Introduction
Osteoporosis, which is defined as a systemic
skeletal impairment characterized by low bone mass, the
deterioration of bone tissues and an increased risk of fracture, is
a universal major public health problem (1,2).
Osteoporosis is affected by numerous factors, including age,
genetic background and sex hormones. Postmenopausal osteoporosis
(PMOP), a type of osteoporosis that is considered to be associated
with an ovarian hormone deficiency, is by far the most common cause
of age-associated bone loss (3). As
estrogen levels decrease, an increase in bone breakdown occurs,
which is associated with bone formation, microarchitectural
deterioration and decreased bone mass (4). Statistics show that ~40% of females
aged >50 years suffer from an osteoporotic fracture during their
lifetime (5). Estrogen plays a
critical role in maintaining bone restoration, particularly for
females, and estrogen hormone replacement therapy (HRT) has been
proposed as the most effective therapeutic method for the
prevention and treatment of PMOP for decades (6). However, prolonged HRT therapy is not
well accepted due to the various side effects, which include higher
occurrences of breast carcinoma, endometrial cancer and
cardiovascular diseases (7).
Therefore, the development of a novel, safer and more effective
bone protective treatment remains a focus of experimental and
clinical research.
Electroacupuncture (EA) is a modified acupuncture
method that is a specified Chinese medical therapy based on the
principles of regulating Yin and Yang and removing Qi stagnation
and blood stasis of the channels and collaterals. In China, EA is
considered to be an effective, alternative and complementary
therapy for the treatment of a multitude of different human
diseases (8). Clinical trials have
indicated that acupuncture is an effective treatment for menopausal
and perimenopausal syndromes, as well as osteoporosis (9–11). In
addition, experimental studies have shown that pretreatment with EA
not only increases the bone mineral density (BMD), but can also
prevent bone loss in ovariectomized (OVX) rats (12,13). In
addition, EA pretreatment has been demonstrated to promote bone
healing and callus formation in a rat model of tibia fracture
(14). However, whether EA treament
at the acupoints of governor vessel (GV) and bladder meridian (BL)
is able to ameliorate the process of PMOP in OVX rats is yet to be
determined, and if so, the underlying mechanisms require
investigation.
Previously, the osteoprotegerin (OPG)/receptor
activator of nuclear factor κB ligand (RANKL) signaling pathway was
found to be involved in the regulation of osteoblastogenesis and
osteoblast activity, which was regarded as a key factor in
inhibiting bone proliferation. Furthermore, the signaling pathway
was found to directly participate in the process of bone formation
and bone resorption (15,16). Homeostatic bone remodeling requires
the coordinated integration of biological signals from numerous
cellular signaling transduction pathways, and these pathways may be
exploited in the development of novel therapies for osteoporosis
(17). The canonical Wnt/β-catenin
signaling pathway, which controls multiple developmental processes
in joint and skeletal patterning, may also be involved in the
progression of osteoporosis (18,19).
In accordance with the aforementioned observations
and studies, the aim of the present study was to investigate the
effects of EA at the acupoints of GV and BL through analyzing the
serum levels of estradiol (E2), the bone turnover markers,
osteocalcin (OC) and tartrate-resistant acid phosphatase 5b (TRACP
5b), as well as the BMD and bone microarchitecture. In addition,
the expression levels of certain important components of the
OPG/RANKL and Wnt/β-catenin signaling pathways were investigated,
with the aim of providing a theoretical understanding for the
clinical treatment of PMOP.
Materials and methods
Reagents
Antibodies targeting low-density lipoprotein
receptor-related protein (LRP) 5 (cat. no. sc-390267), β-catenin
(cat. no. sc-79637), runt-related transcription factor (Runx) 2
(cat. no. sc-10758) and β-actin (cat. no. sc-47778) were obtained
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Clarity™
western enhanced chemiluminescence substrate (cat. no. 170-5060)
was obtained from Bio-Rad Laboratories, Inc., Hercules, CA,
USA.
Animals
In total, 40 healthy and clean, female Sprague
Dawley rats (weight, 326±14 g; age, 6 months) were purchased from
the Shanghai Laboratory Animal Center [Shanghai, China; Laboratory
Animal Use Certificate no. SCXK (SH) 2007-0005]. The rats were
housed in a sterile environment. Experiments involving the animals
complied with Guidance Suggestions for the Care and Use of
Laboratory Animals (2006), administered by the Ministry of Science
and Technology in China (20).
Animal models and experimental
groups
After a week of adaptability feeding, 10 rats were
randomly assigned to the sham-operated (sham) group. The remaining
30 rats were assigned to the OVX group. The OVX rats were
anesthetized with 2% pentobarbital sodium (cat. no. P3761;
Sigma-Aldrich, St. Louis, MO, USA), after which both ovaries were
removed. The sham rats underwent surgery in which the fat tissue
near the ovaries was resected. Following surgery, 50,000 units of
penicillin were administered intramuscularly each day for three
days. Three months after the surgery (when the formation of
osteoporosis was expected), the OVX rats were randomly divided into
three groups of 10 rats each. The ovariectomized (OVX) control
group did not receive any additional treatment. Two EA treatment
groups were established, one group assessing the Mingmen and
Jizhong acupoints (GV group), and the other group assessing the
Pishu and Shenshu acupoints (BL group). The experimental procedures
were in compliance with the Animal Protection Law of China (2001),
and the study was approved by the Ethics Committee at the Fujian
University of Traditional Chinese Medicine (Fuzhou, China).
EA treatment
EA procedures were performed on lightly anesthetized
rats (with 2% pentobarbital sodium), as described previously, to
minimize the stress induced by the animal restraint that was
necessary for needle insertion and stimulation. The selected
acupoints in the GV group were Mingmen (GV4) and Jizhong (GV6),
while in the BL group, Pishu (BL20) and Shenshu (BL23) were
selected. Disposable stainless steel acupuncture needles (0.24×30
mm; Suzhou Huatuo Medical Equipment Co., Ltd., Suzhou, China) were
inserted perpendicularly into the acupuncture points at a depth of
5–10 mm. The stimuli were generated by an EA apparatus (Huatuo
SDZ-V type; Suzhou Huatuo Medical Equipment Co., Ltd.) with 2/100
Hz alternating frequency. The EA treatment was administered once
daily for 20 min each time, with 10 treatments constituting a
therapeutic course. Between each 10-day course, there was an
interval of 5 days. The treatment period lasted for 90 days. The
sham group rats received no treatment.
Bone densitometry analysis
At the end of the study, rats were deeply
anesthetized with 10% chloral hydrate solution (0.3 ml/100g, i.p.).
Following sacrifice of the rats, the lumbar vertebrae (L5) were
removed. BMD was determined by dual-energy X-ray absorptiometry
(Lunar-DPX-IQ; GE Medical Systems, Madison, WI, USA), which was
equipped with small animal measurement software for bone density
assessment.
Bone histomorphology analysis
Histomorphology analysis was performed to observe
the changes in the bone tissue. The left femurs were removed and
fixed with a 10% ethylenediaminetetraacetic acid solution (pH 7.4)
at 4°C for 3 weeks. Following decalcification, each bone sample was
cut into 3-µm coronal planes and embedded in paraffin for tissue
sectioning. The sections were stained with hematoxylin and eosin
(H&E) to evaluate the changes in the bone. A histomorphometric
study of the femurs was performed using image analysis software
(Image-Pro Plus 6.1 for Windows; Media Cybernetics, Inc.,
Rockville, MD, USA). The parameters measured included the mean
thickness of the trabeculae (Tb.Th, µm), the trabecular area
(Tb.Ar, %), the trabecular bone number (Tb.N, mm−1) and
the trabecular separation (Tb.Sp, mm).
Assessment analysis of the serum
levels of E2 and the bone turnover markers, OC and TRACP 5b
Following the 90-day EA treatment period, blood
samples were collected from all the experimental animals and
centrifuged at 3,000 × g for 20 min at 4°C. The supernatants were
stored at −20°C until required for analysis. Subsequently, the
serum E2, OC and TRACP 5b levels were observed using an ELISA kit
(Wuhan Elabscience Biotechnology Co., Ltd., Wuhan, China),
according to the manufacturer's instructions.
Serum OPG and RANKL expression
levels
Serum levels of OPG and RANKL were assessed using an
ELISA (Quantikine; R&D Systems, Inc., Minneapolis, MN, USA),
according to the manufacturer's instructions. The ratio of OPG to
RANKL, expressed as OPG/RANKL, was used as a measure to describe
the process of bone formation coupled with bone resorption.
Western blot analysis for the
determination of LRP5, β-catenin and Runx2 protein expression
Protein expression levels of LRP5, β-catenin and
Runx2 in the femur tissues obtained from the rats in the different
groups were detected by western blot analysis. Protein was
extracted from the femurs using a lysis buffer that contained a
protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim,
Germany). Lysates were centrifuged for 15 min at 12,000 × g to
obtain the supernatants for further analysis. The protein
concentration of the lysates was measured using a bicinchoninic
acid quantification assay (Pierce Biotechnology, Inc., Rockford,
IL, USA). Proteins (50 µg) were separated using 10% SDS-PAGE, and
transferred to polyvinylidene fluoride membranes with a 0.45-µm
pore size (IPVH00010; EMD Millipore, Billerica, MA, USA). The
membranes were incubated with monoclonal primary antibodies
targeting LRP5, β-catenin, Runx2 and β-actin (1:1,000), which were
diluted in immunoblot buffer [Tris-buffered saline containing 0.05%
Tween-20 (TBST) and 5% non-fat dry milk], overnight at 4°C.
Following washing with TBST three times, the membranes were
incubated with an anti-mouse (or rabbit) secondary antibody
(anti-mouse IgG, cat. no. sc-2005; anti-rabbit IgG, cat. no.
sc-2004; Santa Cruz Biotechnology) conjugated to horseradish
peroxidase (1:1,000) for 1 h at room temperature. Following
washing, the blots were visualized with a Clarity™ western enhanced
chemiluminescence substrate (Bio-Rad Laboratories) for 1 min using
a camera along with the ChemiDoc XRS+ System (Bio-Rad Laboratories,
Inc.). The pixel intensities of the immunoreactive bands were
quantified using the percentage-adjusted volume feature of the
Image Lab software (Bio-Rad Laboratories, Inc.). β-actin served as
an internal control.
Statistical analysis
All results are represented as the mean ± standard
deviation of ≥3 experiments. Data were analyzed using one-way
analysis of variance using SPSS statistical package (version 16.0,
SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
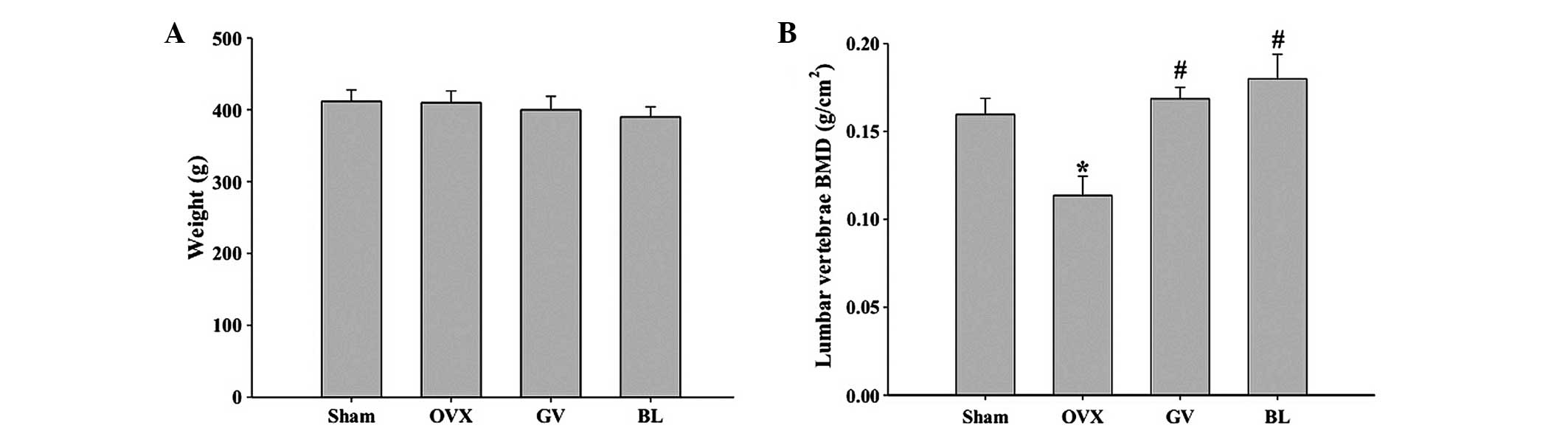
Body weight and BMD
No statistically significant difference was observed
in the body weight among the groups in the initial experimental
period. In addition, at the end of the experimental period, the
body weights in the OVX group and EA treatment groups did not
differ significantly when compared with the sham group, indicating
that no adverse effects were observed as a result of the EA
treatment (Fig. 1A).
As shown in Fig. 1B,
the ovariectomy procedure caused a significant reduction in the BMD
of the lumbar vertebrae in the OVX rats, as compared with that in
the sham-operated group (P<0.01). GV or BL EA treatment was
shown to markedly inhibit the OVX-induced decreases in BMD of the
lumbar vertebrae; however, there was no statistically significant
difference between the GV and BL treatment groups.
Histomorphometry analysis
As shown in Fig. 2A,
H&E staining of the femurs in the sham group revealed complete
trabecular structures in an ordered arrangement, numerous
trabeculae with reasonable diameters, few empty bone lacunae and no
hairline fractures. However, in the OVX group (Fig. 2B), there were fewer trabeculae, which
were disordered and thinning. In addition, large areas of empty
bone lacunae and hairline fractures were observed. In the GV and BL
treatment groups (Fig. 2C and D),
the femurs exhibited a complete trabecular structure, a slightly
ordered arrangement of trabeculae, slight thinning of the
trabeculae, small amounts of empty bone lacunae and no evident
hairline fractures.
Histomorphometric parameters of the trabecular bone
mass and bone architecture in the femur sections are shown in
Fig. 3. The OVX rats exhibited
significant reductions in the Tb.Ar, Tb.Th and Tb.N (P<0.01 for
all) and a significant increase in the Tb.Sp (P<0.01), when
compared with the sham group. However, in the GV and BL treatment
groups, significantly increased values of Tb.Ar, Tb.Th and Tb.N
(P<0.01 for all), and a significantly reduced value of Tb.Sp
(P<0.01), were observed, as compared with the OVX group. No
statistically significant difference was observed between the GV
and BL treatment groups.
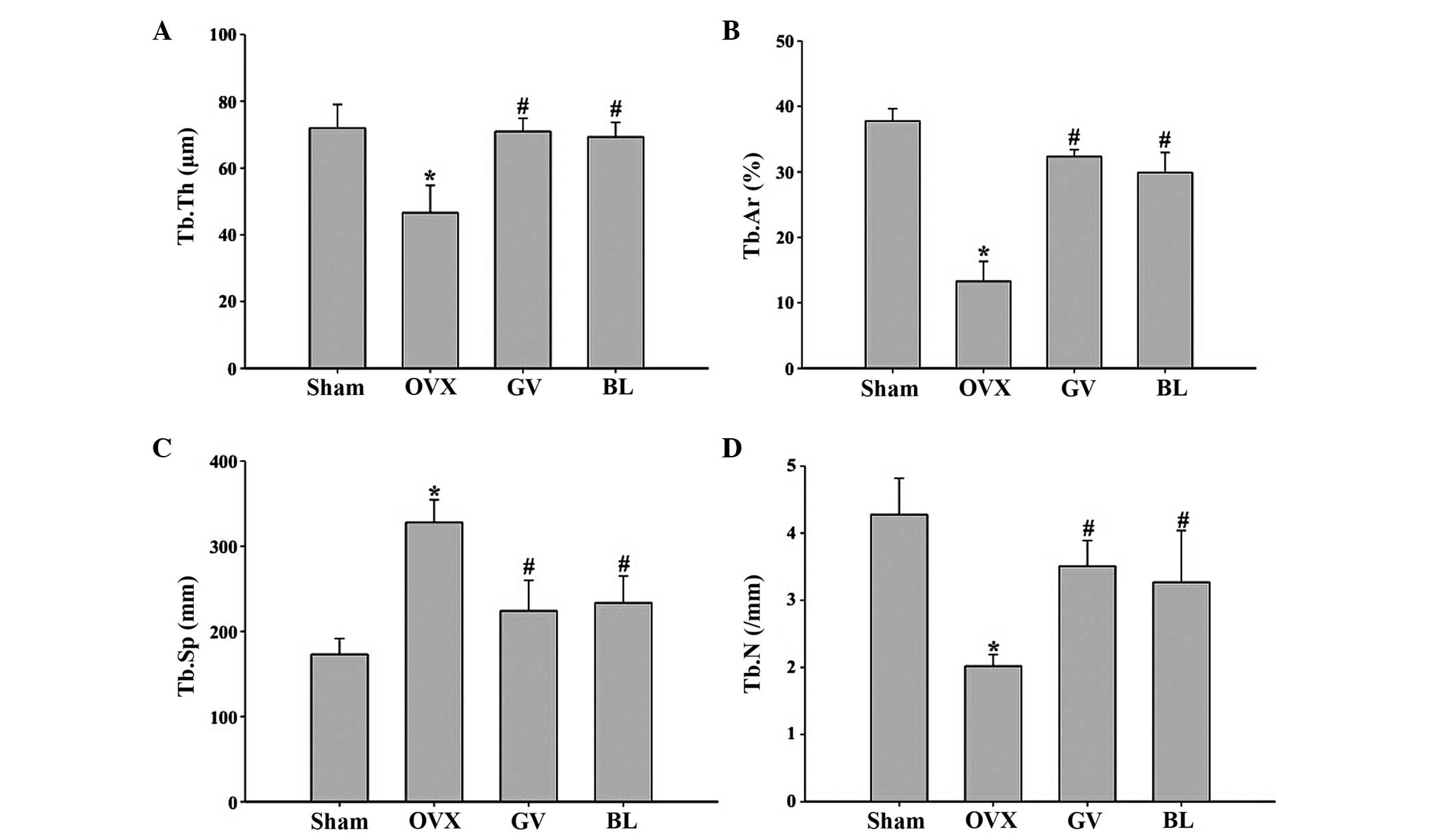 | Figure 3.Histomorphometric analysis of the
femurs in the various groups. Image analysis software was used to
determine the (A) Tb.Th, (B) Tb.Ar percentage; (C) Tb.Sp and (D)
Tb.N. Data are presented as the mean ± standard deviation (n=10).
*P<0.01, vs. sham group; #P<0.01, vs. OVX group.
Tb, trabecular; Th, thickness; Ar, area; Sp; separation; N, number;
OVX, ovariectomized; GV, governor vessel; BL, bladder meridian. |
Serum levels of E2, OC and TRACP
5b
Serum levels of E2, and the bone formation markers,
OC and TRACP 5b, were analyzed using ELISA to provide an evaluation
of the effects of EA on systemic estrogenic and bone metabolism. As
shown in Fig. 4, the serum levels of
E2 were significantly decreased in the OVX group, while the levels
of OC and TRACP 5b were increased when compared with the sham
group. GV and BL EA treatments were shown to significantly increase
the serum levels of E2 and OC, while decreasing the serum levels of
TRACP 5b, when compared with the OVX group (P<0.01).
Interestingly, the EA treatments increased the serum levels of OC
to a greater extent than the OVX rats, as compared with the sham
group. We hypothesise that the high turnover bone metabolism caused
the stress response in the GV and BL rats, which can increase the
serum level of OC.
 | Figure 4.Effects of electroacupuncture on the
serum levels of (A) E2 and the bone turnover markers, (B) OC and
(C) TRACP 5b, as observed by ELISA. Data are presented as the mean
± standard deviation (n=10). *P<0.01, vs. sham group;
#P<0.01, vs. OVX group. E2, estradiol; OC,
osteocalcin; TRACP, tartrate-resistant acid phosphatase; OVX,
ovariectomized; GV, governor vessel; BL, bladder meridian. |
Serum levels of OPG and RANKL
As shown in Fig. 5,
the serum OPG level was lower (P<0.01) in the OVX rats
(481.18±46.08 pg/ml) when compared with the sham-operated animals
(745.0±54.7 pg/ml). By contrast, estrogen deficiency increased the
serum level of RANKL (P<0.01), while the ratio of OPG/RANKL
(P<0.01) was significantly decreased when compared with the sham
group. GV and BL treatment were found to reverse the aforementioned
findings. Thus, the ratio of OPG/RANKL was significantly increased
following treatment with EA.
Western blot analyses of LRP5,
β-catenin and Runx2 protein expression
To investigate the role of EA treatment on the
Wnt/β-catenin signaling pathway and identify the mechanisms
involved in the regulation of bone formation and resorption,
western blot analyses were performed on bone tissues to examine the
protein expression levels of LRP5, β-catenin and Runx2. The results
demonstrated that the femurs from the OVX rats exhibited reduced
levels of LRP5, β-catenin and Runx2 protein expression when
compared with the sham-operated rats. However, following GV and BL
treatments, the protein expression levels of LRP5, β-catenin and
Runx2 significantly increased when compared with the OVX group
(Fig. 6).
 | Figure 6.Effects of electroacupuncture on the
expression levels of various protein involved in the Wnt/β-catenin
signaling pathway. (A) Representative western blot, where β-actin
was used as the internal control. Quantitative determination of the
protein expression levels of (B) LRP5, (C) β-catenin and (D) Runx2
in the femurs of the different groups, as determined by western
blot analysis. Data are presented as the mean ± standard deviation.
*P<0.01, vs. sham group; #P<0.01, vs. OVX group.
LRP5, low-density lipoprotein receptor-related protein 5; Runx2,
runt-related transcription factor 2; OVX, ovariectomized; GV,
governor vessel; BL, bladder meridian. |
Discussion
PMOP is associated with a homeostatic imbalance
between bone modeling and bone resorption (3). For a number of years, HRT has been used
for the treatment and prevention of PMOP; however, this therapeutic
method is associated with serious side effects, including higher
rates of thromboembolism, heart attack, breast and ovarian cancers,
stroke and Alzheimer's disease (6).
Traditionally, acupuncture has been used in Chinese medicine for
the management of disease and pain. Since acupuncture was proposed
as a therapeutic intervention of complementary medicine by a
National Institutes of Health consensus (21), the efficacy of acupuncture has become
more accepted in the western world. EA, which combines the
therapeutic effects of traditional manual acupuncture and
transcutaneous electric nerve stimulation, can be more easily
standardized for frequency, voltage and wave form, in contrast to
manual acupuncture (22). Thus, EA
has been proposed as a potential alternative to estrogen
replacement therapy for the treatment of PMOP due to the decreased
incidence of adverse effects.
According to traditional Chinese medicine theory,
the ‘kidney’ controls the bones. A previous study indicated that
the incidence of kidney deficiency increases with age, causing a
progressive decrease in the BMD (23). In addition, the spleen is considered
to be the acquired foundation and source of Qi and blood
generation. A spleen deficiency may result in malnourishment of the
limbs, other internal organs and bones, with the subsequent result
being bone weakness and osteoporosis (24). Therefore, strengthening the spleen
and tonifying the kidney have become the theoretical basis for the
treatment of PMOP.
In accordance with the traditional Chinese medicine
theory, the present study selected Shenshu (BL23) and Mingmen (GV4)
to tonify the kidneys, while Pishu (BL20) and Jizhong (GV6) were
selected to strengthen the spleen. A previous experimental study
demonstrated that BL20, BL23 and GV4 were the acupoints most
commonly selected for the prevention and treatment of osteoporosis
in postmenopausal individuals (25).
The OVX animal model, which exhibits the majority of
the characteristics of human PMOP (26), has been widely used as a model for
the evaluation of potential osteoporosis treatments (27). The present study was designed to
evaluate the effects of EA at the GV and BL acupoints on
OVX-induced osteoporosis, and to investigate the effects of EA on
the OPG/RANKL/RANK and Wnt/β-catenin signaling pathways. The
results of the present study revealed that EA at the GV4 and GV6 or
BL20 and BL23 acupoints was able to reduce the loss of bone mass,
increase the femoral BMD, regulate the serum levels of the bone
turnover markers, OC and TRACP 5b, and improve the microstructure
of the bone tissue by increasing the Tb.Ar, Tb.N and Tb.Th and
decreasing the Tb.Sp in OVX rats. Furthermore, the EA treatments
did not result in any adverse effects with regard to OVX-induced
increases in body weight, indicating that the GV and BL treatments
exerted beneficial effects on osteoporosis, which may be closely
associated with the function of ‘strengthening the spleen and
tonifying the kidney’.
A number of previous studies (28–30) have
implicated the OPG/RANKL/RANK signaling system as a paracrine
mediator of mechanical strain on bone metabolism. RANKL, which
provides an important signal to osteoclast progenitors, is a
membrane-bound molecule of the tumor necrosis factor ligand family
that promotes the formation of osteoclasts. The binding of RANKL to
its receptor, RANK, which is expressed on osteoclast precursors and
mature osteoclasts, induces osteoclastogenesis and the activation
of mature osteoclasts. Osteoblasts also synthesize and secrete OPG,
a decoy receptor of RANKL, which blocks the interaction between
RANKL and RANK, and thereby inhibits osteoclast formation and the
activation of mature osteoclasts (28–30). The
results of the current study indicated that EA treatment at the GV4
and GV6 or BL20 and BL23 acupoints resulted in the upregulation of
OPG expression and the downregulation of RANKL expression and
secretion from osteoblasts, subsequently inhibiting
osteoclastogenesis and promoting bone formation.
In addition, the Wnt/β-catenin signaling pathway
plays a key role in the regulation of bone growth and remodeling.
When Wnt proteins bind to the Frizzled receptor and the LRP5/6
coreceptor protein, the signaling protein, Dishevelled, becomes
activated. Subsequently, the inhibition of glycogen synthase
kinase-3β occurs, which inhibits the ubiquitination and degradation
of β-catenin. As a consequence, β-catenin accumulates in the
nucleus and binds to lymphoid enhancer-binding
factor-1/transcription factor to upregulate the expression of Wnt
target genes, such as Runx2, which elicits a variety of effects,
including the proliferation and differentiation of osteoblasts. In
accordance with the results of a previous study (31), the present study observed decreased
expression levels of Wnt/β-catenin signaling pathway members,
including LRP5, β-catenin, and Runx2, following an ovariectomy.
Furthermore, the results revealed that EA treatment at the GV4 and
GV6 or BL20 and BL23 acupoints was able to activate the expression
of these members. Therefore, Wnt/β-catenin signaling has become a
focus for the development of targeted therapeutics in PMOP.
In conclusion, to the best of our knowledge, the
data presented in the current study demonstrated for the first time
the multiple working mechanisms of EA treatment at the acupoints of
GV and BL for the therapy of osteoporosis. EA treatment was shown
to function through the OPG/RANKL/RANK and Wnt/β-catenin signaling
pathways, which may be one of the mechanisms through which EA plays
an important role in the treatment of PMOP. Therefore, EA was
demonstrated to be a potential option in the therapeutic strategy
of PMOP, since good tolerance and few undesirable side effects were
observed.
Acknowledgements
The study was supported by a grant from the National
Natural Science Foundation of China (no. 81173282).
Glossary
Abbreviations
Abbreviations:
|
GV
|
governor vessel
|
|
BL
|
bladder meridian
|
|
PMOP
|
postmenopausal osteoporosis
|
|
OVX
|
ovariectomy
|
|
EA
|
electroacupuncture
|
|
BMD
|
bone mineral density
|
|
OC
|
osteocalcin
|
|
TRACP 5b
|
tartrate-resistant acid phosphatase
5b
|
|
OPG
|
osteoprotegerin
|
|
RANKL
|
receptor activator of nuclear
factor-κB ligand
|
|
E2
|
estradiol
|
|
LRP
|
low-density lipoprotein
receptor-related protein
|
|
Runx
|
runt-related transcription factor
|
|
HRT
|
hormone replacement therapy
|
|
H&E
|
hematoxylin and eosin
|
References
|
1
|
NIH Consensus Development Panel on
Osteoporosis Prevention, Diagnosis, and Therapy, . Osteoporosis
prevention, diagnosis, and therapy. JAMA. 285:785–795. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanis JA: Diagnosis of osteoporosis and
assessment of fracture risk. Lancet. 359:1929–1936. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meczekalski B and Czyzyk A: Selective
estrogen receptor modulators in treatment of postmenopausal
osteoporosis. Ginekol Pol. 80:213–217. 2009.(In Polish). PubMed/NCBI
|
|
4
|
Cole Z, Dennison E and Cooper C: Update on
the treatment of post-menopausal osteoporosis. Br Med Bull.
86:129–143. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McNamara LM: Perspective on
post-menopausal osteoporosis: Establishing an interdisciplinary
understanding of the sequence of events from the molecular level to
whole bone fractures. J R Soc Interface. 7:353–372. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nelson HD: Menopause. Lancet. 371:760–770.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rossouw JE, Anderson GL, Prentice RL, et
al Writing Group for the Women's Health Initiative Investigators:
Risks and benefits of estrogen plus progestin in healthy
postmenopausal women: Principal results from The Women's Health
Initiative randomized controlled trial. JAMA. 288:321–333. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han JS: Acupuncture: Neuropeptide release
produced by electrical stimulation of different frequencies. Trends
Neurosci. 26:17–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mason S, Tovey P and Long AF: Evaluating
complementary medicine: Methodological challenges of randomised
controlled trials. BMJ. 325:832–834. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sunay D, Ozdiken M, Arslan H, Seven A and
Aral Y: The effect of acupuncture on postmenopausal symptoms and
reproductive hormones: A sham controlled clinical trial. Acupunct
Med. 29:27–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou ZH, Wang NQ, Pan FF, Wu ZH and Dai
XY: Clinical observation on combined acupuncture and medication for
osteoporosis in postmenopausal women. J Acupunct Tuina Sci.
9:370–375. 2011.(In Chinese). View Article : Google Scholar
|
|
12
|
He J, Yang L, Qing Y and He C: Effects of
electroacupuncture on bone mineral density, oestradiol level and
osteoprotegerin ligand expression in ovariectomised rabbits.
Acupunct Med. 32:37–42. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng Y, Lin H, Zhang Y, Li L, Wu X, Wang
T, Liu Y and Tan Y: Electroacupuncture promotes insulin-like growth
factors system in ovariectomized osteoporosis rats. Am J Chin Med.
36:889–897. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei YF, Liu YL, Zhang SH, Wang ZO, et al:
Effect of electroacupuncture on plasma estrin and bone mineral
density in ovariectomized rats. Zhen Ci Yan Jiu. 32:38–41. 2007.(In
Chinese). PubMed/NCBI
|
|
15
|
Kong YY, Yoshida H, Sarosi I, et al: OPGL
is a key regulator of osteoclastogenesis, lymphocyte development
and lymph-node organogenesis. Nature. 397:315–323. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hofbauer LC and Schoppet M: Clinical
implications of the osteoprotegerin/RANKL/RANK system for bone and
vascular diseases. JAMA. 292:490–495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hartmann C: A Wnt canon orchestrating
osteoblastogenesis. Trends Cell Biol. 16:151–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim W, Kim M and Jho EH: Wnt/β-catenin
signalling: From plasma membrane to nucleus. Biochem J. 450:9–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bodine PV and Komm BS: Wnt signaling and
osteoblastogenesis. Rev Endocr Metab Disord. 7:33–39. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
The Ministry of Science and Technology of
the People's Republic of China, . Guidance Suggestions for the Care
and Use of Laboratory Animals. 2006
|
|
21
|
Vincent CA and Richardson PH: The
evaluation of therapeutic acupuncture: Concepts and methods. Pain.
24:1–13. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lin JG and Chen WL: Acupuncture analgesia:
A review of its mechanisms of actions. Am J Chin Med. 36:635–645.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao Y, Liu KJ, Li JH, Cai XJ and Yang JH:
Bone mineral content and kidney asthenia. Chinese J Osteoporosis.
2:19–21. 1996.(In Chinese).
|
|
24
|
Xie L, Yao GH and Guo ZQ: Preliminary
study on treatment of osteoporosis with spleen-strengthening and
stomach-nourishing method. Hunan Zhongyiyao Xueyuan Xuebao. 41:7–9.
1996.
|
|
25
|
Xu M, Liu BX and Huang CG: The progress of
acupuncture treatment on postmenopausal osteoporosis. Zhejiang
Zhongyiyao Daxue Xuebao. 2:301–302. 2011.
|
|
26
|
Frolik CA, Bryant HU, Black EC, Magee DE
and Chandrasekhar S: Time-dependent changes in biochemical bone
markers and serum cholesterol in ovariectomized rats: Effects of
raloxifene HCl, tamoxifen, estrogen, and alendronate. Bone.
18:621–627. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sato M, Bryant HU, Iversen P, et al:
Advantages of raloxifene over alendronate or estrogen on
nonreproductive and reproductive tissues in the long-term dosing of
ovariectomized rats. J Pharmacol Exp Ther. 279:298–305.
1996.PubMed/NCBI
|
|
28
|
Yasuda H, Shima N, Nakagawa N, et al:
Identity of osteoclastogenesis inhibitory factor (OCIF) and
osteoprotegerin (OPG): A mechanism by which OPG/OCIF inhibits
osteoclastogenesis in vitro. Endocrinology. 139:1329–1337.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bord S, Ireland DC, Beavan SR and Compston
JE: The effects of estrogen on osteoprotegerin, RANKL, and estrogen
receptor expression in human osteoblasts. Bone. 32:136–141. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lacey DL, Timms E, Tan HL, et al:
Osteoprotegerin ligand is a cytokine that regulates osteoclast
differentiation and activation. Cell. 93:165–176. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou J, He H, Yang L, Chen S, Guo H, Xia
L, Liu H, Qin Y, Liu C, Wei X, Zhou Y and He C: Effects of pulsed
electromagnetic fields on bone mass and Wnt/β-catenin signaling
pathway in ovariectomized rats. Arch Med Res. 43:274–282. 2012.
View Article : Google Scholar : PubMed/NCBI
|