Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN)
is a rare entity that was initially identified in 1994 (1). However, the lack of knowledge regarding
the histogenesis of BPDCN led to a succession of different
classifications of this disease as a tumor, blastic natural killer
(NK) leukemia/lymphoma, agranular CD4+ NK cell leukemia
or agranular CD4+ CD56+ hematodermic neoplasm
(2,3). According to the 2008 World Health
Organization (WHO) classification, BPDCN is included in the same
class as the acute myeloid leukemia-associated precursor neoplasms
(4). The disease is characterized by
predominant cutaneous involvement with ensuing or concomitant
spreading to the peripheral blood (PB) and the bone marrow (BM).
BPDCN has an aggressive clinical behavior with poor survival
rates.
BPDCN is characterized by numerous cutaneous lesions
at diagnosis, in addition to extracutaneous involvement of the bone
marrow, peripheral blood and lymph nodes. Patients usually exhibit
asymptomatic, single or numerous skin lesions, which may be
nodules, plaques or bruise-like. Extracutaneous disease is observed
in the majority of patients at diagnosis, frequently involving the
regional lymph nodes. As the BPDCN progresses, the peripheral blood
and bone marrow may be involved (5).
The present study reported the case of a 26-year-old
BPDCN patient, who presented with thrombocytopenia, leukocytosis
and anemia, but with no skin lesions.
Case report
In March 2014, a 26-year-old Chinese female was
admitted to Renji Hospital (Shanghai, China) with pancytopenia. The
patient presented with evident thrombocytopenia, leukocytosis and
anemia. Laboratory results revealed a hemoglobin level of
5.6×1010g/l (normal range, 11.0–15.0×1010
g/l), white blood cell count of 2.83×109/l (normal
range, 4–10×109/l) and platelet count of
8.6×1010/l (normal range, 10.0–30.0×1010/l).
The patient did not have enlarged lymph nodes (LNs), but sternal
tenderness was observed. Symptoms included headache, earache and
cough, with no gum swelling or bleeding. No history of
asymptomatic, solitary or multiple skin lesions, such as nodules,
plaques or bruise-like lesions, was noted. Written informed consent
was obtained from the patient.
The patient presented with evident thrombocytopenia,
leukocytosis and anemia. Therefore, it was suspected that the
patient had pathology of the hematopoietic and lymphoid tissues. PB
and BM smears were performed, and the results demonstrated that
blastic or abnormal cells accounted for 66% of the nucleated cells.
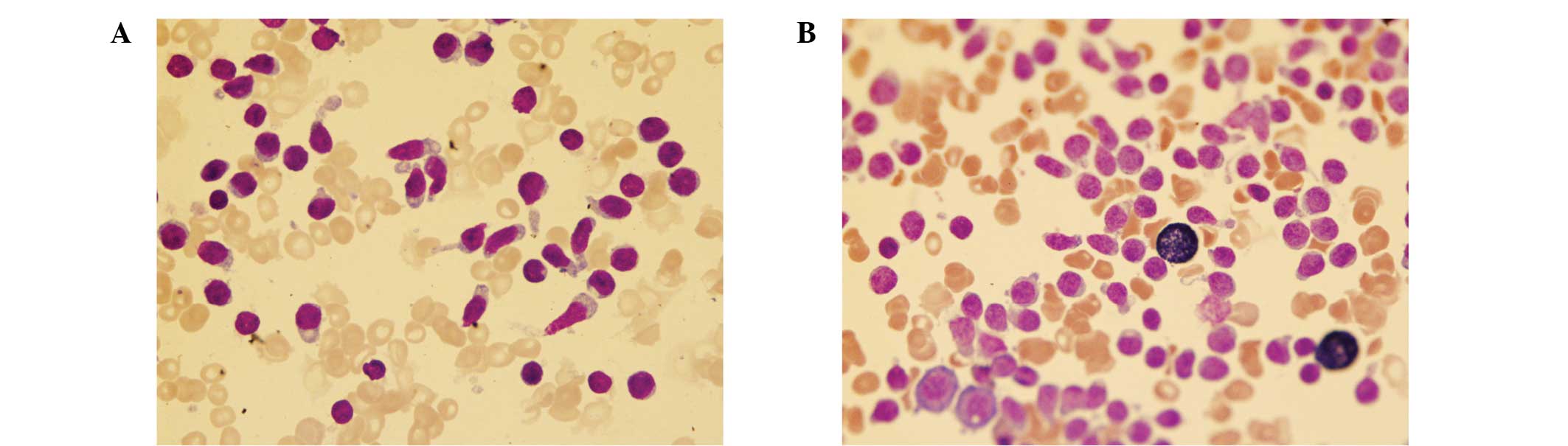
As seen in Fig. 1, the abnormal BM
cells were found to have a relatively moderate nuclear to
cytoplasmic ratio, oval nuclear contours, numerous nuclei with
incisura and rough chromatin. In addition, the majority of cells
exhibited elongated agranular and vacuoles in the cytoplasm
(Fig. 1). Cytochemical staining
revealed that the cells were positive for periodic acid-Schiff and
α-naphthyl acetate esterase, but negative for peroxidase (Fig. 1) and naphthol AS-D chloroacetate
esterase.
BM biopsy demonstrated a markedly hypercellular
marrow, in which the number of diffuse proliferative blastic cells
was significantly higher compared with the number of normal cells.
Based on the results of immunohistochemical analysis, the cells
were found to co-express CD4 and CD56, while they were partially
positive for terminal deoxynucleotidyl transferase and negative for
myeloperoxidase (MPO), CD34, CD15, pericentriolar material 1,
CD234, CD20, CD43 and CD3. In addition, the Ki-67 proliferative
index was ~10% (reference value, <20%), which was within the
normal range.
Flow cytometry was performed on the BM biopsy
sample, and revealed 88.1% abnormal CD45dim+ cells
within the nuclear cell gate. In addition, the blastic plasmacytoid
dendritic cells were positive for CD4 (61.6%), CD7 (17.5%), human
leukocyte antigen (HLA)-DR (94%), CD56 (99%), CD123 (98%) and CD304
(60%), but negative for lineage-associated markers, including CD2,
CD3, CD19, CD79a, CD11b, CD13, CD15, CD33, CD64, CD14, CD117, MPO,
CD34, CD38, CD16, CD57, CD94, CD138 and CD303. Diagnosis of BPDCN
was indicated by positivity for CD4 and CD56, along with other
markers that are more restricted to plasmacytoid dendritic cells
(such as CD123), and negativity for lymphoid, NK and myeloid
lineage-associated antigens. Flow cytometry equipment
(FACSCalibur™) and monoclonal antibodies (Pharmingen™ mouse
anti-human) were purchased from BD Biosciences (San Diego, CA,
USA).
The patient received a single course of
daunorubicin, vincristine and prednisone. The treatment outcome was
promising, with the patient appearing to go into remission and
showing no symptoms at a follow-up examination in September
2015.
Discussion
BPDCN is a rare and aggressive hematodermic
neoplasm, which typically occurs in elderly patients, with a mean
age between 60 and 70 years; however, the disease may present at
any age (2,3,5). BPDCN
is more prevalent in males, with a male to female ratio of 3:1. In
addition, it is characterized by a high frequency of cutaneous
lesions at diagnosis, which is accompanied by extracutaneous
involvement of the LNs, PB and BM (6). Asymptomatic, solitary or multiple skin
lesions, such as plaques, nodules or bruise-like lesions are
commonly detected in these patients (7). BPDCN without cutaneous lesions
typically occurs in young patients that also present evident
leukocytosis anemia and thrombocytopenia, and the median survival
time of these patients is shorter (6). PB and BM involvement is developed along
with the disease progression. Low-level of BM and PB involvement is
identified in the majority of cases (60–90%); however, initial
fulminant leukemia is rare (5–25%). Furthermore, LN involvement at
presentation is common (40–50%), whereas splenomegaly (<20%) or
involvement of other mucosal sites (<10%) are relatively rare.
Systemic B symptoms are also rarely observed at diagnosis (3).
BPDCN can show diffuse involvement of the LNs and
complete effacement of the LN architecture (3). It is characterized by the following
features: A monotonous population of small to medium cells with
irregular nuclear contours, fine to evenly dispersed chromatin, 1–3
small nucleoli, and scant to moderate amount of cytoplasm. The
neoplastic cells can be present in the PB and resemble circulating
leukemic lymphoid or myeloid blasts, particularly in cases with
extensive involvement of the BM. The neoplastic cells in the BM
aspirate tests may exhibit typical pearl necklace-like,
submembranous, cytoplasmic vacuoles and elongated, agranular
cytoplasm. Notably, the residual hematopoietic elements, including
megakaryocytes, granulocytes and erythroid precursors, may present
certain dysplastic alterations in the BM and in the PB (3). In the present case, the morphological
features were in accordance with the typical features observed in
BPDCN.
The diagnosis of BPDCN based on the results of
immunohistochemical analysis requires positivity for CD4 and CD56,
along with other markers that are more restricted to plasmacytoid
dendritic cells (such as CD123), and negativity for lymphoid, NK
and myeloid lineage-associated antigens (8,9). BPDCN
is typically identified in the CD45dim+ flow cytometry
blast gate region; ‘dim’ indicates weakly positive detection. BPDCN
is further phenotypically defined based on the absence of CD34
expression, the co-expression of CD56 and CD4, and the lack of
specific myeloid-, T-, B- or NK-lineage markers (6,10–12).
Furthermore, T-cell leukemia/lymphoma 1 (TCL1) and HLA-DR
expression is typically observed, in addition to marked CD123
expression that is detected in the majority of cases, indicating
the plasmacytoid dendritic cell origin of BPDCN. A previous study
proposed novel markers and extended diagnostic criteria for BPDCN,
including the detection of BDCA-2 (CD303) and BDCA-4 (CD304)
expression (11).
Upon cutaneous presentation, differential diagnoses
may include cutaneous T-cell or nasal-type NK lymphomas,
myeloproliferative disorders with monocytic differentiation, or
leukemia cutis (acute myeloid/lymphocytic leukemia) (6,12). The
diagnosis of BPDCN is facilitated when Epstein-Barr
virus-positivity, absence of lineage-specific markers, specific
dermatopathological features (including angio-invasion/-destruction
in NK/T-cell lymphoma) and T-cell receptor (TCR) gene
rearrangements are observed (4).
In the present case, the diagnosis of BPDCN was
challenging due to the absence of traditional lineage-specific
markers and typical cutaneous presentation. However, the results of
flow cytometric analysis identified the typical CD45dim+
CD4+ CD56+ HLA-DR+
CD123+ CD304+ immunophenotype. These
observations, along with the characteristic morphology and
cytogenetic findings in the present case, indicated that the
disease was consistent with the features of leukemic BPDCN. The
main differential diagnosis for leukemic BPDCN is acute leukemia of
ambiguous lineage, particularly the provisional entity NK-cell
lymphoblastic leukemia/lymphoma (NK-LL/L) (4,13). Based
on the 2008 WHO classification (4),
in cases where BPDCN is excluded, the diagnosis of NK-LL/L may be
considered when absence of B-cell or myeloid cell markers, negative
TCR gene rearrangement and expression of CD56 with immature
T-associated markers (such as cytoplasmic-CD3, CD7 and CD2) are
observed (4). Clearly, BPDCN and
NK-LL/L diagnoses may benefit from extended phenotypic panels that
have been previously proposed (11).
The characteristics observed in the present case are in accordance
with the typical immunophenotypic features.
Patients with BPDCN usually respond well to initial
chemotherapy, with stem cell transplantation in the pediatric
population is reserved for patients who relapse. Outcomes are more
favorable in cases that lack cutaneous disease at presentation,
although a comparison of cutaneous and noncutaneous cases might be
confounded by differences in treatment regimens. In future, it is
imperative to identify the most effective treatment for all
presentations of BPDCN, since the majority of cases are rapidly and
uniformly fatal (14). The majority
of the long-term survivors in the leukemic and cutaneous groups
presented herein represented those who successfully underwent
hematopoietic stem cell transplantation. Indeed, a recent study has
suggested that allogeneic stem cell transplantation with reduced
intensity conditioning should be pursued aggressively in BPDCN
patients (15,16).
References
|
1
|
Lencastre A, Cabete J, João A, Farinha P,
Ferreira G and Lestre S: Blastic plasmacytoid dendritic cell
neoplasm. An Bras Dermatol. 88(Suppl 1): 158–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herling M and Jones D:
CD4+/CD56+ hematodermic tumor: The features
of an evolving entity and its relationship to dendritic cells. Am J
Clin Pathol. 127:687–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feuillard J, Jacob MC, Valensi F, Maynadié
M, Gressin R, Chaperot L, Arnoulet C, Brignole-Baudouin F, Drénou
B, Duchayne E, et al: Clinical and biologic features of
CD4+CD56+ malignancies. Blood. 99:1556–1563.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. (4th).
International Agency for Research on Cancer (IARC) (Lyon).
2008.
|
|
5
|
Shi Y and Wang E: Blastic plasmacytoid
dendritic cell neoplasm: a clinicopathologic review. Arch Pathol
Lab Med. 138:564–569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Petrella T, Bagot M, Willemze R,
Beylot-Barry M, Vergier B, Delaunay M, Meijer CJ, Courville P, Joly
P, Grange F, et al: Blastic NK-cell lymphomas (agranular
CD4+CD56+ hematodermic neoplasms): A review.
Am J Clin Pathol. 123:662–675. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reichard KK, Burks EJ, Foucar MK, Wilson
CS, Viswanatha DS, Hozier JC and Larson RS: CD4+
CD56+ lineage-negative malignancies are rare tumors of
plasmacytoid dendritic cells. Am J Surg Pathol. 29:1274–1283. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Facchetti F, Ungari M, Marocolo D, Lonardi
S and Vermi W: Blastic plasmacytoid dendritic cell neoplasm.
Haematol Meet Rep. 3:1–3. 2009.
|
|
9
|
Pina-Oviedo S, Herrera-Medina H, Coronado
H, Del Valle L and Ortiz-Hidalgo C:
CD4+/CD56+ hematodermic neoplasm:
Presentation of 2 cases and review of the concept of an uncommon
tumor originated in plasmacytoid dendritic cells expressing CD123
(IL-3 receptor alpha). Appl Immunohistochem Mol Morphol.
15:481–486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jegalian AG, Facchetti F and Jaffe ES:
Plasmacytoid dendritic cells: Physiologic roles and pathologic
states. Adv Anat Pathol. 16:392–404. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garnache-Ottou F, Feuillard J, Ferrand C,
Biichle S, Trimoreau F, Seilles E, Salaun V, Garand R, Lepelley P,
Maynadié M, et al: Extended diagnostic criteria for plasmacytoid
dendritic cell leukaemia. Br J Haematol. 145:624–636. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herling M and Jones D:
CD4+/CD56+ hematodermic tumor: The features of an
evolving entity and its relationship to dendritic cells. Am J Clin
Pathol. 127:687–700. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ham MF and Ko YH: Natural killer cell
neoplasm: Biology and pathology. Int J Hematol. 92:681–689. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jegalian AG, Buxbaum NP, Facchetti F,
Raffeld M, Pittaluga S, Wayne AS and Jaffe ESL: Blastic
plasmacytoid dendritic cell neoplasm in children: Diagnostic
features and clinical implications. Haematologica. 95:1873–1879.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dietrich S, Andrulis M, Hegenbart U,
Schmitt T, Bellos F, Martens UM, Meissner J, Krämer A, Ho AD and
Dreger P: Blastic plasmacytoid dendritic cell neoplasia (BPDC) in
elderly patients: Results of a treatment algorithm employing
allogeneic stem cell transplantation with moderately reduced
conditioning intensity. Biol Blood Marrow Transplant. 17:1250–1254.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rauh MJ, Rahman F, Good D, Silverman J,
Brennan MK, Dimov N, Liesveld J, Ryan DH, Burack WR and Bennett JM:
Blastic plasmacytoid dendritic cell neoplasm with leukemic
presentation, lacking cutaneous involvement: Case series and
literature review. Leuk Res. 36:81–86. 2012. View Article : Google Scholar : PubMed/NCBI
|