Introduction
Colorectal cancer (CRC) is one of the most common
malignancies worldwide (1). Over the
last 25 years, the incidence and mortality of CRC in China have
markedly increased (2). CRC is
characterized by invasion and metastasis, and the 5-year survival
rate of patients with distant metastasis is <10% (3).
Previous studies demonstrated that chronic
inflammation is an important initiation event in CRC tumorigenesis
(4). C-X-C motif chemokine ligand 11
(CXCL11), released by immune cells during inflammation, is a small
cytokine that may contribute to progression of colonic
tumorigenesis (5). CXCL11 may
regulate the chemotaxis of cells through interaction with a subset
of 7-transmembrane, G protein-coupled receptors (6). In CRC, CXCL11 induces infiltration by
tumor-associated macrophages and is associated with poor patient
prognosis (7). Colon carcinoma cells
induce the migration of CXCR3-expressing cytotoxic T lymphocytes in
a CXCL11-dependent manner (8). All
evidence suggests that CXCL11 is one of the key cytokines
interlinking inflammation and CRC.
MicroRNAs (miRNAs) are short non-coding RNAs that
regulate the expression of their targets at the mRNA level
(9). Accumulating evidence has
demonstrated that the dysregulation of miRNAs is responsible for
the pathogenesis of CRC (10).
miR-144 is widely present in human tissues and body fluids, but its
levels are abnormal under disease conditions (11). miR-144 is significantly downregulated
in various tumor tissues, including CRC (12,13).
Being a tumor suppressor, miR-144 upregulation may inhibit the
proliferation, invasion and metastasis of cancer cells (14,15).
However, an association between miR-144 and the CXCL11 signaling
pathway has not been reported to date. The present study was
undertaken to analyze the expression profile of CXCL11 in CRC from
Gene Expression Omnibus (GEO) datasets. In addition, we
investigated the expression of CXCL11 and miR-144 in the serum and
tumor specimens of patients with CRC, in order to determine whether
there is a regulatory association between miR-144 and CXCL11.
Materials and methods
Microarray data
Microarray dataset GDS4382, GDS4515, GDS3756 and
GDS2947 were downloaded from the Gene Expression Omnibus database
(GEO DataSets). GDS4382 (16), based
on GPL570 platform, consisted of 17 CRC tumor tissues and 17 normal
tissues; GDS4515 (17), based on
GPL96 platform, included 34 microsatellite-unstable colorectal
tumor samples and 15 normal colonic mucosa samples; 22 rectal tumor
samples and 20 normal rectal tissue samples were selected from
GDS3756 (18) based on GPL2986
platform; the array data of GDS2947 (19), based on GPL570 platform, included 32
adenoma samples and 32 normal mucosa samples. Under the same
experimental conditions, tumor samples and normal samples were
divided into two groups for screening. Data Analysis Tools in GEO
DataSets was used for identifying differentially expressed genes
(DEGs). The cut-off criterion was set as P<0.05 and value means
difference >2+ fold.
Prediction of regulator for
CXCL11
The prediction of regulator for CXCL11 was performed
by miRWalk 1.0 (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk/index.html),
a database that not only documents miRNA binding sites within the
complete sequence of a gene, but also combines this information
with a comparison of binding sites resulting from other existing
miRNA-target prediction programs (20). A total of 10 established miRNA-target
prediction programs (Diana-microT, miRanda, miRDB, miRWalk,
RNAhybrid, PICTAR4, PICTAR5, PITA, RNA22 and TargetScan) were
available in miRWalk. The potential targets of one miRNA are
considered to be the genes that have been predicted by at least 5
programs (21).
Patients and samples
To determine the expression of genes or miRNAs in
CRC tissues, tumor and surrounding normal tissue samples were
collected from 39 patients with CRC (23 men and 16 women; age
range, 25–65 years; median age, 45.3 years). All the patients had
undergone radical surgery at the Department of General Surgery,
West China Hospital, Sichuan University, between May 2010 and
September 2011. Prior to surgery, none of the patients had received
chemotherapy or radiotherapy. The excised tumors and adjacent
tissues were stored in liquid nitrogen for molecular biology
experiments. In addition, peripheral blood was collected from all
39 patients and 26 healthy control subjects (16 men and 10 women;
age range, 23–66 years; median age, 44.8 years). The separation of
serum was performed by centrifugation at 1,000 × g for 10 min at
4°C. All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
Institutional and/or National Research Committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. Tissue and blood samples were collected from consenting
individuals according to the protocols approved by the Ethics
Review Board at Sichuan University.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissue samples using
E.Z.N.A® Total DNA/RNA/Protein kit (Omega Bio-Tek, Inc.,
Norcross, GA, USA). Total RNA was extracted from serum samples
using the GenElute™ Plasma/Serum RNA Purification Mini kit
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). RT for RT-qPCR was
performed with a PrimeScript™ RT reagent kit (Takara Biotechnology
Co., Ltd., Dalian, China) according to the manufacturer's
instructions. The real-time PCR reaction system was prepared using
SYBR® Premix Ex Taq™ II (RR420A; Takara Biotechnology
Co., Ltd.) and the qPCR assay was performed with the CFX96TM
Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The reaction system (20 µl) consisted of 10 µl
SYBR Premix Ex Taq, 0.5 µl forward primer, 0.5 µl reverse primer, 2
µl cDNA and 7 µl double-distilled water (ddH2O). The
reaction protocol was as follows: Initial denaturation at 95°C for
5 min, followed by 40 cycles at 95°C for 15 sec, 60°C for 30 sec
and 72°C for 10 sec. All the reactions were performed in accordance
with the manufacturer's instructions. The primer sequences
(miR-144, CXCL11, GAPDH, U6) for qPCR are listed in Table I. The relative expression of CXCL11
(reference gene GAPDH) and miR-144 (reference gene U6) was analyzed
using the 2−ΔΔCq method (22).
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Primer | Sequences |
|---|
| CXCL11 | F:
5′-ATAGCCTTGGCTGTGATATTGTGTG-3′ |
|
| R:
5′-CCTATGCAAAGACAGCGTCCTC-3′ |
| GAPDH | F:
5′-AGAAGGCTGGGGCTCATTTGC-3′ |
|
| R:
5′-ACAGTCTTCTGGGTGGCAGTG-3′ |
| miR-144 | F:
5′-ACACTCCAGCTGGGGGATATCATCATATACTGT-3′ |
|
| R:
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTTACAG-3′ |
| U6 | F:
5′-CTCGCTTCGGCAGCAC-3′ |
|
| R:
5′-AACGCTTCACGAATTTGCG-3′ |
Immunohistochemistry
Using an immunohistochemical assay kit (BosterBio,
Wuhan, China) according to the manufacturer's instructions, the
tissue samples were fixed in 4% paraformaldehyde and embedded in
paraffin, then cut into 5-µm sections. The slides were treated by a
graded series of alcohol and finally rinsed with ddH2O.
After antigen retrieval, endogenous peroxidase activity was blocked
with 3% hydrogen peroxide at room temperature for 10 min.
Subsequently, the slides were blocked with 10% normal goat serum
(C0265; Beyotime Institute of Biotechnology, Haimen, China) for 1
h, followed by incubation with 1:250 diluted CXCL11 primary
antibody (ab9955, rabbit anti-human; Abcam, Cambridge, MA, USA) at
4°C overnight. After washing off the primary antibody, the second
antibody (ab6721, goat anti-rabbit IgG; Abcam) was applied and
incubated at room temperature for 1 h. After DAB staining (P0203;
Beyotime Institute of Biotechnology) until the appearance of brown
color, the nuclei were stained with hematoxylin (C0107; Beyotime
Institute of Biotechnology) for 1 min. Subsequently, the slides
were washed with ddH2O, sealed with neutral gum and
covered with coverslips. An optical microscope (TS100-F; Nikon
Corporation, Tokyo, Japan) was used for cell observation: Brown
staining was considered as positive; the slides were observed under
a magnification of ×10 and ×40; five non-overlapping fields were
randomly selected from each section and images were captured. The
mean integrated optical density (IOD/area) of the images was
measured by Image-Pro Plus v.6.0 software, which was used for
semi-quantitative analysis.
Western blot analysis
Total protein was extracted with the
E.Z.N.A® Total DNA/RNA/Protein kit (Omega Bio-Tek Inc.).
The BCA protein assay kit (Pierce Biotechnology, Rockford, IL, USA)
was applied for protein concentration detection. The protein
samples were mixed with loading buffer (P0015; Beyotime Institute
of Biotechnology) and boiled for 10 min; then, 50 µg protein from
each sample was subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto a
PVDF membrane (EMD Millipore, Billerica, MA, USA). The blot was
blocked with 5% non-fat milk at room temperature for 2 h and then
incubated with CXCL11 primary antibody (ab9955, rabbit anti-human,
Abcam, USA) for 20 h at 4°C. After TBST washing, the membrane was
incubated with secondary antibody (ab6721, goat anti-rabbit IgG;
Abcam) at room temperature for 1 h. An ECL kit (P0018; Beyotime
Institute of Biotechnology) was used for blot chemiluminescence and
an exposing gel documentation system was applied for imaging the
blots. Image Lab v.3.0 software was used to obtain and analyze the
protein signal.
Enzyme-linked immunosorbent assay
(ELISA)
CXCLL11 in serum specimens was tested using a Human
CXCL11 ELISA kit (ab187392; Abcam); 50 µl serum of each samples was
added into appropriate wells for the detection. All the procedures
were performed according to the manufacturer's instructions. The OD
was measured at 450 nm with a microplate spectrophotometer
(Multiskan™ FC Microplate Photometer; Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Cell culture
293T and HCT116 cells in this study were provided by
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China), and cultured with DMEM high-glucose medium
(HyClone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% FBS (Shengong, Shanghai, China) at 37°C in 5%
CO2.
CRC cell transfection
After growing to 50–70% confluence, HCT116 cells
were transfected with agomiR-144 or agomiR-NC by using ExFect
Transfection Reagent (Vazyme Biotech Co., Ltd., Nanjing, China)
according to the manufacturer's instructions. AgomiR-144 and
agomiR-NC were designed and chemically synthesized by Guangzhou
RiboBio Co., Ltd., Guangzhou, China.
Dual-luciferase reporter assay
According to the bioinformatics analysis results, a
fragment of the CXCL11 3′-untranslated region (UTR) containing the
wild type (WT) or mutant (MT) seed regions of miR-144 was
chemically synthesized in vitro. The products were inserted
into Spe-1 and Hind III restriction sites of pMIR-REPORT luciferase
reporter plasmids (Ambion; Thermo Fisher Scientific, Inc.). The
constructs were co-transfected with agomiR-144 into 293T cells
using ExFect® Transfection Reagent (Vazyme Biotech Co.,
Ltd.); the empty plasmid was set as control. After 48 h of
transfection, the cells were harvested. The luciferase activity was
measured using a dual-luciferase reporter assay kit (Promega
Corporation, Madison, WI, USA) and GloMax 20/20 luminometer
(Promega Corporation). All the procedures were performed according
to the manufacturer's instructions.
Statistical analysis
IBM SPSS software (v.20.0; IBM Corp., Armonk, NY,
USA) was used for statistical analysis. Data are expressed as mean
± standard deviation. Comparisons between two groups were performed
using the Student's t-test, and multi-group measurement data were
analyzed using one-way analysis of variance. The post hoc test was
performed using Student-Newman-Keuls and Least Significant
Difference-t test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression profile of CXCL11 in CRC
tissues form microarray data analysis
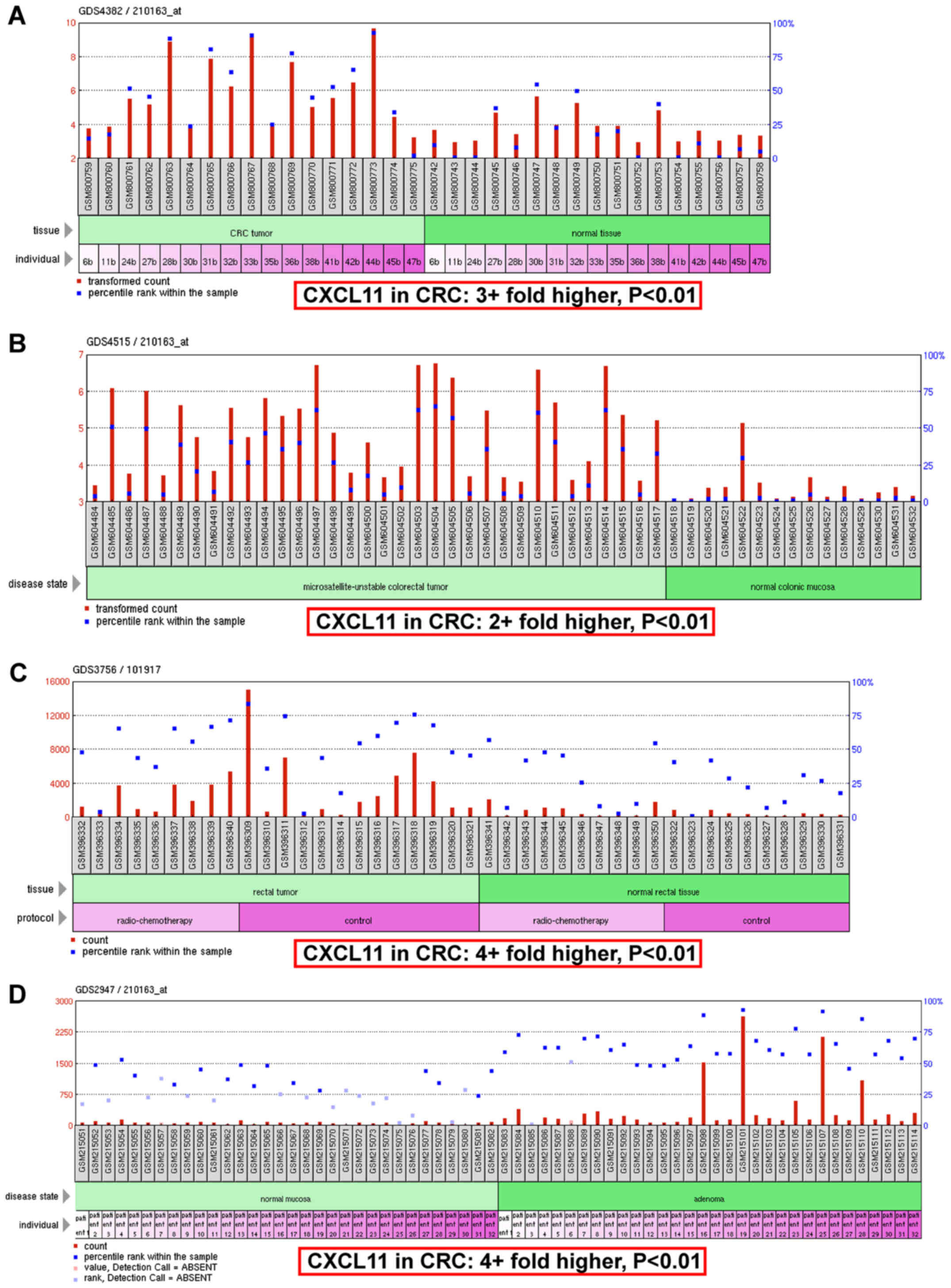
To measure the expression of CXCL11 in CRC tissues,
microarray datasets GDS4382, GDS4515, GDS3756 and GDS2947 were
downloaded from GEO DataSets. The mean values of CXCL11 in CRC
tissues were 3+fold higher compared with normal tissues (Fig. 1A); in microsatellite-unstable
colorectal tumor tissues they were 2+fold higher compared with
normal colonic mucosa tissues (Fig.
1B); in rectal tumor tissues they were 4+fold higher compared
with normal rectal tissues (Fig.
1C); and in adenoma tissues they were 4+fold higher compared
with normal mucosa tissues (Fig.
1D). All the above mentioned data supported the upregulation of
CXCL11 in CRC tissues.
Expression of CXCL11 in the serum and
tumor tissues of patients with CRC
RT-qPCR was used to determine the levels of CXCL11
mRNA in tumor tissue and serum samples; western blotting and
immunohistochemistry were used to detect the expression and
localization of the CXCL11 protein in tumor tissue samples; ELISA
was conducted to measure the concentration of the CXCL11 protein in
serum samples. The data revealed that the levels of CXCL11 mRNA
were significantly upregulated in CRC tissues (2+fold higher,
P<0.05; Fig. 2A) and serum
samples (3+fold higher, P<0.05; Fig.
2D) collected from CRC patients. Upregulation of the CXCL11
protein was detected in CRC tissues (Fig. 2B and C) and the localization of
CXCL11 was in the cytoplasm and on the cell membrane (Fig. 2C). Increased expression of the CXCL11
protein was also found in serum samples from patients with CRC
(Fig. 2E). The expression trend of
CXCL11 in clinical specimens was similar to our bioinformatics
analysis, indicating that increased CXCL11 expression may be
implicated in CRC.
CXCL11 is a potential target of
miR-144
To search for the potential regulator of CXCL11,
miRWalk was used for the bioinformatics predictions. Of the 10 the
established miRNA-target prediction programs, 6 predicted that
CXCL11 was a potential target of miR-144 (Fig. 3A). The miR-144 target site in the
CXCL11 mRNA 3′UTR was shown in some programs (Fig. 3B-D).
Expression of miR-144 in the serum and
tumor tissues of patients with CRC
The levels of miR-144 in the serum and tumor tissues
of patients with CRC were analyzed by RT-qPCR. Compared with
adjacent tissues, miR-144 in CRC tissues was significantly reduced
(P<0.05; Fig. 4A). The expression
of miR-144 was also downregulated in the serum of CRC patients
compared with the control subjects (P<0.05; Fig. 4B). Taking the expression of CXCL11
into account, these results indicated that miR-144 may be a
negative regulator of CXCL11.
CXCL11 is a direct target of
miR-144
To investigate the predicted interaction of miR-144
with CXCL11 mRNA, cell transfection and dual-luciferase reporter
assay were used. HCT116 cells were selected for transfection with
agomiR-144 or agomiR-NC in vitro. Compared with the
agomiR-NC group, agomiR-144 significantly upregulated the
expression of miR-144 (P<0.05; Fig.
5A), while it significantly downregulated the expression of
CXCL11 (P<0.05; Fig. 5B and C) in
HCT116 cells. WT and MT miR-144 binding sites were cloned into the
pMIR-REPORT luciferase reporter plasmids (Fig. 5D) and co-transfected with agomiR-144
into 293T cells; the empty plasmid was used as control. Compared
with the control group, transfection with agomiR-144 resulted in a
significantly reduced fluorescence intensity in the WT group, but
not in the MT group (Fig. 5E). These
observations suggest that miR-144 may directly target the 3′-UTR of
CXCL11 mRNA.
Discussion
The objective of the present study was to elucidate
the role of miR-144 and CXCL11 in CRC progression. We reported the
levels of CXCL11 in clinical specimens obtained from CRC patients.
Then, using bioinformatics analysis, we found that miR-144 is a
potential regulator of CXCL11. Next, we detected the expression of
miR-144 in the serum and tumor tissues of CRC patients. Finally,
luciferase assay verified that miR-144 directly targeted the 3′-UTR
of CXCL11 mRNA.
First, differentially expressed genes in CRC tissues
were identified from microarray data analysis. Microarray analysis
has been applied to investigate the processes involved in CRC, as
it is an effective tool for detecting general genetic alterations
in the study of oncology (23,24). The
most obvious finding to emerge from the analysis was the enhanced
expression of CXCL11 in both CRC and colorectal adenomas, even up
to 4-fold higher compared with normal tissues.
Interferon-γ-inducible chemokines are crucial immunomodulators, not
only in colon cancer, but also in other types of cancer, such as
thyroid cancer (25). Other studies
have also evaluated interferon-γ-inducible chemokines in colon
cancer (26). Interferon-γ-inducible
CXC-chemokines regulate angiogenesis and recruitment of immune
cells in cancer progression (27),
and they are closely associated with CRC. Not only CRC cell lines
were demonstrated to release CXC-chemokines in response to cytokine
stimulation, but also resected tumor explants were found to produce
CXC-chemokines under stimulation, even in cases with initially low
CXC-levels (26). Among these
CXC-chemokines, CXCL11 is deemed to be an important factor during
colonic tumorigenesis (5,28). Similar to the results of microarray
data analysis, we found that the expression levels of CXCL11 were
significantly upregulated in excised tumor specimens compared with
normal tissues. However, there are no sufficiently clear
mechanistic analyses on the regulation of CXCL11 in CRC. We herein
provide evidence that CXCL11 is coordinately regulated by miR-144
in CRC.
It has been demonstrated that miRNAs are crucial for
the occurrence and development of CRC (29,30).
Through bioinformatics analysis, we identified CXCL11 as a
potential target of miR-144. Previous miRNA profiling data revealed
that miR-144 levels were significantly reduced in CRC (12,13). As
a tumor suppressor, miR-144 may inhibit the malignant biological
behavior of CRC by binding to its target mRNAs (31,32).
Thus, we analyzed the levels of miR-144 in excised CRC tissues. The
results revealed that the expression trends of miR-144 were
contrary to those of CXCL11: Compared with normal tissues, the
expression of miR-144 was significantly downregulated in tumor
specimens. In CRC cells, upregulation of miR-144 also significantly
downregulated the expression of CXCL11. Subsequently, the predicted
association between miR-144 and CXCL11 was verified via the
dual-luciferase reporter assay: Changes in relative fluorescence
intensity induced by agomiR-144 indicated their interaction. This
is a novel mechanism of miR-144 regulating the expression of CXCL11
by directly targeting the CXCL11 mRNA 3′-UTR seed sequence. Some
other miRNAs such as miR-128 and miR-376, were also predicted to
interact with CXCL11. We plan to study these miRNAs in future.
CXCL11 plays an important role in chronic
inflammation, which is a common initiating step in tumorigenesis
(23–35). CXCL11 may enhance the chemotaxis of T
cells, particularly Th1 cells highly expressing CXCR3 (8); through the PI3K-AKT and RAS-MAPK
pathways, CXCL11 promotes the proliferation and migration of tumor
cells and exerts anti-apoptosis effects (36). CXCL11 also promotes tumor formation
and development via binding with CXCR7 (37), and it may even be used as an
independent prognostic factor for myeloma, along with CXCL9 and
CXCL10 (38). These previous studies
suggested that CXCL11 may be a key cytokine interlinking
inflammation and tumor development. Moreover, as a regulator of
CXCL11, miR-144 may affect not only colorectal tumorigenesis, but
also chronic inflammation prior to CRC development.
Another novel finding of our study is that decreased
levels of miR-144 are associated with increased levels of CXCL11 in
the serum of patients with CRC, suggesting their potential value as
biomarkers for CRC prediction. However, this study was conducted in
a small sample size of cases and controls, and the findings require
further evaluation in the future.
In conclusion, our study demonstrated that enhanced
expression levels of CXCL11 in patients with CRC were associated
with the decreased expression of miR-144. Through binding to the
CXCL11 mRNA 3′-UTR, miR-144 may regulate the chronic inflammatory
and tumorigenic processes in CRC.
Acknowledgements
The authors are grateful to Dr Fei-Jun Huang of the
Department of Pathology, West China Hospital, for assisting in the
pathological diagnosis of patients with CRC.
Funding
The present study was supported by the Fund of
Health and Family Planning Commission, Sichuan Province (grant no.
16PJ136).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BH and DF analyzed and interpreted the patient data,
conceived and designed the study, and collected and analyzed
experiment data. XY, YQL and MY read and analyzed the documents,
and collected and analyzed data. FLi performed the experiments,
critically revised the manuscript and gave valuable advice for the
study. LMZ and FLu conceived and designed the study, read and
analyzed the documents, drafted and revised the manuscript and gave
final approval of the version to be published. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed involving human
participants were in accordance with the ethical standards of the
Institutional and/or National Research Committee and with the 1964
Helsinki Declaration and its later amendments or comparable ethical
standards. Tissue and blood samples were collected from consenting
individuals according to the protocols approved by the Ethics
Review Board at Sichuan University.
Consent for publication
The patient, parent, guardian or next of kin (in
case of deceased patients) provided written informed consent for
the publication of any associated data and accompanying images.
Conflict of interest
The authors declare that they have no competing
interests.
References
|
1
|
Brenner H, Kloor M and Pox CP: Colorectal
cancer. Lancet. 383:1490–1502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hu T, Li LF, Shen J, Zhang L and Cho CH:
Chronic inflammation and colorectal cancer: The role of vascular
endothelial growth factor. Curr Pharm Des. 21:2960–2967. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rupertus K, Sinistra J, Scheuer C, Nickels
RM, Schilling MK, Menger MD and Kollmar O: Interaction of the
chemokines I-TAC (CXCL11) and SDF-1 (CXCL12) in the regulation of
tumor angiogenesis of colorectal cancer. Clin Exp Metastasis.
31:447–459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kerr JS, Jacques RO, Cardaba Moyano C, Tse
T, Sexton D and Mueller A: Differential regulation of chemotaxis:
Role of Gβγ in chemokine receptor-induced cell migration. Cell
Signal. 25:729–735. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zeng YJ, Lai W, Wu H, Liu L, Xu HY, Wang J
and Chu ZH: Neuroendocrine-like cells-derived CXCL10 and CXCL11
induce the infiltration of tumor-associated macrophage leading to
the poor prognosis of colorectal cancer. Oncotarget. 7:27394–27407.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cole KE, Strick CA, Paradis TJ, Ogborne
KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, et
al: Interferon-inducible T cell alpha chemoattractant (I-TAC): A
novel non-ELR CXC chemokine with potent activity on activated T
cells through selective high affinity binding to CXCR3. J Exp Med.
187:2009–2021. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schneiderova M, Naccarati A, Pardini B,
Rosa F, Gaetano CD, Jiraskova K, Opattova A, Levy M, Veskrna K,
Veskrnova V, et al: MicroRNA-binding site polymorphisms in genes
involved in colorectal cancer etiopathogenesis and their impact on
disease prognosis. Mutagenesis. 32:533–542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Keller A, Leidinger P, Vogel B, Backes C,
El Sharawy A, Galata V, Mueller SC, Marquart S, Schrauder MG,
Strick R, et al: miRNAs can be generally associated with human
pathologies as exemplified for miR-144. BMC Med. 12:2242014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gaedcke J, Grade M, Camps J, Søkilde R,
Kaczkowski B, Schetter AJ, Difilippantonio MJ, Harris CC, Ghadimi
BM, Møller S, et al: The rectal cancer microRNAome-microRNA
expression in rectal cancer and matched normal mucosa. Clin Cancer
Res. 18:4919–4930. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang W, Peng B, Wang D, Ma X, Jiang D,
Zhao J and Yu L: Human tumor microRNA signatures derived from
large-scale oligonucleotide microarray datasets. Int J Cancer.
129:1624–1634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren K, Liu QQ, An ZF, Zhang DP and Chen
XH: MiR-144 functions as tumor suppressor by targeting PIM1 in
gastric cancer. Eur Rev Med Pharmacol Sci. 21:3028–3037.
2017.PubMed/NCBI
|
|
15
|
Iwaya T, Yokobori T, Nishida N, Kogo R,
Sudo T, Tanaka F, Shibata K, Sawada G, Takahashi Y, Ishibashi M, et
al: Downregulation of miR-144 is associated with colorectal cancer
progression via activation of mTOR signaling pathway.
Carcinogenesis. 33:2391–2397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khamas A, Ishikawa T, Shimokawa K, Mogushi
K, Iida S, Ishiguro M, Mizushima H, Tanaka H, Uetake H and Sugihara
K: Screening for epigenetically masked genes in colorectal cancer
Using 5-Aza-2′-deoxycytidine, microarray and gene expression
profile. Cancer Genomics Proteomics. 9:67–75. 2012.PubMed/NCBI
|
|
17
|
Alhopuro P, Sammalkorpi H, Niittymäki I,
Biström M, Raitila A, Saharinen J, Nousiainen K, Lehtonen HJ,
Heliövaara E, Puhakka J, et al: Candidate driver genes in
microsatellite-unstable colorectal cancer. Int J Cancer.
130:1558–1566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Snipstad K, Fenton CG, Kjaeve J, Cui G,
Anderssen E and Paulssen RH: New specific molecular targets for
radio-chemotherapy of rectal cancer. Mol Oncol. 4:52–64. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sabates-Bellver J, Van der Flier LG, de
Palo M, Cattaneo E, Maake C, Rehrauer H, Laczko E, Kurowski MA,
Bujnicki JM, Menigatti M, et al: Transcriptome profile of human
colorectal adenomas. Mol Cancer Res. 5:1263–1275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dweep H, Sticht C, Pandey P and Gretz N:
miRWalk-database: Prediction of possible miRNA binding sites by
‘walking’ the genes of three genomes. J Biomed Inform. 44:839–847.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu Y and Wu F: A new miRNA regulator,
miR-672, reduces cardiac hypertrophy by inhibiting JUN expression.
Gene. 648:21–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Varnholt H, Drebber U, Schulze F,
Wedemeyer I, Schirmacher P, Dienes HP and Odenthal M: MicroRNA gene
expression profile of hepatitis C virus-associated hepatocellular
carcinoma. Hepatology. 47:1223–1232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Yu X, Xu Y and Shen H:
Identification of dysregulated lncRNAs profiling and
metastasis-associated lncRNAs in colorectal cancer by genome-wide
analysis. Cancer Med. 6:2321–2330. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zong S, Li W, Li H, Han S, Liu S, Shi Q
and Hou F: Identification of hypoxia-regulated angiogenic genes in
colorectal cancer. Biochem Biophys Res Commun. 493:461–467. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Antonelli A, Ferrari SM, Fallahi P,
Frascerra S, Piaggi S, Gelmini S, Lupi C, Minuto M, Berti P,
Benvenga S, et al: Dysregulation of secretion of CXC
alpha-chemokine CXCL10 in papillary thyroid cancer: Modulation by
peroxisome proliferator-activated receptor-gamma agonists. Endocr
Relat Cancer. 16:1299–1311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kistner L, Doll D, Holtorf A, Nitsche U
and Janssen KP: Interferon-inducible CXC-chemokines are crucial
immune modulators and survival predictors in colorectal cancer.
Oncotarget. 8:89998–90012. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Keeley EC, Mehrad B and Strieter RM: CXC
chemokines in cancer angiogenesis and metastases. Adv Cancer Res.
106:91–111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin YH, Friederichs J, Black MA, Mages J,
Rosenberg R, Guilford PJ, Phillips V, Thompson-Fawcett M, Kasabov
N, Toro T, et al: Multiple gene expression classifiers from
different array platforms predict poor prognosis of colorectal
cancer. Clin Cancer Res. 13:498–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rahmani F, Avan A, Hashemy SI and
Hassanian SM: Role of Wnt/β-catenin signaling regulatory microRNAs
in the pathogenesis of colorectal cancer. J Cell Physiol.
233:811–817. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Moridikia A, Mirzaei H, Sahebkar A and
Salimian J: MicroRNAs: Potential candidates for diagnosis and
treatment of colorectal cancer. J Cell Physiol. 233:901–913. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cai SD, Chen JS, Xi ZW, Zhang LJ, Niu ML
and Gao ZY: MicroRNA-144 inhibits migration and proliferation in
rectal cancer by downregulating ROCK-1. Mol Med Rep. 12:7396–7402.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sureban SM, May R, Mondalek FG, Qu D,
Ponnurangam S, Pantazis P, Anant S, Ramanujam RP and Houchen CW:
Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144
and inhibits colorectal cancer tumor growth via a Notch-1 dependent
mechanism. J Nanobiotechnology. 9:402011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Afaloniati H, Karagiannis GS, Hardas A,
Poutahidis T and Angelopoulou K: Inflammation-driven colon
neoplasmatogenesis in uPA-deficient mice is associated with an
increased expression of Runx transcriptional regulators. Exp Cell
Res. 361:257–264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mandal P: Molecular mechanistic pathway of
colo-rectal carcinogenesis associated with intestinal microbiota.
Anaerobe. 49:63–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Verbeke H, Geboes K, Van Damme J and
Struyf S: The role of CXC chemokines in the transition of chronic
inflammation to esophageal and gastric cancer. Biochim Biophys
Acta. 1825:117–129. 2012.PubMed/NCBI
|
|
36
|
Miekus K, Jarocha D, Trzyna E and Majka M:
Role of I-TAC-binding receptors CXCR3 and CXCR7 in proliferation,
activation of intracellular signaling pathways and migration of
various tumor cell lines. Folia Histochem Cytobiol. 48:104–111.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Li G, Stanco A, Long JE, Crawford
D, Potter GB, Pleasure SJ, Behrens T and Rubenstein JL: CXCR4 and
CXCR7 have distinct functions in regulating interneuron migration.
Neuron. 69:61–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bolomsky A, Schreder M, Hübl W, Zojer N,
Hilbe W and Ludwig H: Monokine induced by interferon gamma
(MIG/CXCL9) is an independent prognostic factor in newly diagnosed
myeloma. Leuk Lymphoma. 57:2516–2525. 2016. View Article : Google Scholar : PubMed/NCBI
|