Introduction
Postmenopausal osteoporosis (PMO), a common disease
characterized by bone reduction and microarchitectural
deterioration of the bone, has a serious effect on the quality of
life of the patients, particularly the elderly (1,2). PMO is
considered to be the result of an imbalance between bone resorption
and formation, which are regulated by osteoclasts and osteoblasts,
respectively. This imbalance leads to increased bone fragility and
susceptibility to fractures (3–5). The
mechanism underlying the pathogenesis of PMO is multifactorial and
complicated. Gonadal steroids play an important role in bone
remodeling and skeletal structure maintenance (6–8).
According to Traditional Chinese Medicine (TCM)
theory, PMO can be classified into different TCM patterns (Zheng),
including the Kidney-Yang deficiency (KYD), Kidney-Yin deficiency and
Kidney-Yin and Yang deficiency patterns (9–11).
Zheng, the body's overall response to different factors in the
evolution of a disease, is intrinsically linked to a group of signs
and symptoms at a certain stage of the disease (12,13).
Zheng is based on factors including pulse feeling and tongue
appearance and can be used as a guideline in TCM disease
classification; however, it is not simply a collection of disease
symptoms but rather can be defined as the TCM theoretical
abstraction of the symptom profiles of patients (14–16). The
KYD pattern (Shen-Yang-Xu Zheng) is an important syndrome of PMO
(17); while some postmenopausal
women are prone to forming the KYD pattern of osteoporosis, others
of the same age group exhibit no development of osteoporosis. The
underlying mechanism of this phenomenon remains to be elucidated.
We hypothesized that the serum taken from patients with the KYD
pattern of osteoporosis contains bioactive molecules in the
metabolic products of the disease. The collection of this serum is
easy, and the serum can be used to objectively imitate the
interaction between the serum and cells, thus generating an
effective approach for the mechanistic study of the disease. In the
present study, the susceptibility of certain postmenopausal women
of the same age group to the KYD pattern of osteoporosis, as well
as the associated underlying mechanism, was investigated.
Materials and methods
Ethics statement
Ethical approval for the present study was obtained
from the Clinical Trial Ethics Committee of the Second Affiliated
Hospital of Fujian University of TCM (Fuzhou, China), and written
informed consent was obtained from all participants prior to the
experiment.
Participants
A random selection of 30 postmenopausal female
volunteers aged 60–70 years, including 15 women with and 15 without
osteoporosis, was enrolled in the study from the Physical
Examination Center of the Second Affiliated Hospital of Fujian
University of TCM. The diagnosis of PMO was defined by a bone
mineral density (BMD) T-score of ≥2.5 standard deviations below the
young normal gender-matched BMD of the reference database, in
accordance with the World Health Organization criteria (18). Participants receiving any medications
known to affect the calcium or bone metabolism, such as current use
or history of a ≥3-month use of exogenous estrogens, thiazine or
corticosteroids, were excluded from the study. Participants with
any other disorder known to affect bone metabolism were also
excluded.
The TCM diagnosis of the participants was based on
the information obtained from four diagnostic processes, including
looking, smelling, asking and touching. The diagnostic criteria of
the KYD pattern included a sensation of cold and aching in the
loins and knees, cold limbs and body, sexual hypoesthesia,
infertility due to cold in the uterus, dispiritedness and
lassitude, early morning diarrhea or frequent micturition, clear
and profuse urine, profuse nocturnal urine, loose stools, bright
whitish or blackish complexion and a light-colored tongue with
white fur, as well as a deep and weak pulse (19).
Serum preparation
Venous blood was collected in the morning between
8:00 and 9:00 a.m. and centrifuged for 10 min at 1,200 × g within
30 min, and the serum was separated and stored at −80°C.
Cell culture
An hFOB 1.19 human osteoblastic cell line from the
Institute of Biochemistry and Cell Biology (Chinese Academy of
Sciences, Shanghai, China) was cultured in Dulbecco's modified
Eagle's medium (Gibco-BRL, Grand Island, NY, USA), supplemented
with 10% (v/v) fetal bovine serum (FBS) (Gibco-BRL), penicillin
(100 U/ml) and streptomycin (100 µg/ml) at 37°C in humidified
incubator with 5% CO2. When the cells reached 80%
confluence, they were harvested with 0.25% trypsin-EDTA solution and
then seeded in 96- and 12-well plates at a density of
6×103 and 1×105 cells/well, respectively, in
a medium of 10% FBS. Twenty-four hours after stabilization, the
cells were washed in phosphate-buffered saline solution twice and
treated with the KYD pattern-serum or control serum from
postmenopausal women without osteoporosis.
Analysis of cell viability using MTT
assay
The cells were treated with 10% KYD pattern-serum
for different periods of time. The medium was discarded and
replaced with 10 µl MTT (Sigma-Aldrich, St. Louis, MO, USA) at 37°C
for 4 h and then 100 µl dimethylsulfoxide was added. The absorbance
at 490 nm was measured on an ELISA reader (Model EXL800; BioTek
Instruments, Inc., Winooski, VT, USA).
Alkaline phosphatase (ALP) activity
assay
Following treatment with the KYD pattern-serum for
72 h, the cells were lysed with 0.05% Triton X-100 (Amresco, Inc.,
Solon, USA). The activity of ALP was determined by the conversion
of p-nitrophenyl phosphate to p-nitrophenol using a
commercial kit (Nanjing Jiancheng Biological Technology Co., Ltd.,
Nanjing, China). The total protein concentration was evaluated with
a bicinchoninic acid protein assay kit (Bio-Rad, Hercules, CA,
USA). An equal quantity of protein was mixed with 100 µl substrate
at 37°C for 15 min and 80 µl reaction-stop solution was added. The
results were determined at 405 nm. The absorbance was normalized
based on the protein content.
Alizarin red S staining for
mineralization
Calcified nodules of the hFOB 1.19 cells treated
with 10% KYD pattern-serum were demonstrated by Alizarin red S
staining. The cells were seeded into 48-well plates at a density of
2×105 cells per well. The cells were subsequently
treated with 10% KYD pattern-serum for 14 days and then fixed with
0.5 ml/well formalin:methanol:H2O (1:1:1.5) for 30 min
at room temperature. The cells were stained with 0.1% Alizarin red
S (Sigma-Aldrich) at 37°C for 30 min and images of the stained
calcified nodules were captured using microscopy.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
analysis
Total RNA from the cells was isolated using TRIzol®
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA). RT was
performed using random primers and the SuperScript™ III
First-Strand Synthesis system (Invitrogen Life Technologies). qPCR
was conducted in an ABI Prism 7700 Sequence Detection System using
the SYBR® Green PCR Master Mix (Invitrogen Life Technologies). The
sequences of the PCR primers for the amplification of the ALP,
osteocalcin, osteoprotegerin (OPG), receptor activator of nuclear
factor κB ligand (RANKL) and GAPDH transcripts were as follows: ALP
forward, 5′-AGC CCT TCA CTG CCA TCC TGT-3′ and reverse, 5′-ATT CTC
TCG TTC ACC GCC CAC-3′, 68 bp; osteocalcin forward, 5′-CAA AGG TGC
AGC CTT TGT GTC-3′ and reverse, 5′-TCA CAG TCC GGA TTG AGC TCA-3′,
150 bp; OPG forward, 5′-AGT ACG TCA AGC AGG AGT GCA AT-3′ and
reverse, 5′-CCA GCT TGC ACC ACT CCAA-3′, 129 bp; RANKL forward,
5′-AGA GCG CAG ATG GAT CCT AA-3′ and reverse, 5′-TTC CTT TTG CAC
AGC TCC TT-3′, 180 bp; GAPDH forward, 5′-CAA CTA CAT GGT TTA CAT
GTTC-3′ and reverse, 5′-GCC AGT GGA CTC CAC GAC-3′, 163 bp. The
amplification protocol was as follows: Denaturation at 95°C for 10
min and 40 cycles of 95°C for 20 sec, 57°C for 10 sec, and 72°C for
30 sec. The amplification and melting curve data were collected.
Fold-changes of the genes expression were estimated according to
the comparative 2−ΔΔCt method.
Western blot analysis
Total cellular protein was extracted from the cells
using radioimmunoprecipitation assay buffer (Beyotime Biotechnology
Co., Ltd., Shanghai, China), and the total protein concentration
was determined using a Bio-Rad protein assay. Equal quantities of
protein were separated using SDS-PAGE and transferred onto
polyvinylidene fluoride membranes (Invitrogen Life Technologies).
The blots were blocked with 5% skimmed milk powder (Sigma-Aldrich)
for 2 h at room temperature and were incubated with rabbit
polyclonal antibodies against osteocalcin (1:800; sc-30044), OPG
(1:1,000; sc-11383), RANKL (1:800; sc-9073) and β-actin (1:1,000;
sc-130657) antibodies (Santa Cruz Biotechnology Inc., Santa Cruz,
CA, USA) overnight at 4°C followed by a goat anti-rabbit
horseradish peroxidase (HRP)-conjugated secondary antibody IgG
(1:10,000; ZB-2301; Zhongshan Golden Bridge Biotechnology Co.,
Ltd., Beijing, China) at room temperature for 1 h. The
immunoreactive proteins were visualized using Western Blot
Chemiluminescence Luminol Reagent (Santa Cruz Biotechnology, Inc.).
Immunoblot bands were quantified using the Tocan 190 protein assay
system (Bio-Rad). β-actin was used as the loading control.
ELISA
The serum concentration of estradiol
(E2), OPG, and insulin-like growth factor 1 (IGF-1) was
assessed using ELISA (Shanghai Jinma Biological Technology Co.,
Ltd, Shanghai, China). All commercial assays were performed
according to the manufacturer's instructions. Briefly, ELISA plates
were percolated with mouse anti-human immunoglobulin G, and
standards, and samples were loaded into the wells and incubated for
1 h at room temperature. HRP-conjugated anti-human E2,
OPG and IGF-1 detection antibodies were added and incubated at room
temperature for 1 h. The reaction was visualized through color
development and the absorbance (optical density) was measured at a
450-nm wavelength on an ELISA reader (Model EXL800; BioTek
Instruments, Inc.). The conversion of optical density units for the
study samples to concentration was achieved through a computer
software-mediated comparison with a standard curve using the KC
Junior (BioTek Instruments, Inc.).
Statistical analysis
Data were analyzed using the SPSS 19.0 software for
Windows (IBM SPSS, Armonk, NY, USA). The quantitative data are
expressed as the mean ± standard deviation. The statistical
analysis of the data was performed using nonparametric tests for
two independent samples. P<0.05 was considered to indicate a
statistically significant difference.
Results
KYD pattern-serum inhibits cell
viability of the hFOB 1.19 cells
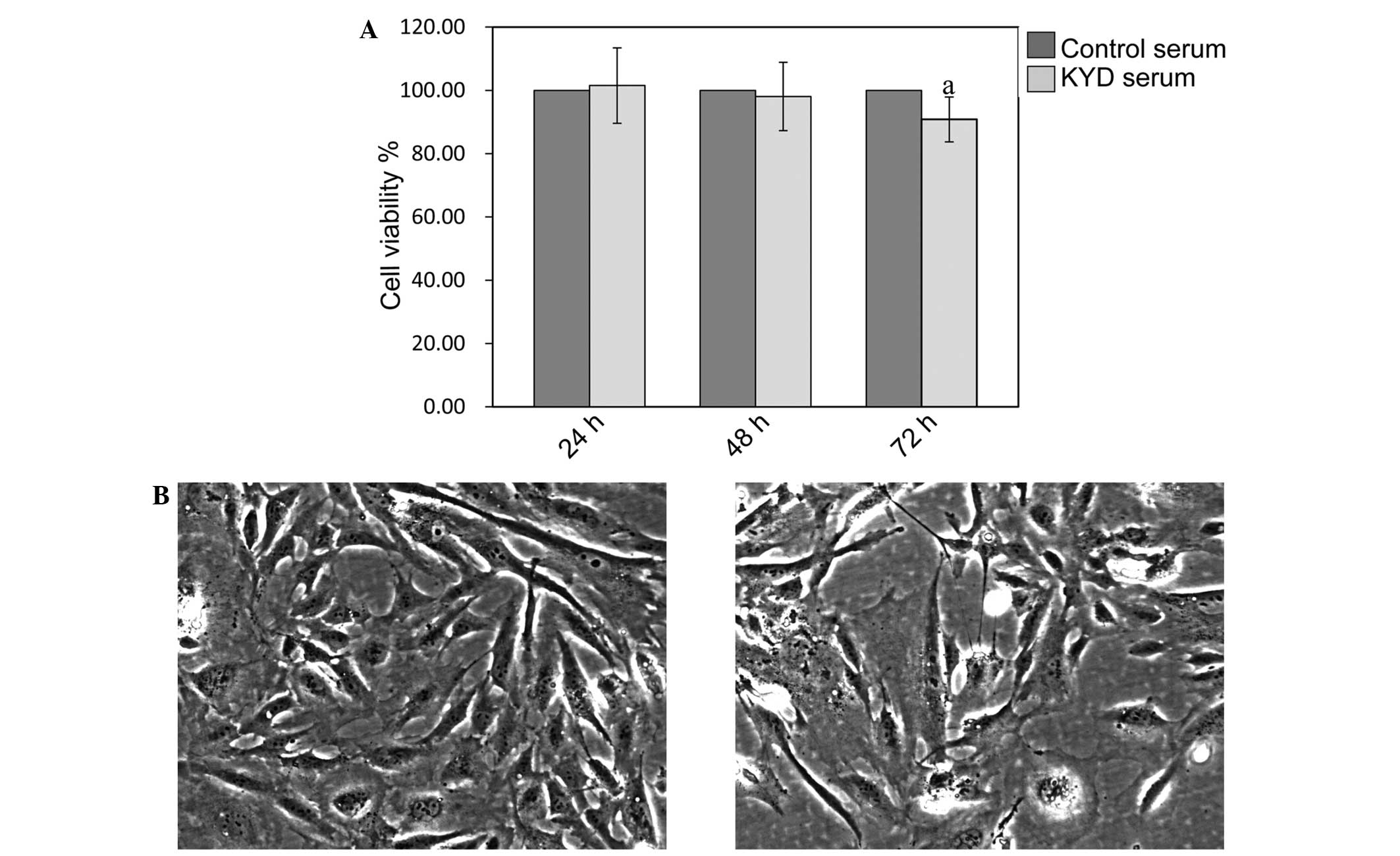
As shown in Fig. 1A,
the viability of the hFOB 1.19 cells was not affected by treatment
with the KYD pattern-serum at 24 and 48 h (P>0.05), but was
significantly decreased at 72 h (P=0.025), compared with the
viability of cells treated with control serum. Cells treated with
the KYD pattern-serum decreased in number following treatment and
underwent morphological changes (Fig. 1B
and C), including cell size and shape, indicating that the KYD
pattern-serum inhibited the osteoblast viability significantly,
contributing to the progression of bone loss in PMO.
KYD pattern-serum decreases ALP
activity and mRNA expression in the hFOB 1.19 cells
The activity of ALP was downregulated in the hFOB
1.19 cells treated with the KYD pattern-serum, compared with that
in the cells treated with control serum (P=0.037) (Fig. 2A). qPCR also showed that the mRNA
expression of ALP was clearly decreased following treatment with
the KYD pattern-serum compared with that following treatment with
control serum (P=0.008) (Fig. 2B).
The calcified nodules appeared bright red in color following
Alizarin red S staining (Fig. 3A–D).
The KYD pattern-serum could significantly inhibit the formation of
calcified nodules compared with the control serum, which suggests
that the KYD pattern-serum reduced bone formation.
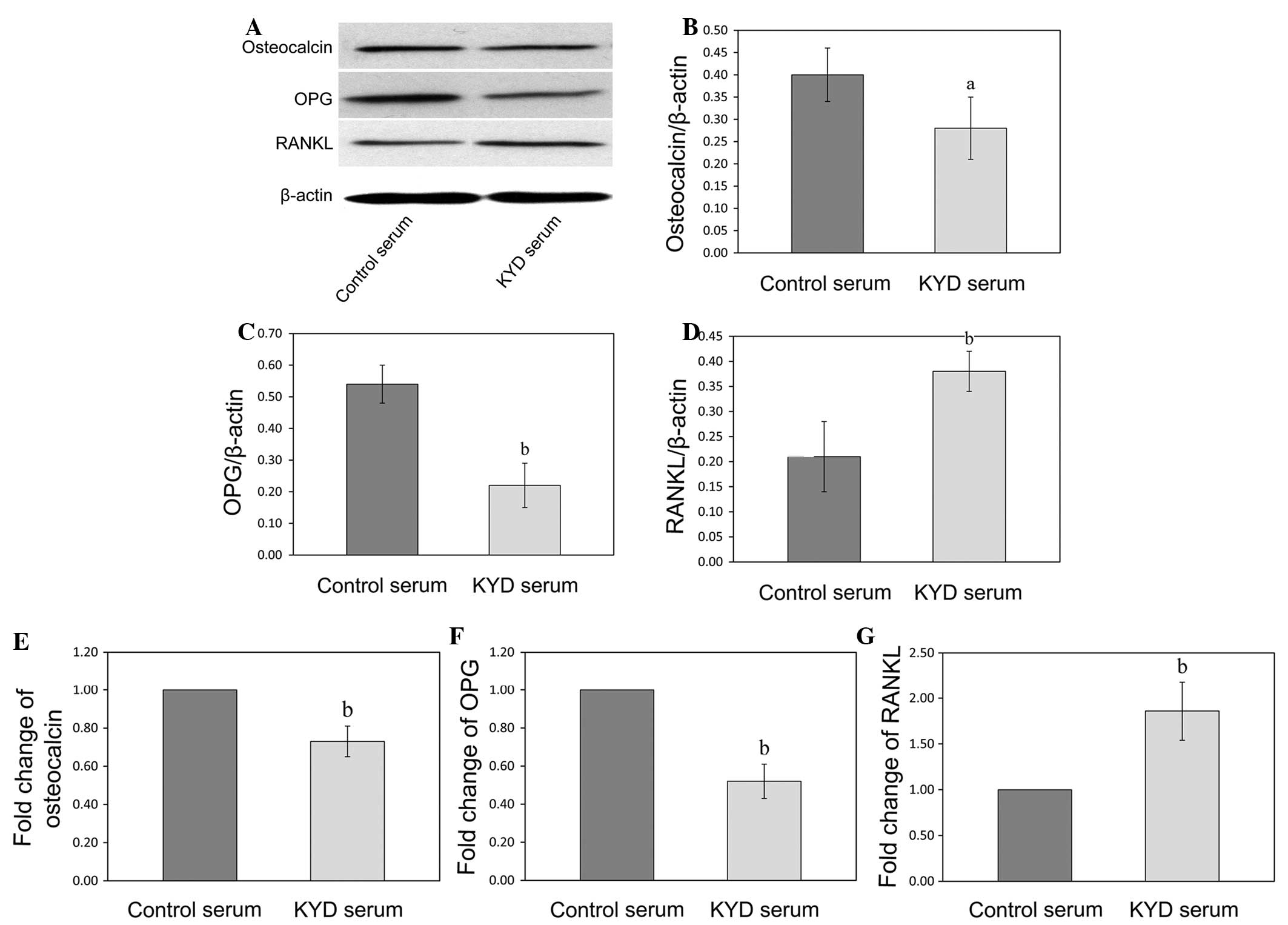
KYD pattern-serum downregulates the
expression of osteocalcin and OPG and upregulates the expression of
RANKL in the hFOB 1.19 cells
In order to further explore the mechanism of the KYD
pattern in bone formation, the mRNA and protein expression of
osteocalcin, OPG and RANKL was analyzed following KYD pattern-serum
treatment using RT-qPCR and western blotting, respectively. The
protein levels of osteocalcin and OPG in the hFOB 1.19 cells
treated with the KYD pattern-serum were downregulated (P=0.047 and
P=0.009), and the protein level of RANKL was upregulated (P=0.006),
compared with the protein levels following treatment with control
serum (Fig. 4A–D). The changes in
the mRNA expression of osteocalcin, OPG and RANKL following
treatment with the KYD pattern-serum were similar to the changes in
the protein levels (Fig. 4E–G)
(P=0.002, P<0.001 and P=0.004 versus control, respectively),
which suggested that the KYD pattern-serum regulated the bone
metabolism via the OPG/RANKL system.
Downregulation of E2, OPG
and IGF-1 in the KYD pattern-serum leads to an inhibition of bone
formation in the hFOB 1.19 cells
In order to obtain some insight into the underlying
mechanism of the inhibition of bone formation by the KYD
pattern-serum, the concentrations of E2, OPG and IGF-1
in the KYD pattern- and control serums were analyzed. As shown in
Fig. 5A–C, the concentrations of
E2, OPG and IGF-1 in the KYD pattern-serum were lower
than those in the control serum (P=0.003, P=0.012 and P=0.001,
respectively), indicating that the alteration in the serum levels
of E2, OPG and IGF-1 may be responsible for the
formation of the KYD pattern in postmenopausal women.
Discussion
According to TCM theory, the kidney regulates bone
formation and development. Kidney deficiency leading to bone loss
is associated with the pathological process of PMO (17,20,21).
Among all kidney deficiency patterns, the KYD pattern is a common
clinical type of PMO; however, the precise mechanism behind its
formation remains unclear. The present results revealed that
alterations in E2, OPG and IGF-1 may account for the
susceptibility of certain postmenopausal women to the KYD pattern
of osteoporosis.
Using the MTT assay, it was shown that the KYD
pattern-serum significantly inhibited the viability of the hFOB
1.19 cells, suggesting that it also inhibited the proliferation of
these cells. The possibility of the KYD pattern-serum controlling
the mineralization of osteoblasts was explored by measuring the ALP
activity, osteocalcin expression and formation of calcified nodules
in the hFOB 1.19 cells. ALP, a classic biomarker of osteoblast cell
differentiation, plays a crucial role in the early stage of
extracellular matrix mineralization (22,23).
When cultured in appropriate osteogenic media, osteoblastic cells
form a calcified extracellular compartment and express osteocalcin;
thus calcified nodules are indicative of osteoblast differentiation
and mineralization (24,25). In the present study, it was found
that the KYD pattern-serum significantly decreased the ALP activity
and formation of calcified nodules and downregulated the expression
of osteocalcin. It has been reported that, in PMO patients, the
altered bone microarchitecture and low BMD result in an increased
risk of bone fractures due to decreased proliferation and
mineralization of osteoblasts (26,27), and
the results of the present study were in accordance with this
conclusion.
Previous studies showing that OPG mediates bone
formation and RANKL mediates bone resorption have enhanced the
understanding of bone remodeling regulation (28–30). A
number of studies have suggested that the binding of RANKL to RANK
results in the activation of signaling pathways, which control the
function of osteoclasts; however, OPG protects the bones from
excessive resorption by inhibiting the binding of RANKL to RANK
(31–33. In order to investigate the effects of the KYD
pattern-serum on the OPG/RANKL system in the hFOB 1.19 cells, the
expression of OPG and RANKL was examined. The present results
showed that the KYD pattern-serum could reduce bone formation
through the downregulation of OPG and upregulation of RANKL.
The risk of PMO develops increasingly with estrogen
deficiency, which causes a series of changes in the blood and
interrupts the balance between bone formation and resorption
(34). The suppression of
E2, OPG and IGF-1 production is closely associated with
an increase in bone turnover and an accelerated bone loss, as shown
by a decrease in the BMD (35,36).
IGF-1, a growth-promoting polypeptide that is essential for normal
growth and development directly regulates bone growth and density;
therefore, the possibility that the changes in the serum levels of
E2, OPG and IGF-1 could account for the formation of the
KYD pattern was explored in the present study by measuring the
concentrations of E2, OPG and IGF-1 in the KYD
pattern-serum and control serum. The findings showed that the
concentrations of E2, OPG and IGF-1 were downregulated
in the KYD pattern-serum, compared with those in the control serum.
Although it is clear that the alterations in the E2, OPG
and IGF-1 serum levels affect bone formation, the other proteins in
the serum may also play a crucial role in bone remodeling and
therefore warrant future investigation.
In conclusion, the present study has provided data
showing that the alterations in the concentrations of
E2, OPG and IGF-1 may account for the susceptibility of
certain postmenopausal women to the KYD pattern of osteoporosis by
inhibiting the OPG/RANKL system, which leads to a reduction in bone
formation. The major limitation of this study was the small sample
size, and thus a randomized, controlled trial with a larger sample
size needs to be conducted. Furthermore, the fact that the KYD
pattern-serum was the only pattern of kidney deficiency
investigated, with regard to its effects on the function of
osteoblasts, could be considered one-sided; therefore experiments
on the other patterns will be carried out in the future.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81202645 and 81230087), and
the Natural Science Foundation of Fujian Province (grant no.
2015J01339).
References
|
1
|
Reid IR: Should we prescribe calcium
supplements for osteoporosis prevention? J Bone Metab. 21:21–28.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lagari VS and Levis S: Phytoestrogens in
the prevention of postmenopausal bone loss. J Clin Densitom.
16:445–449. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liang W, Lin M, Li X, Li C, Gao B, Gan H,
Yang Z, Lin X, Liao L and Yang M: Icariin promotes bone formation
via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19
human osteoblastic cell line. Int J Mol Med. 30:889–895.
2012.PubMed/NCBI
|
|
4
|
Hayden RS, Quinn KP, Alonzo CA,
Georgakoudi I and Kaplan DL: Quantitative characterization of
mineralized silk film remodeling during long-term
osteoblast-osteoclast co-culture. Biomaterials. 35:3794–3802. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang S, Eleniste PP, Wayakanon K, Mandela
P, Eipper BA, Mains RE, Allen MR and Bruzzaniti A: The Rho-GEF
Kalirin regulates bone mass and the function of osteoblasts and
osteoclasts. Bone. 60:235–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao R: Immune regulation of bone loss by
Th17 cells in oestrogen-deficient osteoporosis. Eur J Clin Invest.
43:1195–1202. 2013.PubMed/NCBI
|
|
7
|
Frenkel B, Hong A, Baniwal SK, Coetzee GA,
Ohlsson C, Khalid O and Gabet Y: Regulation of adult bone turnover
by sex steroids. J Cell Physiol. 224:305–310. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baek KH, Oh KW, Lee WY, et al: Changes in
the serum sex steroids, IL-7 and RANKL-OPG system after bone marrow
transplantation: Influences on bone and mineral metabolism. Bone.
39:1352–1360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng M, Wang Q, Fan Y, Liu X, Wang L, Xie
R, Ho CC and Sun W: A traditional Chinese herbal preparation,
Er-Zhi-Wan, prevent ovariectomy-induced osteoporosis in rats. J
Ethnopharmacol. 138:279–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu H and Lawson D: Theories and practice
in prevention and treatment principles in relation to Chinese
herbal medicine and bone loss. J Tradit Chin Med. 24:88–92.
2004.PubMed/NCBI
|
|
11
|
Damiani G: The Yin and Yang of
anti-Darwinian epigenetics and Darwinian genetics. Riv Biol.
100:361–402. 2007.PubMed/NCBI
|
|
12
|
Wang P and Chen Z: Traditional Chinese
medicine ZHENG and Omics convergence: A systems approach to
post-genomics medicine in a global world. OMICS. 17:451–459. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang M, Lu C, Zhang C, Yang J, Tan Y, Lu
A and Chan K: Syndrome differentiation in modern research of
traditional Chinese medicine. J Ethnopharmacol. 140:634–642. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu A, Jiang M, Zhang C and Chan K: An
integrative approach of linking traditional Chinese medicine
pattern classification and biomedicine diagnosis. J Ethnopharmacol.
141:549–556. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li S, Zhang ZQ, Wu LJ, Zhang XG, Li YD and
Wang YY: Understanding ZHENG in traditional Chinese medicine in the
context of neuro-endocrine-immune network. IET Syst Biol. 1:51–60.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang M, Zha Q, Lu C, He Y and Lu A:
Association between tongue appearance in Traditional Chinese
Medicine and effective response in treatment of rheumatoid
arthritis. Complement Ther Med. 19:115–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang W, Li X, Li Y, Li C, Gao B, Gan H,
Li S, Shen J, Kang J, Ding S, et al: Tongue coating microbiome
regulates the changes in tongue texture and coating in patients
with post-menopausal osteoporosis of Gan-shen deficiency syndrome
type. Int J Mol Med. 32:1069–1076. 2013.PubMed/NCBI
|
|
18
|
Méndez JP, Rojano-Mejía D, Pedraza J,
Coral-Vázquez RM, Soriano R, García-García E, Aguirre-García Mdel
C, Coronel A and Canto P: Bone mineral density in postmenopausal
Mexican-Mestizo women with normal body mass index, overweight, or
obesity. Menopause. 20:568–572. 2013.PubMed/NCBI
|
|
19
|
An S, Li E and Tong X: Study on
relationship between estrogen receptor gene polymorphism and
syndrome differentiation typing of female postmenopausal
osteoporosis in Traditional Chinese medicine. Zhongguo Zhong Xi Yi
Jie He Za Zhi. 20:907–910. 2000.(In Chinese). PubMed/NCBI
|
|
20
|
Leung PC and Siu WS: Herbal treatment for
osteoporosis: A current review. J Tradit Complement Med. 3:82–87.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao Z, Lu Y, Halmurat Upur, Jing J and Xu
D: Study of osteoporosis treatment principles used historically by
ancient physicians in Chinese Medicine. Chin J Integr Med.
19:862–868. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Matsumoto Y, Otsuka F, Takano-Narazaki M,
Katsuyama T, Nakamura E, Tsukamoto N, Inagaki K, Sada KE and Makino
H: Estrogen facilitates osteoblast differentiation by upregulating
bone morphogenetic protein-4 signaling. Steroids. 78:513–520. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Coelho MJ and Fernandes MH: Human bone
cell cultures in biocompatibility testing. Part II: Effect of
ascorbic acid, beta-glycerophosphate and dexamethasone on
osteoblastic differentiation. Biomaterials. 21:1095–1102. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Melville KM, Kelly NH, Khan SA, Schimenti
JC, Ross FP, Main RP and van der Meulen MC: Female mice lacking
estrogen receptor-alpha in osteoblasts have compromised bone mass
and strength. J Bone Miner Res. 29:370–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parker BD, Bauer DC, Ensrud KE and Ix JH:
Association of osteocalcin and abdominal aortic calcification in
older women: The study of osteoporotic fractures. Calcif Tissue
Int. 86:185–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sapir-Koren R and Livshits G: Is
interaction between age-dependent decline in mechanical stimulation
and osteocyte-estrogen receptor levels the culprit for
postmenopausal-impaired bone formation? Osteoporos Int.
24:1771–1789. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oh SM, Kim HR and Chung KH: Effects of
ginkgo biloba on in vitro osteoblast cells and ovariectomized rat
osteoclast cells. Arch Pharm Res. 31:216–224. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Botella S, Restituto P, Monreal I, Colina
I, Calleja A and Varo N: Traditional and novel bone remodeling
markers in premenopausal and postmenopausal women. J Clin
Endocrinol Metab. 98:E1740–E1748. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stuss M, Rieske P, Cegłowska A,
Stêpień-Kłos W, Liberski PP, Brzeziańska E and Sewerynek E:
Assessment of OPG/RANK/RANKL gene expression levels in peripheral
blood mononuclear cells (PBMC) after treatment with strontium
ranelate and ibandronate in patients with postmenopausal
osteoporosis. J Clin Endocrinol Metab. 98:E1007–E1011. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shoji S, Tabuchi M, Miyazawa K, Yabumoto
T, Tanaka M, Kadota M, Maeda H and Goto S: Bisphosphonate inhibits
bone turnover in OPG (−/−) mice via a depressive effect on both
osteoclasts and osteoblasts. Calcif Tissue Int. 87:181–192. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mazière C, Salle V, Gomila C and Mazière
JC: Oxidized low density lipoprotein enhanced RANKL expression in
human osteoblast-like cells. Involvement of ERK, NFkappaB and NFAT.
Biochim Biophys Acta. 1832:1756–1764. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yasuda H: RANKL, a necessary chance for
clinical application to osteoporosis and cancer-related bone
diseases. World J Orthop. 4:207–217. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aubin JE and Bonnelye E: Osteoprotegerin
and its ligand: A new paradigm for regulation of osteoclastogenesis
and bone resorption. Osteoporos Int. 11:905–913. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mozaffari-Khosravi H, Hesabgar HA, Owlia
MB, Hadinedoushan H, Barzegar K and Fllahzadeh MH: The effect of
garlic tablet on pro-inflammatory cytokines in postmenopausal
osteoporotic women: A randomized controlled clinical trial. J Diet
Suppl. 9:262–271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yakar S, Rosen CJ, Beamer WG,
Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J,
Boisclair YR, et al: Circulating levels of IGF-1 directly regulate
bone growth and density. J Clin Invest. 110:771–781. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Papierska L, Rabijewski M,
Kasperlik-Załuska A and Zgliczyński W: Effect of DHEA
supplementation on serum IGF-1, osteocalcin and bone mineral
density in postmenopausal, glucocorticoid-treated women. Adv Med
Sci. 57:51–57. 2012. View Article : Google Scholar : PubMed/NCBI
|