Introduction
Intracerebral hemorrhage (ICH) accounts for 10–15%
of all strokes and is associated with high mortality and morbidity
(1). ICH is characterized by the
rupture of cerebral blood vessels and subsequent leakage of blood,
including blood-intrinsic factors, into the brain parenchyma
(2). Currently, no effective
treatment options are available for ICH. Previous studies have
reported that inflammation is a key factor that contributes to
ICH-induced brain injury (2–4). It is thought that resident microglia
and astrocytes are the early inflammatory cells in ICH (4). Activated inflammatory cells release a
variety of cytokines, chemokines, free radicals and other
potentially toxic chemicals (5–7), which
further aggravate brain injury. Therefore, suppressing microglial
function may be a promising novel strategy for ICH therapy.
Artemisia princeps Pampanini (family
Asteraceae) is an herbal medicine widely used in Korea, China and
Japan. Eupatilin, a pharmacologically active flavone derived from
Artemisia sp. has been reported to have antioxidant,
anti-inflammatory, anti-allergy and anti-tumor activities (8–11). Kim
et al (12) reported that
pre-treatment with eupatilin decreased the production of
interleukin (IL)-8 and prostaglandin E2 induced by Bacteroides
fragilis in HT-29 intestinal epithelial cells. Eupatilin has
been reported to exert neuroprotective activities against
ischemia/reperfusion-induced delayed neuronal injury in mice,
increasing the number of viable cells and decreasing the number of
degenerating neuronal cells in the hippocampal CA1 region (13). However, the effect of eupatilin in
ICH has not been well studied. The aim of the present study was to
investigate the effect of eupatilin on ICH-induced microglial
inflammation.
Materials and methods
Cell culture and reagents
The murine microglial cell line BV2 was purchased
from the American Type Culture Collection (ATCC, Manassas, VA, USA)
and cultured in Dulbecco's Modified Eagle Medium (DMEM)/F12
supplemented with 10% fetal bovine serum (FBS; both Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), 2 mM glutamine, 100
U/ml penicillin and 100 mg/ml streptomycin in a humidified
atmosphere containing 5% CO2 at 37°C. Eupatilin was
supplied by Dong-A Pharmaceutical Co. Ltd. (Yong-In, South Korea)
and dissolved in dimethylsulfoxide for treatment.
MTT assay
An MTT assay was performed to assess microglia
viability. In brief, BV2 cells (1×105 cells/well) were
cultured at 37°C with various concentrations of eupatilin (1, 10 or
50 µM) for 24 h. Cells were incubated with MTT solution (5 mg/ml)
at 37°C for 4 h, following which dimethylsulfoxide was added and
shaken at room temperature for 10 min. The optical density was
determined at 570 nm using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Transwell migration assay
The migration assay was performed using a Transwell
system. The lower compartment was filled with 0.5 ml of DMEM
containing 1% FBS with 10 µl erythrocyte lysis buffer (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) alone or together with
eupatilin (1, 10 or 50 µM). BV2 cells (1×105 cells/well)
were resuspended in 0.1 ml of DMEM and placed in the upper
Transwell chamber, which was subsequently incubated for 24 h at
37°C. Cells on the lower surface of the filter were fixed with 3.7%
paraformaldehyde in PBS at 37°C for 2 h. Cells were then stained
with 5% crystal violet for 30 min at 37°C, washed with PBS three
times at room temperature, and the migratory BV2 cells were counted
under a light microscope (Olympus BX51; Olympus Corporation, Tokyo,
Japan; magnification, ×200) in at least six random fields. All
experiments were performed in triplicate.
ELISA
BV2 microglial cells were seeded at a density of
1×105 cells/well in 24-well tissue culture plates and
cultured at 37°C with various concentrations of eupatilin (1, 10 or
50 µM) for 1 h. Subsequently, wells were stimulated with 10 µl
erythrocyte lysis buffer and supernatants were removed 3 days
later. Tumor necrosis factor-α (TNF-α; cat. no. MTA00B), IL-1β
(cat. no. MLB00C) and IL-6 (cat. no. DY406) expression was measured
using ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA)
according to the manufacturer's protocol. The absorbance at 450 nm
was determined using a microplate reader.
Measurement of intracellular reactive
oxygen species (ROS)
BV2 cells (1×105 cells/well) were
pre-treated with various concentrations of eupatilin (1, 10 or 50
µM) for 1 h followed by 10 µl erythrocyte lysis buffer stimulation
for 24 h. Next, 2-,7-dichlorodihydrofluorescein diacetate
(H2DCF-DA, 5 µM) was added to the cells at 37°C for 20
min. Oxidation of the non-fluorescent H2DCF-DA by
intracellular reactive oxygen species (ROS) results in formation of
the fluorescent compound 2-,7-dichlorofluorescein (DCF). DCF mean
fluorescence intensity (MFI) was monitored with a laser confocal
scanning microscope (Leica Microsystems GmbH, Wetzlar,
Germany).
Western blotting
BV2 cells were lysed in radioimmunoprecipitation
assay buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5%
sodium deoxycholate, and 0.1% SDS) containing protease and
phosphatase inhibitors (5 mM EDTA, 1 mM PMSF, and 1 mM sodium
orthovanadate) for 30 min on ice. The protein content was
determined using a BCA protein assay (Pierce; Thermo Fisher
Scientific, Inc.). Proteins (20 µg) were separated by 10% SDS-PAGE
and transferred to polyvinylidene difluoride membranes (EMD
Millipore, Billerica, MA, USA). The membranes were blocked in 5%
skim milk in TBS containing 0.1% Tween-20 (TBST) for 1 h at room
temperature, followed by incubation with primary antibodies: Rabbit
anti-mouse p-NF-κB p65 (1:1,000; cat. no. sc-135768) and NF-κB p65
(1:1,000, cat. no. sc-71675; both Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) overnight at 4°C. Membranes were washed and
incubated with horseradish peroxidase-conjugated secondary
antibodies for 1 h at room temperature, following which bands were
detected using an enhanced chemiluminescent detection kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The relative intensity of protein signals was normalized to the
corresponding β-actin (1:1,000; cat. no. sc-47778; Santa Cruz
Biotechnology, Inc.) intensity and was quantified by densitometric
analysis using ImageQuant software (version 7.0; GE Healthcare Life
Sciences, Little Chalfont, UK).
Statistical analysis
All data are presented as the mean ± standard
deviation. Each experiment was repeated at least three times in
triplicate, unless otherwise stated. Differences between two groups
were analyzed using paired Student's t-tests. One-way analysis of
variance followed by Student-Newman-Keuls post hoc test was used to
compare differences between multiple groups. The results were
analyzed using SPSS software (version 20.0; IBM Corp, Armonk, NY,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of eupatilin on microglia
viability
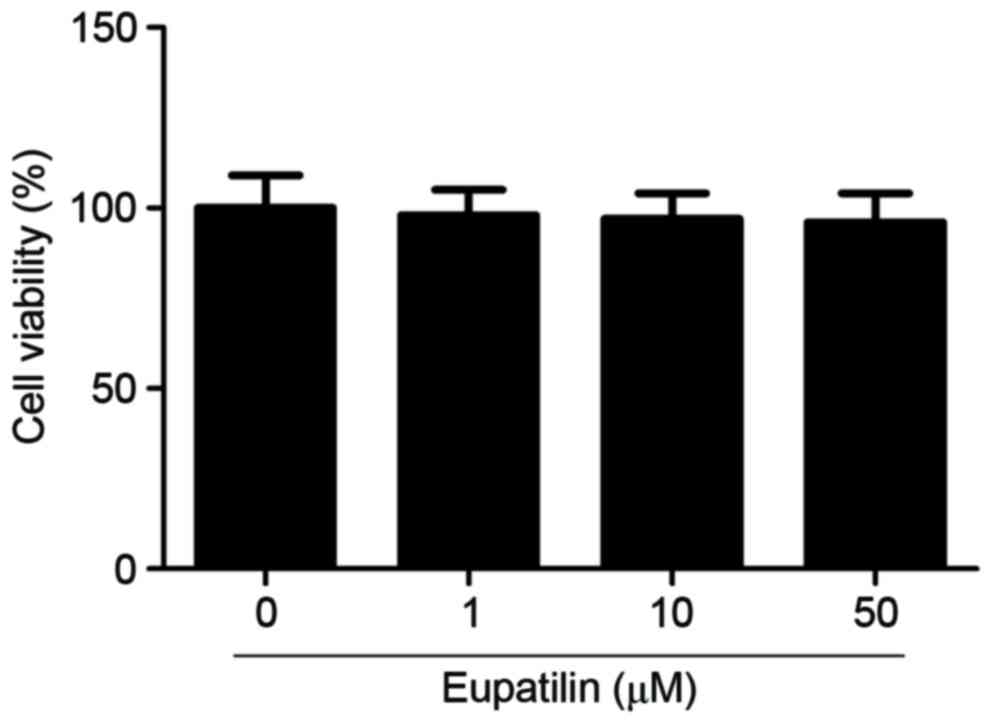
To investigate the effect of eupatilin on microglia
viability, cells were treated with various concentrations of
eupatilin (1, 10 or 50 µM). As indicated in Fig. 1, eupatilin did not significantly
affect microglia viability compared with the control group.
Effects of eupatilin on microglia
migration
The effect of eupatilin on microglia migration was
assessed using a Transwell assay. As indicated in Fig. 2, erythrocyte lysis stimulation
significantly promoted microglia migration compared with the PBS
control, whereas eupatilin significantly suppressed erythrocyte
lysis-induced microglia migration in a dose-dependent manner.
Effects of eupatilin on inflammation
cytokine release
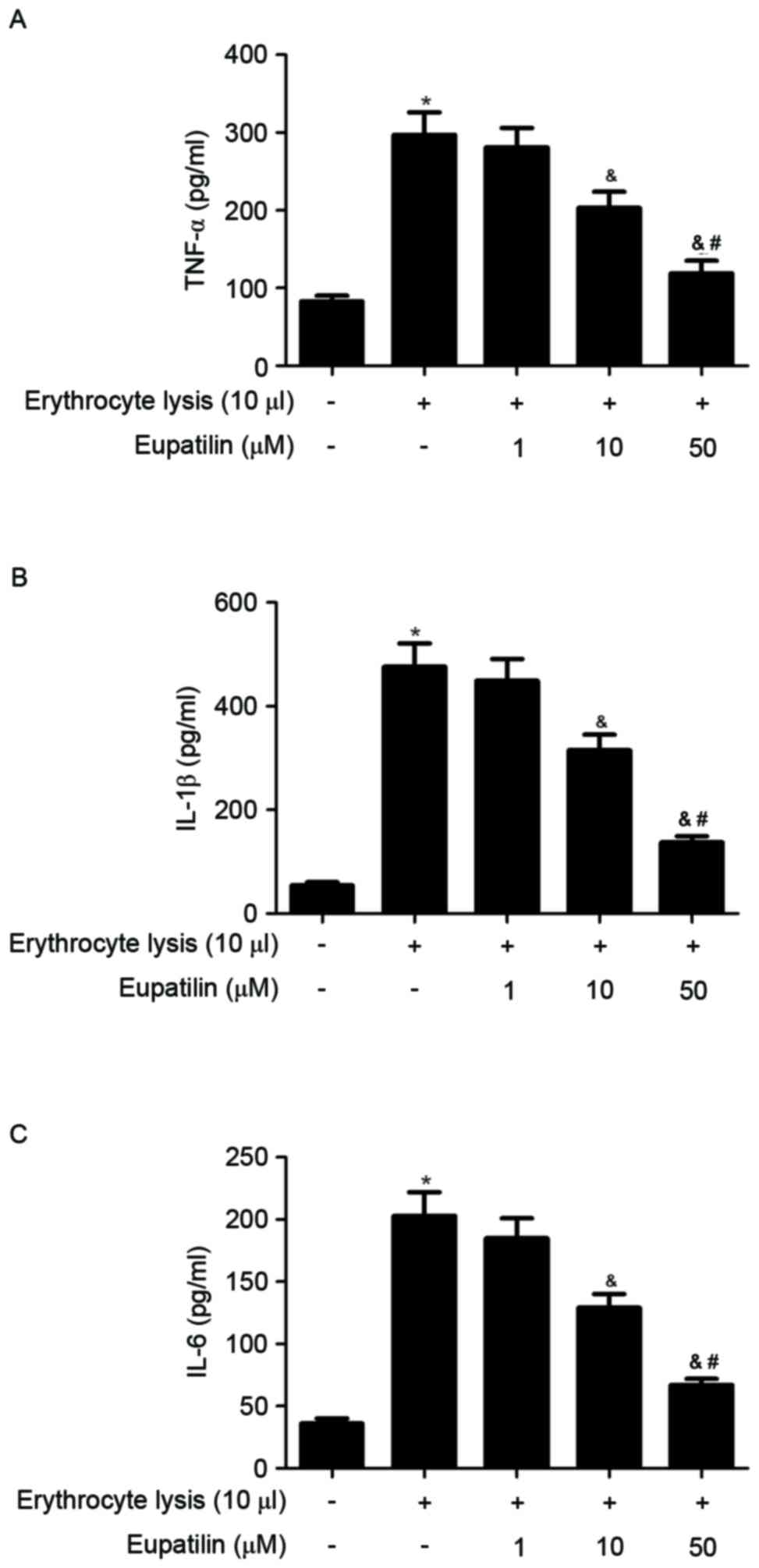
To assess the anti-inflammatory effects of eupatilin
in ICH-induced BV2 microglia, cell culture media were collected and
TNF-α, IL-1β and IL-6 levels were measured in ICH-induced BV2
cells. As indicated in Fig. 3,
erythrocyte lysis stimulation significantly increased the
production of TNF-α, IL-1β and IL-6, whereas eupatilin suppressed
these increases cytokine in a dose-dependent manner.
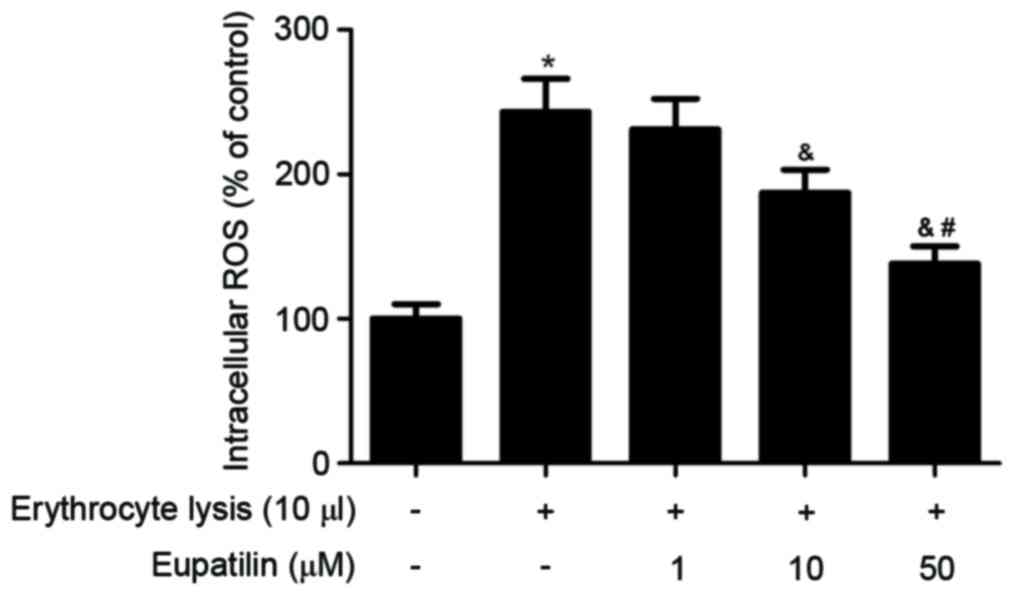
Effect of eupatilin on intracellular
ROS
It has previously been reported that increased
intracellular ROS may serve a critical role in the progression of
ICH (5). The effect of eupatilin on
intracellular ROS production in BV2 cells was therefore
investigated. As indicated in Fig.
4, erythrocyte lysis stimulation significantly increased the
production of ROS. However, eupatilin obviously suppressed
erythrocyte lysis-induced ROS production in BV2 cells.
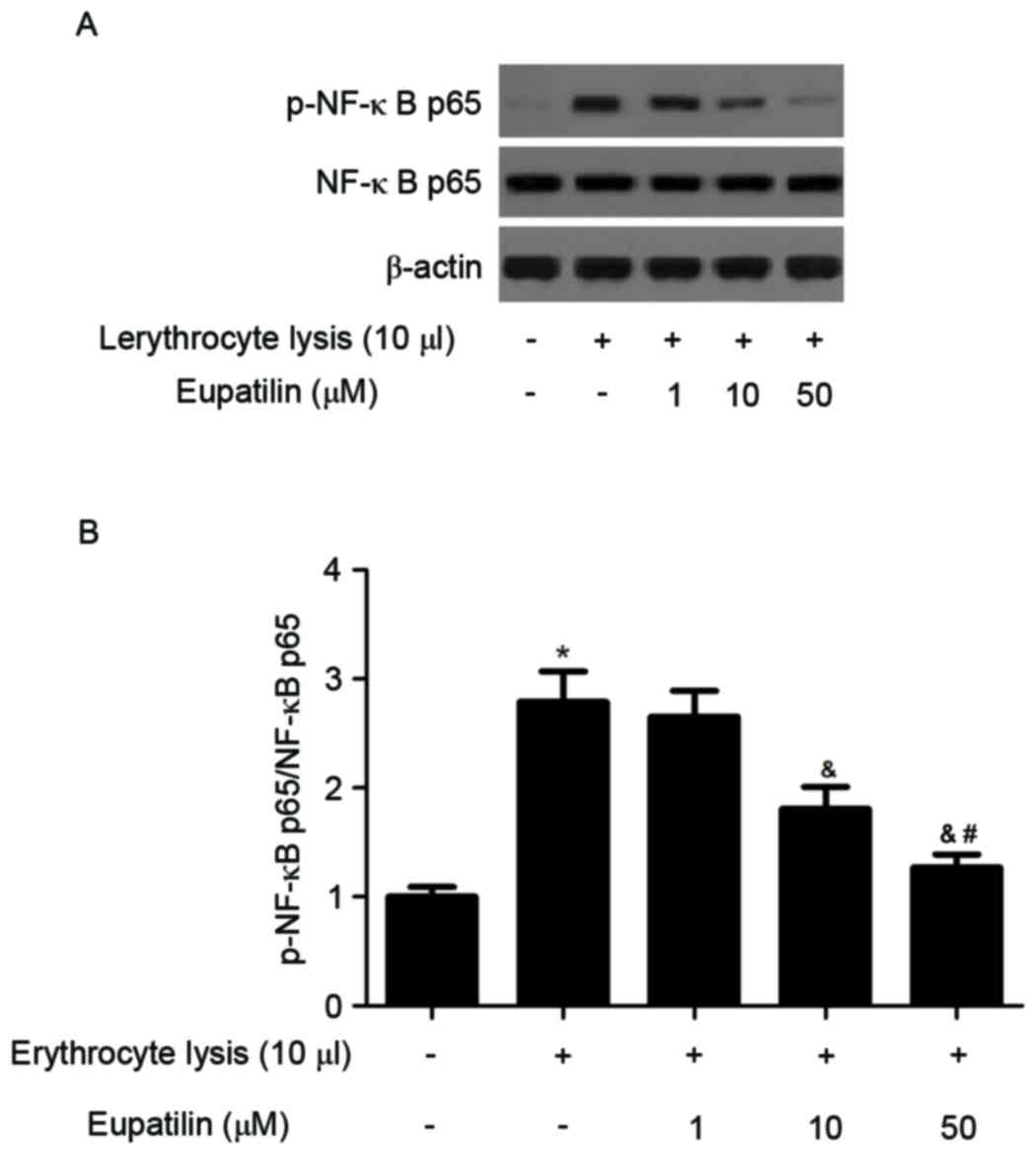
Effect of eupatilin on NF-ĸB
activation
It has been reported that NF-κB is an important
regulator of cell fate and function in the nervous system (7). The effect of eupatilin on NF-κB
activation was investigated using western blotting. As presented in
Fig. 5, erythrocyte lysis
stimulation significantly induced the expression of p-NF-κB p65,
whereas eupatilin reduced erythrocyte lysis-induced NF-κB
activation in BV2 cells.
Discussion
Inflammation serves a critical role in the
pathophysiology of ICH-induced brain injury; however, the mechanism
by which ICH stimulates the inflammatory response remains unclear.
In the present study, it was demonstrated that eupatilin
significantly inhibited microglial migration. It also decreased the
production of inflammatory cytokines and intracellular ROS levels
in erythrocyte lysis-induced BV2 cells. The anti-inflammatory
mechanism of eupatilin was also assessed and the results revealed
that eupatilin was able to inhibit erythrocyte lysis-induced NF-ĸB
activation in BV2 cells.
Microglia are believed to serve a crucial role in
the development ICH (13,14). During ICH, microglia migrate into
damaged tissue to trigger inflammation and wound healing (14). It has been reported that migration
and process motility are typical of activated microglia and serve
to induce a number of regulated cellular functions, including
cytokine production, phagocytosis and antigen production (14). Furthermore, several studies have
reported that microglial migration is increased in erythrocyte
lysis stimulated microglia (15,16). In
the present study, it was demonstrated that erythrocyte lysis
treatment promotes microglial migration, which is consistent with
these previous studies. However, eupatilin significantly inhibited
microglial migration.
In animal models, microglial cells are activated in
the brain following ICH (16).
Microglia are critical regulators of the neuron-inflammatory
response and major contributors to the excessive production of
pro-inflammatory cytokines (2). It
has been reported that pro-inflammatory cytokines serve important
roles in exacerbating ICH-induced brain injury (17). TNF-α is significantly increased in
ICH models and may contribute to the formation of brain edema and
brain injury (4,17), while IL-6 is a multifunctional
cytokine that serves an important role in host defense and has
major regulatory effects in the inflammatory response (18). The results of the present study
demonstrate that erythrocyte lysis stimulation significantly
increases the production of TNF-α, IL-1β and IL-6, whereas
eupatilin suppresses this effect in a dose-dependent manner. These
data suggest that eupatilin may be able to inhibit ICH-induced
microglia mediated inflammation.
ROS production following ICH contributes to ICH
pathogenesis (19). Several lines of
evidence indicate that ROS serve as secondary messengers to encode
and enhance the expression of pro-inflammatory factors (20,21).
Furthermore, intracellular ROS accumulation in microglia has been
reported to trigger the release of inflammatory mediators via the
activation of signaling molecules, including mitogen-activated
protein kinases and NF-κB (22). In
the present study, it was reported that eupatilin downregulates
erythrocyte lysis-induced intracellular ROS in BV-2 cells. These
results suggest that eupatilin has a neuroprotective effect that is
achieved via the inhibition of ROS production in microglia.
NF-κB serves a crucial role in regulating immunity
and inflammation in central nervous system injuries, including ICH
(23–25). Zhang et al (26) reported that NF-κB activation was
increased in perihematomal brain tissue following ICH. It has also
been suggested that NF-ĸB activation in microglia following ICH
results in the upregulation of pro-inflammatory cytokines,
including TNF-α and IL-1β, and contributes to brain injury
(27). The present study
demonstrates that eupatilin is able to prevent erythrocyte
lysis-induced NF-κB activation in BV2 cells. These results suggest
that the anti-inflammatory effect of eupatilin may result from
inhibition of the NF-κB signaling pathway.
In conclusion, the results of the present study
suggest that eupatilin serves a neurological protective effect via
inhibiting microglial inflammation. The present study may provide
an experimental basis for the use of eupatilin as a therapeutic
target for ICH.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed during the
current study are not publicly available due to further research
being performed, but are available from the corresponding author on
reasonable request.
Authors' contributions
HBQ and JL designed the study. LJL, BJN, PL and FX
performed the experiments. ZMZ analyzed the data.
Ethics approval and consent to
participate
All patients were required to provide written
informed consent prior to their inclusion. The study was approved
by the Ethical Committee of Dezhou People's Hospital.
Patients' consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huttner HB, Köhrmann M, Tognoni E, Jüttler
E, Richter G, Dörfler A, Reulbach U, Bassemir T, Staykov D,
Bardutzky J, et al: Clinical severity predicts time to hospital
admission in patients with spontaneous intracerebral hemorrhage.
Cerebrovasc Dis. 25:533–538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang J: Preclinical and clinical research
on inflammation after intracerebral hemorrhage. Prog Neurobiol.
92:463–477. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang X, Li H, Hu S, Zhang L, Liu C, Zhu
C, Liu R and Li C: Brain edema after intracerebral hemorrhage in
rats: The role of inflammation. Neurol India. 54:402–407. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang J and Doré S: Inflammation after
intracerebral hemorrhage. J Cereb Blood Flow Metab. 27:894–908.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Aronowski J and Hall CE: New horizons for
primary intracerebral hemorrhage treatment: Experience from
preclinical studies. Neurol Res. 27:268–279. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao Z, Wang J, Thiex R, Rogove A, Heppner
F and Tsirka S: Microglial activation and intracerebral hemorrhage.
Acta Neurochir Suppl. 105:51–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang J and Tsirka SE: Contribution of
extracellular proteolysis and microglia to intracerebral
hemorrhage. Neurocrit Care. 3:77–85. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Choi EJ, Oh HM, Na BR, Ramesh T, Lee HJ,
Choi CS, Choi SC, Oh TY, Choi SJ, Chae JR, et al: Eupatilin
protects gastric epithelial cells from oxidative damage and
down-regulates genes responsible for the cellular oxidative stress.
Pharm Res. 25:1355–1364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi EJ, Lee S, Chae JR, Lee HS, Jun CD
and Kim SH: Eupatilin inhibits lipopolysaccharide-induced
expression of inflammatory mediators in macrophages. Life Sci.
88:1121–1126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JY, Kwon EY, Lee YS, Kim WB and Ro JY:
Eupatilin blocks mediator release via tyrosine kinase inhibition in
activated guinea pig lung mast cells. J Toxicol Environ Health A.
68:2063–2080. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi EJ, Oh HM, Wee H, Choi CS, Choi SC,
Kim KH, Han WC, Oh TY, Kim SH and Jun CD: Eupatilin exhibits a
novel anti-tumor activity through the induction of cell cycle
arrest and differentiation of gastric carcinoma AGS cells.
Differentiation. 77:412–423. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim J, Lee D, Kim J, Lee J, Park HG, Kim
YJ, Oh YK, Jung H and Kim S: 5,7-dihydroxy-3,4,6-trimethoxyflavone
inhibits the inflammatory effects induced by Bacteroides
fragilis enterotoxin via dissociating the complex of heat shock
protein 90 and I kappaB alpha and I kappaB kinase-gamma in
intestinal epithelial cell culture. Clin Exp Immunol. 155:541–551.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cai M, Phan PT, Hong JG, Kim DH, Kim JM,
Park SJ, Liu X, Han JE, Park H, Choi JW and Ryu JH: The
neuroprotective effect of eupatilin against
ischemia/reperfusion-induced delayed neuronal damage in mice. Eur J
Pharmacol. 689:104–110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Garden GA and Möller T: Microglia biology
in health and disease. J Neuroimmune Pharmacol. 1:127–137. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Z, Zhao T, Zou Y, Zhang JH and Feng
H: Curcumin inhibits microglia inflammation and confers
neuroprotection in intracerebral hemorrhage. Immunol Lett.
160:89–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Z, Liu Y, Yuan F, Li Z, Huang S, Shen
H and Yuan B: Sinomenine inhibits microglia activation and
attenuates brain injury in intracerebral hemorrhage. Mol Immunol.
60:109–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin S, Yin Q, Zhong Q, Lv FL, Zhou Y, Li
JQ, Wang JZ, Su B and Yang QW: Heme activates TLR4-mediated
inflammatory injury via MyD88/TRIF signaling pathway in
intracerebral hemorrhage. J Neuroinflammation. 9:462012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Burton MD, Rytych JL, Freund GG and
Johnson RW: Central inhibition of interleukin-6 trans-signaling
during peripheral infection reduced neuroinflammation and sickness
in aged mice. Brain Behav Immun. 30:66–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nakamura T, Keep RF, Hua Y, Hoff JT and Xi
G: Oxidative DNA injury after experimental intracerebral
hemorrhage. Brain Res. 1039:30–36. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qin L, Liu Y, Wang T, Wei SJ, Block ML,
Wilson B, Liu B and Hong JS: NADPH oxidase mediates
lipopolysaccharide-induced neurotoxicity and proinflammatory gene
expression in activated microglia. J Biol Chem. 279:1415–1421.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee IT and Yang CM: Role of NADPH
oxidase/ROS in pro-inflammatory mediators-induced airway and
pulmonary diseases. Biochem Pharmacol. 84:581–590. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Asehnoune K, Strassheim D, Mitra S, Kim JY
and Abraham E: Involvement of reactive oxygen species in Toll-like
receptor 4-dependent activation of NF-kappaB. J Immunol.
172:2522–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
You WC, Wang C, Pan Y, Zhang X, Zhou X,
Zhang X, Shi JX and Zhou Ml: Activation of nuclear factor-κB in the
brain after experimental subarachnoid hemorrhage and its potential
role in delayed brain injury. PLoS One. 8:e602902013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koedel U, Bayerlein I, Paul R, Sporer B
and Pfister H: Pharmacologic interference with NF-κB activation
attenuates central nervous system complications in experimental
pneumococcal meningitis. J Infect Dis. 182:1437–1445. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kaltschmidt B and Kaltschmidt C: NF-κB in
the nervous system. Cold Spring Harb Perspect Biol. 1:a0012712009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Z, Liu Y, Huang Q, Su Y, Zhang Y,
Wang G and Li F: NF-κB activation and cell death after
intracerebral hemorrhage in patients. Neurol Sci. 35:1097–1102.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wagner KR: Modeling intracerebal
hemorrhage glutamate, nuclear factor-κB signaling and cytokines.
Stroke. 38 Suppl 2:S753–S758. 2007. View Article : Google Scholar
|