Introduction
Acute lung injury (ALI) and acute respiratory
distress syndrome (ARDS), its more severe form, are acute
respiratory failure syndromes, which are characterized by severe
hypoxemia, pulmonary edema and neutrophil accumulation in the lungs
(1). The occurrence of ALI/ARDS may
be a result of critical illnesses of diverse etiologies; these
illnesses may be a result of direct injury to the lung, such as
toxic inhalation, aspiration, pneumonia and near-drowning, or occur
through indirect causes, e.g. sepsis, pancreatitis, burns,
gynecological conditions (amniotic fluid embolism, eclampsia,
placental abruption) and massive blood transfusion. The clinical
incidence of ALI/ARDS is high and the condition is associated with
marked mortality and morbidity (2,3).
Inflammation is an early response of the vascular
tissues to infection, injuries and harmful stimuli, such as
pathogens and irritants; it is involved in non-specific immune
responses for neutralizing invaders and repairing damaged cells and
it initiates the healing processes (4). Lipopolysaccharide (LPS) is a structural
component of the outer membrane of Gram-negative bacteria and is
known to induce inflammation (5).
LPS binds to and signals through the Toll-like receptor 4, leading
to a rapid release of pro-inflammatory cytokines, such as tumor
necrosis factor (TNF). TNF subsequently acts through its membrane
receptor 1 complex I (6–8) to activate multiple downstream
effectors, such as the transcription factor nuclear factor κB
(NF-κB), to further induce the production of pro-inflammatory
cytokines, including interleukin (IL)-6, IL-1β and macrophage
inflammatory protein 2 (MIP-2), thereby amplifying the inflammatory
response (9,10). Excessive inflammatory reactions,
particularly the activation of neutrophils and macrophages, have
been implicated in the pathogenesis of ARDS (11,12). In
addition, increased levels of oxidants, if unchecked, can become
deleterious to the cells, since they are responsible for oxidative
stress (acute or chronic), which can cause tissue damage and
apoptotic cell death. Oxidative stress plays a key role in the
pathogenesis of an extensive range of diseases, including
Alzheimer's and Parkinson's diseases (13), cardiovascular and pulmonary disorders
(14,15) and, in particular, ALI and primary and
secondary infections.
Inflammation and oxidative stress play a critical
role in the pathogenic process, which is generated by the innate
immune response to LPS-induced acute tissue injury. Furthermore,
inflammation and oxidative stress are inextricably linked, as each
generates and reinforces the other; for example, through the
activation of NF-κB, oxidative stress promotes the recruitment and
activation of resident cells and leukocytes, thereby evoking
inflammation (16), while activated
resident cells, leukocytes and macrophages induce oxidative stress
through the generation of reactive oxygen, chlorine and nitrogen
species.
The transcription factor nuclear factor
erythroid-2-related factor 2 (Nrf2) is a basic leucine zipper
transcription factor and a member of the cap ‘n’ collar
transcription factor family (17).
Nrf2 is responsible for both the constitutive and the inducible
expression of antioxidant response element-regulated genes. Nrf2
activation is an effective antioxidant defensive mechanism that is
used by host cells against oxidative stress. Nrf2 has been shown to
be the master regulator of several hundreds of genes that are
involved in the antioxidant defense response (18).
Eriodictyol, a flavonoid isolated from the Chinese
herb Dracocephalum rupestre, has long been established as an
antioxidant and anti-inflammatory agent. Notably, eriodictyol is
distributed in common foods and has been identified to have
beneficial biological activities (19,20).
Eriodictyol has been found to suppress nitric oxide production,
NF-κB activation and mitogen-activated protein kinase
phosphorylation in mouse macrophages (21). Based on these previously observed
properties of eriodictyol, the aim of the present study was to use
a mouse model to investigate whether eriodictyol could exert a
protective effect in LPS-induced ALI.
Materials and methods
Mice
Specific pathogen-free female C57BL/6 mice (8–10
weeks old) were purchased from the SLRC Laboratory Animal Co., Ltd.
(Shanghai, China). The animals were maintained under barrier
conditions and kept at 22–25°C under a 12-h light/dark cycle. The
mice were randomly divided into four groups: Phosphate buffered
saline (PBS)-treated healthy control (n=10), LPS-induced ALI
(n=10), vehicle-treated ALI (LPS + vehicle, n=10) and
eriodictyol-treated ALI (LPS + eriodictyol, n=10). All experimental
procedures were carried out in strict accordance with the NIH
Guidelines for the Care and Use of Laboratory Animals, and animal
handling was performed following the dictates of the National
Animal Welfare Law of China.
Establishment of the ALI mouse
model
Eighty female C57BL/6 mice were anesthetized by an
intraperitoneal injection of 150 mg/kg ketamine HCl and 65 µg/kg
xylazine hydrochloride. E. coli LPS (Sigma-Aldrich, St.
Louis, MO, USA) was instilled intratracheally (25 µg in 50 µl
sterile saline) during inspiration. The control mice received PBS
instillation, while the eriodictyol- and vehicle-treated mice
received eriodictyol (30 mg/kg, dissolved in PBS) and vehicle
(PBS), respectively, orally 2 days prior to the induction of ALI.
The mice were then sacrificed by an intravenous injection of
thiopental 24 h after the induction of ALI. The thorax was opened
and the blood was sampled by cardiac puncture. Simultaneously,
three bronchoalveolar lavage (BAL) procedures were performed, each
using 0.5 ml normal saline. The blood was centrifuged (2,000 × g,
for 10 min at 4°C) and the serum was stored for further processing;
the survival curve was then depicted using the Kaplan-Meier
method.
BAL fluid (BALF) collection and cell
counting
Three simultaneous BAL procedures were carried out
using 0.5 ml sterile saline, and the BALF was collected. The
recovered lavage was centrifuged at 3,000 × g for 10 min at 4°C.
The cell-free supernatants that were obtained from the first
procedure were stored at −20°C for further analysis for
inflammatory cytokine and protein concentration by ELISA. The BALF
cell pellet was resuspended in PBS for cell counting.
Morphological assessment of lung
injury
Mice were anesthetized by an intraperitoneal
injection of 100 mg/kg ketamine mixed with 10 mg/kg xylazine.
Following exposure, the lung tissues were perfused with PBS and
fixed by an injection of 10% formalin into the trachea. Following
tracheal ligation, the lung tissues were incubated in 4% formalin
overnight at 4°C. The lung tissues were then embedded with
paraffin, sectioned at 5-µm thickness and stained with hematoxylin
and eosin.
For the determination of the lung injury score, the
histological images were evaluated by an assessor who was initially
blinded to the study groups. Lung injury was scored based on edema,
neutrophil inflitration, hemorrhage, bronchiole epithelial
desquamation and hyaline membrane formation (22). The score ranged between 0 and 4,
based on severity: 0, indicated no injury; 1, indicated modest
injury (including limited congestion and interstitial edema, but no
interstitial neutrophilic infiltrate with inflammatory cells in the
alveolar spaces); 2, indicated intermediate injury (including mild
congestion, interstitial edema, and interstitial neutrophilic
infiltrate with inflammatory cells in the alveolar spaces); 3,
indicated widespread injury (including more prominent congestion
and interstitial edema with neutrophils partially filling the
alevolar spaces); and 4, indicated widespread injury (including
most prominent, marked congestion, and interstitial edema with
neutrophilic infiltrate nearly filling the alveolar spaces or with
pulmonary consolidation). The average values were considered a
semi-quantitative histological index of lung injury.
Measurement of lung myeloperoxidase
(MPO) activity
The frozen right lower lobe lung samples were
weighed and homogenized in 2 ml of 50 mmol/l phosphate buffer at pH
6.0 containing 0.5% hexadecyltrimethylammonium bromide
(Sigma-Aldrich). The samples were then frozen on dry ice and thawed
at room temperature thrice, following which they were sonicated.
The suspensions were subsequently centrifuged at 2,000 × g for 15
min at 4°C. The MPO activity in the supernatant was assessed by
measuring the H2O2-dependent oxidation of
o-dianisidine dihydrochloride (Sigma-Aldrich), as previously
described (23). A total of 0.1 ml
supernatant was mixed with 2.9 ml phosphate buffer (50 mmol/l, pH
6.0) containing 0.0005% H2O2 (Sigma-Aldrich)
and 0.167 mg/ml o-dianisidine dihydrochloride
(Sigma-Aldrich). The change in absorbance was measured at 460 nm
using a spectrophotometer. The MPO activity was expressed as the
change in absorbance per minute and was further normalized to the
total protein content of the supernatant.
Measurement of lung tissue wet/dry
ratio
The left lungs were extracted and the wet weights
were obtained. The lung tissues were then reweighed 3 days after
drying at 80°C. The wet/dry ratio was calculated as follows:
wet/dry ratio = (wet weight - dry weight)/dry weight (24).
Lactate dehydrogenase (LDH) assay
The LDH activity in the BALF was measured using a
Sigma LDH determination kit (Sigma-Aldrich). Briefly, 200 µl BALF
supernatant was added to 2.5 ml reagent A for 1 min, prior to 100
µl reagent B being added. The absorbance at 340 nm was recorded
every minute for 3 min for the purpose of confirming the linearity
of the reaction. The LDH activity was expressed in U/l. Using LDH
standard (Sigma-Aldrich) it was confirmed that there was a linear
increase in absorbance with increasing LDH concentrations.
Protein concentration in the BALF
BAL was performed as described in a previous study
(25). Briefly, following sacrifice,
the trachea was exposed and intubated using a tracheal cannula. BAL
was performed three times by repeatedly flushing the airways and
lungs with 0.5 ml cold saline. The pooled BALF was collected on ice
and centrifuged at 500 × g for 5 min at 4°C. The supernatant was
then stored at −20°C for further analysis. The determination of
protein concentrations in the cell-free BALF was performed using
Bio-Rad protein assay reagents (Bio-Rad, Hercules, CA, USA). In the
same fashion, a standard curve was generated using bovine serum
albumin.
ELISA
The TNF-α, IL-6, IL-1α and MIP-2 concentrations were
measured by ELISA according to the manufacturer's instructions. The
experiment was repeated thrice and the results were presented as
the mean value of the three experiments.
Survival study of mice with
LPS-induced ALI
The possibility that pretreatment with eriodictyol
could confer protection against LPS-induced ALI was assessed. The
mice were randomly divided into four experimental groups (n=10 per
group), as mentioned previously, and the survival rates were
recorded at 120 h.
Measurement of oxidative stress in
vivo
The lung tissues were homogenized in 0.01 M PBS (pH
7.4) and clarified by centrifugation at 10,000 × g for 10 min at
4°C. The levels of H2O2, •OH and
malondialdehyde (MDA) were subsequently measured in order to
determine the level of oxidative stress in the lungs. The assays
were performed in accordance with the instructions of the
manufacturers of the H2O2 (Abcam, Cambridge,
MA, USA), •OH (Nanjing Jiancheng Bioengineering Research
Institute, Nanjing, China) and MDA kits (Abcam). The
H2O2, •OH and MDA levels
corresponded to the level of oxidative stress in vivo.
Western blot analysis
The tissues and macrophages were immediately
homogenized and the proteins were extracted according to the
instructions of the total protein extraction kit (G-Biosciences,
St. Louis, MO, USA). The protein concentration was determined using
the bicinchoninic acid protein assay kit (Sigma-Aldrich). The
protein samples were then subjected to 10% SDS-PAGE and transferred
to a polyvinylidene difluoride membrane. Following blocking with 5%
milk, the membrane was incubated with the indicated primary
antibodies: Rabbit polyclonal anti-Nrf2 (1:200; cat. no. sc-722;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), mouse monoclonal
anti-thioredoxin 1 (Trx1) (1:200; cat. no. sc-13526; Santa Cruz
Biotechnology, Inc.) and rabbit polyclonal anti-β-actin (1:1,000;
cat. no. A2066; Sigma-Aldrich) at room temperature for 2 h. The
membranes were then incubated with goat anti-rabbit (1:1,000; cat.
no. A0545; Sigma-Aldrich) and goat anti-mouse (1:1,000; cat. no.
HAF007, R&D Systems, Inc., Minneapolis, MN, USA) secondary
antibodies at room temperature for 1 h, and the protein bands were
visualized by the enhanced chemiluminescence system (Pierce
Biotechnology, Inc., Rockford, IL, USA). Blots were analyzed using
ImageJ 1.48V software (National Institutes of Health, Bethesda, MD,
USA).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
The total RNA was isolated from macrophages from
differentially treated ALI mice using TRIzol® reagent (Invitrogen
Life Technologies, Carlsbad, CA, USA), and RT-qPCR was performed as
previously reported (Life Technologies, Carlsbad, CA, USA)
(26). PCR was conducted using a
Plexor® qPCR and qRT-PCR system (Promega Corporation, Madison, WI,
USA) on an ABI 7900 Fast thermal cycler (Applied Biosystems Life
Technologies, Foster City, CA, USA). The following primer pairs
were used in the analysis: Mouse hypoxanthine
phosphoribosyltransferase, 5′-CTG GTG AAA AGG ACC TCT CG-3′
(forward) and 5′-TGA AGT ACT CAT TAT AGT CAA GGG CA-3′ (reverse);
mouse Nrf2, 5′-TTG GCA GAG ACA TTC CCA TTTG-3′ (forward) and 5′-AAA
CTT GCT CCA TGT CCT GCT CTA-3′ (reverse); mouse Trx1, 5′-TGC TAC
GTG GTG TGG ACC TTGC-3′ (forward) and 5′-ACC GGA GAA CTC CCC CAC
CT-3′ (reverse); mouse IL-6, 5′-CCA GAA ACC GCT ATG AAG TTCC-3′
(forward) and 5′-TCA CCA GCA TCA GTC CCA AG-3′ (reverse); mouse
TNF-α, 5′-CTC CAG GCG GTG CCT ATGT-3′ (forward) and 5′-GAA GAG CGT
GGT GGC CC-3′(reverse); mouse MIP-2, 5′-TCC AGA GCT TGA GTG TGA
CG-3′ (forward) and 5′- TCA GGT ACG ATC CAG GCT TC-3′ (reverse);
mouse IL-1β, 5′-CAA CCA ACA AGT GAT ATT CTC CATG-3′ (forward) and
5′-GAT CCA CAC TCT CCA GCT GCA-3′ (reverse) (Sangon Biotech Co.,
Ltd., Shanghai, China). The PCR cycling conditions were as follows:
95°C for 10 min, followed by 40 cycles at 95°C for 15 sec and 60°C
for 60 sec, a final extension step at 95°C for 60 sec, and a
dissociation curve analysis. Relative quantification (RQ) was
determined using the following formula: RQ=
2−∆Ct(∆Ct=Ctgene of
interest-Ctendogenous control).
Macrophage culture
Bone marrow cells from mouse femur or tibia were
harvested and selected using RPMI-1640 medium as previously
described (27). A week later, bone
marrow-derived macrophages were replated, and untreated macrophages
were stimulated with LPS (100 ng/ml) for 24 h or LPS and
eriodictyol or vehicle for 24 h. The cells and culture supernatant
were then collected in order to determine the inflammatory cytokine
expression.
Statistical analysis
All data were analyzed using SPSS 13.0 (SPSS Inc.,
Chicago, IL, USA) software and expressed as the mean ± standard
error of the mean. One-way analysis of variance followed by
Fisher's protected least significant difference test were used to
assess significant differences, and P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of eriodictyol pretreatment on
ALI
The LPS-induced ALI animal model is a common
experimental model used for the investigation of molecular
mechanisms and drug efficacy in ALI (28). To observe the effect of eriodictyol
on ALI, an LPS-induced ALI animal model was therefore established.
As shown in Fig. 1, the
morphological examination following LPS induction revealed that
several histopathological alterations, including cell structure
destruction, neutrophil infiltration, alveolar wall thickening and
lung edema, had occurred in the lung tissue (Fig. 1B), as compared with the PBS-treated
control group (Fig. 1A). When
compared with the vehicle-treated ALI mice (Fig. 1C), however, the LPS-induced
pathological symptoms were markedly improved following pretreatment
with eriodictyol (Fig. 1D),
suggesting that eriodictyol has a protective effect against
LPS-induced ALI.
In order to further evaluate the protective effect
of eriodictyol in ALI, the lung injury index, lung tissue wet/dry
ratio, LDH and protein concentration in the lung BALF of
differentially treated ALI mice were determined. It was found that,
compared with the PBS-treated healthy control group, eriodictyol
pretreatment decreased the lung injury index (Fig. 1E), lung wet/dry ratio (Fig. 1F), LDH level (Fig. 1G) and BALF protein concentration
(Fig. 1H), which had been elevated
due to LPS stimulation. These results were in agreement with the
effect of eriodictyol on lung tissue histology.
Eriodictyol pretreatment decreases the
production of inflammatory cytokines in LPS-induced ALI mice
Pro-inflammatory cytokines, including TNF-α, IL-6,
IL-1β and MIP-2, play a critical role in the pathogenesis of ALI,
and therefore the effect of eriodictyol on TNF-α, IL-6, IL-1β and
MIP-2 production in the serum and BALF of differentially treated
ALI mice was measured by ELISA 24 h after LPS induction. As shown
in Fig. 2, LPS caused a significant
increase in the serum levels of TNF-α (Fig. 2A), IL-6 (Fig. 2B), IL-1β (Fig. 2C) and MIP-2 (Fig. 2D), as well as in the corresponding
lung BALF levels (Fig. 2E–H), of the
differentially treated ALI mice; however, the LPS-induced increase
in inflammatory cytokines observed in the serum and lung BALF of
the vehicle-pretreated ALI mice was reversed by the eriodictyol
pretreatment (Fig. 2). These results
demonstrated that eriodictyol could inhibit the inflammatory
response in LPS-induced ALI.
 | Figure 2.Effect of eriodictyol on LPS-induced
pro-inflammatory cytokine secretion in the serum and BALF of
differentially treated ALI mice. (A-D) Levels of (A) TNF-α, (B)
IL-6, (C) IL-1β and (D) MIP-2 in the serum and (E-H) levels of (E)
TNF-α, (F) IL-6, (G) IL-1β and (H) MIP-2 in the BALF of
differentially treated ALI mice were measured using ELISA. Data are
presented as the mean ± standard error (n=10). *P<0.05 compared
with the LPS-induced ALI group; #P<0.05 compared with
the vehicle-treated ALI group. ALI, acute lung injury; Ctr,
control; LPS, lipopolysaccharide; MIP-2, macrophage inflammatory
protein 2; TNF-α, tumor necrosis factor α; IL, interleukin, BALF,
bronchoalveolar lavage fluid. |
Inhibition of MPO activity and
inflammatory neutrophil accumulation in the lung tissues by
eriodictyol pretreatment
LPS-induced ALI is characterized by an increase in
neutrophils and MPO activity in the lung tissues (29,30).
Furthermore, an increased MPO activity reflects polymorphonuclear
neutrophil accumulation in the lungs. For that reason, the MPO
activity in the lung tissue homogenates and the number of
neutrophils in the lung BALF of differentially treated ALI mice
were examined. Following LPS induction, a significant increase was
observed in the MPO activity (Fig.
3A) and the number of neutrophils (Fig. 3B) in the lung tissues, compared with
the PBS-treated healthy control group; however, this increase in
MPO activity (Fig. 3A) and
neutrophils (Fig. 3B) was eliminated
by the eriodictyol pretreatment, as compared with the LPS-induced
ALI or vehicle-treated groups.
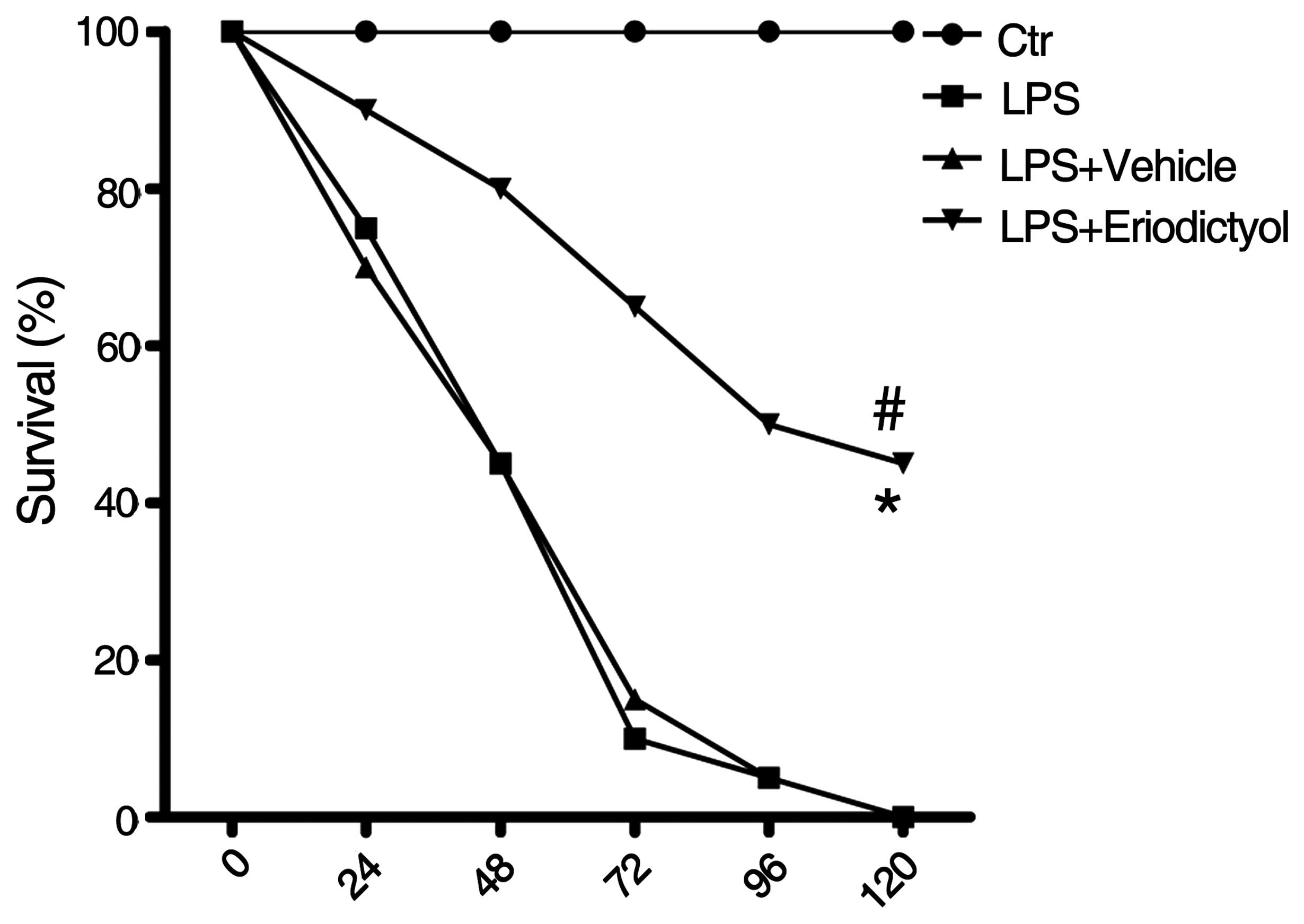
Improved survival rate of LPS-induced
ALI mice following eriodictyol pretreatment
The survival rate, which was strongly indicative of
the protective effect of eriodictyol on LPS-induced ALI, was
examined in differentially treated ALI mice as previously described
(31). It was found that, compared
with the LPS-induced or vehicle-treated ALI groups, the survival
rate of the LPS-induced ALI mice was significantly improved
following eriodictyol administration (Fig. 4).
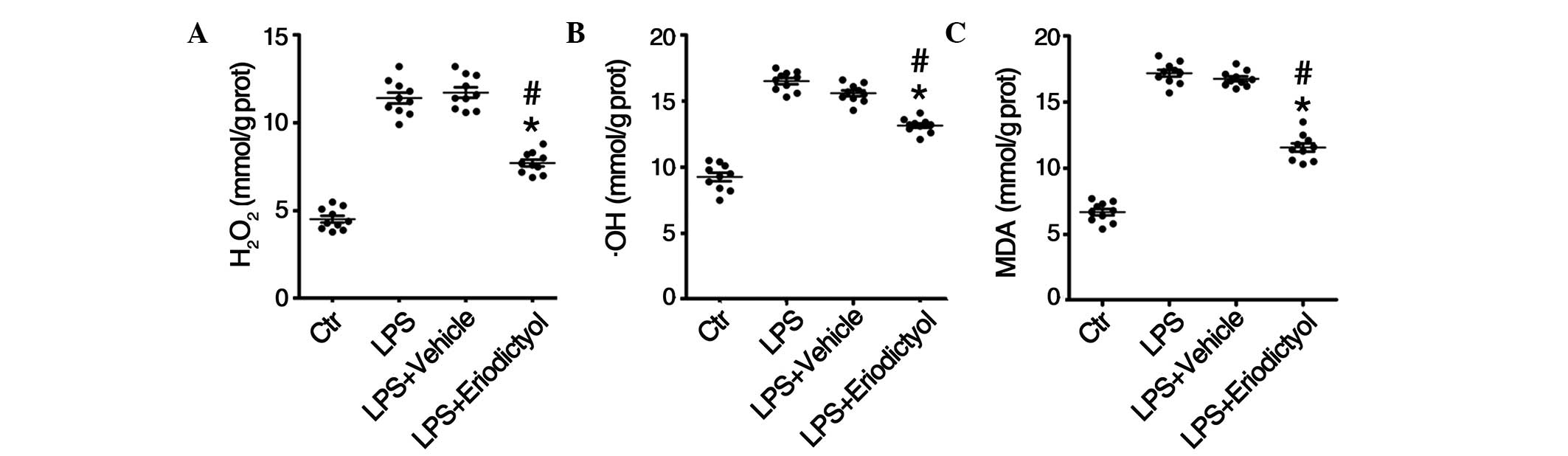
Decrease in oxidative stress levels in
LPS-induced ALI mice following eriodictyol pretreatment
Considerable experimental evidence supports the role
of oxidants, including H2O2, •OH
and MDA, and oxidative injury in the pathogenesis of ALI/ARDS
(32,33); therefore, the levels of
H2O2, •OH and MDA in the lung
tissues from differentially treated ALI mice were determined. The
results of the in vivo assays revealed increased levels of
H2O2 (Fig.
5A), •OH (Fig. 5B)
and MDA (Fig. 5C) in the lung tissue
homogenates from LPS-induced mice, compared with those from the
control group; however the eriodictyol pretreatment decreased the
accumulation of H2O2 (Fig. 5A), •OH (Fig. 5B) and MDA (Fig. 5C) in the lung tissues. In
combination, these results indicated that eriodictyol could
attenuate LPS-induced ALI through its antioxidative activity.
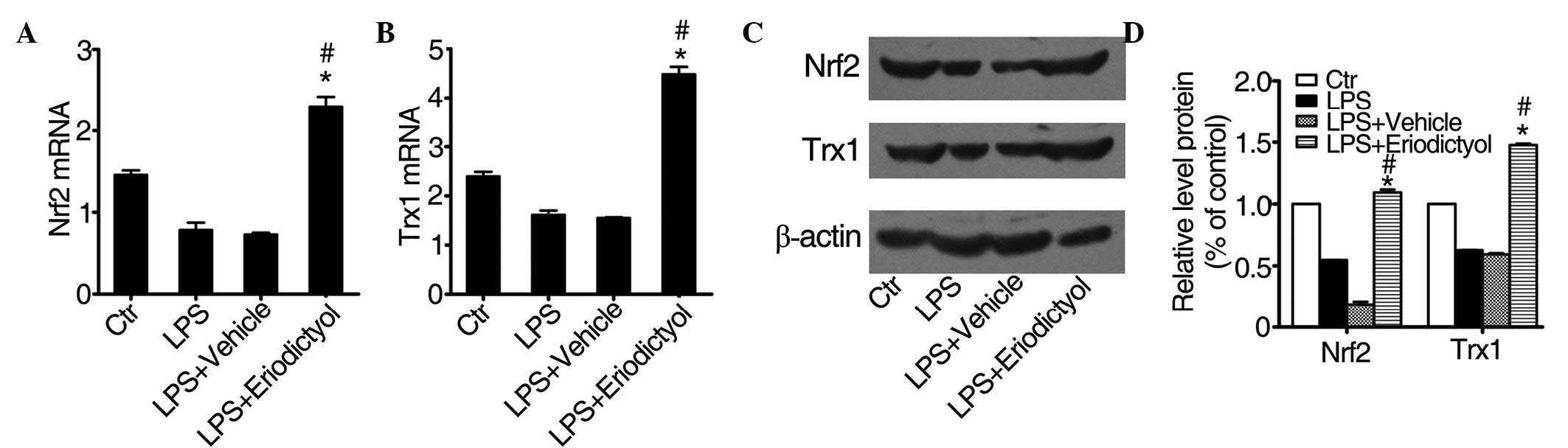
Eriodictyol pretreatment enhances Trx1
expression by regulating the Nrf2 pathway
A previous study reported that eriodictyol could
activate the Nrf2 pathway and enhance the expression of Trx1 in
vitro (34), while a different
study showed that Nrf2 could regulate a series of
antioxidant-responsive genes (35).
Trx1 is a small, ubiquitous protein that acts as a redox protein in
response to various oxidative stress conditions (36). As shown in Fig. 6, treatment with eriodictyol
significantly increased the mRNA expression of Nrf2 (Fig. 6A) and Trx1 (Fig. 6B) in the eriodictyol-treated ALI
group, as compared with the expression in the LPS-induced or
vehicle-treated ALI groups. The protein expression levels of Nrf2
and Trx1 (Fig. 6C and D) were
consistent with the mRNA expression levels. These results also
indicated that eriodictyol could alleviate LPS-induced ALI through
its antioxidative activity.
Expression of inflammatory cytokines
in macrophages is inhibited by eriodictyol pretreatment
Previous studies have demonstrated that macrophages
play a critical role in the pathogenesis of lung injury, since they
initiate the inflammatory response and promote neutrophil
infiltration and tissue damage in the lungs (37–39).
In vitro, eriodictyol has been shown to inhibit the
expression of inflammatory cytokines (21); therefore, in the present study,
macrophages from C57BL/6 mice were isolated and stimulated with LPS
and eriodictyol or vehicle, and then the mRNA and protein levels of
inflammatory cytokines produced in the macrophages were detected.
It was found that the expression levels of inflammatory cytokines,
such as TNF-α (Fig. 7A), IL-6
(Fig. 7B), IL-1β (Fig. 7C), and MIP-2 (Fig. 7D), were decreased in
eriodictyol-treated macrophages, as compared with the LPS-treated
or vehicle-treated macrophages. The mRNA expression levels of these
inflammatory cytokines: TNF-α (Fig.
7E), IL-6 (Fig. 7F), IL-1β
(Fig. 7G), and MIP-2 (Fig. 7H), were similar to the protein
expression levels (Fig. 7A–D).
Discussion
The occurrence of ALI is strongly associated with
infection with LPS-containing Gram-negative bacteria (40). In the LPS-induced ALI mouse model,
the manifestations are similar to the pathological characteristics
of ALI in humans (41); therefore,
this model appears to be well suited for the study of potential
preliminary preventive or therapeutic compounds against ALI in
humans. Eriodictyol, a flavonoid found in citrus fruits, has been
reported to have a potential antioxidative and anti-inflammatory
activity in vitro (34,42). In
the present study, it was demonstrated that eriodictyol could
attenuate the LPS-induced ALI in mice, thus prolonging their
survival time, as well as that eriodictyol treatment could protect
mice from LPS-induced ALI via inhibiting the expression of
inflammatory cytokines in macrophages and activating the Nrf2
pathway to suppress oxidative injury in ALI mice.
The exudation in the early phase of LPS-induced ALI
is characterized by increased levels of neutrophils, proteins and
inflammatory cytokines and chemokines in the BALF and serum,
depending on the severity of the disease (43,44). In
both systemic and local both information, edema is a typical
symptom. Widespread alveolar epithelial destruction and neutrophil
infiltration in the alveolar spaces, in association with high
volumes of proteinaceous exudate, comprise a typical ALI lesion.
MPO is an enzyme located mainly in the primary granules of the
neutrophils and, thus, the activity of MPO in the parenchyma
reflects the adhesion and margination of neutrophils in the lung
tissues; therefore, to investigate the protective effect of
eriodictyol on LPS-induced ALI, LPS-induced ALI mice were
pretreated with eriodictyol, and the effect of eriodictyol
pretreatment on the histology, lung tissue wet/dry ratio, protein
concentration in the BALF, MPO activity and neutrophil count in the
ALI mice was examined. The results of the histopathological
examination showed that eriodictyol pretreatment improved the
LPS-induced histological manifestations (Fig. 1A–D), including inflammatory cell
infiltration, increased alveolar septum thickness, hyaline membrane
formation, alveolar congestion and hemorrhage. It was also found
that eriodictyol treatment decreased the lung wet/dry ratio
(Fig. 1F), protein concentration
(Fig. 1H) and neutrophil count
(Fig. 3B) in the BALF and the MPO
activity in the homogenates (Fig.
3A), compared with the LPS-induced or vehicle-treated ALI
groups. These results suggest a potential therapeutic effect of
eriodictyol on LPS-induced ALI.
It is believed that a sustained and uncontrolled
pulmonary inflammation plays an important role in the pathogenesis
of ALI (45,46) and, therefore, a potential strategy to
attenuate the progression of ALI could be based on suppressing the
immune system-mediated inflammatory responses. It was found in the
present study that eriodictyol exerts a powerful anti-inflammatory
activity in the ALI mouse model. Compared with the LPS-induced or
vehicle-treated ALI groups, the levels of inflammatory cytokines in
the serum and BALF were significantly reduced in the ALI mice that
received eriodictyol pretreatment (Fig.
2). Since the primary source of inflammatory cytokines in the
ALI mice was macrophages, the macrophages were isolated and
cultured with eriodictyol or vehicle, and it was found that the
increase in inflammatory cytokines, such as TNF-α, IL-6, IL-1β and
MIP-2, which was caused by LPS stimulation, was inhibited following
the administration of eriodictyol (Fig.
7). These data suggested that eriodictyol reduced LPS-induced
ALI by inhibiting the expression of inflammatory cytokines in
macrophages.
Excessive oxidative injury is another factor
involved in the pathogenesis of ALI (47,48). A
previous study indicated that eriodictyol is capable of exhibiting
antioxidative activity in vitro (34). In the present study it was observed
that eriodictyol could enhance the activation of the Nrf2 pathway
and increase the expression of antioxidant response
element-regulated genes, such as Trx1, in the lung tissues from
LPS-induced ALI mice. These results were supported by a study on
the effects of eriodictyol in ARPE-19 cells in vitro
(34).
In conclusion, the present results demonstrated that
pretreatment with eriodictyol significantly attenuated pulmonary
inflammation and lung injury in mice with LPS-induced ALI, and that
the protective effect of eriodictyol could be, at least partly,
attributed to its abilities to alleviate the excessive oxidative
injury and inhibit the production of inflammatory cytokines,
including TNF-α, IL-6, IL-1β and MIP-2, in macrophages. In
addition, the protective effect of eriodictyol in ALI may be
associated with the suppression of NF-κB signaling and the
activation of the Nrf2 pathway, which subsequently causes a marked
reduction in inflammatory responses and oxidative injury in lung
tissue.
Acknowledgements
This study was partly supported by a grant from the
Beijing Municipal Commission of Education (grant no. M200810025006)
and a grant from the Beijing Natural Science Foundation (grant no.
7112039).
References
|
1
|
Luh SP and Chiang CH: Acute lung
injury/acute respiratory distress syndrome (ALI/ARDS): The
mechanism, present strategies and future perspectives of therapies.
J Zhejiang Univ Sci B. 8:60–69. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rubenfeld GD: Epidemiology of acute lung
injury. Crit Care Med. 31(Suppl): S276–S284. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Song K, Xiong H, Li H, Chu X and
Deng X: Protective effect of florfenicol on acute lung injury
induced by lipopolysaccharide in mice. Int Immunopharmacol.
9:1525–1529. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrero-Miliani L, Nielsen OH, Andersen PS
and Girardin SE: Chronic inflammation: Importance of NOD2 and NALP3
in interleukin-1beta generation. Clin Exp Immunol. 147:227–235.
2007.PubMed/NCBI
|
|
5
|
Aderem A and Ulevitch RJ: Toll-like
receptors in the induction of the innate immune response. Nature.
406:782–787. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsu H, Shu HB, Pan MG and Goeddel DV:
TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF
receptor 1 signal transduction pathways. Cell. 84:299–308. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karin M and Lin A: NF-kappaB at the
crossroads of life and death. Nat Immunol. 3:221–227. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu J, Minemoto Y and Lin A: c-Jun
N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for
tumor necrosis factor alpha-induced c-Jun kinase activation and
apoptosis. Mol Cell Biol. 24:10844–10856. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J and Lin A: Wiring the cell signaling
circuitry by the NF-kappa B and JNK1 crosstalk and its applications
in human diseases. Oncogene. 26:3267–3278. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang G, Minemoto Y, Dibling B, et al:
Inhibition of JNK activation through NF-kappaB target genes.
Nature. 414:313–317. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aldridge AJ: Role of the neutrophil in
septic shock and the adult respiratory distress syndrome. Eur J
Surg. 168:204–214. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martin TR: Cytokines and the acute
respiratory distress syndrome (ARDS): A question of balance. Nat
Med. 3:272–273. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calkins MJ, Johnson DA, Townsend JA, et
al: The Nrf2/ARE pathway as a potential therapeutic target in
neurodegenerative disease. Antioxid Redox Signal. 11:497–508. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Touyz RM: Reactive oxygen species,
vascular oxidative stress and redox signaling in hypertension: What
is the clinical significance? Hypertension. 44:248–252. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Van Eeden S, Leipsic J, Man Paul SF and
Sin DD: The relationship between lung inflammation and
cardiovascular disease. Am J Respir Crit Care Med. 186:11–16. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cachofeiro V, Goicochea M, de Vinuesa SG,
Oubiña P, Lahera V and Luño J: Oxidative stress and inflammation, a
link between chronic kidney disease and cardiovascular disease.
Kidney Int Suppl. 74:S4–S9. 2008. View Article : Google Scholar
|
|
17
|
Pedruzzi LM, Stockler-Pinto MB, Leite M Jr
and Mafra D: Nrf2-keap1 system versus NF-kappaB: The good and the
evil in chronic kidney disease? Biochimie. 94:2461–2466. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deramaudt TB, Dill C and Bonay M:
Regulation of oxidative stress by Nrf2 in the pathophysiology of
infectious diseases. Med Mal Infect. 43:100–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ismaili H, Sosa S, Brkic D, et al: Topical
anti-inflammatory activity of extracts and compounds from Thymus
broussonettii. J Pharm Pharmacol. 54:1137–1140. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Minato K, Miyake Y, Fukumoto S, et al:
Lemon flavonoid, eriocitrin, suppresses exercise-induced oxidative
damage in rat liver. Life Sci. 72:1609–1616. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JK: Anti-inflammatory effects of
eriodictyol in lipopolysaccharide-stimulated raw 264.7 murine
macrophages. Arch Pharm Res. 34:671–679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou ZH, Sun B, Lin K and Zhu LW:
Prevention of rabbit acute lung injury by surfactant, inhaled
nitric oxide, and pressure support ventilation. Am J Respir Crit
Care Med. 161:581–588. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bradley PP, Priebat DA, Christensen RD and
Rothstein G: Measurement of cutaneous inflammation: Estimation of
neutrophil content with an enzyme marker. J Invest Dermatol.
78:206–209. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Numata M, Suzuki S, Miyazawa N, et al:
Inhibition of inducible nitric oxide synthase prevents LPS-induced
acute lung injury in dogs. J Immunol. 160:3031–3037.
1998.PubMed/NCBI
|
|
25
|
Lee JP, Li YC, Chen HY, et al: Protective
effects of luteolin against lipopolysaccharide-induced acute lung
injury involves inhibition of MEK/ERK and PI3K/Akt pathways in
neutrophils. Acta Pharmacol Sin. 31:831–838. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nosotti M, Falleni M, Palleschi A, et al:
Quantitative real-time polymerase chain reaction detection of lymph
node lung cancer micrometastasis using carcinoembryonic antigen
marker. Chest. 128:1539–1544. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Manicone AM, Birkland TP, Lin M, et al:
Epilysin (MMP-28) restrains early macrophage recruitment in
Pseudomonas aeruginosa pneumonia. J Immunol. 182:3866–3876. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kabir K, Gelinas JP, Chen M, et al:
Characterization of a murine model of endotoxin-induced acute lung
injury. Shock. 17:300–303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Abraham E: Neutrophils and acute lung
injury. Crit Care Med. 31(4 Suppl): S195–S199. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grommes J and Soehnlein O: Contribution of
neutrophils to acute lung injury. Mol Med. 17:293–307. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng PY, Lee YM, Wu YS, Chang TW, Jin JS
and Yen MH: Protective effect of baicalein against endotoxic shock
in rats in vivo and in vitro. Biochem Pharmacol. 73:793–804. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Baldwin SR, Simon RH, Grum CM, Ketai LH,
Boxer LA and Devall LJ: Oxidant activity in expired breath of
patients with adult respiratory distress syndrome. Lancet. 1:11–14.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kietzmann D, Kahl R, Müller M, Burchardi H
and Kettler D: Hydrogen peroxide in expired breath condensate of
patients with acute respiratory failure and with ARDS. Intensive
Care Med. 19:78–81. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Johnson J, Maher P and Hanneken A: The
flavonoid, eriodictyol, induces long-term protection in ARPE-19
cells through its effects on Nrf2 activation and phase 2 gene
expression. Invest Ophthalmol Vis Sci. 50:2398–2406. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang B, Zhu X, Kim Y, et al: Histone
deacetylase inhibition activates transcription factor Nrf2 and
protects against cerebral ischemic damage. Free Radic Biol Med.
52:928–936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Furukawa M, Tanaka R, Chuang VT, et al:
Human serum albumin-thioredoxin fusion protein with long blood
retention property is effective in suppressing lung injury. J
Control Release. 154:189–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Frank JA, Wray CM, McAuley DF, Schwendener
R and Matthay MA: Alveolar macrophages contribute to alveolar
barrier dysfunction in ventilator-induced lung injury. Am J Physiol
Lung Cell Mol Physiol. 291:L1191–L1198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Eyal FG, Hamm CR and Parker JC: Reduction
in alveolar macrophages attenuates acute ventilator induced lung
injury in rats. Intensive Care Med. 33:1212–1218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Johnston LK, Rims CR, Gill SE, McGuire JK
and Manicone AM: Pulmonary macrophage subpopulations in the
induction and resolution of acute lung injury. Am J Respir Cell Mol
Biol. 47:417–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Matthay MA, Ware LB and Zimmerman GA: The
acute respiratory distress syndrome. J Clin Invest. 122:2731–2740.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rojas M, Woods CR, Mora AL, Xu J and
Brigham KL: Endotoxin-induced lung injury in mice: Structural,
functional and biochemical responses. Am J Physiol Lung Cell Mol
Physiol. 288:L333–L341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu Y, Singer M, Thouron F, Alaoui-El-Azher
M and Touqui L: Effect of surfactant on pulmonary expression of
type IIA PLA(2) in an animal model of acute lung injury. Am J
Physiol Lung Cell Mol Physiol. 282:L743–750. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jerala R: Structural biology of the LPS
recognition. Int J Med Microbiol. 297:353–363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Goodman RB, Pugin J, Lee JS and Matthay
MA: Cytokine-mediated inflammation in acute lung injury. Cytokine
Growth Factor Rev. 14:523–535. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Manicone AM: Role of the pulmonary
epithelium and inflammatory signals in acute lung injury. Expert
Rev Clin Immunol. 5:63–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ward PA: Oxidative stress: Acute and
progressive lung injury. Ann NY Acad Sci. 1203:53–59. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Imai Y, Kuba K, Neely GG, et al:
Identification of oxidative stress and Toll-like receptor 4
signaling as a key pathway of acute lung injury. Cell. 133:235–249.
2008. View Article : Google Scholar : PubMed/NCBI
|