Introduction
Pregnancy is a physiological condition accompanied
by dynamic metabolic changes in multiple organ systems, resulting
in increased oxygen consumption. In the first weeks after
conception, embryonic development occurs in a primarily hypoxic
environment; however, during pregnancy a highly vascular placenta
develops, which is rich in mitochondria and influences maternal
homeostasis. After the first trimester of pregnancy, the formation
and development of placental blood flow results in a rapid increase
in partial oxygen pressure (1,2).
Increased metabolic activity and reduced antioxidative activity in
uncomplicated pregnancy may lead to exaggerated oxidative stress
(OS) (3–5).
OS is defined as an imbalance between oxidants and
antioxidants in favour of reactive oxygen species (ROS), and may
lead to a disruption of redox signalling and control, and/or to
molecular damage (6). ROS are
produced endogenously by various physiological processes and in
response to external factors, such as pathogens and environmental
risk factors. Excessive physiological levels of ROS may lead to an
increase in damage to the cellular components, including lipids,
nucleic acids and proteins (7).
Oxidative damage to lipids is a complex process,
which modifies cellular membranes and has an adverse effect on
cellular function. The lipids that are most vulnerable to lipid
peroxidation (LP) are polyunsaturated fatty acids. Lipid
hydroperoxide and its corresponding aldehydes are generated at an
early stage of LP. Hydroperoxides and aldehydes are highly reactive
and may interact with proteins, nucleic acids or
amino-phospholipids (8). Stable
amide-type adducts, which are a product of lipid
hydroperoxide-derived protein modification, have been recently
identified, including hexanoyl-lysine (HEL) and propanoyl-lysine
(PRL). HEL is formed via the reaction of peroxidised n-6 fatty acid
with protein lysine residues, while PRL is generated through the
reaction of n-3 fatty acid with these residues (9). Previous studies have suggested that HEL
levels are elevated in the urine of patients with diabetes
(10–12), metabolic syndrome (13), rheumatoid arthritis (14) and systemic sclerosis (15).
Oxidative damage to proteins may alter various
levels of the protein structure and activity. Aromatic amino acids,
such as tyrosine, are highly susceptible to oxidation. The
nitration of tyrosine in proteins by peroxynitrite, which is a
strong oxidising agent generated during the reaction of nitric
oxide and the superoxide anion, generates 3-nitrotyrosine (NY). NY
is a promising biomarker for protein tyrosine nitration in
vivo, and elevated levels of NY have been detected in the urine
of diabetic patients (16). An
additional stable and potential marker of protein oxidation is
dityrosine (DiY), the formation of which is initiated with a
tyrosyl radical and concludes with the intermolecular cross-linkage
of two tyrosine-containing proteins (17,18).
Exaggerated oxidation of any of the aforementioned
substrates may indicate and theoretically contribute to various
complications and chronic diseases, thus posing a health risk to
the mother and the fetus. Previous studies have demonstrated an
association between exaggerated OS markers and pregnancy
complications, including gestational diabetes, pre-eclampsia,
intrauterine growth restriction, preterm birth and low birth weight
(19–23). The extent of physiologically
acceptable OS and the critical concentrations affecting fetal
growth and health outcomes are unclear.
The aim of the present study was to investigate
relatively novel potential oxidative markers in pregnant patients
and to evaluate their association with and effect on the pregnancy
outcome. The identification of novel biomarkers is a crucial
challenge for the early diagnosis, prediction and prevention of
pregnancy complications. The use of any marker requires knowledge
of normal baseline levels and their association with clinical
changes.
We hypothesised that the urine levels of markers of
oxidative damage to lipids and proteins in the second trimester of
pregnancy were associated with maternal and fetal characteristics
and with an increased risk of adverse infant features. To the best
of our knowledge, the present study is the first to investigate
markers of the initial stages of lipid peroxidation (HEL and PRL)
and of tyrosine moiety oxidation (NY and DiY) in the urine of
pregnant women.
Materials and methods
Study procedure and subjects
The present study is part of a larger study with the
aim of investigating the markers of OS in maternal urine, blood and
amniotic fluid. The study was conducted at the Department of
Obstetrics and Gynaecology of University Medical Centre Ljubljana
(UKCLJ; Ljubljana, Slovenia). The study cohort consisted of women
undergoing amniocentesis and a routine examination of karyotype
during the second trimester of pregnancy, and that intended to
deliver their infants at the UKCLJ. All of the subjects were
previously assigned for the amniocentesis due to their own decision
or increased risk for Down's syndrome, due to advanced maternal
age, increased nuchal translucency and/or inadequate levels of
serum Down's syndrome markers (β-hCG, AFP and UE3) or family
history of karyotype abnormalities.
A total of 130 women were prospectively recruited
into the study between January 2011 and December 2012. The study
group consisted of healthy, singleton pregnant women, between 15
and 26 weeks of gestation, with no detectable structural or genetic
fetal abnormalities. Urine samples were collected at enrolment and
analysed for the selected biomarkers. All women signed written
informed consent and completed a questionnaire, which included
demographic and biological data, such as gynaecological history,
smoking status and maternal body mass and height information.
Infant status data was collected at delivery. Apgar scores were
evaluated at 1 and 5 min after birth (24), while birth weight and gestation were
recorded at delivery. Certain subjects were excluded due to missing
data for essential variables, including maternal weight, infant
birth weight, birth length, Apgar scores and biomarker
concentrations, resulting in a final sample size of 114 women.
The study protocol was approved by the National
Medical Ethics Committee of Slovenia (no. 108/09/09).
Sample preparation and quantitative
analysis
Urine samples were collected at enrolment. Samples
were immediately delivered to the laboratory of the Clinical
Institute of Clinical Chemistry and Biochemistry (University
Medical Centre Ljubljana, Ljubljana, Slovenia) and centrifuged at
219 × g for 10 min at 4°C, then divided into 200 µl single-use
aliquots and stored at −20°C prior to analysis.
Biochemical analysis was performed at the laboratory
of the School of Human Science and Environment (University of
Hyogo, Himeji, Japan). Prior to the analysis, urine samples were
defrosted and centrifuged at 877 × g for 10 min at 4°C to remove
all insoluble particles, and the supernatant was collected. The
concentration of urinary PRL, HEL, NY and DiY was measured by
liquid chromatography-tandem mass spectrometry (LC-MS/MS), using a
multiple reaction monitoring technique with stable-isotope
dilution, as previously described (11,16).
LC-MS/MS analysis was conducted using an API-3000 electrospray
ionisation/quadrupole tandem mass spectrometer (Applied Biosystems;
Thermo Fisher Scientific, Inc., Foster City, CA, USA). HEL, PRL and
NY were not detectable in their intact form and were therefore
butylated (n-butanol/HCl) prior to the analysis, as previously
described (11,16). Chromatography was performed using a
Develosil C30-UG-5 (2×150 mm) column for HEL and PRL, an ODS-HG-3
(2×50 mm) column for DiY and an ODS-SR-5 (2×150 mm) for NY (all
columns from Nomura Chemical Co., Seto, Japan), using an Agilent
1100 high-performance liquid chromatography system (Agilent
Technologies, Inc., Santa Clara, CA, USA).
Urinary concentrations of the biomarkers were
normalised against creatinine to account for the variations in
urine flow and expressed as µmol/mol creatinine (µmol/mol Cr).
Creatinine levels in the urine were evaluated using a Roche/Hitachi
917 automated chemistry analyser (Roche Diagnostics GmbH, Mannheim,
Germany).
Statistical analysis
Statistical analysis was conducted using SPSS for
Windows, version 17.0 (SPSS Inc., Chicago, IL, USA) and MedCalc
version 14.12.0 (MedCalc Software, Mariakerke, Belgium) for
receiver operating characteristic (ROC) curve. General descriptive
statistics were calculated for selected characteristics. Continuous
variables are reported as the mean ± standard deviation and
dichotomous as counts and percentages. Normal distribution was
tested using the Kolmogorov-Smirnov test. All skewed variables are
expressed as the median value with the interquartile range.
Creatinine-corrected concentrations (µmol/mol) were
calculated by dividing the HEL, PRL, DiY and NY concentrations
(µmol/l) by the creatinine concentration. A number of maternal
characteristics, such as maternal age, parity, smoking status and
prepregnancy body mass index (BMI) were categorised (Table I). Prepregnancy height and weight
were used to calculate prepregnancy BMI, which was categorised
according to the WHO classification as underweight (<18.5
kg/m2), normal weight (18.5–24.9 kg/m2),
overweight (25–29.9 kg/m2) and obese (≥30
kg/m2). Smoking status was defined as active smoker or
non-smoker. Parity was defined as nulliparous or parous (second
pregnancy or more). Statistical differences between parameters for
each characteristic were evaluated using the χ2 test for
dichotomous variables. Continuous variables were analysed using the
independent sample t-test in the case of Gaussian, and
non-parametric Mann-Whitney test in the case of non-Gaussian
distribution.
 | Table I.Maternal and infant characteristics
of the study cohort. |
Table I.
Maternal and infant characteristics
of the study cohort.
| Characteristic | n (%) | Mean ± SD |
|---|
| Maternal age,
years |
| 36.70±3.65 |
|
<35 | 19
(16.7) |
|
|
≥35 | 95
(83.3) |
|
| Parity |
|
2.82±1.39 |
|
Nulliparous | 26
(22.8) |
|
|
Parous | 88
(77.2) |
|
| Smoking status |
|
|
|
Yes | 19
(16.7) |
|
| No | 95
(83.3) |
|
| Prepregnancy
BMI |
| 23.83±3.86 |
|
Underweight | 2
(1.8) | 18.14±0.21 |
|
Normal | 76
(66.7) | 21.82±1.81 |
|
Overweight | 28
(24.6) | 27.10±1.48 |
|
Obese | 8
(7.0) | 32.81±3.09 |
| Infant gender |
|
|
|
Female | 55
(48.2) |
|
|
Male | 59
(51.8) |
|
| Gestation at
delivery, weeks |
| 38.97±2.22 |
| Preterm
birth (<37 weeks) | 13
(11.4) | 34.18±3.04 |
| Term
birth | 101 (88.6) | 39.59±1.05 |
| Birth weight,
g |
|
3,308.19±654.82 |
| Low
(<2,500 g) | 10 (8.7) |
1,693.75±596.29 |
|
Normal | 104 (91.3) |
3,431.19±471.25 |
| Apgar1 and
Apgar5 |
|
8.77±1.00 |
| Low
(<7) | 6
(5.3) |
|
| Normal
(≥7) | 108 (94.7) |
|
As not all the parameters were normally distributed
and certain parameters were ordinal, Spearman's correlation
coefficient was calculated to determine the association between the
maternal and infant characteristics and OS biomarker
concentrations. To examine the association and adjust for
confounding variables, odds ratios (OR) and 95% confidence interval
(CI) for OS markers and dichotomous Apgar score at 1 and 5 min
after birth (≥7 or <7), preterm birth (<37 weeks of
gestation) and low birth weight (<2,500 g) were calculated using
logistic regression. The results are shown as unadjusted and
adjusted for confounding factors.
ROC analysis was performed to determine the overall
discriminatory value, sensitivity, specificity and optimal cut-off
for Apgar scores and gestation binary outcome. Youden's index was
used to determine the optimal cut-off level on the probability
scale for distinguishing between women with normal and adverse
pregnancy outcomes. Two-tailed P=0.05 was considered to indicate a
statistically significant difference.
Results
Population characteristics
The overall maternal and infant characteristics are
summarised in Table I. The present
prospective study included pregnant women between 15 and 26 weeks
of gestation. Markers of OS were measured in the final cohort of
114 urine samples. The mean maternal age was 36.7±3.6 years (age
range, 22–44 years). A total of 26 subjects (22.8%) were
nulliparous, 23 subjects were primiparous (20.2%) and the remainder
were multiparous (57%). A total of 19 women (16.7%) were active
smokers. Based on the prepregnancy BMI, 28 women (24.6%) were
overweight and 8 women (7%) were obese. The mean infant birth
weight, birth length and gestation duration were 3,308±655 g
(range, 680–4,590 g), 50.54±3.52 cm (range, 32–56 cm) and
38.97±2.22 weeks (range, 27.86–41.43 weeks), respectively. There
were 59 male and 54 female infants. The mean Apgar score at 1 min
was 8.77±1.0 (range, 3–9) and at 5 min was 8.93±0.05 (range,
6–10).
Urinary biomarkers of OS measured in the overall
cohort of mothers in the second trimester of pregnancy are shown in
Table II. Only DiY values were
normally distributed. A statistically significant difference was
detected in PRL levels between the smoker and non-smoker women
(P=0.034). The levels of all other biomarkers did not vary based on
the smoking status or on the prepregnancy BMI and infant
gender.
 | Table II.Concentrations of oxidative stress
markers in the second trimester of pregnancy in the maternal urine
(µmol/mol Cr). |
Table II.
Concentrations of oxidative stress
markers in the second trimester of pregnancy in the maternal urine
(µmol/mol Cr).
| Parameter | HEL | PRL | DiY | NY |
|---|
| Total cases | 3.18
(2.32–5.19) | 26.33
(20.78–33.42) | 9.11
(8.17–10.28) | 0.61
(0.37–1.12) |
| Smoking status |
|
|
|
|
|
Yes | 3.57
(2.84–5.22) | 29.87a (23.89–37.65) | 8.56
(7.96–10.09) | 0.79
(0.35–1.26) |
| No | 2.99
(2.23–5.19) | 25.52a (20.15–32.72) | 9.16
(8.17–10.43) | 0.59
(0.37–1.10) |
| Prepregnancy
BMI |
|
|
|
|
|
Underweight | 6.28
(5.61–6.95) | 27.19
(23.44–30.93) | 11.05
(10.70–11.40) | 0.49
(0.43–0.54) |
|
Normal | 3.29
(2.21–5.11) | 27.02
(21.54–34.25) | 9.11
(8.24–10.36) | 0.62
(0.40–1.17) |
|
Overweight | 2.86
(2.43–5.31) | 25.71
(20.04–32.71) | 8.89
(7.89–10.08) | 0.53
(0.32–0.97) |
|
Obese | 2.74
(2.53–2.95) | 23.61
(19.71–28.82) | 9.16
(7.40–10.05) | 0.80
(0.61–1.16) |
| Infant gender |
|
|
|
|
|
Female | 3.24
(2.46–5.30) | 26.50
(21.53–34.51) | 9.07
(7.88–10.77) | 0.59
(0.37–1.07) |
|
Male | 3.09
(2.19–4.94) | 25.52
(20.28–30.92) | 9.15
(8.42–10.00) | 0.62
(0.37–1.14) |
Association of urinary biomarkers of
OS with maternal and infant characteristics
Urinary OS marker levels were correlated with each
other and with maternal and infant parameters (Table III). Maternal age, parity and
prepregnancy BMI negatively correlated with PRL levels. Neonatal
Apgar score at 1 min after birth was negatively correlated with
PRL, while Apgar score at 1 and 5 min was negatively correlated
with NY.
 | Table III.Correlation between urinary markers
of oxidative stress and maternal and infant characteristics. |
Table III.
Correlation between urinary markers
of oxidative stress and maternal and infant characteristics.
| Characteristic | HEL | PRL | DiY | NY |
|---|
| Maternal age | −0.051 (0.589) | −0.188 (0.046) | −0.119 (0.206) | 0.007
(0.938) |
| Parity | −0.038 (0.688) | −0.200 (0.033) | −0.059 (0.536) | −0.107 (0.259) |
| Maternal
height | −0.052 (0.579) | −0.076 (0.421) | −0.123 (0.192) | 0.112
(0.237) |
| Prepregnancy
BMI | −0.117 (0.215) | −0.193 (0.040) | −0.115 (0.221) | −0.059 (0.532) |
| HEL | 1 | 0.294
(0.002) | 0.249
(0.008) | −0.026 (0.783) |
| PRL | 0.294
(0.002) | 1 | 0.113
(0.232) |
0.367
(<0.001) |
| DiY | 0.249
(0.008) | 0.113
(0.232) | 1 | −0.105 (0.267) |
| NY | −0.026 (0.783) |
0.367
(<0.001) | −0.105 (0.267) | 1 |
| Gestation at
delivery | 0.002
(0.981) | 0.002
(0.986) | −0.145 (0.124) | 0.087
(0.358) |
| Birth weight | −0.123 (0.193) | −0.039 (0.679) | 0.079
(0.406) | −0.073 (0.441) |
| Birth length | −0.137 (0.148) | −0.036 (0.702) | 0.055
(0.563) | −0.015 (0.871) |
| Apgar1 | −0.009 (0.928) | −0.234 (0.013) | −0.120 (0.204) | −0.250 (0.008) |
| Apgar5 | −0.002 (0.984) | −0.158 (0.094) | −0.122 (0.197) | −0.244 (0.009) |
The association between OS markers and pregnancy
outcome, Apgar score at 1 and 5 min, preterm birth and low birth
weight was estimated using logistic regression. A crude and
adjusted logistic regression model was used to assess the
likelihood of an adverse outcome as a function of OS and covariates
(Table IV). The concentration of
the markers was treated as a continuous variable, and the simple
probabilities of Apgar score of <7, a birth weight of <2,500
g and preterm birth were estimated based on the marker levels.
Increasing PRL and NY levels by one unit would on average increase
the infant probability of having low Apgar score at 1 and 5 min
after birth by 1.098 (95% CI, 1.024–1.178) and 2.084 (95% CI,
1.157–3.853), respectively. The significant association persisted
after controlling for confounding factors. For other markers, no
statistical significance was observed in the logistic regression
models.
 | Table IV.Logistic regression assessing
independent association between each urinary marker of oxidative
stress and adverse pregnancy outcome. |
Table IV.
Logistic regression assessing
independent association between each urinary marker of oxidative
stress and adverse pregnancy outcome.
| Marker | Apgar1 or Apgar5
(<7; n=6) | P-value | Birth weight
(<2,500 g; n=10) | P-value | Gestation at
delivery (<37 weeks; n=13) | P-value |
|---|
| HEL |
|
|
|
|
|
|
|
Unadjusted | 0.984
(0.690–1.402) | 0.928 | 1.105
(0.799–1.530) | 0.546 | 0.835
(0.683–1.021) | 0.079 |
|
Adjusted | 1.014
(0.638–1.611) | 0.953 | 1.351
(0.764–2.388) | 0.301 | 0.881
(0.701–1.107) | 0.276 |
| PRL |
|
|
|
|
|
|
|
Unadjusted | 1.098
(1.023–1.178) | 0.009a | 0.984
(0.926–1.047) | 0.614 | 1.018
(0.953–1.087) | 0.596 |
|
Adjusted | 1.113
(1.009–1.227) | 0.032a | 1.021
(0.936–1.114) | 0.635 | 1.043
(0.962–1.132) | 0.307 |
| DIY |
|
|
|
|
|
|
|
Unadjusted | 1.257
(0.826–1.911) | 0.286 | 0.990
(0.690–1.422) | 0.958 | 0.703
(0.512–0.963) | 0.028a |
|
Adjusted | 1.623
(0.884–2.977) | 0.118 | 0.903
(0.476–1.713) | 0.755 | 0.632
(0.422–0.948) | 0.027a |
| NY |
|
|
|
|
|
|
|
Unadjusted | 2.078
(1.127–3.833) | 0.019a | 0.818
(0.431–1.553) | 0.539 | 1.385
(0.542–3.538) | 0.496 |
|
Adjusted | 4.414
(1.107–17.599) | 0.035a | 1.235
(0.446–3.416) | 0.684 | 1.009
(0.392–2.596) | 0.985 |
Notably, in cases of preterm birth, the statistical
results indicated that for each one-unit increase in the level of
DiY, there was a reduction in the probability of preterm birth in
the unadjusted and adjusted model (OR=0.703; 95% CI, 0.512–0.963);
thus, the DiY level exhibited negative association with preterm
birth. The distribution of PRL, DiY and NY levels between groups
with adverse and normal pregnancy outcomes for significant logistic
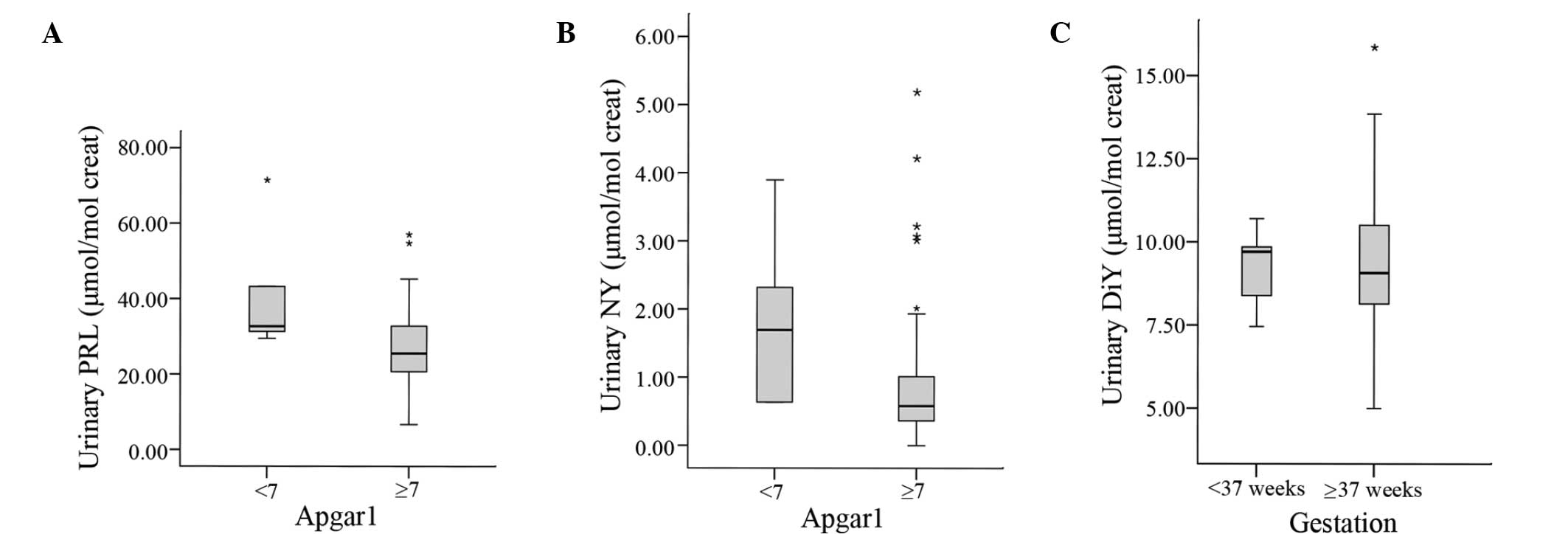
regression results are presented in Fig.
1.
The urinary markers significantly associated with
Apgar scores at 1 and 5 min after birth were analysed for their
ability to predict low Apgar score, and the optimal cut-off urinary
PRL and NY concentrations were determined using ROC curve analysis
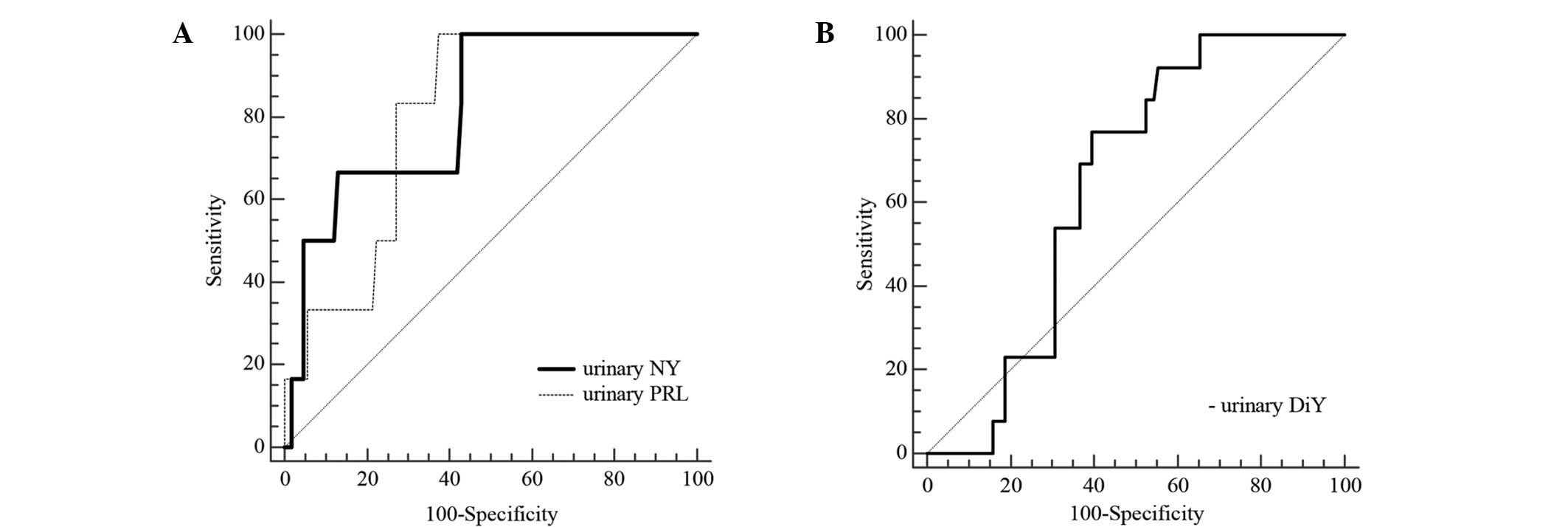
(Fig. 2A). The urine NY
concentration had the highest area under the curve (AUC), with a
value of 0.818 (95% CI, 0.734–0.884; P=0.0001) for Apgar score at 1
and 5 min after birth. In addition, the predictive cut-off for NY
in urine was 0.62 µmol/mol Cr, with a sensitivity of 100% and a
specificity of 57%. The positive predictive value (PPV) and
negative predictive value (NPV) were 11.54 and 100%, respectively.
An appropriate cut-off level for PRL in maternal urine was 29.15
µmol/mol Cr, with a sensitivity of 100% and a specificity of 62.6%,
and an AUC of 0.802 (95% CI, 0.717–0.871; P<0.0001) for the
prediction of a low Apgar score at 1 and 5 min. The positive
predictive value reached 13.05% and the negative predictive value
was 100%. Inclusion of additional confounding factors, which were
used in logistic regression, did not substantially alter the
positive predictive value. The results obtained for NY were as
follows: AUC, 0.928; P<0.001; sensitivity, 100%; specificity,
75.5%; and PPV, 20.69. Similarly, the results obtained for PRL were
as follows: AUC, 0.899; P=0.001; sensitivity, 100%; specificity,
79.4%; and PPV, 21.40. ROC curve analysis was also performed for
the DiY level in order to evaluate its ability to identify pregnant
women with a reduced risk of preterm birth (Fig. 2B). The AUC was 0.645 (95% CI,
0.550–0.732; P=0.010), the observed cut-off value was 9.33 µmol/mol
Cr, the diagnostic specificity was 60.40% and the sensitivity was
76.92%. Furthermore, positive and negative predictive values
reached 20 and 95.31%, respectively.
Discussion
To the best of our knowledge, the present study is
the first to investigate biomarkers of the initial stages of lipid
peroxidation (HEL and PRL) and of tyrosine residue oxidation (DiY
and NY) in the urine of pregnant women.
HEL, PRL, DiY and NY are relatively novel biomarkers
for OS monitoring, and their concentration in the general pregnant
population has not been determined yet. Although 83.3% of the women
included in the present study were ≥35 years old, the data may
provide initial indicative values.
The levels of the measured concentrations of the
markers correspond to ranges identified in previously published
data, which were measured on the different groups of healthy,
non-pregnant subjects (11,12,16,25,26). The
concentrations of the markers vary between studies. These studies
in healthy non-pregnant women reported that the urinary HEL levels
were between 1.58±0.23 and 2.3±1.2 µmol/mol Cr (11,12,16), PRL
levels were 21.6±10.6 µmol/mol Cr (11), DiY levels were between 8.8±0.6 and
10.1±0.4 µmol/mol Cr (16,25) and NY levels were between 0.46±0.49
and 1.4±0.4 µmol/mol Cr (16,26).
Oxidative damage may be triggered by internal and
external factors. Potential confounders in the present study
include factors that alter the oxidative environment and thus
influence birth outcomes. Therefore, we assessed maternal
characteristics and environmental risk factors.
The influence of active maternal perinatal smoking
as a confounding factor on pregnancy development and outcome has
been well-acknowledged. Smoking is associated with increased risk
of perinatal mortality and congenital abnormalities, preterm birth
and low birth weight (27–30). Tobacco smoke contains a number of
oxidising species capable of producing ROS that may be associated
with OS damage (31,32). Although a previous study involving
non-pregnant subjects did not confirm the correlation of HEL and
PRL concentrations with smoking (11), in the present study cohort of
pregnant women, PRL concentrations were significantly higher in the
subgroup of smokers compared with those in non-smokers. In
addition, studies have also investigated the association of NY and
DiY levels with smoking status in healthy subjects (32–34).
Urinary levels of DiY did not differ between smokers and
non-smokers, while plasma NY studies have presented conflicting
results (32–34). Increased HEL concentration was
detected in the tears of subject following passive exposure to
cigarette smoke in healthy non-smokers compared with non-exposed
subjects (35). No statistically
significant association of HEL, NY and DiY levels with smoking
status was identified in the present study. However, smoking status
was included in the adjusted model of logistic regression for
assessing biomarkers for adverse pregnancy outcome.
Increased levels of oxidative damage were expected
to be associated with ageing and maternal weight, on the basis of
previously reported associations (34–39).
However, no correlation was detected between maternal age and the
levels of HEL, DiY and NY. Notably, PRL showed a weak negative
correlation with age (rho=−0.188; P=0.046). The absence of
correlation of HEL and PRL levels with age has been noted
previously in a healthy non-pregnant population (11). A similar difference between age
groups applies to NY. The present results are consistent with the
findings of the Framingham Heart Study, in which urinary
concentrations of the oxidative marker 8-isoprostane were found to
decrease with age, suggesting that LP is not a significant feature
of normal ageing (37). Due to the
selection of study cohort in the present study, the cohort age
represented a relative narrow range and this may limit the
application of the present results to a wider population. However,
these results represent the effect of age on the markers' behaviour
in the present study.
Subjects that are obese and overweight may present
an increased risk of pregnancy complications. According to previous
results, obesity is a state of chronic increased OS and
inflammation (37); therefore, we
expected to observe a positive correlation and an increased LP in
the overweight and obese group. However, the present results were
contrary to our expectations, as no correlation was detected
between prepregnancy BMI and HEL, DiY and NY levels. A weak
negative correlation was observed between BMI and PRL (rho=−0.193;
P=0.040). The linkage between increased extent of adipose tissue
and decreased OS in pregnancy may be due to the hormone estradiol,
which is generated and secreted by the placenta. In obese patients,
estradiol is secreted by adipocytes. Estradiol has been
demonstrated to exhibit an antioxidative activity, and may modulate
OS by inhibiting the generation of ROS and scavenging ROS (40). The present results are consistent
with this possibility.
In the case of individual markers that were
correlated with each other, a positive correlation was observed
between HEL and PRL, as well as between HEL and DiY, which is
consistent with the findings of previous studies (11,16).
However, no correlation was detected between PRL and DiY, which is
inconsistent with previous results (11). The absence of a correlation between
NY and the other markers has been noted previously in a healthy
non-pregnant population (16);
however, we detected the most marked positive correlation between
PRL and NY (rho=0.367; P<0.001). Positive correlations between
the selected biomarkers suggest that OS is a complex process,
involving simultaneous oxidation pathways of various
macromolecules. However, the biochemical pathway of the in
vivo generation of these markers is different, and each marker
can provide independent information. It is difficult to compare and
simplify the results to general systemic stress, as different
markers exhibit differing stability, accumulation, susceptibility
to metabolism and excretion in the urine. Furthermore, this
investigation of correlation serves only as an indicator of a
plausible association between OS markers and does not conclusively
demonstrate the existence of a causal association. Further studies
are required to fully elucidate the mechanism underlying this
dependence.
Urinary OS profile in the second trimester of
pregnancy may be used to assess fetal exposure and probability for
infant outcomes at birth. The most notable observation of the
present study is that low Apgar scores at 1 and 5 min after birth
are associated with high levels of PRL and NY. Apgar score is a
strong routine indicator of infant survival, and is based on the
evaluation of the physical condition of infants immediately after
birth, including estimation of heart rate, respiratory effort,
muscle tone, reflex irritability and skin colour (41). In the present study a significant
negative linear correlation of urinary levels of PRL and NY with
Apgar score was identified among pregnant women.
The majority of the mothers with the highest
concentrations of PRL and NY in the second trimester of pregnancy
gave birth to infants with a low Apgar score; however, no
differences in birth weight, birth length and gestational age were
observed at delivery.
Adjusted logistic regression analysis of birth
outcomes revealed that high urinary PRL and NY concentrations in
the second trimester of pregnancy increased the probability (OR) of
a low Apgar score. By contrast, the other markers did not increase
the risk of an adverse pregnancy outcome. Following adjustment of
the logistic regression model, increased levels of DiY were
associated with a reduced risk of preterm birth.
ROC analysis was performed on the OS markers that
were significantly associated with low Apgar and preterm birth in
logistic regression in order to detect possible predictors of
pregnancy outcome that may be used to identify pregnancies at high
risk of complications. In addition, markers for low risk estimation
are valuable for avoiding unnecessary intensive monitoring and
interventions.
The AUC values obtained for PRL, NY and DiY were
moderate but statistically significant. The cut-off values for PRL
and NY were evaluated to predict low Apgar score at 1 and 5 min
after birth and the optimal value of DiY to assess probability for
the normal duration of pregnancy. Despite significant AUC and high
sensibility, the specificity and positive predictive value of the
markers were low and insufficient for a reliable diagnostic marker.
Inclusion of additional confounding factors used in logistic
regression did not improve the results. The ROC curve of NY and PRL
demonstrated poor positive predictive accuracy for a low Apgar
score, suggesting that screening for enhanced levels of NY and PRL
in the second trimester of pregnancy would be a poor approach for
identifying women at risk. The presence of increased NY and PRL
levels in the urine should not be interpreted as indicating an
increased risk for an adverse outcome; however, levels under the
cut-off value are able to indicate the absence of a low Apgar
score. The negative predictive values for NY and PRL markers were
100%. The high negative predictive value reflects test performance
and the low overall prevalence of low Apgar score in the study
population. Unless a low Apgar score is relatively frequent, there
is no substantial benefit in having a high negative predictive
value. Furthermore, NY and PRL screening may represent a useful
tool for excluding the risk of a poor Apgar score.
The reason why a high positive predictive value was
not observed may be due to the infrequency of a low Apgar score, in
addition to the heterogeneous and multifactorial causes of adverse
pregnancy outcomes. The results of the present pilot study cannot
be applied to laboratory and clinical practice; however, certain
indicative values were provided. Individual positive predictive
values for NY and PRL are too low, but in combination with other
biochemical markers and maternal parameters they may provide
improved predictive efficacy. Further studies for these markers and
widespread screening for high levels of PRL and NY are
required.
There are a number of limitations associated with
the present study. The first limitation is that the study group
consisted primarily of pregnant women with an elevated Down's
syndrome risk, due to the increased nuchal translucency, triple
test measurements and/or higher maternal age. Therefore, the
possibility of a certain population selection bias cannot be
excluded. Secondly, valid statistical analyses are difficult to
perform on a small number of study subjects. Small data sets may
possess restricted statistical accuracy and produce primarily
exploratory results. The present study was a part of a larger study
aimed at investigating the markers of OS in maternal urine, blood
and amniotic fluid and was considered to be prospective. Therefore,
the incidence of adverse pregnancy outcome in the present
population was relatively low. Furthermore, the low number of cases
with a low Apgar score has an effect on the accuracy of the
statistical analysis performed in the study and may negatively
affect the accuracy of a statistical significant association
observed between PRL, NY and Apgar score. Thus, these findings
cannot be generalized to a broader population.
OS is a complex process and it may be difficult to
determine whether OS is a cause of complications or a consequence
of internal or external interferences. Although the present study
controlled for the associations with a range of potential
confounders, a possibility that the observed association of OS is
due to unmeasured or residual confounding factors remained.
Additional confounding variables that may cause the alternation of
oxidative status, such as nutritional habits, antioxidant and
vitamin supplementation and genetic factors, should be included in
future investigations. Similarly, food-derived substances may be a
source of markers present in urine. However, these markers still
have a potential value. Furthermore, the present exploratory study
is unique and has generated a number of potential biomarker
candidates for future investigation.
In conclusion, the present results indicated that
high levels of urinary PRL and NY may be associated with low Apgar
scores, while high DiY levels are associated with a reduced risk of
preterm birth. Cigarette smoking was confirmed to be a confounding
factor for the urinary excretion of PRL and should be factored into
the investigation of PRL. This study identified statistically
significant correlations among urinary markers and detected markers
with a high negative predictive value. Further studies involving
larger cohorts are required to elucidate the mechanism underlying
the association of PRL, NY and DiY with pregnancy outcome.
Acknowledgements
This study was supported by grant a from the
Slovenian Research Agency (no. P3-0124). The authors would like to
thank Mr. Ryo Matsumoto for his technical assistance with LC-MS/MS
measurements and Mrs. Sara Hajdarević for the revision of the
English text.
References
|
1
|
Jauniaux E, Poston L and Burton GJ:
Placental-related diseases of pregnancy: Involvement of oxidative
stress and implications in human evolution. Hum Reprod Update.
12:747–755. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Myatt L and Cui XL: Oxidative stress in
placenta. Histochem Cell Biol. 122:369–382. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toescu V, Nuttall S, Martin U, Kendall M
and Dunne F: Oxidative stress and normal pregnancy. Clin Endocrinol
(Oxf). 57:609–613. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hung TH, Lo LM, Chiu TH, Li MJ, Yeh YL,
Chen SF and Hsieh TT: A Longitudinal study of oxidative stress and
antioxidant status in women with uncomplicated pregnancies
throughout gestation. Reprod Sci. 17:401–409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Osredkar J: Oxidative stress. Zdrav Vestn.
81:393–406. 2012.(In Slovenian).
|
|
6
|
Sies H: Oxidative stress: Oxidants and
antioxidants. Exp Physiol. 82:291–295. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burton GJ and Jauniaux E: Oxidative
stress. Best Pract Res Clin Obstet Gynaecol. 25:287–299. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Halliwell B and Chirico S: Lipid
peroxidation: Its mechanism, measurement and significance. Am J
Clin Nutr. 57(Suppl 5): 715S–724S. 1993.PubMed/NCBI
|
|
9
|
Kato Y and Osawa T: Detection of
lipid-lysine amide-type adduct as a marker of PUFA oxidation and
its applications. Arch Biochem Biophys. 501:182–187. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato Y and Osawa T: Detection of a
lipid-lysine adduct family with an amide bond as the linkage: Novel
markers for lipid-derived protein modifications. Methods Mol Biol.
580:129–141. 2009.PubMed/NCBI
|
|
11
|
Hisaka S, Kato Y, Kitamoto N, Yoshida A,
Kubushiro Y, Naito M and Osawa T: Chemical and immunochemical
identification of propanoyllysine derived from oxidized n-3
polyunsaturated fatty acid. Free Radic Biol Med. 46:1463–1471.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kato Y, Yoshida A, Naito M, Kawai Y, Tsuji
K, Kitamura M, Kitamoto N and Osawa T: Identification and
quantification of N(epsilon)-(Hexanoyl)lysine in human urine by
liquid chromatography/tandem mass spectrometry. Free Radic Biol
Med. 37:1864–1874. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tokuda F, Sando Y, Matsui H and Yokoyama
T: N epsilon-(hexanoyl) lysine, a new oxidative stress marker, is
increased in metabolic syndrome, but not in obstructive sleep
apnea. Am J Med Sci. 338:127–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kageyama Y, Takahashi M, Nagafusa T,
Torikai E and Nagano A: Etanercept reduces the oxidative stress
marker levels in patients with rheumatoid arthritis. Rheumatol Int.
28:245–251. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimizu K, Ogawa F, Akiyama Y, Muroi E,
Yoshizaki A, Iwata Y, Komura K, Bae S and Sato S: Increased serum
levels of N(epsilon)-(hexanoyl)lysine, a new marker of oxidative
stress, in systemic sclerosis. J Rheumatol. 35:2214–2219. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kato Y, Dozaki N, Nakamura T, Kitamoto N,
Yoshida A, Naito M, Kitamura M and Osawa T: Quantification of
modified tyrosines in healthy and diabetic human urine using liquid
chromatography/tandem mass spectrometry. J Clin Biochem Nutr.
44:67–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DiMarco T and Giulivi C: Current
analytical methods for the detection of dityrosine, a biomarker of
oxidative stress, in biological samples. Mass Spectrom Rev.
26:108–120. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Giulivi C, Traaseth NJ and Davies JA:
Tyrosine oxidation products: Analysis and biological relevance.
Amino Acids. 25:227–232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen X and Scholl TO: Oxidative stress:
Changes in pregnancy and with gestational diabetes mellitus. Curr
Diab Rep. 5:282–288. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Min J, Park B, Kim YJ, Lee H, Ha E and
Park H: Effect of oxidative stress on birth size: Consideration of
window from mid pregnancy to delivery. Placenta. 30:418–423. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Longini M, Perrone S, Kenanidis A, Vezzosi
P, Marzocchi B, Petraglia F, Centini G and Buonocore G:
Isoprostanes in amniotic fluid: A predictive marker for fetal
growth restriction in pregnancy. Free Radic Biol Med. 38:1537–1541.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stein TP, Scholl TO, Schulter MD, Leskiw
MJ, Chen X, Spur BW and Rodriguez A: Oxidative stress early in
pregnancy and pregnancy outcome. Free Radic Res. 42:841–848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsieh TT, Chen SF, Lo LM, Li MJ, Yeh YL
and Hung TH: The association between maternal oxidative stress at
mid-gestation and subsequent pregnancy complications. Reprod Sci.
19:505–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Apgar V: A proposal for a new method of
evaluation of the newborn infant. Originally published in July
1953, volume 32, pages 250–259. Anesth Analg. 120:1056–1059. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Orhan H, Coolen S and Meerman JH:
Quantification of urinary o,o-dityrosine, a biomarker of oxidative
damage to proteins, by high performance liquid chromatography with
triple quadrupole tandem mass spectrometry. A comparison with
ion-trap tandem mass spectrometry. J Chromatogr B Analyt Technol
Biomed Life Sci. 827:104–108. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsikas D, Mitschke A, Suchy MT, Gutzki FM
and Stichtenoth DO: Determination of 3-nitrotyrosine in human urine
at the basal state by gas chromatography-tandem mass spectrometry
and evaluation of the excretion after oral intake. J Chromatogr B
Analyt Technol Biomed Life Sci. 827:146–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Figueras F, Meler E, Eixarch E, Francis A,
Coll O, Gratacos E and Gardosi J: Association of smoking during
pregnancy and fetal growth restriction: Subgroups of higher
susceptibility. Eur J Obstet Gynecol Reprod Biol. 138:171–175.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Raatikainen K, Huurinainen P and Heinonen
S: Smoking in early gestation or through pregnancy: A decision
crucial to pregnancy outcome. Prev Med. 44:59–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Windham GC, Hopkins B, Fenster L and Swan
SH: Prenatal active or passive tobacco smoke exposure and the risk
of preterm delivery or low birth weight. Epidemiology. 11:427–433.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yerushalmy J: The relationship of parents'
cigarette smoking to outcome of pregnancy-implication as to the
problem of inferring causation from observed associations. Am J
Epidemiol. 93:443–456. 1971.PubMed/NCBI
|
|
31
|
Seet RC, Lee CY, Loke WM, Huang SH, Huang
H, Looi WF, Chew ES, Quek AM, Lim EC and Halliwell B: Biomarkers of
oxidative damage in cigarette smokers: Which biomarkers might
reflect acute versus chronic oxidative stress? Free Radic Biol Med.
50:1787–1793. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Campos C, Guzmán R, López-Fernández E and
Casado Á: Urinary biomarkers of oxidative/nitrosative stress in
healthy smokers. Inhal Toxicol. 23:148–156. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sakano N, Takahashi N, Wang DH, Sauriasari
R, Takemoto K, Kanbara S, Sato Y, Takigawa T, Takaki J and Ogino K:
Plasma 3-nitrotyrosine, urinary 8-isoprostane and 8-OHdG among
healthy Japanese people. Free Radic Res. 43:183–192. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang WZ, Lang C and Kaye DM:
Determination of plasma free 3-nitrotyrosine and tyrosine by
reversed-phase liquid chromatography with
4-fluoro-7-nitrobenzofurazan derivatization. Biomed Chromatogr.
21:273–278. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rummenie VT, Matsumoto Y, Dogru M, Wang Y,
Hu Y, Ward SK, Igarashi A, Wakamatsu T, Ibrahim O, Goto E, et al:
Tear cytokine and ocular surface alterations following brief
passive cigarette smoke exposure. Cytokine. 43:200–208. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Malti N, Merzouk H, Merzouk SA, Loukidi B,
Karaouzene N, Malti A and Narce M: Oxidative stress and maternal
obesity: Feto-placental unit interaction. Placenta. 35:411–416.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Keaney JF Jr..Larson MG, Vasan RS, Wilson
PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA and
Benjamin EJ: Framingham Study: Obesity and systemic oxidant stress:
Clinical correlates of oxidative stress in the Framingham Study.
Arteroscler Thromb Vasc Biol. 23:434–439. 2003. View Article : Google Scholar
|
|
38
|
Fernández-Sánchez A, Madrigal-Santillán E,
Bautista M, Esquivel-Soto J, Morales-González A, Esquivel-Chirino
C, Durante-Montiel I, Sánchez-Rivera G, Valadez-Vega C and
Morales-González JA: Inflammation, oxidative stress and obesity.
Int J Mol Sci. 12:3117–3132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jacob KD, Hooten Noren N, Trzeciak AR and
Evans MK: Markers of oxidant stress that is clinically relevant in
aging and age-related disease. Mech Ageing Dev. 134:139–157. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Reyes MR, Sifuentes-Alvarez A and Lazalde
B: Estrogens are potentially the only steroids with an antioxidant
role in pregnancy: In vitro evidence. Acta Obstet Gynecol
Scand. 85:1090–1093. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Casey BM, McIntire DD and Leveno KJ: The
continuing value of the Apgar score for the assessment of newborn
infants. N Engl J Med. 344:467–471. 2001. View Article : Google Scholar : PubMed/NCBI
|