Introduction
Mesenchymal stem cells (MSCs) are self-renewable
multipotent stromal cells that are capable of differentiation into
cells having a mesodermal lineage (1); in addition, in their undifferentiated
state they exhibit immunosuppressive properties that can be
exploited in the treatment of various inflammatory states. On this
basis, MSC administration has the potential to ameliorate
inflammation occurring on a wide range of conditions, including
heart and brain injury, joint damage, Crohn's disease, multiple
sclerosis (2) and acute
graft-versus-host disease (GVHD) (3).
The usual source of MSCs is the bone marrow;
however, MSCs are also obtainable from adult adipose tissues and
whereas MSCs from the latter source [adipose tissue-derived MSCs
(AMSCs)] may not be fully identical with bone marrow MSCs, their
immunosuppressive properties are thought to be similar or even
greater than those of bone marrow MSCs (4,5). Adipose
tissue is also quite a useful MSC source since this type of tissue
can yield ~500-fold larger (4–6) MSC
numbers than those obtainable from bone marrow (7). Additionally, adipose tissue is readily
available given the large number of liposuctions performed each
year (400,000 in the USA alone), and aspirated adipose tissue
obtained in this way is regarded as waste (5,6); thus,
no specific tissue donors have to be identified (8).
Acute GVHD is one of the major risks of allogeneic
hematopoietic stem cell transplantation therapy in patients
suffering from malignant hematopoietic neoplasms and severe bone
marrow failure (1). This
complication is due to the activation of donor CD8+ T
cells by recipient human leucocyte antigen (HLA)-mismatched tissue
cells that are thus stimulated to produce a number of
pro-inflammatory cytokines, including interferon (IFN)-γ,
interleukin (IL)-1β and tumor necrosis factor (TNF)-α (9). This then leads to the various GVHD
manifestations, including inflammation of the skin, liver and
gastrointestinal tract characterized by rash or erythema, jaundice
or diarrhea (10–12). In spite of the fact that MSCs express
HLA major histocompatibility complex (MHC) class I molecules that
could be recognized by alloreactive T cells mediating GVHD, they
appear to escape alloantigen-induced immune destruction following
their injection as third party allogeneic cells (13). In addition to the avoidance of
alloantigen-induced immune destruction as alluded to above, MSCs
exert immunoregulatory properties that lead to the inhibition
effector T cells causing the GVHD (14). However, the mechanism of such
suppression and whether or not it requires MHC compatibility awaits
further investigation (15).
In the present study, an in vitro GVHD model
in mice was established, and this model was then used to determine
whether AMSCs are able to suppress inflammatory cytokine secretion
to the same degree as bone marrow MSCs. In addition, whether MHC
class I expression on MSCs is involved in MSC-mediated
immunosuppression in GVHD was examined using β2-microglobulin (β2m)
knockout mice, which are completely deficient in cell surface
expression of MHC class I molecules.
Materials and methods
Animals
A total of 30 male C57BL/6-Ly5.1 mice (age, 6–8
weeks; weight 18–20 g) were obtained from RIKEN BioResource Center
(Tsukuba, Japan), and they were maintained in the animal facility
at Asahi University (Mizuho, Japan) for 22–24 weeks. A total of 30
male BALB/cCr, 20 male C3H/HeN and 20 male C57BL/6J mice (age, 5
weeks; weight 18–20 g) were purchased from Shimizu Laboratory
Supplies (Shizuoka, Japan). A total of 10 male β2m-deficient
C57BL/6 (β2m−/−) mice (age, 5 weeks; weight 18–20 g)
were purchased from the Jackson Laboratory (Ben Harbor, ME, USA).
Animals were maintained at 22–26°C and 50% humidity with a 12-h
light/dark cycle and were fed a standardized diet and had ad
libitum access to autoclaved tap water. All animal experiments
were reviewed and approved by the ethics committee for animal
experiments of Asahi University under the ID 12–009.
Monoclonal antibodies (mAbs)
CD4 mAb (cat no. 553047) was purchased from BD
Biosciences (San Jose, CA, USA); anti-mouse CD25 mAb (cat no.
12-0251) was obtained from eBioscience, Inc. (San Diego, CA, USA);
and anti-mouse forkhead box P3 (Foxp3) mAb (cat no. 20-5773-U100)
were from Tonbo Biosciences (San Diego, CA, USA).
Isolation of AMSCs
AMSCs were isolated from various male 6-week-old
mice (BALB/cCr, C3H/HeN, C57BL/6 J and β2m−/−) as
described previously (16). AMSCs of
the third passage were used in the present study.
Mixed lymphocyte reaction (MLR)
Spleen cells were isolated as described previously
(17). To decide the optimal cell
number of spleen cells for MLR to detect IFN-γ production, spleen
cells (1×105−16×105) from recipients
(30-week-old male C57BL/6-Ly5.1 mice) were mixed with spleen cells
(1×105−16×105) from allogenic donors
(30-week-old male BALB/c mice). To decide optimal cell number of
AMSCs for the MLR, spleen cells (4×105) from recipients
(30-week-old male C57BL/6-Ly5.1 mice) were mixed with spleen cells
(4×105) from allogenic donors (30-week-old male BALB/c
mice) in the presence or absence of AMSCs (0–4×103) or
BALB-3T3 cells (0–4×103). To observe the
immunosuppression effect of AMSCs for the MLR, spleen cells
(4×105) from recipients (30-week-old male C57BL/6-Ly5.1
mice) were mixed with spleen cells (4×105) from
allogenic donors (30-week-old male BALB/c mice) in the presence of
AMSCs (1×103) from various mice (BALB/c, C3H/HeN,
C57BL/6 and β2m−/−) or BALB-3T3 cells. MLR was performed
in 200 µl of RPMI-1640 medium (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) containing 10% fetal bovine serum (FBS; cat no.
F4135; Sigma-Aldrich, Merck KGaA) and 50 µM 2-mercaptoethanol on
flat-bottom 96-well plates in 5% CO2 at 37°C for 48 h.
The levels of IFN-γ produced in the culture supernatants were
determined using mouse IFN-γ ELISA sets (cat no. 555138; BD
Biosciences) according to the manufacturer's protocol.
A Transwell assay was performed using a
Transwell® insert (polycarbonate membrane with 0.4-µm
pore size; Costar; Corning, Inc.; Corning, NY, USA). Spleen cells
(8×105) from recipients (30-week-old male C57BL/6-Ly5.1
mice) and spleen cells (8×105) from allogenic donors
(30-week-old male BALB/c mice) were cultured on Transwell inserts
in the presence or absence of AMSCs (4×103) or BALB-3T3
cells (4×103) in 800 µl of RPMI-1640 medium containing
10% FBS and 50 µM 2-mercaptoethanol on flat-bottom 24-well plates
in 5% CO2 at 37°C for 48 h.
Flow cytometric analysis
Following MLR, the cells (8×105) were
washed once with ice-cold staining buffer (PBS containing 2% FBS, 1
mM Na2EDTA and 0.1% sodium azide), incubated with
anti-CD16/CD32 mAb for 20 min at 4°C and then stained with mAbs
against CD4 (fluorescein isothiocyanate; diluted 1:50), CD25
(phycoerythrin; diluted 1:50) for 30 min at 4°C. Following a single
wash step with ice-cold staining buffer, the cells were fixed and
permeabilized with BD FACS lysis solution and BD FACS
permeabilizing solution 2 (both from BD Biosciences) according to
the manufacturer's protocol, and then stained with anti-Foxp3 mAb
(allophycocyanin; diluted 1:50) for 30 min at room temperature. The
viability marker 7-amino actinomycin D (7AAD; cat no. 13-6993-T500;
Tonbo Biosciences) was used to eliminate dead cells. Stained cells
were analyzed using an Accuri C6 cytometer (BD Biosciences). Data
were analyzed using FlowJo software (version 7.6; Tree Star, Inc.,
Ashland, OR, USA).
Statistical analysis
All quantified results are presented as the mean ±
standard deviation. Each experiment was repeated at least 3 times.
Statistical analyses were performed using Prism version 6 software
(GraphPad Software, Inc., La Jolla, CA, USA). An unpaired t-test or
a one-way analysis of variance followed by Dunnett or
Tukey-Kramer's post-test analysis were used. P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphology, differentiation and
immunophenotype characteristics of AMSCs
As previously shown (16), passage-3 AMSCs from 6 week-old
BALB/c, C3H/HeN, C57BL/6 and β2m−/− mice all exhibited a
similar spindle-shape morphology, ability to differentiate
appropriately into adipogenic and osteogenic cells and to express
CD44, CD105 and stem cell antigen-1 but not CD45 in flow cytometric
analysis regardless of the mouse strain (data not shown) (8).
Suppression of MLR-induced IFN-γ
production by AMSCs
To model the capacity of AMSCs to suppress GVHD
in vivo, their ability to suppress MLRs in vitro was
assessed. First, increasing numbers of whole spleen cells from
C57BL/6-Ly5.1 and BALB/c mice were cultured and the culture
supernatants were harvested after 48 h to examine whether IFN-γ
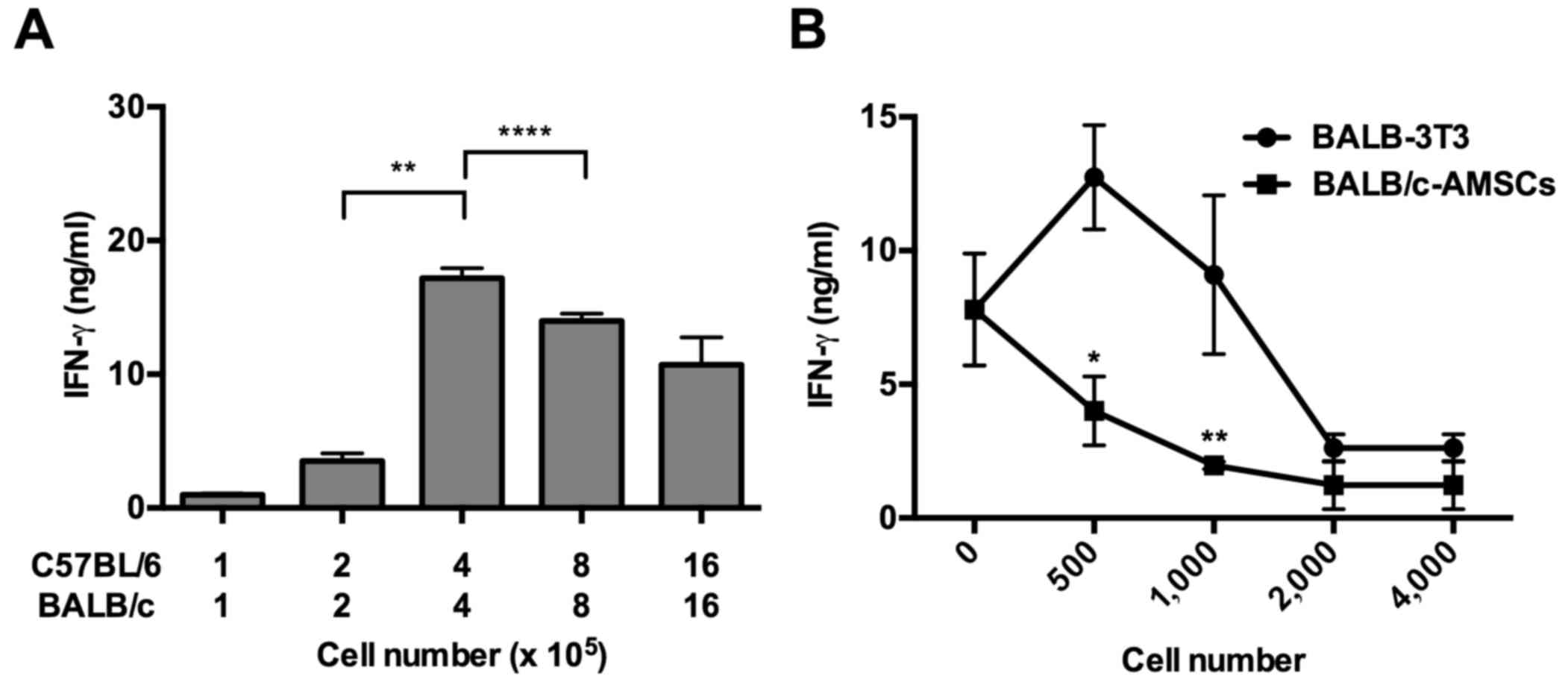
secretion was generated by MLR. It was observed that a mixture of
4×105 spleen cells exhibited the highest IFN-γ secretion
(Fig. 1A). Next, 4×105
whole spleen cells from C57BL/6-Ly5.1 mice were cultured with
4×105 whole spleen cells from BALB/c mice in the
presence and absence of BALB/c-AMSCs or BALB-3T3 cells. The
addition of increasing numbers of AMSCs to the culture caused
increasing suppression of IFN-γ secretion; the addition of a low
number of BALB-3T3 cells caused an increase in IFN-γ secretion,
whereas the addition of a high number of BALB-3T3 cells suppressed
IFN-γ secretion, with the latter being possible due to nutrient
depletion and/or cell overgrowth (Fig.
1B). In any case, addition of only 1×103 AMSCs to
the culture caused significant suppression of baseline IFN-γ
secretion occurring in the absence of AMSCs whereas addition of the
same number of BALB-3T3 cells caused no significant suppression of
baseline secretion.
Immunosuppression by AMSCs derived
from various mouse strains
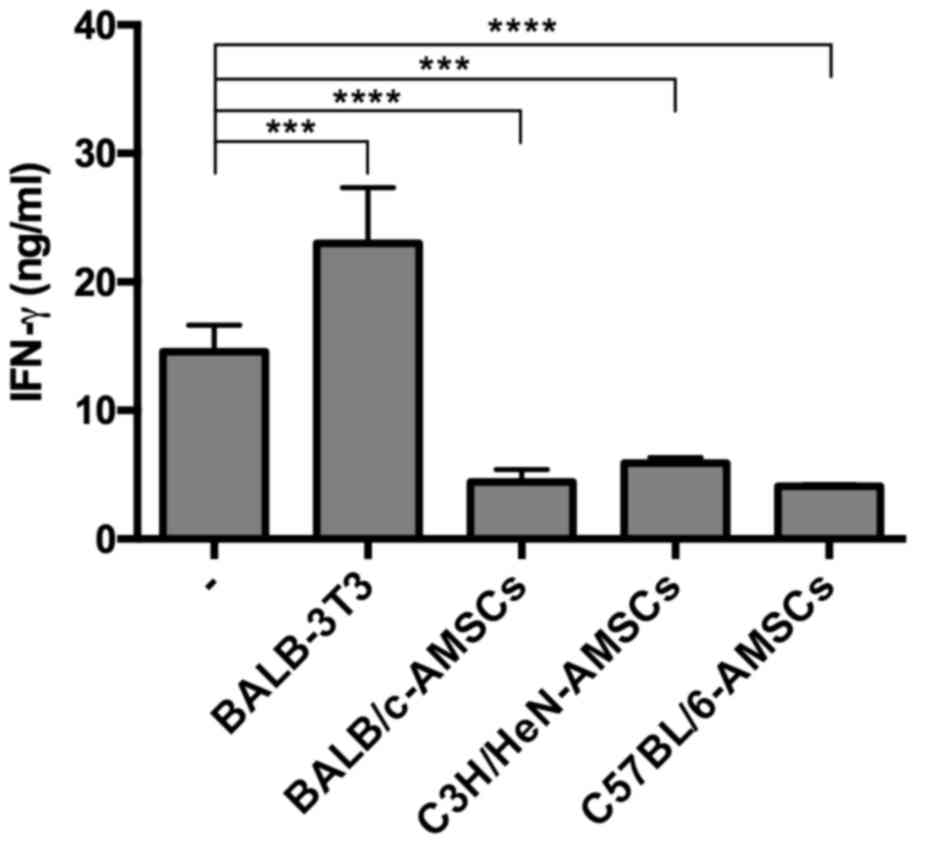
In further experiments, the capacity of AMSCs
derived from various mouse strains to suppress IFN-γ production in
the C57BL/6-BALB/c MLR cultures was determined. As before, the
addition of 1×103 BALB-3T3 cells to the MLR cultures did
not suppress MLR-generated IFN-γ production whereas addition of the
same number of AMSCs from BALB/c, C3H/HeN and C57BL/6 mice to the
culture all led to significant suppression of IFN-γ (Fig. 2). In related ELISA experiments, it
was revealed that IL-1β, TNF, and IL-10 production in the same MRL
cultures did not reach detectable levels in the presence or absence
of AMSCs (data not shown).
Lack of Foxp3+ Treg cell
expansion in MLR cultures containing AMSCs
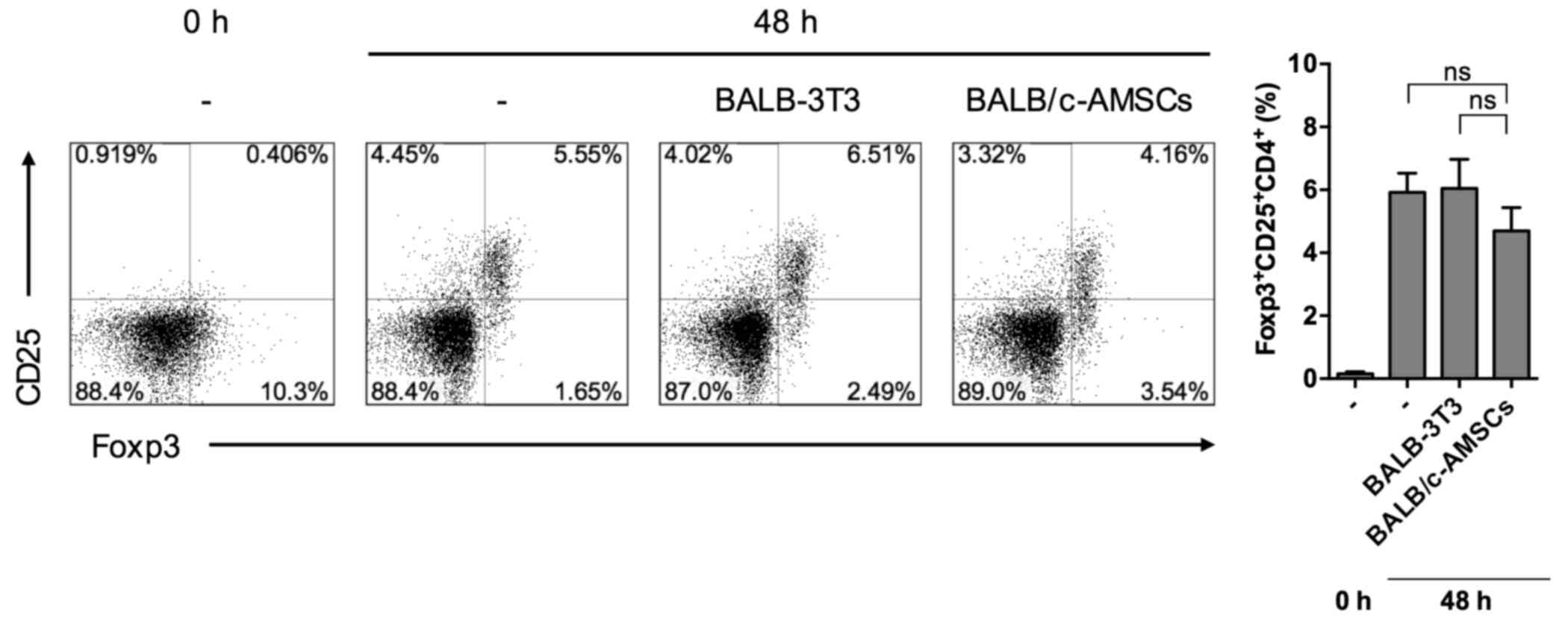
It was hypothesized that the suppressive effect of
AMSC addition may have been due to the induction of
Foxp3+ CD25+ CD4+ regulatory T
cells (Treg). However, as determined using flow cytometry, the
percentage of Tregs among splenocytes in MLR cultures containing
BALB/c-AMSCs was equivalent to that in MLR cultures containing
BALB/c-3T3 cells (Fig. 3).
Immunosuppression by AMSCs from
β2m−/− mice
The suppressive capacity of AMSCs from mice lacking
β2m expression was then detected, as such mice do not express MHC
class I on the cell surface. However, addition of AMSCs from
β2m−/− mice exhibited the ability to suppress IFN-γ
secretion. In addition, the level of suppression obtained with
wild-type (WT) and β2m−/− AMSCs was equivalent, with no
significant difference (Fig. 4).
Suppression of IFN-γ secretion by AMSC
secretions
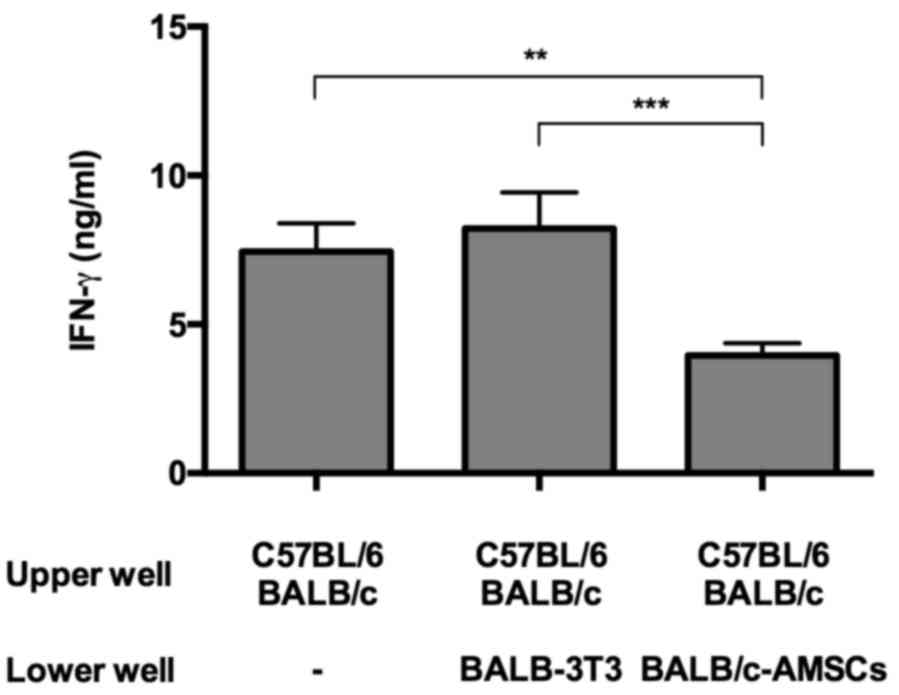
Finally, to determine if the suppressive function
exerted by AMSCs required cell contact in the MLR cultures, AMSCs
and MLR cells were cultured separately in the upper and lower
Transwell compartments, respectively. Under these conditions AMSCs
but not 3T3 cells significantly suppressed IFN-γ secretion,
indicating that AMSC suppression is mediated by a soluble factor,
at least partly (Fig. 5).
Discussion
MSCs are a highly heterogeneous population of cells
whose precise phenotype culture remains debated (18). Despite this heterogeneity, it was
established in the present study that AMSCs harvested from adipose
tissue obtained from three different mouse strains were
morphologically and phenotypically identical and had an equal
capacity to suppress IFN-γ production generated in MLR cultures
serving as an in vitro model of GVHD. In addition, it was
revealed that such suppression was also observed with AMSCs
obtained from mice deficient in β2m, an MHC class I subunit that is
required for cell surface MHC class I molecule expression. These
observations indicate that MHC class I expression on the AMSCs is
dispensable for AMSC suppressor activity. Notably, in a previous
study it was revealed that downregulation of β2m by exposure of
AMSC-containing cultures to β2m-specific small interfering RNA led
to a modest reduction in AMSC suppression (16). However, in showing that AMSCs from
mice that are genetically deficient in β2m have an equal ability to
mediate suppression as compared with WT cells, the present study is
more definitive in showing that cell-cell contact involving MHC
class I is not, in fact, involved in AMSC immunosuppression.
IFN-γ is the major type 1 T helper (Th1)-inducing or
associated cytokine responsible for the inflammatory state caused
by acute GVHD (19). Splenocytes of
aged mice produce more IFN-γ than splenocytes of young mice since
the spleens of aged mice contain markedly increased numbers of
CD8+ CD122+ T cells (20). The AMSC suppressor activity was
therefore assessed in MLR cultures containing splenocytes from
30-week-old BALB/c and C57BL/6-Ly5.1 mice in the present study, and
robust IFN-γ production was thereby obtained in the MLR cultures,
albeit less than that obtained in anti-CD3ε/anti-CD28-stimulated
cultures (16,20). The data of the present study revealed
that despite this robust Th1-inducing MLR response, addition of
only 1×103 AMSCs suppressed IFN-γ production in the MLR.
These results indicate that AMSCs have an immunosuppressive
capacity equal to that of bone marrow derived MSCs (8).
One possible mechanism of AMSC suppression in the
absence of AMSCs MHC class I expression is that AMSCs secrete
HLA-G, a non-classical MHC class I molecule with suppressor
function that was originally thought to explain the ability of
cells in human trophoblasts to evade maternal immunorejection
(21). HLA-G secreted by human bone
marrow MSCs has also been reported to serve a pivotal role in the
ability of human MSCs to suppress proliferation of cells in
allogeneic MLR cultures (21,22).
Murine Qa-2 is considered to be the functional homolog of human
HLA-G and has been demonstrated to be involved in natural killer
cell-mediated suppression of CD4+ T cell responses
(23). Since an alternative
(soluble) form of HLA-G can be secreted in the absence of β2m
(24,25), it is possible that a similar form of
Qa-2 secreted by β2m−/− murine AMSCs may account for
AMSC regulatory function in mice (26,27).
Exosomes budding from the plasma membrane are known
to contain immune modulators, including HLA-G, IL-10 and
transforming growth factor-β (28,29).
Thus, while the MLR supernatants in the present study contained
little if any IL-10, the immunosuppression mediated by these MLR
supernatants may have been due to immune modulators associated with
exosomes.
In summary, the observations reported in the present
study indicate that AMSCs are cells with potent suppressor activity
for the allogeneic interactions that accompany GVHD and that such
suppressor activity does not require MHC complementarity. Thus,
AMSCs may serve as a useful source for the control of GVHD in
humans.
Acknowledgements
The present study was supported in part by the
Grants-in-Aid for Scientific Research from the Japan Society for
the Promotion of Science (grant nos. 24592851 and 15K11096),
Program to Disseminate Tenure Tracking System, The Ministry of
Education, Culture, Sports, Science and Technology (MEXT), Japan,
Okayama Foundation for Science and Technology, Ryobi Teien Memory
Foundation and Sanyo Hohso Foundation.
References
|
1
|
Abdi R, Fiorina P, Adra CN, Atkinson M and
Sayegh MH: Immunomodulation by mesenchymal stem cells: A potential
therapeutic strategy for type 1 diabetes. Diabetes. 57:1759–1767.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shen H: Stricter standards sought to curb
stem-cell confusion. Nature. 499:3892013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miura Y, Yoshioka S, Yao H, Takaori-Kondo
A, Maekawa T and Ichinohe T: Chimerism of bone marrow mesenchymal
stem/stromal cells in allogeneic hematopoietic cell
transplantation: Is it clinically relevant? Chimerism. 4:78–83.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fraser JK, Wulur I, Alfonso Z and Hedrick
MH: Fat tissue: An underappreciated source of stem cells for
biotechnology. Trends Biotechnol. 24:150–154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Melief SM, Zwaginga JJ, Fibbe WE and
Roelofs H: Adipose tissue-derived multipotent stromal cells have a
higher immunomodulatory capacity than their bone marrow-derived
counterparts. Stem Cells Transl Med. 2:455–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gimble JM, Katz AJ and Bunnell BA:
Adipose-derived stem cells for regenerative medicine. Circ Res.
100:1249–1260. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ning H, Yang F, Jiang M, Hu L, Feng K,
Zhang J, Yu Z, Li B, Xu C, Li Y, et al: The correlation between
cotransplantation of mesenchymal stem cells and higher recurrence
rate in hematologic malignancy patients: Outcome of a pilot
clinical study. Leukemia. 22:593–599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yañez R, Lamana ML, García-Castro J,
Colmenero I, Ramírez M and Bueren JA: Adipose tissue-derived
mesenchymal stem cells have in vivo immunosuppressive properties
applicable for the control of the graft-versus-host disease. Stem
Cells. 24:2582–2591. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang Y, Ma S, Zhang Y, Wang Y, Cheng Q,
Wu Y, Jin Y, Zheng D, Wu D and Liu H: IL-1β and TLR4 signaling are
involved in the aggravated murine acute graft-versus-host disease
caused by delayed bortezomib administration. J Immunol.
192:1277–1285. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McDonald GB, Shulman HM, Sullivan KM and
Spencer GD: Intestinal and hepatic complications of human bone
marrow transplantation. Part I. Gastroenterology. 90:460–477. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deeg HJ and Antin JH: The clinical
spectrum of acute graft-versus-host disease. Semin Hematol.
43:24–31. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McDonald GB: Hepatobiliary complications
of hematopoietic cell transplantation, 40 years on. Hepatology.
51:1450–1460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tse WT, Pendleton JD, Beyer WM, Egalka MC
and Guinan EC: Suppression of allogeneic T-cell proliferation by
human marrow stromal cells: Implications in transplantation.
Transplantation. 75:389–397. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Le Blanc K, Rasmusson I, Sundberg B,
Götherström C, Hassan M, Uzunel M and Ringdén O: Treatment of
severe acute graft-versus-host disease with third party
haploidentical mesenchymal stem cells. Lancet. 363:1439–1441. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi SW and Reddy P: Current and emerging
strategies for the prevention of graft-versus-host disease. Nat Rev
Clin Oncol. 11:536–547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagaya R, Mizuno-Kamiya M, Takayama E,
Kawaki H, Onoe I, Tanabe T, Nagahara K and Kondoh N: Mechanisms of
the immunosuppressive effects of mouse adipose tissue-derived
mesenchymal stromal cells on mouse alloreactively stimulated spleen
cells. Exp Ther Med. 7:17–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Masuda J, Takayama E, Strober W, Satoh A,
Morimoto Y, Honjo Y, Ichinohe T, Tokuno SI, Ishizuka T, Nakata T,
et al: Tumor growth limited to subcutaneous site vs tumor growth in
pulmonary site exhibit differential effects on systemic immunities.
Oncol Rep. 38:449–455. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu
W, Shen C, Liu J and Ren X: Myeloid-derived suppressor cells
suppress antitumor immune responses through IDO expression and
correlate with lymph node metastasis in patients with breast
cancer. J Immunol. 190:3783–3797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lim JY, Park MJ, Im KI, Kim N, Jeon EJ,
Kim EJ, Cho ML and Cho SG: Combination cell therapy using
mesenchymal stem cells and regulatory T-cells provides a
synergistic immunomodulatory effect associated with reciprocal
regulation of TH1/TH2 and th17/treg cells in a murine acute
graft-versus-host disease model. Cell Transplant. 23:703–714. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takayama E, Seki S, Ohkawa T, Ami K, Habu
Y, Yamaguchi T, Tadakuma T and Hiraide H: Mouse CD8+
CD122+ T cells with intermediate TCR increasing with age
provide a source of early IFN-gamma production. J Immunol.
164:5652–5658. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Favier B, LeMaoult J and Carosella ED:
Functions of HLA-G in the immune system. Tissue Antigens. 69 Suppl
1:S150–S152. 2007. View Article : Google Scholar
|
|
22
|
Nasef A, Mathieu N, Chapel A, Frick J,
François S, Mazurier C, Boutarfa A, Bouchet S, Gorin NC, Thierry D
and Fouillard L: Immunosuppressive effects of mesenchymal stem
cells: Involvement of HLA-G. Transplantation. 84:231–237. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Melo-Lima BL, Evangelista AF, de Magalhães
DA, Passos GA, Moreau P and Donadi EA: Differential transcript
profiles of MHC class Ib(Qa-1, Qa-2, and Qa-10) and Aire genes
during the ontogeny of thymus and other tissues. J Immunol Res.
2014:1592472014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shiroishi M, Kuroki K, Rasubala L, Tsumoto
K, Kumagai I, Kurimoto E, Kato K, Kohda D and Maenaka K: Structural
basis for recognition of the nonclassical MHC molecule HLA-G by the
leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d). Proc Natl
Acad Sci USA. 103:16412–16417. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carosella ED, Gregori S and LeMaoult J:
The tolerogenic interplay(s) among HLA-G, myeloid APCs, and
regulatory cells. Blood. 118:6499–6505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ulker N, Lewis KD, Hood LE and Stroynowski
I: Activated T cells transcribe an alternatively spliced mRNA
encoding a soluble form of Qa-2 antigen. EMBO J. 9:3839–3847. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Comiskey M, Goldstein CY, De Fazio SR,
Mammolenti M, Newmark JA and Warner CM: Evidence that HLA-G is the
functional homolog of mouse Qa-2, the Ped gene product. Hum
Immunol. 64:999–1004. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ludwig AK and Giebel B: Exosomes: Small
vesicles participating in intercellular communication. Int J
Biochem Cell Biol. 44:11–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alegre E, Rebmann V, Lemaoult J, Rodriguez
C, Horn PA, Díaz-Lagares A, Echeveste JI and González A: In vivo
identification of an HLA-G complex as ubiquitinated protein
circulating in exosomes. Eur J Immunol. 43:1933–1991. 2013.
View Article : Google Scholar : PubMed/NCBI
|