Introduction
Malignant melanoma (MM) is a common malignant tumor
of the skin that is increasing in incidence (1). MM, developed from moles or pigment
spots, is fast growing and presents with a poor prognosis and a
high mortality (2). Additionally, MM
is more prevalent in adults (age, 18–60 years) and has an average
survival period of ~30.3 months (2)
due to it being prone to metastasis and recurrence (3,4). At
present, the primary method of MM treatment is surgery as the tumor
grows rapidly, meaning that it is often found in the growth
(5). In cases where no distant
metastasis is identified prior to surgery, MM may be fully resected
with tumor margins removed; however, recurrence and metastasis may
still result following surgery (6,7).
Furthermore, postoperative adjuvant antitumor therapy has little
effect on the prognosis of the disease (8). In addition to its strong propensity
towards invasion and metastasis, MM is not sensitive to traditional
anti-tumor therapy, including surgical excision (9). It has been demonstrated that signal
transduction networks, particularly the Wnt/β-catenin pathway,
serve crucial roles in the pathogenesis of MM (9). Previous studies have demonstrated that
Wnt/n-catenin signaling serves a vital role in embryonic
development, cell differentiation and proliferation and in the
self-renewing capacity of stem and progenitor cells (10–12). In
addition, it has been indicated that the Wnt/β-catenin pathway is
involved in the regulation of neural crest melanophore formation
and melanoblast development (13,14). The
present study therefore examined the influence of β-catenin
downregulation on the biological behavior of MM cells in
vitro.
Materials and methods
Cell culture and transfection
The human MM A375 cell line was purchased from the
Biovector Science Lab, Inc. (Beijing, China). There were 3 groups
included in the present study: The experimental group consisted of
interfering lentivirus infected A375 cells, marked as A375-RNAi;
the negative control group consisted of A375 cells infected using a
lentivirus empty vector, marked as A375-negative and the blank
control group consisted of uninfected A375 cells, marked as A375.
MM A375 cells were cultured in Dulbecco's Modified Eagle's medium
(DMEM; Beijing Transgen Biotech Co., Ltd., Beijing, China)
containing 1% penicillin, streptomycin and 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and
incubated at 37°C and 5% CO2. The medium was replenished
every 2–3 days depending on cell growth, which was monitored using
an inverted microscope. When 80–90% confluence was reached, cells
were passaged. To achieve this, the medium was carefully discarded
and the cells were washed 2–3 times with PBS prior to the addition
of 1 ml 0.25% pancreatin (Shanghai Suer Biotechnology Co., Ltd.,
Shanghai, China). Cells were observed using an inverted microscope
(magnification, ×20) and when the cells had become rounded in shape
but remained attached to the cell wall, 2 ml complete medium
(Dulbecco's modified Eagles' medium; DMEM; Beijing Transgen Biotech
Co., Ltd.) was added to terminate digestion. A 3 ml sterile straw
was then used to lightly agitate the bottom of the Culture bottle
of the culture flask to detach cells from their cell walls. A 3 ml
sterile straw was utilized to gently blow the cell culture at the
bottom wall of the flask, to ensure for complete wall
separation.
The cell suspension was then centrifuged for 3 min
at 252 × g 37°C and the supernatant was then removed. A total of 3
ml complete medium was used to re-suspend the cells and an
appropriate quantity of medium was subsequently added to the
culture flask at a ratio of 1:3. (Cells were then marked, cultured
and incubated. Following incubation, cells were then counted using
a hemocytometer (Shanghai Biochemical Reagent Refinement Instrument
Co., Ltd., Shanghai, China).) and sterile glass coverslips, on
which the cells were mounted on. A total of four fields were
counted. The following formula was used to calculate the total
number of cells: Total cellular score of the counting block
grids/4×104 (cells/ml). Lentivirus small interfering RNA
(siRNA) packaging was completed by Cyagen Biosciences Inc. (Santa
Clara, CA, USA). The interference sequences of β-catenin
interfering lentivirus liquid (β-catenin-RNAi-LV) and β-catenin
empty vector lentivirus liquid (β-catenin-negative-LV; negative
control) utilized in the present study were
5′-GGATGTGGATACCTCCCAAGT-3′ and 5′-TTCTCCGAACGTGTCACGT-3′,
respectively, the latter being used as a control. The multiplicity
of infection value of infected A375 cells was ~100. siRNA was
transfected as a volume of 100 µl and the time interval between
transfection and subsequent experimentation was ٢٤ h. Cells were
then planted in the 24 hole culture medium; Shanghai Bai Li
Biological, Shanghai, China) one day in advance, at a confluence of
30% with 0.45 ml of 24 hole culture medium. Using the SiRNA living
transfection kit (Guangzhou Bobai Trading Co., Ltd., Guangzhou,
China) (http://www.chem17.com/st301933/Product_21772496.html)
efficiency was calculated using the formula: (Number of green
fluorescent cells/total number of cells) ×100. A375 cells in the
logarithmic growth phase were harvested and counted, and the cell
suspension was diluted to 1×104/ml. The A375 cells that
demonstrated good growth (determined by the absence of suspension
or microsuspension in the culture medium and even growth of cells)
were routinely processed and counted, and the cell suspension was
diluted to 1×104/ml. Following dilution, cells were
seeded in three wells of a 6-well plate plate. A375 cells were
incubated in one well, A375 cells + β-catenin-negative-LV were
incubated in a second well and A375 cells + β-catenin-RNAi-LV were
incubated in a third well. Each well contained 2×104
cells in 2 ml complete culture medium. Then, the cells were placed
in an orifice plate (consisting of 3 holes with A375 seeded into A,
A375 cell + β-catenin-negative-LV into B and A375 cell +
β-catenin-RNAi-LV into hole C; invitrogen; Thermo Fisher
Scientific, Inc.). When cell confluence reached 30–40%, cells were
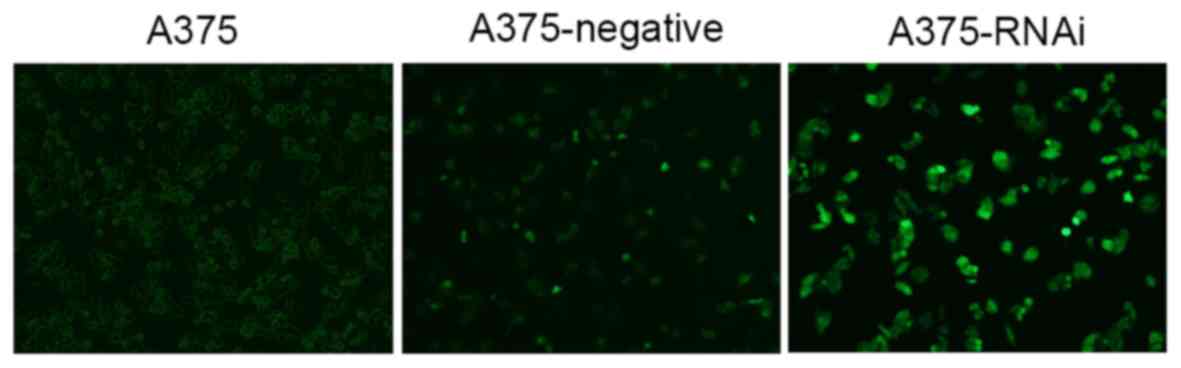
infected and observed using a fluorescent microscope
(magnification, ×200). (Shenzhen Topco Industrial Co., Ltd.,
Senzhen, China). Each of the three cell groups were observed 4 days
following treatment using a fluorescent microscope (Shenzhen Topco
Industrial Co., Ltd., Shenzhen, China) At 4 days following
transfection, the level of fluorescence was observed under an
inverted fluorescence microscope. When the transfection efficiency
was >70%, cells in each of the three groups were passaged and
cultured for 24 h at 5% CO2 and 37°C for use in
subsequent experiments.
Western blotting
Cells in each group included in the current study
were first digested and centrifuged. The supernatant was then
removed and cells were re-suspended. Cells were inoculated in 6-cm
cell culture dishes. During total cell protein extraction, cells
were centrifuged at a speed of 36,800 × g for 10 min at 4°C to
analyze the expression of β-catenin and β-actin. Following gel
electrophoresis, the gel was cut between 34 and 130 kDa to
determine the interlude of the marker. The separated proteins were
subsequently transferred onto a nitrocellulose membrane. The
constant current was adjusted to 200 mA and the membrane was
rotated for 90 min. The membranes were subsequently incubated with
1:1,000 primary antibodies against β-catenin and β-actin overnight
at 4°C. Membranes were then washed three times using Tris-buffered
saline with 0.1% Tween-20 (TBST) for 10 min/wash. Subsequently,
membranes were incubated with 1:2,500 goat-anti-rabbit and
goat-anti-rat immunoglobulin G for 1 h at the room temperature.
TBST was used to wash the membranes three times for 10 min/wash.
Protein bands were visualized using a configured illuminant
solution. Proteins were then analyzed using ImageJ 2.0 (National
Institutes of Health, Bethesda, MD, USA) to assess the differential
expression of β-catenin.
Detection of cell proliferation using
MTT
The 3 cell groups in the logarithmic phase were
selected and processed as previously described. A total of
0.75×104/ml cells in suspension were inoculated onto a 96-well
plate (200 µl/well). The 96-well plate was then incubated at 37°C
in 5% CO2. Cells were removed and observed using an inverted phase
contrast microscope (magnification, ×200). Cells were then cultured
for a further 24 h at 37°C and 5% CO2. Cultivation was
terminated after a 4 h incubation with 5 mg/ml MTT at room
temperature for 4 h. Each experiment was repeated three times.
Detection of cell clone formation
The 3 cell groups exhibiting logarithmic phase
growth were selected and processed as aforementioned. A total of
1×103/ml cells were inoculated onto a 6-cm sterile petri-dish and
200 µl suspension was added. Each group treatment was then repeated
twice. Petri-dishes were incubated at 37°C in 5% CO2 for 14 days.
Following visible clone growth, culture medium was removed and the
remaining cells were washed three times with PBS. Cells were then
stained with crystal violet for 10 min. Following staining, dishes
were placed on a transparent grid and the number of clone cells was
counted using a WTDS-1 inverted microscope (magnification, ×400;
Shenzhen weite photoelectric instrument sales department, Shenzhen,
China). Clone formation rate was calculated using the formula:
Clone formation rate=number of clones formed/Inoculation cell
number × 100.
Transwell invasion and migration
assays
The 3 cell groups in the logarithmic growth phase
were selected and processed as aforementioned. A total 1×105/ml of
cells in suspension were plated in the upper chambers of Transwell
plates in DMEM containing 10% bovine serum albumin (BSA; BioWit
Technologies Ltd., Shenzhen, China). Transwell membranes were
pre-coated with Matrigel and diluted with 10% BSA in a ratio of
1:6. A total 50 µl solution was added to each well of the upper
chamber. Each cell group was allocated a total of ٤ chambers,
placed in a 24-well plate and incubated at 37°C with 5%
CO2 for 1 h to solidify the gel. Following incubation,
chambers were removed and 50 µl DMEM with ١٠٪ BSA was added. This
was incubated for a second time at 37°C for 30 min. The lower
chambers of the Transwell plate were plated with 500 µl DMEM
containing ١٠٪ BSA. Matrigel-coated chambers were subsequently
placed into the ٢٤-well plate and incubated for ٢٤ h. Following
incubation, migratory cells were stained with 0.5% crystal violet
at 37°C for 20 min and counted in nine randomly-selected fields
using an inverted microscope (magnification, ×400; Shenzhen Weite
Photoelectric Instrument Sales Department). An average cell count
was then calculated. A Transwell migration experiment All
incubation and staining conditions used during this procedure were
the same as those used for the Transwell invasion. The steps of
this experiment were the same as aforementioned Transwell invasion
method, however, in this protocol, Matrigel was not used. A total
of 5×105/ml cells were directly inoculated onto the upper chambers
of the Transwell plate. Lower chambers were inoculated with DMEM
with 10% BSA. Cell staining was performed following 8 h
culture.
Detection of cell apoptosis
The 3 groups of cells in the logarithmic phase were
selected. Following conventional processing, an appropriate
quantity of 1× Buffer A was used to wash cells, once. Following
treatment, cells were centrifuged at a speed of 7,300 × g for 5 min
at room temperature so that the transfer buffer (Beijing Transgen
Biotech Co., Ltd.). Cells were stored in a refrigerator for 16 h at
20°C Samples were then removed and centrifuged at a speed of 448 ×
g and a temperature of 37°C for 5 min. Following centrifugation,
ethyl alcohol was removed and cells were re-suspended in 500 µl
Buffer A. RNaseA was then added to adjust cell concentration to
٠.٢٥ mg/ml and cells were incubated at 37°C for 30 min. A total of
5 ml 0.5% crystal violet temperature according to the kit
requirement (apoptosis PI staining kit; Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China) was then added and left for 30 min at 22°C.
Flow cytometry (Flowjo software; version 10.4.2; BD Biosciences,
Franklin Lakes, NJ, USA) was used to detect cell apoptosis rate,
which was analyzed at a wavelength of 488 nm. A flow cytometer was
used to analyze cell DNA content. The presence of a sub G1 peak
indicated that a cell was apoptotic.
Statistical analysis
Data are presented as the mean ± standard deviation.
SPSS 19.0 (IBM Corp., Armonk, NY, USA) was utilized to analyze the
results of this study. One-way analysis of variance was performed
to assess differences among groups and a student Newman-Keuls test
was performed if variance was consistent. If discrepancies between
groups were identified, the Games-Howell variance method was
performed. P<0.05 was considered to indicate a statistically
significant difference.
Results
Cellular morphology
Strengthened adherence and an increase in cell
number was observed in A375-RNAi cells compared with A375 and
A375-negative cells (Fig. 1). The
doubling time of A375-negative and A375-RNAi cells was also
prolonged. The size and morphology of uninfected control cells was
normal: The cytoplasm was evenly distributed, good refractivity was
observed and cell reproduction was fast. No significant differences
were identified between the morphologies of A375-negative and
A375-RNAi. There was no significant difference between
A375-negative and A375 cells.
Western blot analysis and β-catenin
expression
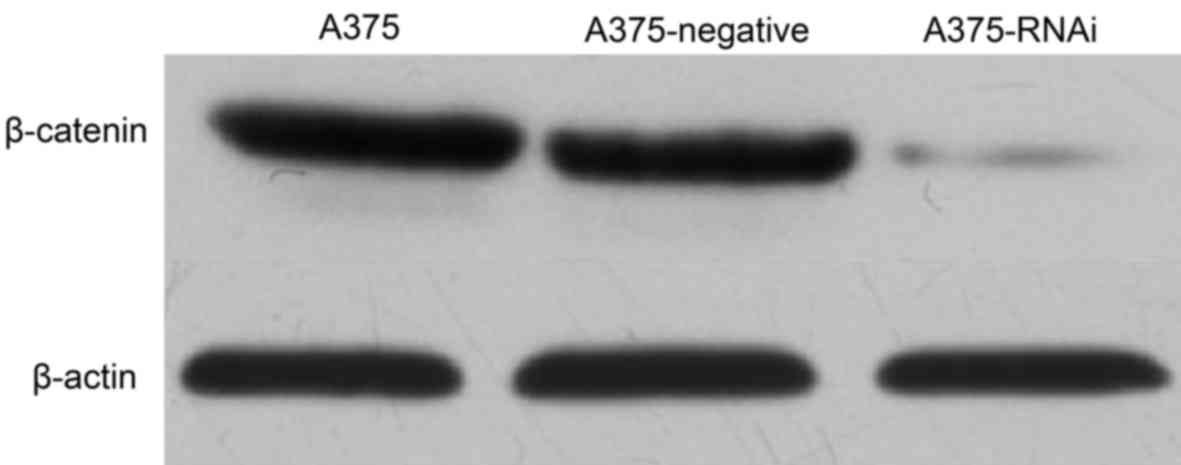
As presented in Fig.
2, β-catenin expression in A375-RNAi cells decreased markedly
compared with that of A375 cells indicating that the lentiviral
vector-mediated siRNA silenced β-catenin expression. No marked
differences between the expression of β-catenin in A375-negative
cells and A375 cells were identified. The lentiviral empty vector
therefore did not significa1ntly influence the expression of
β-catenin.
Cell proliferation
The results of the MTT assay (Table I) demonstrated that the proliferation
of A375-RNAi cells was significantly inhibited at all time points,
compared with A375-negative cells (P<0.05; Table I).
 | Table I.Influence of lentiviral
vector-mediated siRNA on A375 cell proliferation. |
Table I.
Influence of lentiviral
vector-mediated siRNA on A375 cell proliferation.
| Time (h) | A375 | A375-negative | A375-RNAi |
|---|
| 24 | 0.215±0.082 | 0.214±0.085 |
0.184±0.077a |
| 48 | 0.371±0.025 | 0.356±0.019 |
0.292±0.082b |
| 72 | 0.593±0.039 | 0.572±0.027 |
0.499±0.033b |
Invasion and migration of A375
cells
The invasive abilities of A375-RNAi cells in the
experiment group were significantly inhibited in vitro
compared with A375-negative cells (P<0.05; Table II; Fig.
3A). The invasiveness of A375 and A375-negative cells was
similar. The migration ability of A375-RNAi cells in the experiment
group was also significantly inhibited compared with A375-negative
cells (P<0.05; Table III;
Fig. 3B). A375 cells in the blank
control group and A375-negative cells in the negative control group
exhibited similar migration abilities in vitro.
 | Table II.Influence of lentiviral
vector-mediated siRNA on A375 cell invasion. |
Table II.
Influence of lentiviral
vector-mediated siRNA on A375 cell invasion.
| Group | A375 | A375-negative | A375-RNAi |
|---|
| Number of penetrating
cells | 44.54±3.47 | 41.15±3.64 |
22.58±2.20a |
 | Table III.Influence of lentiviral
vector-mediated siRNA on A375 cell migration. |
Table III.
Influence of lentiviral
vector-mediated siRNA on A375 cell migration.
| Group | A375 | A375-negative | A375-RNAi |
|---|
| Number of
penetrating cells | 71.13±5.46 | 70.72±4.51 |
53.33±49.15a |
Cellular apoptosis
As presented in Table
IV, the apoptosis rate of A375-negative cells was 4.02±0.59%.
However, the apoptosis rate of A375-RNAi cells in the experiment
group increased significantly to 20.57±3.26% (P<0.05). The cell
apoptosis rate of A375 cells was 3.13±0.46%, similar to that of
A375 cells.
 | Table IV.Influence of lentiviral
vector-mediated siRNA on A375 cell apoptosis. |
Table IV.
Influence of lentiviral
vector-mediated siRNA on A375 cell apoptosis.
| Group | A375 | A375-negative | A375-RNAi |
|---|
| Sub G1
phase (%) | 3.13±0.46 | 4.02±0.59 |
20.57±3.26a |
Discussion
Excessive and fast cell proliferation is one of the
primary causes of high-grade MM malignancy. Katiyar and Vaid
(15) and Widlund et al
(16) demonstrated that activation
of the Wnt/β-catenin signaling pathway increased MM cell clone
formation and proliferation in vitro. Previous studies have
also demonstrated that the upregulation of Dickhopf-related protein
1 (DKK1) significantly downregulates β-catenin, thus reversing the
inhibitory effect of DKK1 on A375 cell proliferation (17,18). In
MM tissue, DKK1 expression is significantly lower than in normal
human epidermal tissue (19). In
addition, the expression of β-catenin in A375 cells is
significantly higher than that of epidermal cells (20). Therefore, it was hypothesized that
the downregulation of β-catenin may inhibit cell proliferation and
clone formation. However, other studies (21–23) have
demonstrated that β-catenin is found in the majority of benign
melanocytic nevi and that a decrease in β-catenin content may cause
the development of MM. Cell cultures performed in this study
revealed that A375 cells grow actively. The results of the current
study also demonstrated that the downregulation of β-catenin
inhibits A375 cell proliferation and clone formation. However, the
specific mechanism that underlies this process requires further
study.
Another cause of high grade malignancy is the
invasive and migratory abilities of MM tumor cells. Tobias et
al (24) demonstrated that the
transcriptional activity of β-catenin increases gradually as MM
develops. Previous studies have revealed that β-catenin serves a
vital role in two primary processes: β-catenin/E-cadherin mediated
cell adhesion and the transcription and regulation of classical
Wnt/β-catenin signaling in the cell nucleus (25,26). As
MM develops, the expression of E-cadherin gradually decreases,
which inhibits the formation of β-catenin/E-cadherin cell adhesion
compounds, resulting in cell invasion and migration (27). However, previous studies have
demonstrated that the upregulation of β-catenin may improve the
prognosis of patients with MM (28,29).
Murine studies have demonstrated that the downregulation of
β-catenin may accelerate the metastasis of MM. Invasion and
migration experiments in the present study demonstrated that
β-catenin silencing significantly decreases the invasion and
migration of A375-RNAi cells, compared with that of A375 cells
in vitro. However, no significant differences in the
migration and invasion between A375-negative and A375 cells were
identified. In benign melanocytic nevi and pre-invasive MM cells,
β-catenin is primarily located in the cell membrane and in
E-cadherin cell adhesion compounds. This serves to increase
intercellular adhesion and limit cell invasion (30). As MM increases in malignant grade due
to the loss of casein kinase 1α activity, the expression and
transcriptional activity of intracellular β-catenin increases
(31). Vaid et al (32) revealed that the downregulation of
β-catenin effectively inhibits the invasion and migration of A375
cells. This may be due to the downregulation of β-catenin
decreasing the formation of E-cadherin cell adhesion compounds,
thus resulting in easily detachable cells that break away from the
tumor. It has also been demonstrated that the downregulation of
β-catenin in A375 cells effectively inhibits cell invasion
(33).
Cellular apoptosis is primary regulated by the B
cell lymphoma 2 (Bcl-2) protein family. Bcl-2 can inhibit apoptosis
by attenuating the activation of Bax and Bak. It has been
demonstrated that β-catenin may regulate the expression of Bax and
Bcl-2 (34). c-Myc is one of the
primary target genes of β-catenin, which influences cell
proliferation and apoptosis by activating or inhibiting genetic
transcription. c-Myc may become overexpressed and induce apoptosis
when there is a lack of cell growth and activation due to nutrient
deficiencies (35,36). In the present study, the apoptosis
rate of A375-RNAi cells was significantly higher than that of A375
cells. However, no significant differences in the apoptosis rates
of A375-negative cells and A375 cells was identified. Schittek
et al (37) assessed multiple
MM cells and the apoptosis of MM cells and demonstrated that
β-catenin regulates the apoptosis of MM cells. This may be due to a
decrease in the expression of Bcl-2 and c-Myc and an increase of
Bax, (mediated by the inhibition of β-catenin), reversing the
inhibition of apoptosis, thus accelerating apoptosis.
In conclusion, the inhibition of β-catenin
expression or activity may inhibit the proliferation, invasion and
migration of cells and may induce the downregulation of
anti-apoptosis genes, thus accelerating apoptosis. The results of
the present study also indicate that β-catenin may be an effective
target for the treatment of patients with MM.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Erdmann F, Lortettieulent J, Schüz J, Zeeb
H, Greinert R, Breitbart EW and Bray F: International trends in the
incidence of malignant melanoma 1953–2008-are recent generations at
higher or lower risk? Int J Cancer. 132:385–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang JW, Yeh KY, Wang CH, Yang TS, Chiang
HF, Wei FC, Kuo TT and Yang CH: Malignant melanoma in Taiwan: A
prognostic study of 181 cases. Melanoma Res. 14:537–541. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perlis C and Herlyn M: Recent advances in
melanoma biology. Oncologist. 9:182–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Miao Y, Owen NK, Whitener D, Gallazzi F,
Hoffman TJ and Quinn TP: In vivo evaluation of 188Re-labeled
alpha-melanocyte stimulating hormone peptide analogs for melanoma
therapy. Int J Cancer. 101:480–187. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Delmas V, Beermann F, Martinozzi S,
Carreira S, Ackermann J, Kumasaka M, Denat L, Goodall J, Luciani F,
Viros A, et al: Beta-catenin induces immortalization of melanocytes
by suppressing p16INK4a expression and cooperates with N-Ras in
melanoma development. Genes Dev. 21:2923–2935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chin L, Garraway LA and Fisher DE:
Malignant melanoma: Genetics and therapeutics in the genomic era.
Genes Dev. 20:2149–2182. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lens MB, Dawes M, Goodacre T and Bishop
JA: Excision margins in the treatment of primary cutaneous
melanoma: A systematic review of randomized controlled trials
comparing narrow vs. wide excision. Arch Surg. 137:1101–1105. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Qian QH, Yao X, Zhu M and Jiang Y:
Clinical pathological analysis of 29 cases of cutaneous malignant
melanoma. Guide of China Medicine. 6:20–22. 2008.(In Chinese).
|
|
9
|
Chien AJ, Moore EC, Lonsdorf AS,
Kulikauskas RM, Rothberg BG, Berger AJ, Major MB, Hwang ST, Rimm DL
and Moon RT: Activated Wnt/ß-catenin signaling in melanoma is
associated with decreased proliferation in patient tumors and a
murine melanoma model. Proc Natl Acad Sci USA. 106:1193–1198. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ciolczyk-Wierzbicka D, Gil D and Laidler
P: The inhibition of cell proliferation using silencing of
N-cadherin gene by siRNA process in human melanoma cell lines. Curr
Med Chem. 19:145–151. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Niu LL and Hao J: Rosiglitazone inhibition
of A375 human malignant melanoma cell invasion. Chinese J Dermatol.
42:831–834. 2009.
|
|
12
|
Riccardo F, Iussich S, Maniscalco L,
Mayayo SL, Rosa GL, Arigoni M, Maria RD, Gattino F, Lanzardo S,
Lardone E, et al: CSPG4-specific immunity and survival prolongation
in dogs with oral malignant melanoma immunized with human CSPG4
DNA. Clin Cancer Res. 20:3753–3762. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rappa G, Mercapide J, Anzanello F, Le TT,
Mary G, Johlfs RRF, Wilsch-Bräuninger M, Corbeil D and Loricoa A:
Wnt interaction and extracellular release of prominin-1/CD133 in
human malignant melanoma cells. Exp Cell Res. 319:810–819. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tarapore RS, Siddiqui IA, Saleem M,
Spiegelman V and Mukhtar H: Abstract 3794: Growth inhibition of
human melanoma cells in vitro and in vivo by lupeol is associated
with inhibition of Wnt/β-catenin signaling. Cancer Res. 70
Suppl:S37942010. View Article : Google Scholar
|
|
15
|
Katiyar SK and Vaid M: Abstract 3683:
Bioactive phytochemical proanthocyanidins target β-catenin
signaling in preventing invasive potential of human melanoma cells.
Cancer Res. 73 Suppl:S36832013. View Article : Google Scholar
|
|
16
|
Widlund HR, Horstmann MA, Price ER, Cui J,
Lessnick SL, Wu M, He X and Fisher DE: β-Catenin-induced melanoma
growth requires the downstream target Microphthalmia-associated
transcription factor. J Cell Biol. 158:1079–1087. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Beck D, Niessner H, Krieg K, Gogel J,
Bonin M, Garbe C and Meier F: Chemosensitizing activity of the mTOR
inhibitor temsirolimus (Torisel) in metastatic melanoma involves
DKK1. Journal der Deutschen Dermatologischen Gesellschaft. 11:6.
2013.
|
|
18
|
Mikheev AM, Mikheeva SA, Rostomily R and
Zarbl H: Dickkopf-1 activates cell death in MDA-MB435 melanoma
cells. Biochem Biophys Res Commun. 352:675–680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pawlikowski JS, Mcbryan T, Van TJ, Drotar
ME, Hewitt RN, Maier AB, King A, Blyth K, Wu H and Adams PD: Wnt
signaling potentiates nevogenesis. Proc Natl Acad Sci USA.
110:16009–16014. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mælandsmo GM, Holm R, Nesland JM, Fodstad
Ø and Flørenes VA: Reduced β-catenin expression in the cytoplasm of
advanced-stage superficial spreading malignant melanoma. Clin
Cancer Res J Am Assoc Cancer Res. 9:3383–3388. 2003.
|
|
21
|
Cimetta E, Cannizzaro C, James R, Biechele
T, Moon RT, Elvassore N and Vunjak-Novakovic G: Microfluidi device
generating stable concentration gradients for long term cell
culture: Application to Wnt3a regulation of β-catenin signaling.
Lab Chip. 10:3277–3283. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Comodo AN, Bachi ALL, Soares MF, Franco M,
de Paulo V and Teixeira C: Galectin-3 expression favors metastasis
in murine melanoma. Adv Biosci Biotechnol. 4:55–62. 2013.
View Article : Google Scholar
|
|
23
|
Shah PK, Walker MP, Sims CE, Major MB and
Allbritton NL: Dynamics and evolution of β-catenin-dependent Wnt
signaling revealed through massively parallel clonogenic screening.
Integr Biol. 6:673–684. 2014. View Article : Google Scholar
|
|
24
|
Tobias S, Moritz M, Daniel E and Birgit S,
Michael S, Martin S, Claus G and Birgit S: β-catenin signaling
increases during melanoma progression and promotes tumor cell
survival and chemoresistance. PloS One. 6:e234292011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dong F and Herlyn M: The dynamic roles of
cell-surface receptors in melanoma development. From Melanocytes to
Melanoma. 169–181. 2006.
|
|
26
|
Hsu MY, Ling L and Herlyn M: Cultivation
of normal human epidermal melanocytes in the absence of phorbol
esters. Methods Mol Med. 107:13–28. 2005.PubMed/NCBI
|
|
27
|
Tucci MG, Lucarini G, Brancorsini D, Zizzi
A, Pugnaloni A, Giacchetti A, Ricotti G and Biagini G: Involvement
of E-cadherin, β-catenin, Cdc42 and CXCR4 in the progression and
prognosis of cutaneous melanoma. Br J Dermatol. 157:1212–1216.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuno T, Tsukamoto T, Hara A and Tanaka T:
Cancer chemoprevention through the induction of apoptosis by
natural compounds. J Biophys Chem. 3:156–173. 2012. View Article : Google Scholar
|
|
29
|
Novellino L, De FA, Deho P, Perrone F,
Pilotti S, Parmiani G and Castelli C: PTPRK negatively regulates
transcriptional activity of wild type and mutated oncogenic
beta-catenin and affects membrane distribution of
beta-catenin/E-cadherin complexes in cancer cells. Cell Signal.
20:872–883. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee DJ, Kang DH, Choi M, Choi YJ, Lee JY,
Park JH, Park YJ, Lee KW and Kang SW: Peroxiredoxin-2 represses
melanoma metastasis by increasing E-Cadherin/β-Catenin complexes in
adherens junctions. Cancer Res. 73:4744–4757. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pećina-Šlaus N, Žigmund M, Kušec V, Martić
TN, Čačić M and Šlaus M: E-cadherin and β-catenin expression
patterns in malignant melanoma assessed by image analysis. J Cutan
Pathol. 34:239–246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vaid M, Prasad R, Sun Q and Katiyar SK:
Silymarin targets β-catenin signaling in blocking
migration/invasion of human melanoma cells. PloS One. 6:e230002010.
View Article : Google Scholar
|
|
33
|
Damsky WE, Curley DP, Santhanakrishnan M,
Rosenbaum LE, Platt JT, Rothberg Gould BE, Taketo MM, Dankort D,
Rimm DL, McMahon M and Bosenberg M: β-catenin signaling controls
metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell.
20:741–754. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chon E, Thompson V, Schmid S and Stein TJ:
Activation of the canonical Wnt/β-catenin signalling pathway is
rare in canine malignant melanoma tissue and cell lines. J Comp
Pathol. 148:178–187. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dang CV, O'Donnell KA, Zeller KI, Nguyen
T, Osthusa RC and Lia F: The c-Myc target gene network. Semin
Cancer Biol. 16:253–264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeller KI, Zhao X, Lee CWH, Chiu KP, Yao
F, Yustein JT, Ooi HS, Orlov YL, Shahab A, Yong HC, et al: Global
mapping of c-Myc binding sites and target gene networks in human B
cells. Proc Natl Acad Sci USA. 103:17834–17839. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schittek B and Sinnberg T: Biological
functions of casein kinase 1 isoforms and putative roles in
tumorigenesis. Mol Cancer. 13:2312014. View Article : Google Scholar : PubMed/NCBI
|