Failure to obtain complete disease clearance due to
incomplete resection, including positive tumor margins or
metastatic cancer cells in lymph nodes, is a major challenge in
tumor surgery and occurs in 20–60% of operations (2). Tumor cells may spread to distant host
tissues, leading to metastatic disease, a well-known cause of
mortality in patients with cancer (4). Following treatment, high levels of
metastasis and the recurrence of cancer may be observed due to
incomplete removal of the edges of the primary tumor (4). Surgery holds different challenges,
including identifying small lesions, locating metastases, as well
as confirming complete tumor removal (5–9).
To improve surgical accuracy, fluorescence guidance
is an advisable approach. Near-infrared fluorescence (NIRF) imaging
displays promising results in preclinical studies, allowing for
real-time early diagnosis and intraoperative imaging lesion tissue
(10–12). It describes the non-invasive use of
near-infrared light to excite the contrast agent, after which the
intensity of contrast agent fluorescence can be detected. Thus, the
fluorescence represents the transformation of the molecular
structure of the contrast agent, which is tissues in different
diseases (4). NIRF guidance was
introduced to improve the identification of lesions and guide the
removal of these lesions (13).
Compared with the more traditional approach of molecular imaging,
which involves a radioactive tracer at cm resolution, NIRF provides
higher resolution, allowing the identification of numerous details
on the surface of the tissue (14).
The fluorescence guidance technology is limited by the strong
attenuation of the signal, meaning the technology would lose be
less accurate with increasing depth (15). Intraoperative fluorescent molecular
imaging agents have emerged as an innovative approach to guide
surgical resection. There are three types of fluorescent molecular
imaging agents, which include passive fluorescent dyes, ‘pro-dye’
fluorescent agents and biomarker-targeted fluorescent dyes. The
current review introduces these types kinds of fluorescent contrast
agents by using examples of each.
The mechanism of ICG accumulation in a tumor remains
elusive. Previous studies have demonstrated that ICG undergoes
hepatobiliary excretion (25–28). The
excretion of ICG into the liver then bile may impact its clearance
in different types of tumors. For hepatic tumors, it is assumed
that organic-anion transporting polypeptides expressed on liver
cells, transporter proteins and intracellular transporter proteins
give rise to the tumor contrast (24,29). For
non-liver tumors, the enhanced permeability and retention (EPR)
effect is the primary mechanism for the accumulation of ICG in
solid carcinomas (30–33). The EPR mechanism has been associated
with tumor environments, such as blood pressure, pH, vascular
endothelial cell separation, differences in local prostaglandins
and bradykinin levels and the lack of angiogenesis in lymphatic
vessels (24,34).
It was further indicated that the intracellular
accumulation of ICG may increase as the temperature increases;
also, the authors suggested that ICG may be absorbed into the cells
by binding to the cell membrane (43). Two molecules may influence the
process of ICG uptake: Phospholipids and Pitstop2. The ability of
ICG to interact with phospholipids allows it to bind to the cell
membrane, the cells then uptake ICG; Pitstop2, the grid
protein-dependent endocytosis inhibitor, is activated through the
binding of extracellular molecules to the cell membrane and
inhibits the uptake of ICG (44).
Furthermore, the authors recommended that ICG may be absorbed into
the cells by binding to the cell membrane (43).
The conflicting results of the fluorescence imaging
often depend on tumor type, staging and microenvironment. The
fluorescence emitted by ICG only penetrates 5–10 mm into the
tissue, so the depth of the tumor influences the imaging result
(45). Hill et al (46) stated that human leukocyte antigen
(HLA) is a natural, biodegradable substance; ICG (0.0026–0.0052
mmol, 2.0–4.0 mg) loaded into HLA may become a nanoparticle. Hill
et al (46) also indicated
that tumor contrast with ICG nanoparticles was significantly
improved compared with the use of regular ICG. This indicates that
the size of ICG may influence the fluorescence image results.
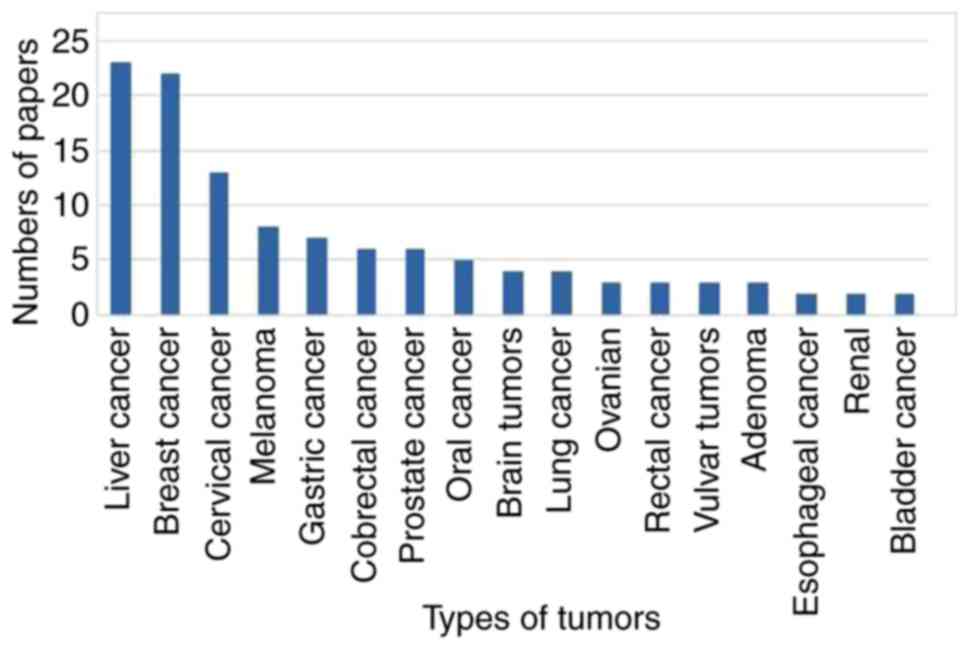
A PubMed analysis of papers published over the last
five years using ICG in surgery by tissue or cell type is given in
Fig. 3. Liver cancer exhibits the
highest publication numbers describing the use of ICG in surgery,
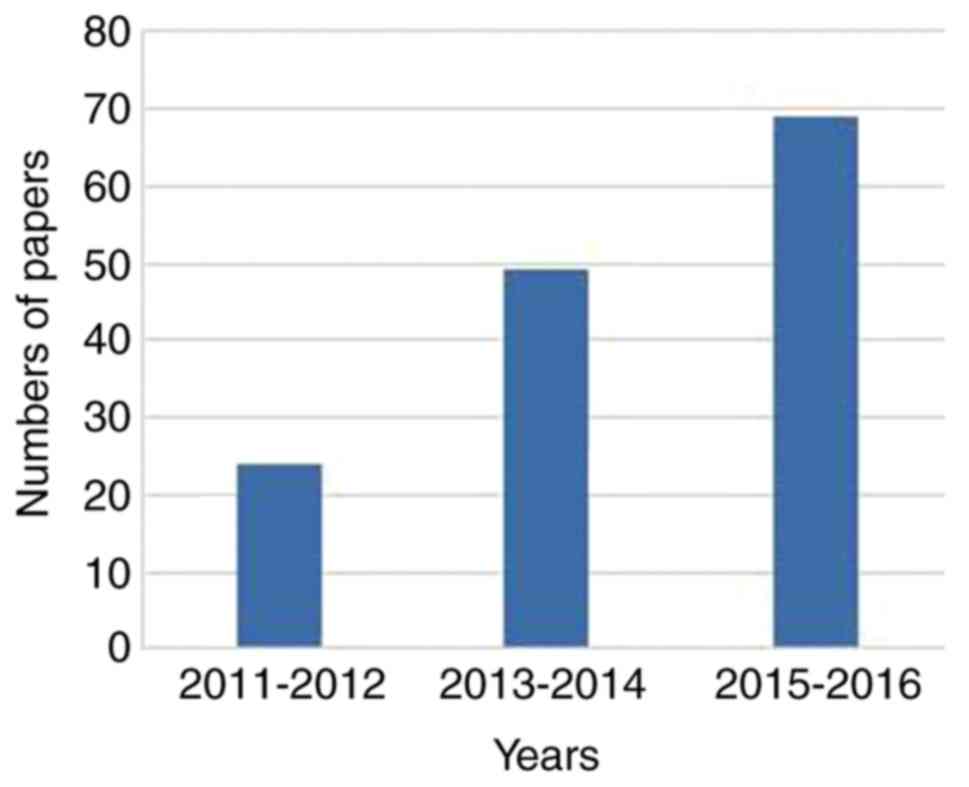
followed by breast and cervical cancer. Publications describing the
use of ICG in surgery have increased between 2011 and 2016, as
Fig. 4 indicates.
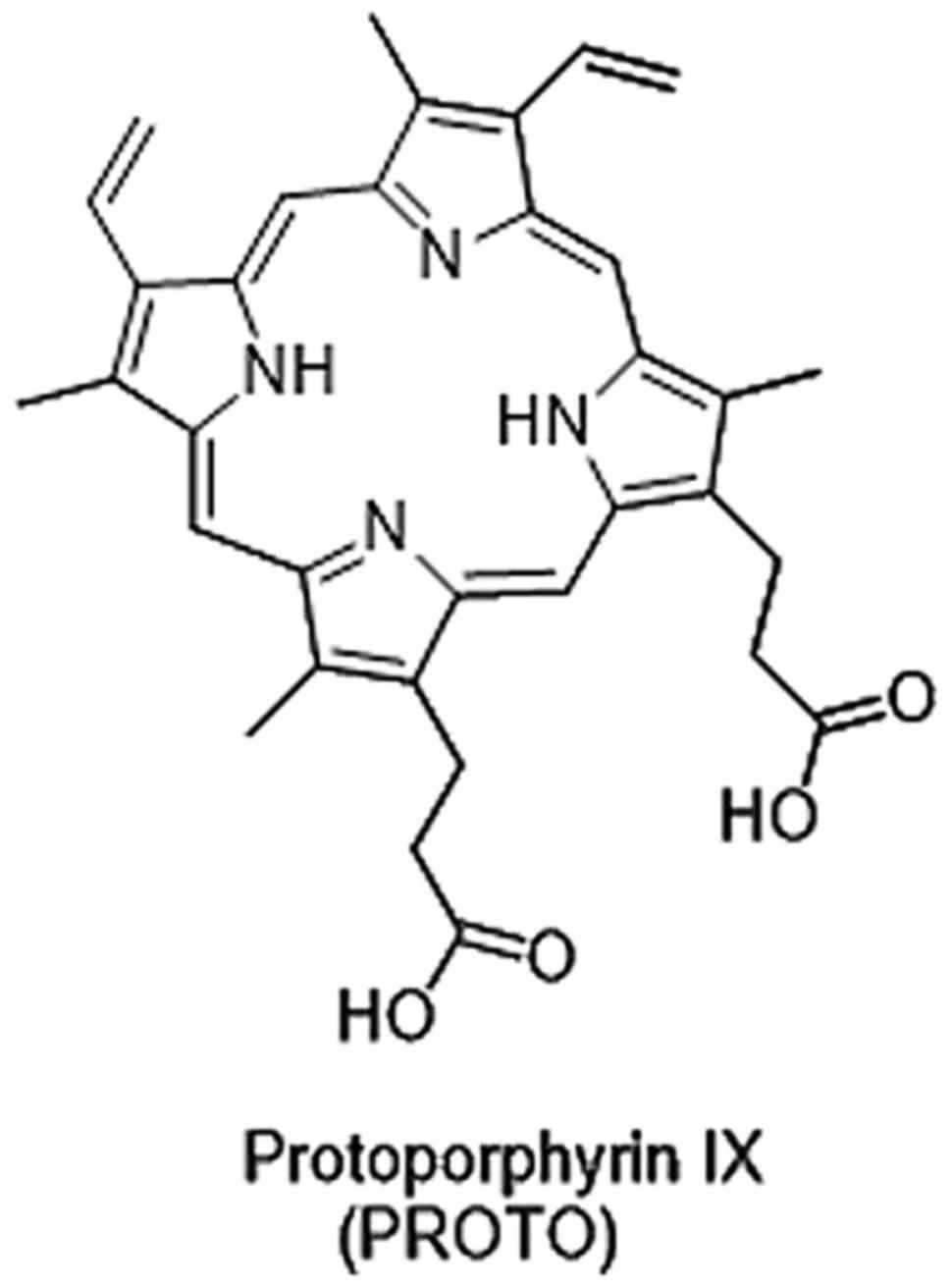
Improved PpIX fluorescence following 5-ALA treatment
is observed in different types of tumor cells and tissues (Fig. 7), validated through a comparison with
a control group (55). Extensive
research has demonstrated that increased PpIX fluorescence in tumor
cells may be the result of influencing various tumor-associated
properties, including heme biosynthesis, mitochondrial function and
changes in porphyrin transporters (56).
The activity and expression profile of enzymes
participating in heme biosynthesis differ between tumor and healthy
cells or tissues. Eight enzymes were included in the heme
biosynthesis pathway (57).
Comparing the expression level of genes or activity of enzymes
involved in heme biosynthesis between tumor, normal cells and
tissues from studies indicated that the following enzymes exhibited
significant differences in activity (21,58–72). The
first enzyme in the heme biosynthesis is called ALA synthase, which
catalyzes the formation of 5-ALA from glycine and succinyl-coenzyme
A (CoA). Following the migration of 5-ALA from the mitochondrial
matrix to the cytoplasm, ALA dehydratase, also referred to as
porphobilinogen synthase, catalyzes the formation of
porphobilinogen (PBG), by combining two molecules of 5-ALA. The
connection of four PBG molecules yielding in hydroxymethyl bilane
is catalyzed by the porphobilinogen deaminase (also known as
hydroxylmethylbilane synthase). Uroporphyrinogen III decarboxylase
(UROD) is involved in the fifth enzymatic step of the heme
biosynthesis pathway, where uroporphyrinogen III is decarboxylated
by UROD giving proporphyrinogen III. Ferrochelatase (FECH)
catalyzes the conversion of PpIX to heme b, making it the last
enzyme utilized in the heme biosynthesis.
In order to make it clear that these enzymes are
differentially expressed in the tumors, Table I (21,58–72)
illustrates the changes observed in gene expression and enzyme
activity linked to the heme biosynthesis pathway of various tumor
tissues compared to normal tissue. The data provided may further be
used a guide aiding the decision as to which tumor types may
exhibit improved surgical results through the use of 5-ALA-mediated
PpIX florescence.
Succinyl-CoA, one of the two starting materials of
the PpIX/heme biosynthesis, is a metabolite produced in the
tricarboxylic acid (TCA) cycle. In order to prevent the
accumulation of metabolites from the TCA cycle, as well as
mitochondrial NADH, a connection between the glucose metabolism,
TCA cycle and the heme biosynthesis has been established (73). That is to say, Succinyl-CoA, a
metabolite produced in the TCA cycle, participated in the first
step of PpIX/heme biosynthesis.
In cancer cells, metabolic reprogramming from the
TCA cycle into aerobic glycolysis, generating glutamine for energy,
may lead to the accumulation of TCA cycle metabolites and the
activation of the heme biosynthesis to remove those metabolites
(74). Activation of heme
biosynthesis may lead to PpIX accumulation due to FECH saturation
(56). Effective 5-ALA absorption
and the transport of different porphyrin metabolites may further
affect the PpIX accumulation in cells. In theory, the improved
5-ALA-PpIX in tumor cells may be triggered by certain processes,
including elevated ALA uptake, improved porphyrin activity and
reduced PpIX activity. An increase in 5-ALA uptake has been
identified through elevated levels of PpIX in tumor cells (75). Furthermore, studies have indicated
that high and low PpIX 5-ALA absorption were not significantly
different between cell lines (64,76,77).
Nakanishi et al (78)
demonstrated that there was no correlation between 5-ALA-induced
PpIX accumulation and the uptake clearance of 5-ALA. The
aforementioned study also revealed that ALA uptake rates were far
greater than maximum conversion rates of ALA to PpIX in LS-180,
T24, A2780, DU145 and MCF-7 cell lines. ALA uptake is not the only
decisive factor to enhance ALA-PpIX fluorescence in tumor cells. A
porphyrin transporter that is postulated to be linked to the
porphyrin synthesis is the adenosine 5′-triphosphate-binding
cassette subgroup B member 6 (ABCB6), which was originally
described as a transporter protein on the outer mitochondrial outer
membrane (79). ABCB6 interacts with
various porphyrins, including coproporphyrinogen III, PpIX and
hemoglobin, with the highest affinity recorded for
coproporphyrinogen III (46).
Therefore, ABCB6 was thought to be primarily involved in the
transporting coproporphyrin III into the mitochondria for PpIX/heme
b synthesis (79). Increased ABCB6
expression has been linked to an increase in fluorescence in human
glioma tissues allowing for better contrast in fluorescence-guided
surgery, via more sufficient PpIX accumulation (80). ABCB6 is located in the cell membrane
and Golgi apparatus, and transports coproporphyrinogen III between
the cellular departments (81–83). An
enhanced ABCB6 function may be observed at increased
coproporphyrinogen III concentration, reducing the intracellular
concentration of PpIX/hemoglobin (46). The net influence of ABCB6 on
5-ALA-PpIX levels in the cells may depend on the relative ABCB6
activity in the mitochondria and cell membranes (56).
In the plasma membrane, ATP-binding cassette
sub-family G member 2 (ABCG2), a transporter, serves the most
important role in transporting PpIX. Studies have demonstrated that
increased ABCG2 activity reduces the intracellular PpIX level
following 5-ALA stimulation, and the cell lines with high ABCG2
expression or activity often exhibit decreased 5-ALA-PpIX
fluorescence (84,85). Robey et al (84) indicated that the use of ABCG2
transport inhibitors would enhance 5-ALA-PpIX fluorescence.
Several NIR fluorescent dyes have been developed and
incorporated, for example with antibodies (86,87),
nanoparticles (88) or encapsulated
within nanomaterials (89,90), to be used as contrast agents for
molecular imaging of different tumors (4). Researchers have identified that
elevated levels of fibroblast activation protein (FAP) in stromal
fibroblasts are associated with aggressive cancer types (91–95). FAP
is a type II salivary glycoprotein with the ability to cleave
biological peptides, including collagen and proteolytic enzymes,
and serve a central role in the aggressiveness of the solid tumors.
FAP is expressed in stromal fibroblasts of several types of cancer,
but not in healthy tissue; it is used as a tumor marker that has
drawn increasing attention (91,96).
Rüger et al (90) linked
anti-single-chain variable fragment directed against FAP antibody
fragments to quenched liposomes, which became a novel fluorescence
diagnostic contrast dye termed anti-FAP-IL. Anti-FAP-IL antibodies
were used to ensure the specificity and fluorescence imaging of FAP
expression cells and tumor muscle fibroblasts in mice
xenotransplantation (96).
Carbohydrate antigen 19.9 (CA19.9) is a ligand of
epithelial leukocyte adhesion molecules and its overexpression has
been found in some malignancies as well as in some non-malignant
conditions (97–101). CA19.9 is an attractive target for
pancreatic ductal adenocarcinoma (PDAC) imaging, due to its high
expression on tumors, compared with healthy pancreatic tissue
(102,103). The usage of CA19.9 as biomarkers
for PDAC led to the identification of several antibodies, including
the characterization of the fully human monoclonal antibody 5B1,
which binds to extracellular epitopes of CA19.9 with low nanomolar
affinity (104–106). So Houghton et al (107) generated three modular tools,
including 89Zr-ssDFO-5B1, ssFL-5B1
and 89Zr-ssdual-5B1. These modular tools can
target CA19.9, which is an important molecule in invasion and
metastasis of many cancers, including PDAC (103). The results revealed that the three
modular tools evaluated displayed excellent uptake in the CA19.9
positive xenograph model of PDAC, indicating that each of them is
likely to improve the detection rates of tumor of patients with
PDAC (107).
Cysteine protease is another biomarker that is
highly upregulated in the tumor cells and the surrounding matrix of
tumor support cells in multiple types of cancers (109). Fluorescent contrast agents that may
be helpful for dynamic monitoring in vivo and used as
imaging contrast agents for FGS may improve the detection rates for
tumors (110). Researchers designed
and synthesized a series of NIR fluorescent probes, using the
latent lysosomotropic effect to promote the cell retention of
protease activation. These probes exhibit tumor-specific retention,
rapid activation kinetics and rapid system distribution.
Furthermore, they may be used to detect multiple types of cancer,
including breast, colon and lung cancer (110).
The most common biomarker used for targeted
fluorescence is folate, a B vitamin involved in metabolic
processes, including DNA and RNA synthesis, epigenetic processes,
cell proliferation and lung adenocarcinoma survival (111). The folate receptor (FR) family
consists of four members, with only FR-α and FR-β displaying high
affinities for folic acid. When expressed in the cavity surface of
polarized epithelial cells, FR-α is able to prevent binding of the
serum folate salt (111–114). The FR-α expression in lung
adenocarcinoma (1–3,000,000 receptor/cancer cells) appears more
connected with serum folic acid than in normal pulmonary epithelial
cells (115–118). For the purpose of diagnosis, FR-α
provides a reasonable molecular target for lung adenocarcinoma. Two
contrast agents, EC17 and OTL38, have been proposed to image
ovarian and lung adenocarcinomas during surgery (12,119).
These agents are similar in that they target FR-α via a folate
ligand. Although EC17 and OTL38 use the same ligand, they have two
different fluorochromes: EC17 contains a fluorescein dye and OTL38
contains a cyanine dye (118).
Fluorescein is in the visible wavelength and the cyanine is in the
NIR range. De Jesus et al (118) revealed that OTL38 appears to have
superior sensitivity and brightness compared to EC17 in a
preclinical testing. This conclusion is consistent with the
accepted belief that NIR dyes exhibit less auto fluorescence and
scattering compared with visible wavelength fluorochromes (118).
Fluorescent contrast agents may guide surgeons in
making real-time decisions during surgery. ICG is an NIR contrast
dye, which was approved by the FDA for clinical use in the USA. It
is a water-soluble organic compound, which may easily penetrate
tissues and cells with an adverse reaction rate of <0.1%. The
EPR influence is the major mechanism by which ICG accumulates in
solid cancer. ICG is processed by the excretory pathways of the
biliary system and may offer superiority in some tumor nodules
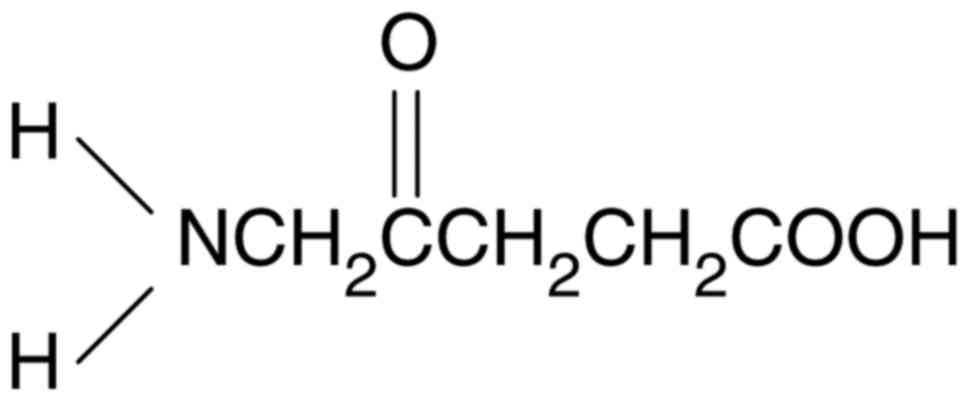
during surgery. 5-ALA is a natural amino acid and a natural prodrug
that metabolizes to the heme precursor PpIX. 5-ALA, through oral
administration, increases the PpIX accumulation in the tumor tissue
and subsequent photosensitizing may guide tumor resection.
Fluorescent dyes have been developed and combined with antibodies
or nanoparticles to function as contrast agents for molecular
imaging by increasing the binding to the target site and providing
more accurate information during tumor resection. Increasing
studies focus on combining these different advantages into one dye,
which it is believed will further the development of fluorescent
contrast agents. Table II provides
a brief summary of targeting abilities and methods of
administration of the three types fluorescent contrast agents
discussed in the current review. Combining these imaging agents for
clinical use may provide more options in tumor surgeries. The
application of contrast agents may significantly improve the
surgery outcome.
Not applicable.
The present study was supported by the Key
Development Plan for Social Development of Jiangsu Province (grant
no. SBE2016750057), and Jiangsu Provincial Key R & D Program
Social Development Clinical Frontier Technology Project
(Application of Image-guided Precise Tumor Surgical Equipment in
Esophageal Cancer Surgery) (grant no. BE2016731).
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
QX contributed to the conception and design of the
study, analysis of data, and drafting and revising of the
manuscript. TC was involved in analyzing the data, as well as
drafting the manuscript and revising it critically for important
intellectual content. SC contributed to the conception of the
study, provided financial support for the paper, gave approval of
the version to be published, and supervised and directed the
research group. All authors agreed to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aliperti LA, Predina JD, Vachani A and
Singhal S: Local and systemic recurrence is the Achilles heel of
cancer surgery. Ann Surg Oncol. 18:603–607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang JX, Keating JJ, Jesus EM, Judy RP,
Madajewski B, Venegas O, Okusanya OT and Singhal S: Optimization of
the enhanced permeability and retention effect for near-infrared
imaging of solid tumors with indocyanine green. Am J Nucl Med Mol
Imaging. 5:390–400. 2015.PubMed/NCBI
|
|
4
|
Tansi FL, Rüger R, Böhm C, Kontermann RE,
Teichgraeber UK, Fahr A and Hilger I: Potential of activatable
FAP-targeting immunoliposomes in intraoperative imaging of
spontaneous metastases. Biomaterials. 88:70–82. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fedor D, Johnson WR and Singhal S: Local
recurrence following lung cancer surgery: Incidence, risk factors,
and outcomes. Surg Oncol. 22:156–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zaman M, Bilal H, Woo CY and Tang A: In
patients undergoing video-assisted thoracoscopic surgery excision,
what is the best way to locate a subcentimetre solitary pulmonary
nodule in order to achieve successful excision? Interact Cardiovasc
Thorac Surg. 15:266–272. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chella A, Lucchi M, Ambrogi MC, Menconi G,
Melfi FM, Gonfiotti A, Boni G and Angeletti CA: A pilot study of
the role of TC-99 radionuclide in localization of pulmonary nodular
lesions for thoracoscopic resection. Eur J Cardiothoracic Surg.
18:17–21. 2000. View Article : Google Scholar
|
|
8
|
Powell TI, Jangra D, Clifton JC,
Lara-Guerra H, Church N, English J, Evans K, Yee J, Coxson H, Mayo
JR and Finley RJ: Peripheral lung nodules: Fluoroscopically guided
video-assisted thoracoscopic resection after computed
tomography-guided localization using platinum microcoils. Ann Surg.
240:481–489. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eichfeld U, Dietrich A, Ott R and Kloeppel
R: Video-assisted thoracoscopic surgery for pulmonary nodules after
computed tomography-guided marking with a spiral wire. Ann Thorac
Surg. 79:313–317. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Licha K, Riefke B, Ebert B and Grötzinger
C: Cyanine dyes as contrast agents in biomedical optical imaging.
Acad Radiol. 9 Suppl 2:S320–S322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luker GD and Luker KE: Optical imaging:
Current applications and future directions. J Nucl Med. 49:1–4.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Dam GM, Themelis G, Crane LM, Harlaar
NJ, Pleijhuis RG, Kelder W, Sarantopoulos A, de Jong JS, Arts HJ,
van der Zee AG, et al: Intraoperative tumor-specific fluorescence
imaging in ovarian cancer by folate receptor-α targeting: First
in-human results. Nat Med. 17:1315–1319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Polom K, Murawa D, Rho YS, Nowaczyk P,
Hünerbein M and Murawa P: Current trends and emerging future of
indocyanine green usage in surgery and oncology: A literature
review. Cancer. 117:4812–4822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Leeuwen FW, Hardwick JC and van Erkel
AR: Luminescence-based imaging approaches in the field of
interventional molecular imaging. Radiol. 276:12–29. 2015.
View Article : Google Scholar
|
|
15
|
KleinJan GH, Bunschoten A, van den Berg
NS, Olmos RA, Klop WM, Horenblas S, van der Poel HG, Wester HJ and
van Leeuwen FW: Fluorescence guided surgery and tracer-dose, fact
or fiction? Eur J Nucl Med Mol Imaging. 43:1857–1867. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brülisauer M, Moneta G, Jager K and
Bollinger A: Infrared fluorescence videomicroscopy with indocyanine
green (Cardiogreen). Adv Exp Med Biol. 220:219–221. 1987.PubMed/NCBI
|
|
17
|
Chen CY, Fancher RM, Ruan Q, Marathe P,
Rodrigues AD and Yang Z: A liquid chromatography tandem mass
spectrometry method for the quantification of indocyanine green in
dog plasma and bile. J Pharm Biomed Anal. 47:351–359. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Engel E, Schraml R, Maisch T, Kobuch K,
König B, Szeimies RM, Hillenkamp J, Bäumler W and Vasold R:
Light-induced decomposition of indocyanine green. Invest Ophthalmol
Vis Sci. 49:1777–1783. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van der Vorst JR, Schaafsma BE, Hutteman
M, Verbeek FP, Liefers GJ, Hartgrink HH, Smit VT, Löwik CW, van de
Velde CJ, Frangioni JV and Vahrmeijer AL: Near-infrared
fluorescence-guided resection of colorectal liver metastases.
Cancer. 119:3411–3418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yokoyama N, Otani T, Hashidate H, Maeda C,
Katada T, Sudo N, Manabe S, Ikeno Y, Toyoda A and Katayanagi N:
Real-time detection of hepatic micrometastases from pancreatic
cancer by intraoperative fluorescence imaging: Preliminary results
of a prospective study. Cancer. 118:2813–2819. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rajaraman P, Schwartz BS, Rothman N,
Yeager M, Fine HA, Shapiro WR, Selker RG, Black PM and Inskip PD:
Delta-aminolevulinic acid dehydratase polymorphism and risk of
brain tumors in adults. Environ Health Perspect. 113:1209–1211.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iida G, Asano K, Seki M, Ishigaki K,
Teshima K, Yoshida O, Edamura K and Kagawa Y: Intraoperative
identification of canine hepatocellular carcinoma with indocyanine
green fluorescent imaging. J Small Anim Pract. 54:594–600. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gotoh K, Yamada T, Ishikawa O, Takahashi
H, Eguchi H, Yano M, Ohigashi H, Tomita Y, Miyamoto Y and Imaoka S:
A novel image-guided surgery of hepatocellular carcinoma by
indocyanine green fluorescence imaging navigation. J Surg Oncol.
100:75–79. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishizawa T, Masuda K, Urano Y, Kawaguchi
Y, Satou S, Kaneko J, Hasegawa K, Shibahara J, Fukayama M, Tsuji S,
et al: Mechanistic background and clinical applications of
indocyanine green fluorescence imaging of hepatocellular carcinoma.
Ann Surg Oncol. 21:440–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cherrick GR, Stein SW, Leevy CM and
Davidson CS: Indocyanine green: Observations on its physical
properties, plasma decay, and hepatic extraction. J Clin Invest.
39:592–600. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cornelius CE, Ben-Ezzer J and Arias IM:
Binding of sulfobromophthalein sodium (BSP) and other organic
anions by isolated hepatic cell plasma membranes in vitro.
Proc Soc Exp Biol Med. 124:665–667. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hunton DB, Bollman JL and Hoffman HN:
Studies of hepatic function with indocyanine green.
Gastroenterology. 39:713–724. 1960.PubMed/NCBI
|
|
28
|
Leevy CM and Bender J: Physiology of dye
extraction by the liver: Comparative studies of sulfobromophthalein
and indocyanine green. Ann NY Acad Sci. 111:161–176. 1963.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shibasaki Y, Sakaguchi T, Hiraide T,
Morita Y, Suzuki A, Baba S, Setou M and Konno H: Expression of
indocyanine green-related transporters in hepatocellular carcinoma.
J Surg Res. 193:567–576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Holt D, Okusanya O, Judy R, Venegas O,
Jiang J, DeJesus E, Eruslanov E, Quatromoni J, Bhojnagarwala P,
Deshpande C, et al: Intraoperative near-infrared imaging can
distinguish cancer from normal tissue but not inflammation. PLoS
One. 9:e1033422014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kosaka N, Mitsunaga M, Longmire MR, Choyke
PL and Kobayashi H: Near infrared fluorescence-guided real-time
endoscopic detection of peritoneal ovarian cancer nodules using
intravenously injected indocyanine green. Int J Cancer.
129:1671–1677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maeda H, Nakamura H and Fang J: The EPR
effect for macromolecular drug delivery to solid tumors:
Improvement of tumor uptake, lowering of systemic toxicity, and
distinct tumor imaging in vivo. Adv Drug Deliv Rev. 65:71–79. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Madajewski B, Judy BF, Mouchli A, Kapoor
V, Holt D, Wang MD, Nie S and Singhal S: Intraoperative
near-infrared imaging of surgical wounds after tumor resections can
detect residual disease. Clin Cancer Res. 18:5741–5751. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shin EH, Li Y, Kumar U, Sureka HV, Zhang X
and Payne CK: Membrane potential mediates the cellular binding of
nanoparticles. Nanoscale. 5:5879–5886. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: Mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46:6387–6392. 1986.PubMed/NCBI
|
|
36
|
Heneweer C, Holland JP, Divilov V, Carlin
S and Lewis JS: Magnitude of enhanced permeability and retention
effect in tumors with different phenotypes: 89Zr-albumin as a model
system. J Nucl Med. 52:625–633. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fang C, Wang K, Zeng C, Chi C, Shang W, Ye
J, Mao Y, Fan Y, Yang J, Xiang N, et al: Illuminating necrosis:
From mechanistic exploration to preclinical application using
fluorescence molecular imaging with indocyanine green. Sci Rep.
6:210132016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hyun H, Park MH, Owens EA, Wada H, Henary
M, Handgraaf HJ, Vahrmeijer AL, Frangioni JV and Choi HS:
Structure-inherent targeting of near-infrared fluorophores for
parathyroid and thyroid gland imaging. Nat Med. 21:192–197. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoneya S, Saito T, Komatsu Y, Koyama I,
Takahashi K and Duvoll-Young J: Binding properties of indocyanine
green in human blood. Invest Ophthalmol Vis Sci. 39:1286–1290.
1998.PubMed/NCBI
|
|
40
|
Baker KJ: Binding of sulfobromophthalein
(BSP) sodium and indocyanine green (ICG) by plasma alpha-1
lipoproteins. Proc Soc Exp Biol Med. 122:957–963. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Janecki J and Krawcynski J: Labeling with
indocyanine green of serum protein from normal persons and patients
with acute viral hepatitis. Clin Chem. 16:1008–1011.
1970.PubMed/NCBI
|
|
42
|
Desmettre T, Devoisselle JM and Mordon S:
Fluorescence properties and metabolic features of indocyanine green
(ICG) as related to angiography. Surv Ophthalmol. 45:15–27. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Onda N, Kimura M, Yoshida T and Shibutani
M: Preferential tumor cellular uptake and retention of indocyanine
green for in vivo tumor imaging. Int J Cancer. 139:673–682. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
von Kleist L, Stahlschmidt W, Bulut H,
Gromova K, Puchkov D, Robertson MJ, MacGregor KA, Tomilin N,
Pechstein A, Chau N, et al: Role of the clathrin terminal domain in
regulating coated pit dynamics revealed by small molecule
inhibition. Cell. 146:471–484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kaibori M, Matsui K, Ishizaki M, Iida H,
Okumura T, Sakaguchi T, Inoue K, Ikeura T, Asano H and Kon M:
Intraoperative detection of superficial liver tumors by
fluorescence imaging using indocyanine green and 5-aminolevulinic
acid. Anticancer Res. 36:1841–1849. 2016.PubMed/NCBI
|
|
46
|
Hill TK, Abdulahad A, Kelkar SS, Marini
FC, Long TE, Provenzale JM and Mohs AM: Indocyanine green-loaded
nanoparticles for image-guided tumor surgery. Bioconjug Chem.
26:294–303. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ishizuka M, Abe F, Sano Y, Takahashi K,
Inoue K, Nakajima M, Kohda T, Komatsu N, Ogura S and Tanaka T:
Novel development of 5-aminolevurinic acid (ALA) in cancer
diagnoses and therapy. Int Immunopharmacol. 11:358–365. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nakamura M, Nishikawa J, Hamabe K, Goto A,
Nishimura J, Shibata H, Nagao M, Sasaki S, Hashimoto S, Okamoto T
and Sakaida I: Preliminary study of photodynamic diagnosis using
5-aminolevulinic acid in gastric and colorectal tumors. World J
Gastroenterol. 21:6706–6712. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Leroy HA, Vermandel M, Lejeune JP, Mordon
S and Reyns N: Fluorescence guided resection and glioblastoma in
2015: A review. Lasers Surg Med. 47:441–451. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kitada M, Ohsaki Y, Matsuda Y, Hayashi S
and Ishibashi K: Photodynamic diagnosis of pleural malignant
lesions with a combination of 5-aminolevulinic acid and intrinsic
fluorescence observation systems. BMC Cancer. 15:1742015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Friesen SA, Hjortland GO, Madsen SJ,
Hirschberg H, Engebraten O, Nesland JM and Peng Q: 5-Aminolevulinic
acid-based photodynamic detection and therapy of brain tumors
(review). Int J Oncol. 21:577–582. 2002.PubMed/NCBI
|
|
52
|
Colditz MJ and Jeffree RL: Aminolevulinic
acid (ALA)-protoporphyrin IX fluorescence guided tumour resection.
Part 1: Clinical, radiological and pathological studies. J Clin
Neurosci. 19:1471–1474. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Colditz MJ, Leyen K and Jeffree RL:
Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided
tumour resection. Part 2: Theoretical, biochemical and practical
aspects. J Clin Neurosci. 19:1611–1616. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Eljamel S: 5-ALA fluorescence image guided
resection of glioblastoma multiforme: A meta-analysis of the
literature. Int J Mol Sci. 16:10443–10456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Nokes B, Apel M, Jones C, Brown G and Lang
JE: Aminolevulinic acid (ALA): Photodynamic detection and potential
therapeutic applications. J Surg Res. 181:262–271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang X, Palasuberniam P, Kraus D and Chen
B: Aminolevulinic acid-based tumor detection and therapy: Molecular
mechanisms and strategies for enhancement. Int J Mol Sci.
16:25865–25880. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ponka P: Cell biology of heme. Am J Med
Sci. 318:241–256. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kemmner W, Wan K, Rüttinger S, Ebert B,
Macdonald R, Klamm U and Moesta KT: Silencing of human
ferrochelatase causes abundant protoporphyrin-IX accumulation in
colon cancer. FASEB J. 22:500–509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hooda J, Cadinu D, Alam MM, Shah A, Cao
TM, Sullivan LA, Brekken R and Zhang L: Enhanced heme function and
mitochondrial respiration promote the progression of lung cancer
cells. PloS One. 8:e634022013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gonçalves TL, Erthal F, Corte CL, Müller
LG, Piovezan CM, Nogueira CW and Rocha JB: Involvement of oxidative
stress in the pre-malignant and malignant states of cervical cancer
in women. Clin Biochem. 38:1071–1075. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Neslund-Dudas C, Levin AM, Rundle A,
Beebe-Dimmer J, Bock CH, Nock NL, Jankowski M, Datta I, Krajenta R,
Dou QP, et al: Case-only gene-environment interaction between ALAD
tagSNPs and occupational lead exposure in prostate cancer.
Prostate. 74:637–646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Navone NM, Polo CF, Frisardi AL, Andrade
NE and Battle AM: Heme biosynthesis in human breast cancer-mimetic
‘in vitro’ studies and some heme enzymic activity levels. Int J
Biochem. 22:1407–1411. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Krieg RC, Fickweiler S, Wolfbeis OS and
Knuechel R: Cell-type specific protoporphyrin IX metabolism in
human bladder cancer in vitro. Photochem Photobiol.
72:226–233. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Krieg RC, Messmann H, Rauch J, Seeger S
and Knuechel R: Metabolic characterization of tumor cell-specific
protoporphyrin IX accumulation after exposure to 5-aminolevulinic
acid in human colonic cells. Photochem Photobiol. 76:518–525. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hinnen P, de Rooij FW, van Velthuysen ML,
Edixhoven A, van Hillegersberg R, Tilanus HW, Wilson JH and
Siersema PD: Biochemical basis of 5-aminolaevulinic acid-induced
protoporphyrin IX accumulation: A study in patients with
(pre)malignant lesions of the oesophagus. Br J Cancer. 78:679–682.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hinnen P, de Rooij FW, Terlouw EM,
Edixhoven A, van Dekken H, van Hillegersberg R, Tilanus HW, Wilson
JH and Siersema PD: Porphyrin biosynthesis in human Barrett's
oesophagus and adenocarcinoma after ingestion of 5-aminolaevulinic
acid. Br J Cancer. 83:539–543. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Misawa Y, Tojo A and Shibuya M: Isolation
of genes highly expressed in early and late stages of Friend
virus-induced erythroleukemia in mice. Biochem Biophys Res Commun.
170:39–45. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ito E, Yue S, Moriyama EH, Hui AB, Kim I,
Shi W, Alajez NM, Bhogal N, Li G, Datti A, et al: Uroporphyrinogen
decarboxylase is a radiosensitizing target for head and neck
cancer. Sci Transl Med. 3:67ra672011. View Article : Google Scholar
|
|
69
|
Dailey HA and Smith A: Differential
interaction of porphyrins used in photoradiation therapy with
ferrochelatase. Biochem J. 223:441–445. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Miyake M, Ishii M, Kawashima K, Kodama T,
Sugano K, Fujimoto K and Hirao Y: siRNA-mediated knockdown of the
heme synthesis and degradation pathways: Modulation of treatment
effect of 5-aminolevulinic acid-based photodynamic therapy in
urothelial cancer cell lines. Photochem Photobiol. 85:1020–1027.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Teng L, Nakada M, Zhao SG, Endo Y,
Furuyama N, Nambu E, Pyko IV, Hayashi Y and Hamada JI: Silencing of
ferrochelatase enhances 5-aminolevulinic acid-based fluorescence
and photodynamic therapy efficacy. Br J Cancer. 104:798–807. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang X, Li W, Palasuberniam P, Myers KA,
Wang C and Chen B: Effects of silencing heme biosynthesis enzymes
on 5-aminolevulinic acid-mediated protoporphyrin IX fluorescence
and photodynamic therapy. Photochem Photobiol. 91:923–930. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Frezza C, Zheng L, Folger O, Rajagopalan
KN, MacKenzie ED, Jerby L, Micaroni M, Chaneton B, Adam J, Hedley
A, et al: Haem oxygenase is synthetically lethal with the tumour
suppressor fumarate hydratase. Nature. 477:225–228. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ward PS and Thompson CB: Metabolic
reprogramming: A cancer hallmark even warburg did not anticipate.
Cancer Cell. 21:297–308. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ohgari Y, Nakayasu Y, Kitajima S, Sawamoto
M, Mori H, Shimokawa O, Matsui H and Taketani S: Mechanisms
involved in delta-aminolevulinic acid (ALA)-induced
photosensitivity of tumor cells: Relation of ferrochelatase and
uptake of ALA to the accumulation of protoporphyrin. Biochem
Pharmacol. 71:42–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Gibson SL, Nguyen ML, Havens JJ, Barbarin
A and Hilf R: Relationship of delta-aminolevulinic acid-induced
protoporphyrin IX levels to mitochondrial content in neoplastic
cells in vitro. Biochem Biophys Res Commun. 265:315–321.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gibson SL, Havens JJ, Foster TH and Hilf
R: Time-dependent intracellular accumulation of
delta-aminolevulinic acid, induction of porphyrin synthesis and
subsequent phototoxicity. Photochem Photobiol. 65:416–421. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nakanishi T, Ogawa T, Yanagihara C and
Tamai I: Kinetic evaluation of determinant factors for cellular
accumulation of protoporphyrin IX induced by external
5-aminolevulinic acid for photodynamic cancer therapy. J Pharm Sci.
104:3092–3100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Krishnamurthy PC, Du G, Fukuda Y, Sun D,
Sampath J, Mercer KE, Wang J, Sosa-Pineda B, Murti KG and Schuetz
JD: Identification of a mammalian mitochondrial porphyrin
transporter. Nature. 443:586–589. 2006.PubMed/NCBI
|
|
80
|
Zhao SG, Chen XF, Wang LG, Yang G, Han DY,
Teng L, Yang MC, Wang DY, Shi C, Liu YH, et al: Increased
expression of ABCB6 enhances protoporphyrin IX accumulation and
photodynamic effect in human glioma. Ann Surg Oncol. 20:4379–4388.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Paterson JK, Shukla S, Black CM, Tachiwada
T, Garfield S, Wincovitch S, Ernst DN, Agadir A, Li X, Ambudkar SV,
et al: Human ABCB6 localizes to both the outer mitochondrial
membrane and the plasma membrane. Biochemistry. 46:9443–9452. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Tsuchida M, Emi Y, Kida Y and Sakaguchi M:
Human ABC transporter isoform B6 (ABCB6) localizes primarily in the
Golgi apparatus. Biochem Biophys Res Commun. 369:369–375. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Matsumoto K, Hagiya Y, Endo Y, Nakajima M,
Ishizuka M, Tanaka T and Ogura S: Effects of plasma membrane ABCB6
on 5-aminolevulinic acid (ALA)-induced porphyrin accumulation in
vitro: Tumor cell response to hypoxia. Photodiagnosis Photodyn
Ther. 12:45–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Robey RW, Steadman K, Polgar O and Bates
SE: ABCG2-mediated transport of photosensitizers: Potential impact
on photodynamic therapy. Cancer Biol Ther. 4:187–194. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Barron GA, Moseley H and Woods JA:
Differential sensitivity in cell lines to photodynamic therapy in
combination with ABCG2 inhibition. J Photochem Photobiol B.
126:87–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ogawa M, Kosaka N, Choyke PL and Kobayashi
H: H-type dimer formation of fluorophores: A mechanism for
activatable, in vivo optical molecular imaging. ACS Chem Biol.
4:535–546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tansi F, Kallweit E, Kaether C, Kappe K,
Schumann C, Hilger I and Reissmann S: Internalization of
near-infrared fluorescently labeled activatable cell-penetrating
peptide and of proteins into human fibrosarcoma cell line HT-1080.
J Cell Biochem. 116:1222–1231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Rizzo LY, Theek B, Storm G, Kiessling F
and Lammers T: Recent progress in nanomedicine: Therapeutic,
diagnostic and theranostic applications. Curr Opin Biotechnol.
24:1159–1166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tansi FL, Rüger R, Rabenhold M, Steiniger
F, Fahr A, Kaiser WA and Hilger I: Liposomal encapsulation of a
near-infrared fluorophore enhances fluorescence quenching and
reliable whole body optical imaging upon activation in vivo. Small.
9:3659–3669. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Rüger R, Tansi FL, Rabenhold M, Steiniger
F, Kontermann RE, Fahr A and Hilger I: In vivo near-infrared
fluorescence imaging of FAP-expressing tumors with activatable
FAP-targeted, single-chain Fv-immunoliposomes. J Control Release.
186:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kalluri R and Zeisberg M: Fibroblasts in
cancer. Nat Rev Cancer. 6:392–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Huang Y, Simms AE, Mazur A, Wang S, León
NR, Jones B, Aziz N and Kelly T: Fibroblast activation
protein-alpha promotes tumor growth and invasion of breast cancer
cells through non-enzymatic functions. Clin Exp Metastasis.
28:567–579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Lee HO, Mullins SR, Franco-Barraza J,
Valianou M, Cukierman E and Cheng JD: FAP-overexpressing
fibroblasts produce an extracellular matrix that enhances invasive
velocity and directionality of pancreatic cancer cells. BMC Cancer.
11:2452011. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhi K, Shen X, Zhang H and Bi J:
Cancer-associated fibroblasts are positively correlated with
metastatic potential of human gastric cancers. J Exp Clin Cancer
Res. 29:662010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tommelein J, Verset L, Boterberg T,
Demetter P, Bracke M and De Wever O: Cancer-associated fibroblasts
connect metastasis-promoting communication in colorectal cancer.
Front Oncol. 5:632015. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Garin-Chesa P, Old LJ and Rettig WJ: Cell
surface glycoprotein of reactive stromal fibroblasts as a potential
antibody target in human epithelial cancers. Proc Natl Acad Sci
USA. 87:7235–7239. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Albert MB, Steinberg WM and Henry JP:
Elevated serum levels of tumor marker CA19-9 in acute cholangitis.
Dig Dis Sci. 33:1223–1225. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Benamouzig R, Buffet C, Fourre C, Ink O,
Moati F and Etienne JP: Serum levels of carbohydrate antigenic
determinant (CA 19.9) in obstructive jaundice. Dig Dis Sci.
34:1640–1642. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Encabo G and Ruibal A: Seric CA 19.9
levels in patients with non tumoral pathologies. Our experience in
892 cases. Bull Cancer. 73:256–259. 1986.PubMed/NCBI
|
|
100
|
Gupta MK, Arciaga R, Bocci L, Tubbs R,
Bukowski R and Deodhar SD: Measurement of a
monoclonal-antibody-defined antigen (CA19-9) in the sera of
patients with malignant and nonmalignant diseases. Comparison with
carcinoembryonic antigen. Cancer. 56:277–283. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Haglund C, Roberts PJ, Jalanko H and
Kuusela P: Tumour markers CA 19-9 and CA 50 in digestive tract
malignancies. Scand J Gastroenterol. 27:169–174. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Loy TS, Sharp SC, Andershock CJ and Craig
SB: Distribution of CA 19-9 in adenocarcinomas and transitional
cell carcinomas. An immunohistochemical study of 527 cases. Am J
Clin Pathol. 99:726–728. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Makovitzky J: The distribution and
localization of the monoclonal antibody-defined antigen 19-9
(CA19-9) in chronic pancreatitis and pancreatic carcinoma. An
immunohistochemical study. Virchows Arch B Cell Pathol Incl Mol
Pathol. 51:535–544. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Magnani JL, Steplewski Z, Koprowski H and
Ginsburg V: Identification of the gastrointestinal and pancreatic
cancer-associated antigen detected by monoclonal antibody 19-9 in
the sera of patients as a mucin. Cancer Res. 43:5489–5492.
1983.PubMed/NCBI
|
|
105
|
Girgis MD, Kenanova V, Olafsen T, McCabe
KE, Wu AM and Tomlinson JS: Anti-CA19-9 diabody as a PET imaging
probe for pancreas cancer. J Surg Res. 170:169–178. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Sawada R, Sun SM, Wu X, Hong F, Ragupathi
G, Livingston PO and Scholz WW: Human monoclonal antibodies to
sialyl-Lewis (CA19.9) with potent CDC, ADCC, and antitumor
activity. Clin Cancer Res. 17:1024–1032. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Houghton JL, Zeglis BM, Abdel-Atti D,
Aggeler R, Sawada R, Agnew BJ, Scholz WW and Lewis JS:
Site-specifically labeled CA19.9-targeted immunoconjugates for the
PET, NIRF, and multimodal PET/NIRF imaging of pancreatic cancer.
Proc Natl Acad Sci USA. 112:15850–15855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Li CH, Kuo TR, Su HJ, Lai WY, Yang PC,
Chen JS, Wang DY, Wu YC and Chen CC: Fluorescence-guided probes of
aptamer-targeted gold nanoparticles with computed tomography
imaging accesses for in vivo tumor resection. Sci Rep. 5:156752015.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Góra J and Latajka R: Involvement of
cysteine proteases in cancer. Curr Med Chem. 22:944–957. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ofori LO, Withana NP, Prestwood TR,
Verdoes M, Brady JJ, Winslow MM, Sorger J and Bogyo M: Design of
protease activated optical contrast agents that exploit a latent
lysosomotropic effect for use in fluorescence-guided surgery. ACS
Chem Biol. 10:1977–1988. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Kelemen LE: The role of folate receptor
alpha in cancer development, progression and treatment: Cause,
consequence or innocent bystander? Int J Cancer. 119:243–250. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Low PS and Antony AC: Folate
receptor-targeted drugs for cancer and inflammatory diseases. Adv
Drug Deliv Rev. 56:1055–1058. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Low PS, Henne WA and Doorneweerd DD:
Discovery and development of folic-acid-based receptor targeting
for imaging and therapy of cancer and inflammatory diseases. Acc
Chem Res. 41:120–129. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Low PS and Kularatne SA: Folate-targeted
therapeutic and imaging agents for cancer. Curr Opin Chem Biol.
13:256–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lu Y, Sega E and Low PS: Folate
receptor-targeted immunotherapy: Induction of humoral and cellular
immunity against hapten-decorated cancer cells. Int J Cancer.
116:710–719. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lu Y, Xu LC, Parker N, Westrick E, Reddy
JA, Vetzel M, Low PS and Leamon CP: Preclinical pharmacokinetics,
tissue distribution, and antitumor activity of a folate-hapten
conjugate-targeted immunotherapy in hapten-immunized mice. Mol
Cancer Ther. 5:3258–3267. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
O'Shannessy DJ, Yu G, Smale R, Fu YS,
Singhal S, Thiel RP, Somers EB and Vachani A: Folate receptor alpha
expression in lung cancer: Diagnostic and prognostic significance.
Oncotarget. 3:414–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
De Jesus E, Keating JJ, Kularatne SA,
Jiang J, Judy R, Predina J, Nie S, Low P and Singhal S: Comparison
of folate receptor targeted optical contrast agents for
intraoperative molecular imaging. Int J Mol Imaging.
2015:4690472015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Srinivasarao M, Galliford CV and Low PS:
Principles in the design of ligand-targeted cancer therapeutics and
imaging agents. Nat Rev Drug Discov. 14:203–219. 2015. View Article : Google Scholar : PubMed/NCBI
|