Introduction
There are few specific early symptoms or sensitive
biomarkers for the screening of ovarian cancer. Thus, the majority
of patients with ovarian cancer are not diagnosed until stage
III–IV when they are no longer eligible for the most effective
surgical interventions. Platinum-based chemotherapy has been a
critical treatment for ovarian cancer since the late 1970s and has
improved overall survival significantly (1). However, initial chemotherapy resistance
and platinum-resistant relapse occur frequently, causing increased
mortality due to progressive disease (2). It is therefore imperative to find an
approach to overcome resistance to cisplatin (CDDP).
The aquaporins (AQPs) are a family of small
transmembrane proteins that primarily facilitate the rapid, passive
movement of water or small sugar alcohol molecules, such as
glycerol, across cell plasma membranes and are essential for
cellular water homeostasis. AQPs are ubiquitously expressed in all
types of organisms from bacteria to plants, insects and mammals
(3,4). Amino acid sequence and molecular
function have been used to categorize AQPs into three distinct
subgroups: Classical aquaporins, aquaglyceroporins and unorthodox
aquaporins (5–8). The first group includes AQP0, AQP1,
AQP2, AQP4 and AQP5, which have highly selective permeability to
water but not other molecules. The aquaglyceroporin group includes
AQP3, AQP7, AQP9 and AQP10, which are permeable to water, as well
as glycerol, urea and other small non-electrolytes. AQP6, AQP8,
AQP11 and AQP12 are classified as unorthodox aquaporins, and their
functions remain under investigation (8).
It has been revealed that AQPs are unusually
expressed in numerous kinds of human cancers. According to the
review by Ribatti et al, AQP1, AQP3, AQP4, AQP5, AQP8 and
AQP9 are closely associated with various kinds of tumors (9). In ovarian tumors, the localization and
expression patterns of AQP1–9 have been investigated using
immunohistochemistry; AQP1, AQP3, AQP5, AQP6, AQP8 and AQP9 were
identified in epithelial ovarian cancer, while AQP1, AQP5 and AQP9
were significantly overexpressed in malignant and borderline tumors
compared with benign tumors and normal ovarian tissue (10). Another study revealed that the
expression of AQP1 was not significantly associated with the
clinicopathological stage in serous epithelial ovarian cancer
(11), although AQP1 expression was
associated with ascites, intratumoral microvessel density (IMD) and
clinicopathological variables. AQP5 is highly expressed in lymph
node metastasis cases and in abundant ascites, and previous studies
determined that AQP9 expression correlated with the degree of
histological malignancy (10,12,13).
It has also been reported that AQP3 facilitates ovarian cancer cell
migration and correlates with epidermal growth factor (EGF)-induced
cell metastasis (14). A previous
study by our group demonstrated that CDDP downregulates AQP5 in a
concentration-dependent manner in CAOV3 cells, and that nuclear
factor (NF)-κB is involved in AQP5 regulation (15). Epigallocatechin gallate, which
inhibits proliferation and induces apoptosis of SKOV3 cells,
decreased the expression of AQP5, suggesting a possible association
between ovarian cancer cell proliferation and AQP5 protein
expression (16). These studies
indicate that AQP might be a new therapeutic target for ovarian
cancer.
AQPs are involved in the fluidity and integrity of
cell membranes, angiogenesis, cell migration and cell volume
regulation (9,17). It has been recognized that water
transport across cytomembranes could be modified by osmotic stress
through AQP proteins, and that an increase in AQP3 or AQP9
expression is associated with increased chemoresistance to arsenite
in melanoma, lung cancer, primary cultured chorion and amnion cells
(18–20). AQP expression has also been found to
affect chemosensitivity in ovarian carcinoma (21), which may be associated with
osmosis.
To demonstrate whether chemotherapeutic drug
sensitivity and resistance are affected by water permeability and
AQP expression in ovarian cancer, the effects of extracellular
hyperosmotic stress on AQP expression and sensitivity to CDDP were
investigated in the present study.
Materials and methods
Cell culture and reagents
Ovarian cancer cell line 3AO was obtained from the
Institute of Cancer Research, Chinese Academy of Medical Sciences
(Beijing, China). 3AO cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco BRL, Gaithersburg, MD, USA)
supplemented with 15% fetal bovine serum and maintained at 37°C and
5% CO2 in a humid environment. For osmotic stress,
hyperosmotic medium was made by adding various concentrations of
D-sorbitol (Sigma-Aldrich, St. Louis, MO, USA) to regular DMEM for
different times. Standard DMEM was used as isosmotic media.
Cell growth and inhibition rate
assay
Cells were treated with the following solutions: i)
DMEM medium alone (the control group); ii) 0.625, 1.25, 2.5, 5, 10
or 20 µg/ml CDDP (Sigma-Aldrich) for 24, 48 or 72 h; iii) 100, 200,
300, 400, 500, 600 or 800 mM D-sorbitol for 24, 48 or 72 h; iv) 200
mM D-sorbitol and 0.625, 1.25, 2.5, 5, 10 or 20 µg/ml CDDP for 24,
48 or 72 h. Cell growth and inhibition rate was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay.
3AO cells were seeded in 96-well plates (5,000 cells
per well) for 24 h and exposed to various concentrations of CDDP or
D-sorbitol for 24–72 h. Following treatment, the cells were
incubated with 10 µl 5 mg/ml MTT (Sigma-Aldrich) 4 h at 37°C in the
dark. The formazan crystals were lysed with 150 µl dimethyl
sulfoxide (DMSO; Sigma-Aldrich) for 10 min. Absorbance values
(optical density; OD) at a wavelength of 490 nm were obtained using
a microplate reader (model 680: Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Wells containing cells in DMEM and wells
containing no cells served as the normal control and background
control, respectively. To convert OD values to a growth inhibition
rate, the following equation was used: Inhibition rate = (OD of
control – OD of test concentration)/(OD of control – OD of
cell-free wells). Each concentration was evaluated in 3–5 repeated
wells, and every assay was performed at ≥3 times.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The expression of AQP5 mRNA was examined by RT-qPCR.
Total RNA was isolated from 3AO cells with TRIzol reagent (Thermo
Fisher Scientific, Inc., Waltham, MA, USA). RNA was quantified
using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher
Scientific, Inc.). Those samples whose
A260/A280 ratios were 1.8–2.0 were used for
further analysis. Then, 1 µg RNA was used for reverse transcription
conducted using a PrimeScript™ RT reagent kit with gDNA eraser
(Takara Bio, Tokyo, Japan) according to the instructions provided
by the manufacturer. qPCR was carried out in a 20-µl reaction
volume containing 1 µl cDNA with SYBR Premix Ex Taq™ (Takara Bio)
using an Applied Biosystems StepOne Fast Real-Time PCR system
(Thermo Fisher Scientific, Inc.). The sequences of the primers used
were as follows: AQP1 (NM_198098), sense: 5′-ATC CTC TCA GGC ATC
ACC TC-3′ and antisense: 5′-GGT AGT AGCC AGC ACG CAT A-3′; AQP3
(NM_004925), sense: 5′-CAG TGG GAC GTG TTT CTG TC-3′ and antisense:
5′-CCC GGA TCC CTA AGA CTG TA-3′; AQP5 (NM_001651), sense: 5′-CTG
TCC ATT GGC CTG TCT GTC-3′ and antisense 5′-GGC TCA TAC GTG CCT TTG
ATG-3′; AQP9 (NM_020980), sense: 5′-CCT GAA ACA GCC TTC TCT CC-3′
and antisense: 5′-AAA CCA CCC AAA TGG GAC TA-3′; glyceraldehyde
3-phosphate dehydrogenase (GAPDH), sense: 5′-CAT CAA TGG AAA TCC
CATCA-3′ and antisense: 5′-TTCTCCATGGTGGTGAAGAC-3′.
The specificity of primers was tested by running a
regular PCR followed by agarose gel electrophoresis. The conditions
of the amplification process were: 95°C for 30 sec, 95°C for 5 sec
and 60°C for 30 sec, for 45 cycles. Each cDNA sample was analyzed
in triplicate, and GAPDH primer was included in every plate as an
internal control. Melting curve data were collected to assure PCR
specificity. All qPCRs were performed in more than triplicate.
Relative quantification of AQP5 mRNA expression was calculated
using the 2−ΔΔCq method.
Western blot analysis
Cells with or without treatments were washed with
cold phosphate-buffered saline and harvested by scraping in
radioimmunoprecipitation assay (RIPA) buffer containing protease
inhibitor cocktail (Sigma-Aldrich). Total protein was extracted
with RIPA buffer containing protease inhibitor for 30 min on ice.
After centrifugation at 13,000 × g for 15 min at 4°C, protein
concentrations were determined by Bio-Rad protein assay (Bio-Rad
Laboratories, Inc.). A 40-µg quantity of total protein was
subjected to 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred onto a
polyvinylidene (PVDF) membrane (Millipore, Bedford, MA, USA). After
being blocked for 1 h with 5% non-fat milk in Tris-buffered saline
and Tween 20 (TBS-T) at room temperature, the membranes were
incubated with rabbit polyclonal anti-AQP5 (1:200; BA2200-2; Boster
Biological Technology, Wuhan, China) or mouse monoclonal anti-GAPDH
(1:5,000; TA-08; Zhongshan Golden Bridge Biotechnology Co., Ltd.,
Beijing, China) primary antibody at 4°C overnight. The membranes
were then washed three times for 10 min each with TBS-T, and
incubated with a 1:5,000 dilution of goat anti-rabbit (ZB-5301) or
goat anti-mouse (ZB-5305) secondary antibody labeled with
horseradish peroxidase (Zhongshan Golden Bridge Biotechnology Co.,
Ltd.) for 1 h at room temperature. Antibody binding was detected
using an enhanced chemiluminescence (ECL; Millipore) detection
system following the manufacturer's instructions.
Small interfering (si)RNA
transfection
A lentiviral vector (LV) expressing AQP5-siRNA and
equipped with green fluorescence protein (GFP) and puromycin
acetyltransferase protein (PACP) was constructed by Shanghai
GenePharma Co., Ltd. (Shanghai, China). The provided control siRNA
was used as a negative control (mock). 3AO cells were transfected
with 30 multiplicity of infection (MOI) of AQP5-siRNA in the
presence of 6 ng/ml Polybrene (Sigma-Aldrich). The medium
containing the siRNA was replaced with fresh medium after 24 h
transfection. Green fluorescence was observed after 48 h
incubation. Then, cells were screened in medium containing
puromycin (3 µg/ml; Sigma-Aldrich) for 3 weeks. Following the
selection of transfected 3AO cells, in order to test the effects of
AQP5-siRNA, AQP5 expression was detected by RT-PCR and western
blotting.
Statistical analysis
The data are expressed as mean ± standard deviation
(SD). Values were analyzed by one-way analysis of variance (ANOVA)
and Student's unpaired t-test. P<0.05 was considered to indicate
a statistically significant result.
Results
Effects of hyperosmotic stress on the
proliferation of ovarian cancer cells
3AO cells were incubated with various concentrations
of D-sorbitol, representing different osmotic pressures, for
various times, and the inhibition of cell proliferation was
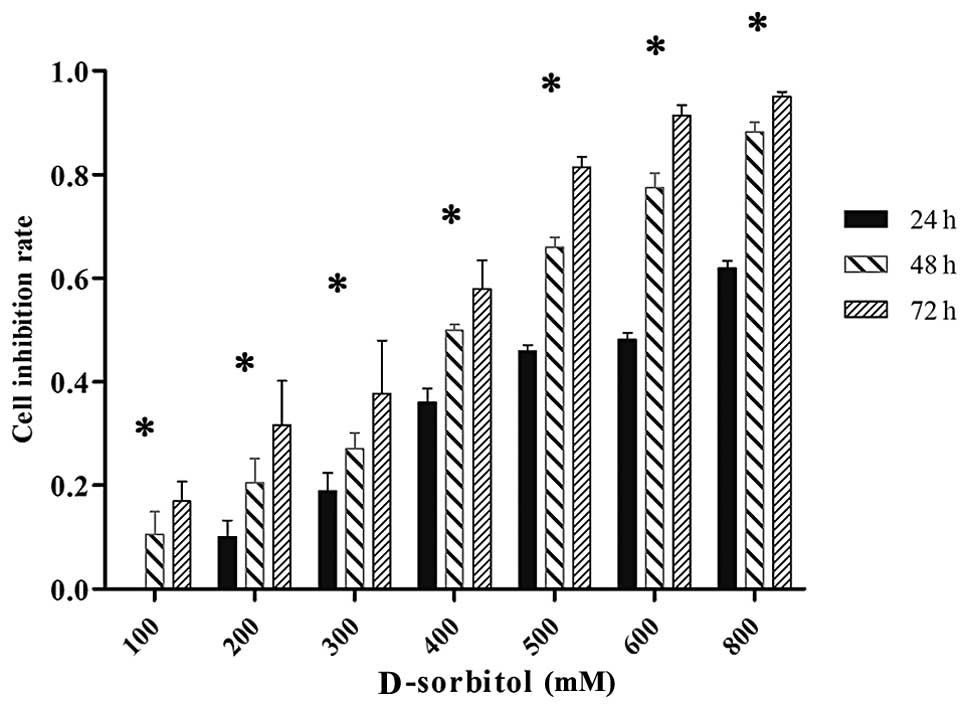
measured by MTT assay. Results showed that 3AO cell proliferation
was reduced in a dose- and time-dependent manner in hypertonic
culture medium. When 3AO cells were treated with 200 mM D-sorbitol
for 24, 48 or 72 h, the inhibition rate of cell proliferation was
10.08, 20.52 and 31.63%, respectively (Fig. 1).
Effect of hyperosmotic stress on the
mRNA expression of AQPs in ovarian cancer cells
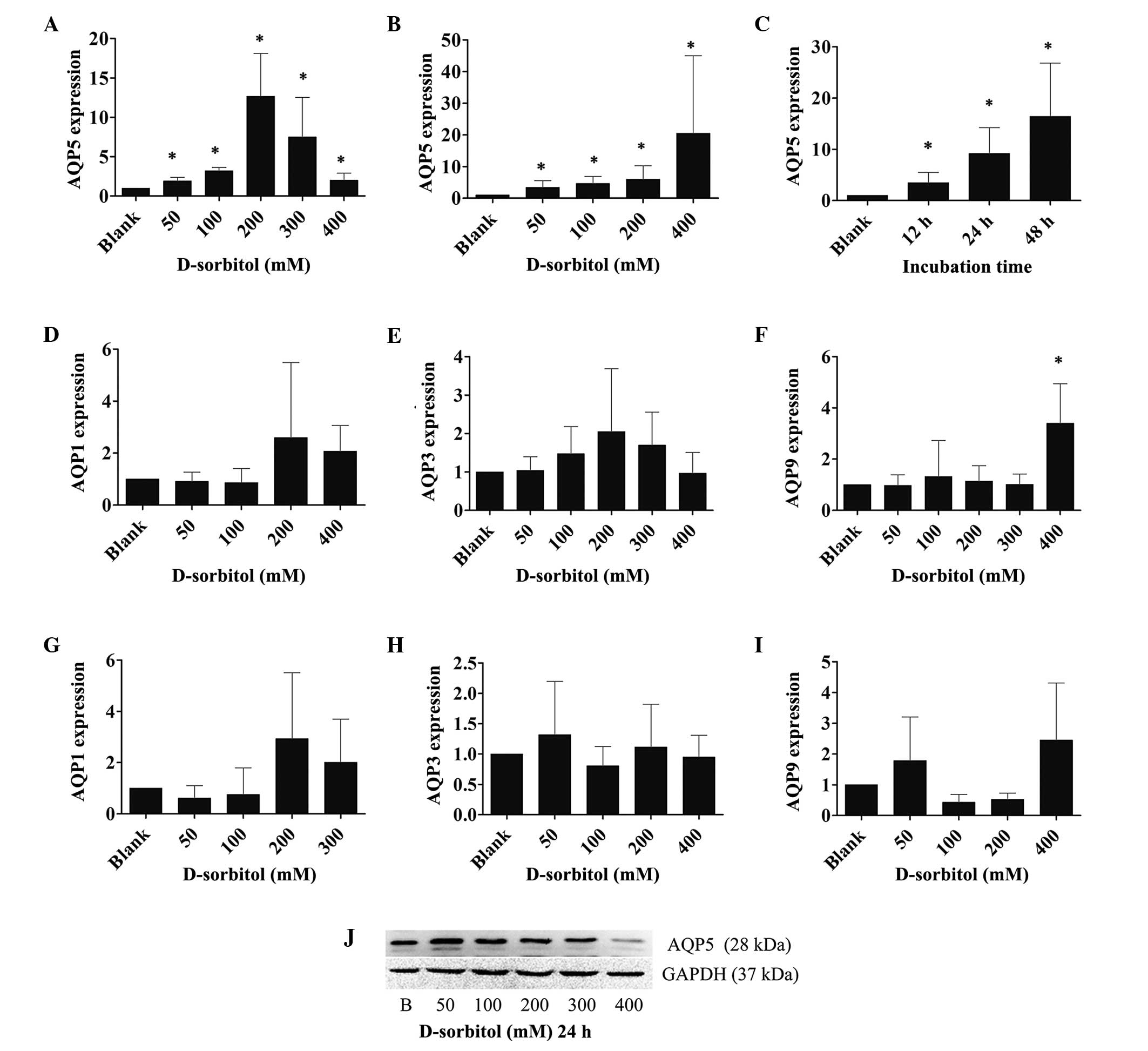
AQP expression is affected by hyperosmotic stress in
several cell types. To examine the AQP expression in response to
extracellular hyperosmotic stress in ovarian cancer, the mRNA
expression levels of various AQPs were measured by RT-qPCR in 3AO
cells that were incubated with 50, 100, 200, 300 or 400 mM
D-sorbitol (hypertonic medium) for 12, 24 or 48 h. It was found
that AQP5 mRNA expression levels increased significantly when the
cells were treated with hyperosmotic medium for 24 h. However, AQP5
mRNA expression peaked at the 200 mM concentration of D-sorbitol;
at higher concentrations of D-sorbitol, the expression of AQP5 was
reduced, yet it remained higher than that in control cultures
(P<0.05; Fig. 2A). By contrast,
the expression levels of AQP1, AQP3 and AQP9 mRNA were only
slightly elevated by hypertonic sorbitol-containing medium
(Fig. 2D–F).
When cells were incubated with hypertonic medium for
48 h, the expression levels of AQP1, AQP3, AQP9 mRNA were still
only slightly increased (Fig. 2G–I);
however, AQP5 expression was increased continuously and markedly
(P<0.05; Fig. 2B) as the osmotic
pressure increased. To examine the time course of AQP5 expression,
3AO cells were treated with 200 mM D-sorbitol for 12, 24 and 48 h,
followed by the analysis of AQP5 mRNA expression levels using
RT-qPCR. The outcomes showed that AQP5 mRNA expression was
increased in a time-dependent manner in hypertonic medium
(P<0.05; Fig. 2C). Western blot
analysis indicated that post-transcriptional AQP5 expression was
induced in a similar manner to transcriptional level expression by
hyperosmotic pressure (Fig. 2J).
Effects of AQP5 silencing on the
response to hyperosmotic stress in 3AO cells
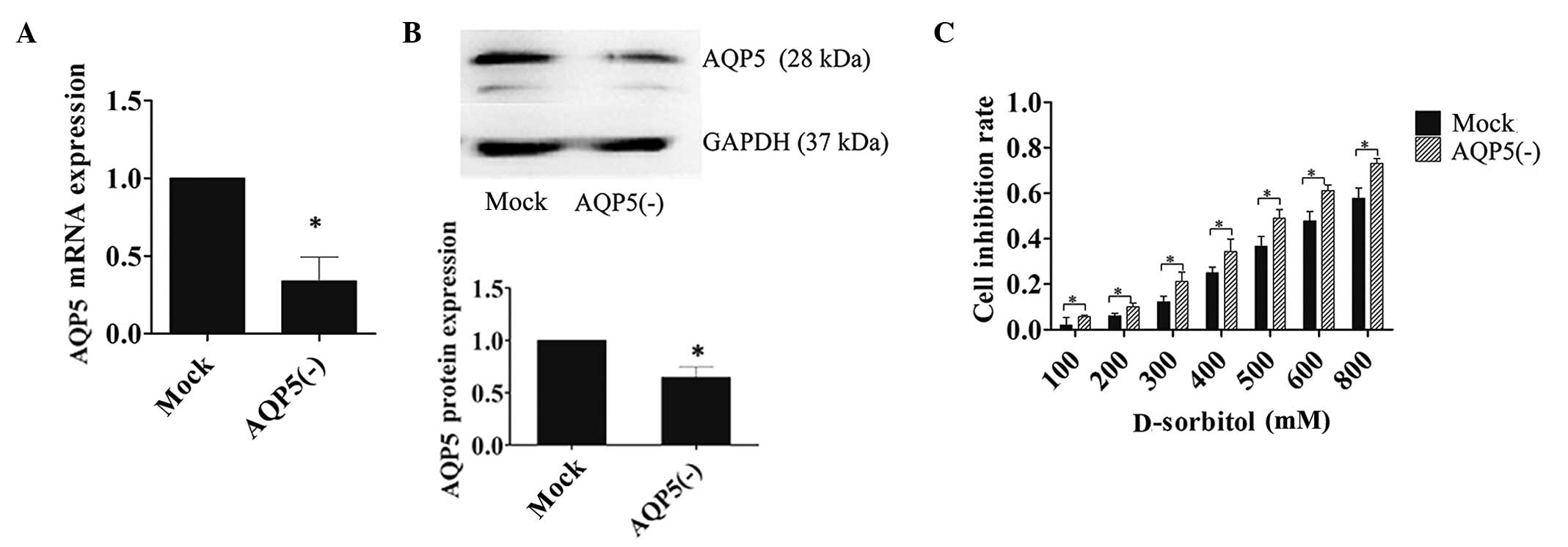
To determine the contribution of AQP5 to
hyperosmotic stress, 3AO cells were transfected with LV-siRNA-AQP5
or LV-siRNA-mock siRNA constructs. Transfection efficiency was
confirmed by RT-PCR and western blotting (Fig. 3A and B). MTT assays were performed on
transfected cells incubated in D-sorbitol-containing medium for 24
h. The inhibition rate of cell proliferation was significantly
increased in cells transfected with AQP5 siRNA compared with that
in mock-transfected controls in response to hypertonic medium
(Fig. 3C). The attenuated reactivity
of ovarian cancer cells to hyperosmotic pressure increased
incrementally with escalating osmotic concentrations in the cells
transfected with AQP5 siRNA, which indicated that AQP5 expression
facilitated protective mechanisms in response to hypertonic
conditions.
Effect of hyperosmotic stress on cell
sensitivity to CDDP
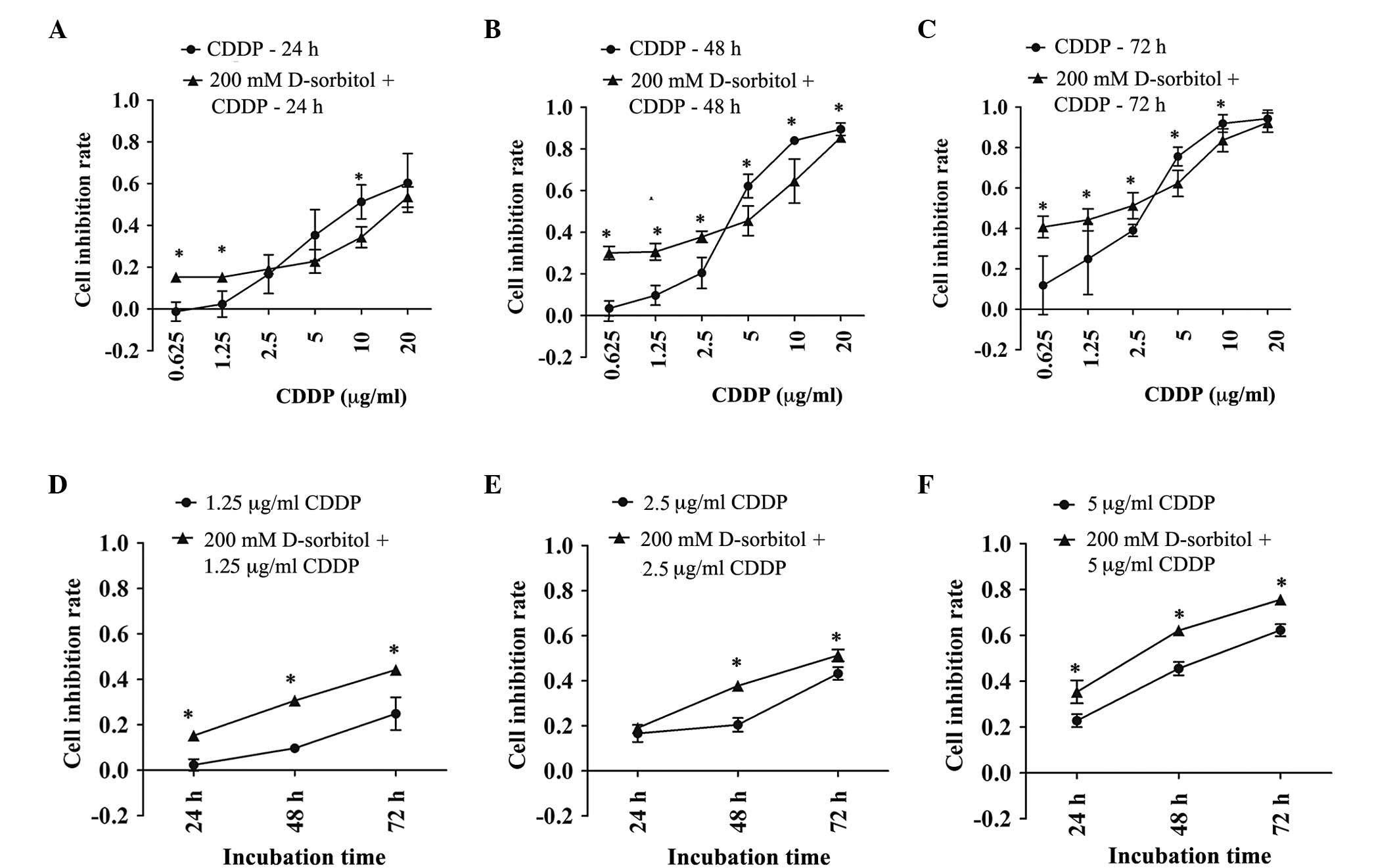
The addition of 200 mM D-sorbitol to the
extracellular environment induced hyperosmotic pressure, which
elevated AQP5 expression levels and caused slight cytotoxicity
(Figs. 1 and 2C). CDDP had variable inhibitory effects on
cell proliferation in response to either hypertonic or isotonic
conditions. MTT results showed that the inhibition rate of cell
proliferation induced by CDDP was increased in extracellular
hyperosmotic medium when the CDDP concentration was <2.5 µg/ml
and decreased in hyperosmotic medium with CDDP concentrations of
5–20 µg/ml (Fig. 4). These results
were more pronounced when the incubation time was prolonged to 48
or 72 h. Even though hyperosmosis or CDDP alone can lead to
cellular damage, together they exerted an additive effect on
cytotoxicity for CDDP concentrations of 0.625 and 1.25 µg/ml.
Furthermore, it was found that the decreased sensitivity to CDDP
caused by extracellular hyperosmosis at 5 and 10 µg/ml CDDP was
significant. In general, the sensitivity of ovarian cancer cells to
CDDP was changed by exposure to hypertonic medium. Moreover,
CDDP-induced cell death had dose- and time-dependent effects that
were independent of a hypertonic or isotonic extracellular
environment.
Effect of hyperosmotic stress on
sensitivity to CDDP is associated with AQP5 expression in ovarian
tumors
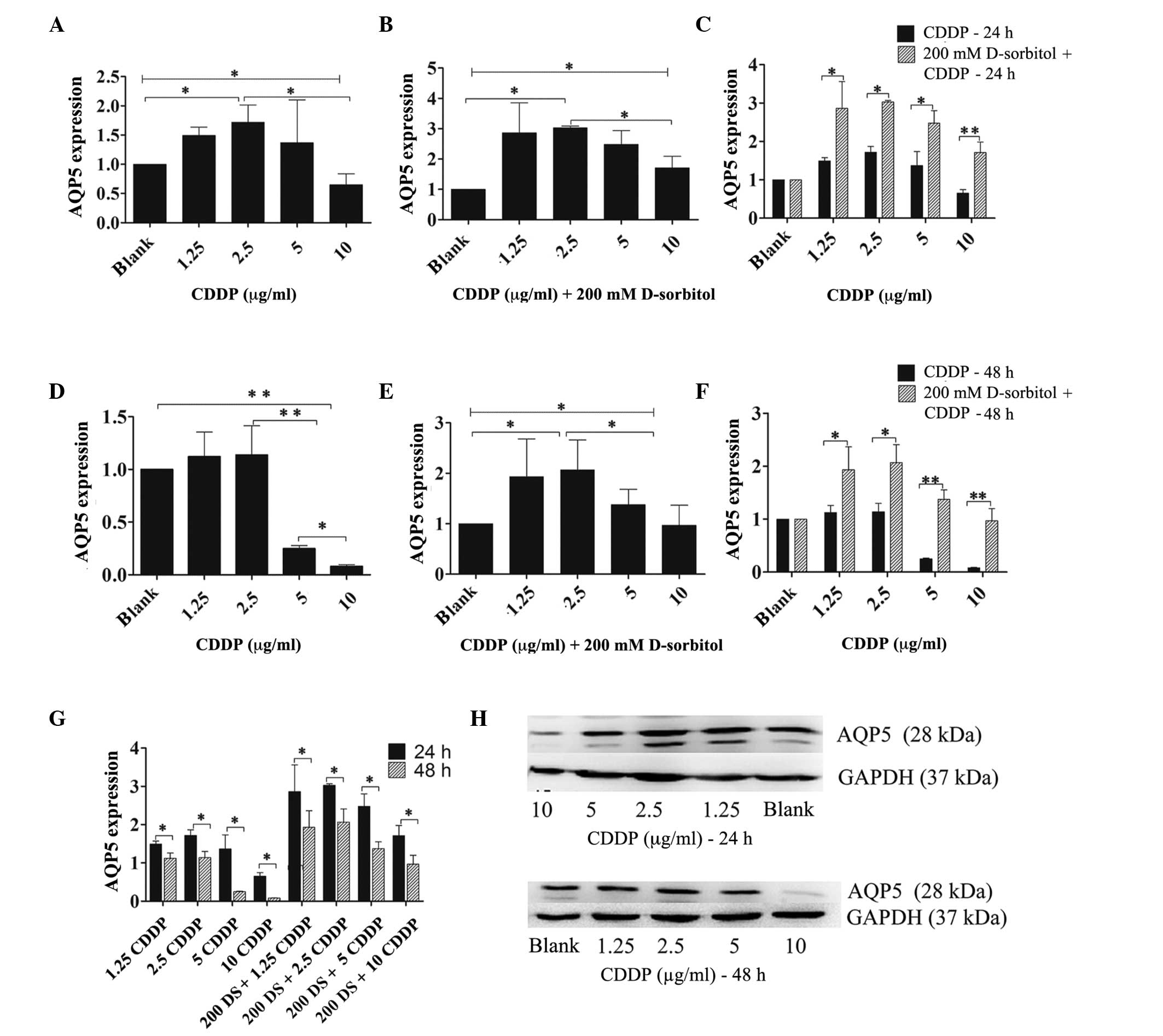
To examine the effect of CDDP on AQP5 expression
under conditions of hyperosmotic stress, hyperosmosis was induced
by adding 200 mM D-sorbitol to the normal culture medium and
treating 3AO cells with increasing concentrations of CDDP (1.25–10
µg/ml) in hypertonic or isotonic medium for 24 h. The RT-qPCR
results demonstrated that CDDP had similar effects on AQP5 mRNA
expression in hypertonic or isotonic external environments
(Fig. 5A–F). Following a 24-h
incubation, a low-dose of CDDP (<2.5 µg/ml) enhanced AQP5 mRNA
expression under hypertonic and isotonic extracellular conditions.
At CDDP concentrations ≥5 µg/ml, AQP5 expression was inversely
proportional to the CDDP dose, and was reduced compared with that
of 2.5 µg/ml CDDP (Fig. 5A and B).
After 48 h of incubation, ≥5 µg/ml CDDP attenuated AQP5 expression
levels by a large margin in an isotonic environment when compared
with those in the blank cells or the cells treated with 2.5 µg/ml
CDDP (Fig. 5D). However, when cells
were treated with CDDP in hypertonic medium for 48 h different
consequences were observed, with CDDP at doses ≤2.5 µg/ml inducing
intensified AQP5 mRNA expression levels, while AQP5 mRNA expression
levels were reduced at CDDP concentrations ≥5 µg/ml compared with
those observed for 2.5 µg/ml CDDP (Fig.
5E). Furthermore, as the incubation time was prolonged, AQP5
expression levels were reduced by CDDP, regardless of whether the
extracellular medium was hypertonic or isotonic (Fig. 5G), and the changes in AQP5 protein
expression in response to CDDP were synchronized with those of AQP5
mRNA (Fig. 5H).
Nevertheless, the primary distinction between the
two kinds of extracellular medium was that hyperosmosis enhanced
the expression level of AQP5 mRNA at every dose of CDDP tested
compared with those in isotonic medium, regardless of whether the
incubation time was prolonged (48 h) or not (24 h) (Fig. 5C and F).
Discussion
Members of the AQP family have been associated with
several types of tumors and can affect cell migration,
proliferation, and angiogenesis (9).
AQPs allow water to rapidly penetrate the cell membrane and are the
primary determinants of membrane permeability to water. There is
evidence that osmotic pressure can modify the expression of AQPs.
In MLE-15 mouse lung epithelial cells, AQP5 was induced by
hypertonic sorbitol-containing medium (22,23),
while in human airway epithelial cells, it was induced by
hyperosmotic stress (24). AQP5
abundance decreased in a dose-dependent manner when MLE-12 mouse
lung epithelial cells were exposed to hypotonic medium (25). In human keratinocytes, AQP3 mRNA
expression was increased by hypertonic sorbitol-containing medium;
however, AQP1, AQP4 and AQP9 mRNA expression remained unchanged
(26). Moreover, hypertonicity
promoted AQP2 expression in mouse principal kidney cortical
collecting duct cells (27), while
hypotonicity reduced it by attenuating cAMP-induced AQP2 promoter
activity, a process mediated by TonE-mediated c-Jun N-terminal
kinase activation (28). In mouse
brain tissue, a 3% NaCl hypertonic saline solution inhibited AQP4
mRNA and protein expression in astrocytes (29). Despite these results, to the best of
our knowledge, AQP expression in response to osmotic stress has not
been reported in ovarian cancer.
A previous study reported that AQP1, AQP5 and AQP9
expression levels were significantly increased in malignant ovarian
cancer (10). In the present study,
the effect of hypertonic sorbitol-containing medium on the
proliferation of ovarian cancer cells and expression of AQP1, AQP3,
AQP5, and AQP9 was determined. The results confirmed that ovarian
cancer cell proliferation was dose- and time-dependently inhibited
by a hypertonic extracellular environment, and that AQP5 mRNA and
protein expression levels were induced by hypertonic stress. This
provides the first evidence that AQP mRNA can be induced by
hypertonic pressure in epithelial ovarian carcinoma, which is
consistent with results in airway epithelial cells (22–24).
Reduced induction of AQP5 by 300 or 400 mM D-sorbitol-containing
medium after 24 h could be associated with diminished cell
viability at that level of osmotic stress (22). However, in the present study, a
greater increase in AQP5 mRNA expression was observed with 400 mM
sorbitol medium after 48 h. We propose that AQP5 expression can be
regulated by osmotic pressure and is associated with cell
viability, and the enhancing effects of hypertonic D-sorbitol on
AQP5 expression were particularly evident when the incubation time
was 48 h.
Previous studies have determined that AQPs are
important during membrane osmosis (30–32). In
the current study, the MTT assay results showed that ovarian cancer
cells were more susceptible to hypertonic medium after AQP5
expression was reduced by siRNA knockdown. The fact that AQP5
knockdown can reduce both osmotic water permeability and regulatory
volume in human lung adenocarcinoma cells (30) may explain the effects observed in
AQP5-knockdown ovarian cancer cells in response to hyperosmotic
pressure. This indicates that AQP5 has an important role in osmotic
homeostasis and that hypertonic stress is able to regulate AQP5
expression in ovarian cancer. Therefore, we speculate that ovarian
cancer cells adapt to hypertonic pressure based on changes in AQP5
expression, since water permeability is affected by increased AQP5
expression, which allowed the cells to adapt to the extracellular
hypertonic state.
The results of the present study also indicated that
a D-sorbitol-mediated extracellular hypertonic environment can
modify AQP1, AQP3, and AQP9 mRNA expression; however, the changes
were not determined to be statistically significant. There are
several factors that might explain this result: i) AQP1 is
expressed primarily in the microvascular endothelium in ovarian
cancer (10,11); ii) AQP3 and AQP9 are
aquaglyceroporins permeable to glycerol, urea, other small
non-electrolytes and water (8); iii)
AQP1 and AQP9 are expressed at low levels in 3AO cells (Fig. 2); and iv) AQPs respond differently to
osmotic pressure in various tissues (22–29).
Our previous study revealed that AQP5 expression was
decreased by CDDP in the CAOV3 cell line (15), which correlates with the present
study, and chemosensitivity was influenced by AQPs in the SKOV3
cell line (21). The present study
sought to determine the associations among CDDP sensitivity, AQP
expression and osmotic pressure in the 3AO cell line. The influence
of hyperosmotic pressure on sensitivity to CDDP and its association
with the expression of AQP5 is clarified by the current
results.
Resistance to CDDP is affected by many factors,
including changes in drug uptake and efflux, increased drug
metabolism in tumor cells, and DNA repair. Reduced uptake and
enhanced efflux of drugs from cells mediated by membrane
transporters and ion channels play an important role in drug
sensitivity and resistance. Osmotic stress controls water influx
and efflux across cells and may have an impact on drug metabolism
to further affect drug sensitivity. However, there are few reports
that have investigated this mechanism. We have previously
demonstrated that hyperosmotic stress induced by sorbitol increases
the sensitivity of SKOV3 cells to CDDP (21). The present study revealed that
sensitivity to CDDP was modified by hypertonic pressure in the 3AO
cell line. Moreover, AQP5 expression was modified significantly by
hypertonic sorbitol medium and was essential for the response of
ovarian cancer cells to extracellular hypertonic medium.
Our results indicate that 3AO cell sensitivity to
CDDP is enhanced by extracellular hyperosmosis when the CDDP
concentration is low, which may contribute to an induction of
CDDP-mediated AQP5 expression, further increased by hypertonic
stress. In addition, inhibition of cell proliferation at a high
CDDP dose was decreased in hyperosmotic medium compared with that
in isotonic medium, and this could be attributed to the
downregulation of AQP5 expression caused by a high-dose CDDP being
antagonized by hypertonic pressure. Accordingly, we hypothesized
that the changes in sensitivity to CDDP induced by hypertonic
medium were caused by an increase in AQP5 expression. In addition,
the abnormal expression of AQP3 or AQP9 affects chemoresistance to
arsenite in melanoma cells, lung cancer, primary cultured chorion
and amnion cells (18–20), and sensitivity to CDDP is associated
with AQPs in the SKOV3 cell line (21). On the basis of the present study,
sensitivity to CDDP is closely associated with AQP5 expression in
ovarian cancer. However, additional studies are necessary to
determine the association between CDDP sensitivity and AQP5
expression and to elaborate on the regulatory mechanism
involved.
In summary, the present study demonstrated that
extracellular hypertonic stress inhibits the proliferation of 3AO
cells, and that increased expression of AQP5 plays an important
role in the response of ovarian cancer cells to hypertonic medium,
which regulates CDDP sensitivity in ovarian cancer. Changes in CDDP
sensitivity induced by hyperosmosis were found to be associated
with changes in AQP5 expression, indicating that AQP5 expression is
relevant to CDDP sensitivity. The results show that CDDP
sensitivity was affected by extracellular hyperosmosis in an
ovarian cancer cell line, which suggests a novel direction for
ovarian cancer research. In addition, the important role of AQP5
expression in the regulation of osmotic pressure and sensitivity to
chemotherapy suggest it may be a new focus for ovarian
cancer-targeted therapy.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81202064).
References
|
1
|
Wiedemeyer WR, Beach JA and Karlan BY:
Reversing platinum resistance in high-grade serous ovarian
carcinoma: Targeting BRCA and the homologous recombination system.
Front Oncol. 4(34)2014.PubMed/NCBI
|
|
2
|
Bogliolo S, Cassani C, Gardella B,
Musacchi V, Babilonti L, Venturini PL, et al: Oxaliplatin for the
treatment of ovarian cancer. Expert opinion on investigational
drugs. 2015.24(9): 1275–86. PubMed/NCBI
|
|
3
|
Benga G: Water channel proteins (later
called aquaporins) and relatives: Past, present and future. IUBMB
Life. 61:112–133. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gomes D, Agasse A, Thiébaud P, Delrot S,
Gerós H and Chaumont F: Aquaporins are multifunctional water and
solute transporters highly divergent in living organisms. Biochim
Biophys Acta. 1788:1213–1228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agre P and Kozono D: Aquaporin water
channels: Molecular mechanisms for human diseases. FEBS Lett.
555:72–78. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nozaki K, Ishii D and Ishibashi K:
Intracellular aquaporins: Clues for intracellular water transport?
Pflugers Arch. 456:701–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zardoya R: Phylogeny and evolution of the
major intrinsic protein family. Biol Cell. 97:397–414. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang D, Tan YJ, Qu F, Sheng JZ and Huang
HF: Functions of water channels in male and female reproductive
systems. Mol Aspects Med. 33:676–690. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ribatti D, Ranieri G, Annese T and Nico B:
Aquaporins in cancer. Biochim Biophys Acta. 1840:1550–1553. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang JH, Yu YQ and Yan CX: Localisation
and expression of aquaporin subtypes in epithelial ovarian tumours.
Histol Histopathol. 26:1197–1205. 2011.PubMed/NCBI
|
|
11
|
Takal MK, Baykal C, Başer E, Kaya MD,
Dursun P, Ozen O, Haberal AN and Ayhan A: Does Aquaporin-1
expression have clinical significance in serous epithelial ovarian
cancer? J Turk Ger Gynecol Assoc. 14:130–135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang JH, Shi YF, Cheng Q and Deng L:
Expression and localization of aquaporin-5 in the epithelial
ovarian tumors. Gynecol Oncol. 100:294–299. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang JH, Shi YF, Chen XD and Qi WJ: The
influence of aquaporin-1 and microvessel density on ovarian
carcinogenesis and ascites formation. Int J Gynecol Cancer.
16(Suppl 1): 400–405. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji C, Cao C, Lu S, Kivlin R, Amaral A,
Kouttab N, Yang H, Chu W, Bi Z, Di W and Wan Y: Curcumin attenuates
EGF-induced AQP3 up-regulation and cell migration in human ovarian
cancer cells. Cancer Chemother Pharmacol. 62:857–865. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang J, Yan C, Zheng W and Chen X:
Proliferation inhibition of cisplatin and aquaporin 5 expression in
human ovarian cancer cell CAOV3. Arch Gynecol Obstet. 285:239–245.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yan C, Yang J, Shen L and Chen X:
Inhibitory effect of Epigallocatechin gallate on ovarian cancer
cell proliferation associated with aquaporin 5 expression. Arch
Gynecol Obstet. 285:459–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Day RE, Kitchen P, Owen DS, Bland C,
Marshall L, Conner AC, Bill RM and Conner MT: Human aquaporins:
Regulators of transcellular water flow. Biochim Biophys Acta.
1492–1506. 1840:2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao L, Gao Y, Li X, Howell P, Kumar R, Su
X, Vlassov AV, Piazza GA, Riker AI, Sun D and Xi Y: Aquaporins
mediate the chemoresistance of human melanoma cells to arsenite.
Mol Oncol. 6:81–87. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miao ZF, Chang EE, Tsai FY, Yeh SC, Wu CF,
Wu KY, Wang CJ and Tsou TC: Increased aquaglyceroporin 9 expression
disrupts arsenic resistance in human lung cancer cells. Toxicol In
Vitro. 23:209–216. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yoshino Y, Yuan B, Kaise T, Takeichi M,
Tanaka S, Hirano T, Kroetz DL and Toyoda H: Contribution of
aquaporin 9 and multidrug resistance-associated protein 2 to
differential sensitivity to arsenite between primary cultured
chorion and amnion cells prepared from human fetal membranes.
Toxicol Appl Pharmacol. 257:198–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen XJ, Chen WM, Ding XY, Zheng W, Zhang
Q and Yang JH: Effects of aquaporins on chemosensitivity to
cisplatin in ovarian cancer cells. Arch Gynecol Obstet.
290:525–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoffert JD: Hypertonic induction of
aquaporin-5 expression through an ERK-dependent pathway. J Biol
Chem. 275:9070–9077. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou B, Ann DK, Li X, Kim KJ, Lin H, Minoo
P, Crandall ED and Borok Z: Hypertonic induction of aquaporin-5:
Novel role of hypoxia-inducible factor-1alpha. Am J Physiol Cell
Physiol. 292:C1280–C1290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pedersen PS, Braunstein TH, Jørgensen A,
Larsen PL, Holstein-Rathlou NH and Frederiksen O: Stimulation of
aquaporin-5 and transepithelial water permeability in human airway
epithelium by hyperosmotic stress. Pflugers Arch. 453:777–785.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sidhaye VK, Güler AD, Schweitzer KS,
D'Alessio F, Caterina MJ and King LS: Transient receptor potential
vanilloid 4 regulates aquaporin-5 abundance under hypotonic
conditions. Proc Natl Acad Sci USA. 103:4747–4752. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sugiyama Y, Ota Y, Hara M and Inoue S:
Osmotic stress up-regulates aquaporin-3 gene expression in cultured
human keratinocytes. Biochim Biophys Acta. 1522:82–88. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li SZ, McDill BW, Kovach PA, Ding L, Go
WY, Ho SN and Chen F: Calcineurin-NFATc signaling pathway regulates
AQP2 expression in response to calcium signals and osmotic stress.
Am J Physiol Cell Physiol. 292:C1606–C1616. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Saito T, Saito T, Kasono K, Tamemoto H,
Kawakami M, Sasaki S and Ishikawa SE: Hypotonicity reduces the
activity of murine aquaporin-2 promoter induced by dibutyryl cAMP.
Exp Physiol. 93:1147–1156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao C, Yu X, Liao Z, Zhu N, Huo H, Wang M,
Ji G, She H, Luo Z and Yue S: Hypertonic saline reduces
lipopolysaccharide-induced mouse brain edema through inhibiting
aquaporin 4 expression. Crit Care. 16:R1862012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen Z, Zhang Z, Gu Y and Bai C: Impaired
migration and cell volume regulation in aquaporin 5-deficient
SPC-A1 cells. Respir Physiol Neurobiol. 176:110–117. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Solenov E, Watanabe H, Manley GT and
Verkman AS: Sevenfold-reduced osmotic water permeability in primary
astrocyte cultures from AQP-4-deficient mice, measured by a
fluorescence quenching method. Am J Physiol Cell Physiol.
286:C426–C432. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thiagarajah JR and Verkman AS: Aquaporin
deletion in mice reduces corneal water permeability and delays
restoration of transparency after swelling. J Biol Chem.
277:19139–19144. 2002. View Article : Google Scholar : PubMed/NCBI
|