesthesia of 10% chloral hydrate (3
ml/kg) was administered. After fixing the rats into position, a
catheter was placed into the bladder through the urethra, and the
bladder was emptied. The urine was collected and stored at −80°C. A
middle abdominal incision with a length of 3.5 cm was made, after
which the bladder was found and the cecum was exposed to the
appendix and protected with non-sterile (NS) gauzes (Kanjie,
Zhejiang, China). A segment of the terminal ileum (2.5–3.0 cm),
that was 2–3 cm away from the cecum and had an adequate blood
supply (with the vascular pedicle), was removed and daubed with
iodophor (Sigma-Aldrich, St. Louis, MO, USA). NS gauzes were again
used to protect the surgical site. Ileal continuity was restored
and the ileal was put into the abdomen. The two ends of the
pedicled ileal segment were closed and an arc incision measuring ∼1
cm in length was made at the central part of the bowel loops in the
mesenteric margin (vascular pedicle contra lateral). The majority
of the bladder was removed up until 0.3 cm away from the bladder
triangle, and the arc margin of the neobladder was connected with
the remaining bladder (needle distance, 1 mm). The abdominal cavity
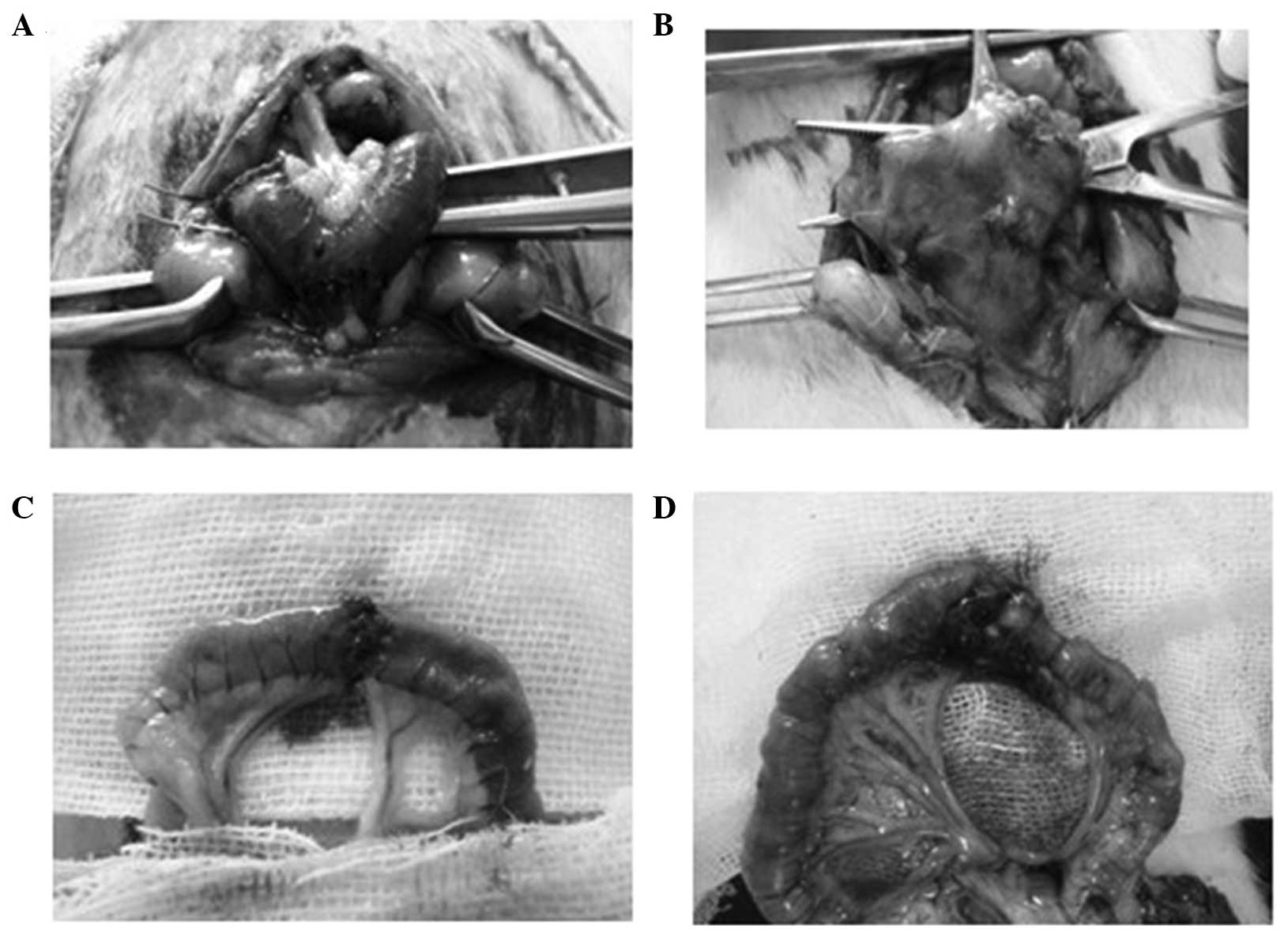
was closed and the surgery time was recorded (Fig. 1). After 6 h of fasting, the rats were
fed 5% GNS solution by injection, and subsequently received a
normal diet at 24 h after the surgery. In addition, the rats were
administered anti-infective therapy (cefminox, 20 mg/kg·day) for 3
days. With regard to the sham-operated group, the incision was
closed following the opening of the cavity, with the remaining
procedure the same as aforementioned.
Collection of urine and tissue
specimens
Urine specimens were collected prior to the
irrigation of the ileal neobladder on days 8, 11, 14 and 17.
Intraperitoneal anesthesia was induced using 10% chloral hydrate (3
ml/kg), and the rats were fixed in position. Urine was collected by
inserting a catheter into the bladder through the urethra. The
upper fluid layer was separated by centrifugation for 5 min at room
temperature, and the specimens were stored at −80°C. On day 17,
following urine specimen collection, a mid-abdominal incision was
made to retrieve the substituted bladder and normal ileum tissues.
The specimens were divided into two pieces, one section was stored
at −80°C and the other section was fixed in 4% formalin.
Bicinchoninic acid (BCA) assay of the
urine protein concentration
A BCA protein assay kit was purchased from Pierce
Biotechnology, Inc. (Rockford, IL, USA). Urine samples containing
0.2–50 µg/µl protein were established, and 1 ml standard working
solution from the BCA kit was added to each 20-µl sample and mixed.
The solutions were incubated for 30 min at 60°C, after which the
samples were cooled for a minimum of 1 h and the absorbance was
measured at 562 nm using a visible light spectrophotometer (Thermo
Fisher Scientific, Waltham, MA, USA). A standard curve of
absorbance against protein (µg) was constructed, and the amount was
determined from the curve. Subsequently, the protein concentration
in the urine was determined.
Assay of sialic acid in the urine
A sialic acid assay kit (Abcam, Cambridge, UK) was
used to measure the concentration of sialic acid in the urine.
Briefly, 0.1 ml periodic acid (0.04 M) was added to the samples and
thoroughly stirred, after which the samples were incubated in an
ice bath for 20 min. Next, 1.25 ml resorcinol reagent was added and
the samples were placed in an ice bath for a further 5 min. The
solution was heated at 100°C for 15 min and cooled in tap water,
following which 1.25 ml tertbutyl alcohol was added and mixed
vigorously to produce a single-phase solution. The sample tubes
were placed in a 37°C water bath for 3 min to stabilize the color,
and the absorbance was read at 560 nm.
Hematoxylin and eosin (H&E) and
Alcian blue-periodic acid Schiff (AB-PAS) staining
Neobladder specimens were formalin-fixed and
embedded in paraffin, and H&E staining was performed as
previously described (3,4). A scoring system was used to categorize
the mucus membrane of the intestines, which was evaluated
independently by two pathologists who were blinded to the specimen
data. The scoring system was recorded as follows: 0, normal tissue;
1, slight intestinal epithelium desquamation that is restricted to
the mucus membrane cupula; 2, villi necrosis that is restricted to
the mucus membrane cupula; 3, central villi; 4, complete villi
necrosis.
With regard to the AB-PAS staining,
paraffin-embedded sections were treated with Schiff reagents, as
previously described. Sialic acid mucus protein appeared as a blue
stain (4). Five villi in every
sample were selected and the number of goblet cells with sialic
acid mucus were counted using a microscope (Olympus Corporation,
Tokyo, Japan), along with the total number of goblet cells, to
acquire the mean value.
Immunohistochemical staining
Immunohistochemical staining of the specimens was
performed using an anti-rat mucin 2 (MUC-2) antibody (AB11197;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), as previously
described (4). The expression of
MUC-2 was observed as buffy particles in the cytoplasm of the
goblet cells.
Statistical analysis
Data were analyzed using SPSS software, version 19.0
(IBM SPSS, Armonk, NY, USA), and are presented as the mean ±
standard deviation. Statistical significance was determined by
one-way analysis of variance, the Student's t-test, the
Kruskal-Wallis test and the χ2 test, where P≤0.05 was
considered to indicate a statistically significant difference.
Results
Morphological effects of the ileal
neobladder and normal ileal mucus membrane in the different
groups
Damage to the villi on the small intestinal
structures was most significant in the DA group. The mucus and
submucus membrane understructure exhibited varying degrees of
destruction, while the muscular layer exhibited tears and
hemorrhage. When compared with the DA group, the damage caused by
HMMC and LMMC was minimal. The majority of the mucus and submucus
membrane and the muscular layer remained normal. However, in the
LMMC, HMMC and DA groups, the goblet cells were predominantly
scattered in the root of villus, and did not present with the
typical goblet shape (Fig. 2).
Scoring of the ileal neobladder and normal ileal
mucus membrane revealed that the average scores of the HMMC and
LMMC groups were much lower compared with those of the DA group
(P<0.01), which indicated that MMC resulted in significantly
less damage compared with DA.
Effects on goblet cells in the
different groups
AB-PAS staining results revealed that the quantity
of goblet cells in the HMMC and LMMC groups increased when compared
with the sham and NS groups (P<0.05); however the percentage of
mature goblet cells decreased (P<0.05; Table I).
 | Table I.Quantities of goblet cells in the
ileal neobladder mucus villi. |
Table I.
Quantities of goblet cells in the
ileal neobladder mucus villi.
|
|
| AB-PAS coloring |
|---|
|
|
|
|
|---|
| Group | Cases (n) | Blue-colored cells
(n) | Total number (n) | Blue-colored/total
number (%) |
|---|
| NS | 10 |
13.63±2.33 |
32.63±6.26a |
42.96±9.51a |
| HMMC | 10 |
5.88±1.25 |
38.10±5.89b |
15.73±4.10b |
| LMMC | 10 |
7.75±8.82 |
38.63±5.95b |
20.90±5.74b |
| DA | 10 |
3.13±1.13 |
1.14±0.35a,b |
38.33±10.80a |
Urine protein concentrations in the
different groups
Urine protein concentrations in the HMMC and DA
groups on days 11, 14 and 17 following surgery were shown to
decrease when compared with the NS group (P<0.05). In the LMMC
group, the urine protein concentrations decreased on days 14 and 17
following surgery when compared with the NS group (P<0.05). When
compared with the LMMC group, the urine protein concentrations in
the HMMC group on days 11 and 17 following surgery were
significantly lower (P<0.05; Table
II).
 | Table II.Changes in the urine protein
concentrations following surgery (mg/ml). |
Table II.
Changes in the urine protein
concentrations following surgery (mg/ml).
| Group | Cases (n) | Preoperative | Day 8 | Day 11 | Day 14 | Day 17 |
|---|
| Sham | 10 |
0.035±0.0063 |
0.046±0.0069 |
0.044±0.0060 |
0.040±0.0059 |
0.041±0.0062 |
| NS | 10 |
0.043±0.0068 |
7.91±1.30 |
9.19±1.35a |
10.31±1.85a,b |
11.63±2.31a,b |
| HMMC | 10 |
0.041±0.060 |
8.27±1.63 |
6.18±1.14b,c |
5.20±1.33b,c |
4.16±0.97b,c |
| LMMC | 10 |
0.045±0.0080 |
8.41±1.04 |
7.38±0.84a,b |
6.11±1.04b,c |
5.59±0.73a,b,c |
| DA | 10 |
0.040±0.0098 |
8.53±1.94c |
5.96±1.04b–c |
3.82±1.61a,b,c |
2.56±1.08a,b,c |
Urine sialic acid concentrations in
the different groups
When compared with the NS group, the sialic acid
concentration in the HMMC, LMMC and DA groups were significantly
different on days 11, 14 and 17 following surgery, respectively
(P<0.05). Compared with the LMMC group, the urine sialic acid
concentration in the HMMC group on day 17 following surgery was
significantly lower (P<0.05).
Discussion
To date, numerous methods have been adopted to
prevent early mucus plug formation following orthotopic neobladder
urinary diversion surgery. In one method, pure saline is repeatedly
injected into a new bladder through a large-diameter catheter or
fistula to prevent the accumulation of mucus. In addition, the
dissolution of mucus can be promoted through the intravesical
instillation of urea to disrupt the mucin molecule hydrogen bonds
and to reduce the viscosity of mucin (5). Furthermore, a variety of agents, such
as ethanol, formaldehyde, silver nitrate and octreotide, have been
reported to reduce mucus secretion (6–8). Early
mucus plug formation may also be prevented through the mechanical
removal of intestinal mucosa (9), or
through the use of an artificial bladder (10).
The intestinal epithelium of mammals is composed of
five types of cell (3), including
absorption, goblet, Paneth, undifferentiated and endocrine cells.
The proliferation and mitosis of goblet cells are active, and the
cytotoxic function of MMC is much greater for cells with short
cellular cycles. Therefore, the present study was designed on the
basis that MMC is more suitable for goblet cells, and the toxic
effect for other epithelial cells is weaker. Thus, investigations
into the effects on goblet cells can be performed without damaging
other cell types.
The H&E staining results revealed that DA
inflicts serious damage to the intestinal epithelium within a short
time period, indicating that DA is not an ideal bladder
instillation agent. By contrast, the intestinal mucosa injury of
the HMMC and LMMC groups was confined to the upper intestinal
villi, while the glands and muscles remained normal. The scores
from the ileum mucosa Kruskal-Wallis tests have further confirmed
these conclusions.
The results of the present study demonstrate that
urine protein levels in the HMMC and LMMC groups tend to decrease
following the addition of MMC into the bladder, and the degree is
associated with the dose. By contrast, the protein concentration in
the NS group increased. Urine protein concentrations in the normal
bladder tissues were lower compared with those in the experimental
groups; thus, it was concluded that the protein concentration in
the urine can reflect the level of mucus secretion; the lower the
concentration, the lower the level of secretion.
Sialic acid has an evident effect on the space
conformation of protein molecules (11), and the amount of sialic acid reflects
the maturity of goblet cells (12,13).
Previous studies have indicated that the use of enzymes or a
combination of acid hydrolysis can significantly reduce the
viscosity of mucus (14,15). The results of the present study
demonstrated that experimental dosages of 0.6 and 0.4 mg/ml MMC can
decrease the level of sialic acid in the urine and the number of
mature goblet cells, with the effect dose-dependent. Decreasing the
sialic acid level results in decreased mucosity of the mucus, which
subsequently prevents early mucus plug formation following
surgery.
H&E and AB-PAS histochemical staining revealed
that the HMMC and LMMC groups exhibited intestinal epithelium
swelling, hyperplasia and hypertrophy of the goblet cells when
compared with the NS group. According to these results, the
mechanisms underlying the phenomenon were summarized as follows.
Firstly, MMC may interfere with the cell cycle of goblet cells,
causing mitosis to be blocked at the late G1 and early S
phase, and the cell volume to increase. In addition, the quantity
of immature goblet cells was elevated, which further indicated that
MMC can inhibit the development of goblet cells. Secondly, MMC may
reduce mucin exocytosis by disturbing organelles. In the urine
environment, under the premise of surgical stress, MMC inhibits the
DNA replication of goblet cells by provoking the cross-linking of
DNA, and the inhibition of DNA replication can lead to a
dysfunction of the endoplasmic reticulum, Golgi apparatus and other
organelles. Subsequently, protein molecules are no longer assembled
properly and are not transferred out of the cells. The
aforementioned changes may also be the result of ischemia and
anoxia; thus, enlargement and swelling of the goblet cells are
observed.
In conclusion, the establishment of experimental
models in the present study was simple and time effective. When
compared with the DA group, the MMC dosages administered were shown
to decrease the early postoperative mucus secretions, with the
effect associated with the dose and the percentage of mature goblet
cells. However, the mechanism underlying the increased size and
immaturity of the goblet cells in the ileal neobladder remains
unclear and requires further investigation.
References
|
1
|
Kashif KM and Holmes SA: The use of small
intestine in bladder reconstruction. Int Urogynecol J Pelvic Floor
Dysfunct. 9:275–280. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun X, Song M, Bai R, Cheng S, Xing Y,
Yuan H, Wang P and Zhou L: Ileal interposition surgery-induced
improvement of hyperglycemia and insulin resistance in
Goto-Kakizaki rats by upregulation of TCF7L2 expression. Exp Ther
Med. 5:1511–1515. 2013.PubMed/NCBI
|
|
3
|
Hautmann RE, de Petriconi RC and Volkmer
BG: Lessons learned from 1,000 neobladders: The 90-day complication
rate. J Urol. 184:990–994. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu Yang: The experimental studies of
anti-tumor agents to intestinal mucus secretion in vitro.
(unpublished PhD thesis)Dalian Medical University2006, (In
Chinese).
|
|
5
|
Demirbilek S, Uğuralp S, Gürbüz N, Sezgin
N and Kirimlioĝlu H: The use of silver nitrate for chemical
de-epithelialization and urothelialization of intestine in a rabbit
model of augmentation cystoplasty. Urol Res. 31:236–241. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leknes IL: Histochemical study on the
intestine goblet cells in cichlid and poecilid species (Teleostei).
Tissue Cell. 42:61–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khorrami MH, Salehi P, Nouri-Mahdavi K,
Ghalamkari A and Tadayyon F: Dramatic effect of a somatostatin
analogue in decreasing mucus production by the intestinal segment
after enterocystoplasty. J Urol. 180:2501–2503. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang T and Zhou X: Clinical application
of expectorant therapy in chronic inflammatory airway diseases
(Review). Exp Ther Med. 7:763–767. 2014.PubMed/NCBI
|
|
9
|
Falke G, Caffaratti J and Atala A: Tissue
engineering of the bladder. World J Urol. 18:36–43. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Loeffler M, Birke A, Winton D and Potten
C: Somatic mutation, monoclonality and stochastic models of stem
cell organization in the intestinal crypt. J Theor Biol.
160:471–491. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shen G-J, Datta AK, Izumi M, Koeller KM
and Wong C-H: Expression of α2, 8/2, 9-polysialyltransferase from
Escherichia coli K92. Characterization of the enzyme and its
reaction products. J Boil Chen. 274:35139–35146. 1999. View Article : Google Scholar
|
|
12
|
Levine MJ, Aguirre A, Hatton MN and Tabak
LA: Artifical salivas: Present and future. J Dent Res. 66:693–698.
1987.PubMed/NCBI
|
|
13
|
Matsuo K, Ota H, Akamatsu T, Sugiyama A
and Katsuyama T: Histochemistry of the surface mucous gel layer of
the human colon. Gut. 40:782–789. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Baylin SB: Reversal of gene silencing as a
therapeutic target for cancer-roles for DNA methylation and its
interdigitation with chromatin. Novartis Found Symp. 259:226–233.
2004.PubMed/NCBI
|
|
15
|
Ozdemir F, Altinisik J, Karateke A,
Coksuer H and Buyru N: Methylation of tumor suppressor genes in
ovarian cancer. Exp Ther Med. 4:1092–1096. 2012.PubMed/NCBI
|