Introduction
Currently, it is suggested that the main reason for
aseptic loosening (AL) of an artificial joint prosthesis is due to
an inflammatory osteolytic reaction induced by wear particles
(1,2). Studies have suggested that wear
particles, including metal particles (titanium, cobalt),
ultra-high-molecular-weight polyethylene (UHMWPE), and
polymethylmethacrylate, are largely responsible for the initial
stage and progression of an inflammatory osteolytic reaction
(3–6). A chronic inflammatory reaction induced
by wear particles triggers macrophage infiltration and cytokine
release associated with osteolysis, suppression of osteoblast
proliferation and differentiation, and promotion of osteoclast
activation. Therefore, osteoclast differentiation induced by wear
particles is a determinant of inflammatory osteolysis (7–10). The
receptor activator of nuclear factor-κB (RANK)-RANK ligand
(RANKL)-osteoprotegerin (OPG) signal transduction pathway is a
crucial signaling pathway for osteoclast differentiation and
maturation and influences the key process of osteolysis around the
prosthesis. Medication inhibiting osteoclast activity is an
effective method of treating osteolysis. Drugs, including estrogen
and bisphosphonates, can inhibit bone resorption; however,
significant side effects limit their long-term use. There is a
herbal decoction that inhibits osteoclast action in the treatment
of osteoporosis, in which neomangiferin is one of the important
active ingredients. Neomangiferin, derived from Anemarrhena
plants, has a molecular formula of
C25H28O16 and molecular weight of
584.4802. In addition, neomangiferin has anti-inflammatory,
antioxidant, anti-osteoporotic, and liver and kidney protective
biological activities (11–14). In our previous experiments (Wang
et al; unpublished data), it was found that neomangiferin
inhibited osteoclast differentiation in vitro, however, the
exact mechanism remains to be elucidated. Therefore, the purpose of
the present study was to investigate the role of neomangiferin in
the inhibition of inflammatory osteolysis through in vivo
animal experiments and to examine the possible mechanism of action
of neomangiferin, thereby providing therapeutic options for the
prevention or treatment of metabolic bone diseases induced by wear
particles.
Materials and methods
Preparation of the UHMWPE particle
suspension
Pure UHMWPE particles were purchased from Germany
Clariant (Gersthofer, Germany). The average particle diameter was
1.84±1.50 µm. It was estimated that >32% of the particles were
<1 µm in size. The UHMWPE particles were soaked in 75% ethanol
for 48 h to remove toxins; subsequently, the cells were
cryogenically sealed with standard ethylene oxide. The UHMWPE
particles were cleaned three times with phosphate-buffered saline
(PBS) and formulated into a 100-mg/ml UHMWPE particle suspension
with cryogenic PBS prior to use.
Animals
The animal model was designed according to previous
reports (15,16). The neomangiferin was screened for
osteoclast differentiation, and it was found that the neomangiferin
inhibited the formation of osteoclasts at a concentration of 2.5
µmol/l (data not shown). The concentration of Chinese herbs
required to inhibit osteoclasts in vivo is a low
concentration of 1–10 mg/kg and a high concentration of 2–30 mg/kg
(17–18). In the present study, the doses in
vivo were calculated (low and high concentrations of 2.5 and 5
mg/kg, respectively) according to the weight of mice and the
content of body fluid (data not shown). A total of 24, 8 week old
C57BL/6 mice (specific pathogen free grade) were provided by the
Experimental Animal Center of Guangxi Medical University (Nanning,
China). Mice were housed at a temperature of 22–24°C, a humidity of
56% and interval lighting (12 h dark/light cycle), with regular
ventilation. All mice were fed standard laboratory chow with ad
libitum water, but were fasted from 10:00 to 15:00 prior to
experimentation. The animals were randomly divided into four groups
(n=6 per group): Negative control group (injected with PBS only);
positive control group (UHMWPE particles + PBS); neomangiferin
(purity >98% by high-performance liquid chromatography; Chengdu
Manster Biotechnology Co., Ltd., Sichuan, China), low-dose group
(UHMWPE particles + 2.5 mg/kg neomangiferin), and neomangiferin
high-dose group (UHMWPE particles + 5 mg/kg neomangiferin). The
animal experimental protocol was approved by the Animal Ethics
Committee of Guangxi Medical University (approval no. 201707006),
and the Guidelines for Care and Use of Laboratory Animals were
strictly followed.
According to the body weight of each mouse,
anesthesia was induced intraperitoneally with 4% chloral hydrate
(400 mg/kg mouse body weight). Following successful anesthesia, a
depilatory agent was used to adequately remove iodine from the hair
of the mouse. Following placement on a disposable sterile towel,
the mouse skull sagittal line and mouse bilateral external auditory
canal connection was selected as a reference point. Subsequently,
via the midpoint of the connection, incisions of the skin and
subcutaneous tissue were made using 15 small circular knives. Mouse
calvarial bone was fully exposed in an area of ~1×1 cm of
full-range periosteum. A 2×2 mm periosteum defect area was created
using a small round knife at the top of the cranium. The control
group was syringe-injected with sterile PBS (100 µl); the other
three groups were injected with UHMWPE particles (100 µl, 100
mg/ml). The skin wound was closed with sutures postoperatively to
prevent drug spillover. Following these procedures, the mice were
housed separately. Penicillin was routinely used to ensure
anti-infective surgery. At 2 days post-surgery, the negative
control group and the positive control group were injected with 100
µl PBS; the other two groups were injected with neomangiferin at
2.5 or 5 mg/kg. The drug was injected every other day for 21 days.
None of the mice died; therefore, the model was successful
(19,20). At 3 weeks post-surgery, each group of
mice was anesthetized with ether, and blood samples from the celiac
artery were collected for ELISA assays. The mice were sacrificed by
cervical dislocation, following which the intact skull was
collected, trimmed, and immersed in 10% paraformaldehyde for fixing
and later use.
Serum ELISA
In each group, blood was collected from the celiac
artery of the mouse. Subsequently, serum was prepared via
centrifugation (350 × g for 10 min at 4°C) and analyzed using an
ELISA kit (Wuhan Landing Medical Hi-Tech Co., Ltd., Hubei, China).
The levels of osteoclast-related receptor (OSCAR), RANKL,
cross-linked C-telopeptide of type I collagen (CTX-1), OPG,
interleukin lβ (IL-1β), and tumor necrosis factor (TNF)-α were
assayed.
Micro-computed tomography (micro-CT)
examination
Following fixing in paraformaldehyde (40 g/l)
solution for 1 day, the skulls from each group, with UHMWPE
particles removed, were scanned by micro-CT (Skyscan1176; Bruker
microCT, Kontich, Belgium). The parameters were set as follows:
Resolution 18 µm, current 100 mA, voltage 80 kV, and exposure time
100 ms. Bone mineral density (BMD) and the bone volume/tissue
volume ratio (BV/TV) were analyzed by software measurement
(Skyscan1176; Skyscan CT analyser v1.115.2.2+; Bruker microCT).
Bone resorption pits and porosity were quantified using Image J
software (version 1.36; NIH, Bethesda, MA, USA).
Histological analysis
Following micro-CT examination, the skulls in each
group were placed in an EDTA solution for decalcification. The
decalcification solution was replaced every 2 days. The samples
were embedded in paraffin and sectioned (section thickness 4 µm).
Five consecutive sections were stained with hematoxylin and eosin
(H&E) for each specimen to observe the inflammatory osteolysis
necrotic responses. Subsequently, tartrate-resistant acid
phosphatase (TRAP) reagent (Sigma, EMD Millipore, Billerica, MA,
USA) staining was applied. The sections were placed in the TRAP
scanning liquid and incubated for 50 min at 37°C, until red
wine-colored staining of the osteoclasts was observed under an
optical microscope (DM4000B; Leica Microsystems GmbH, Wetzlar,
Germany). High-power microscopic examination of images was
performed as follows: Each section was subjected to osteoclast
counting in five visual fields. Using Image Pro-Plus 6 software
(Media Cybernetics, Inc., Bethesda, MD, USA), TRAP staining
analysis (+) was performed to determine the number of osteoclasts
and region of osteolysis.
Statistical analysis
Experimental results are represented as the mean ±
standard deviation. Statistical analyses were performed with the
SPSS Statistics Package 19.0 (IBM SPSS, Armonk, NY, USA). One-way
analysis of variance (ANOVA) was performed to compare groups. If
P<0.05 in AVONA, the SNK-q test was used for any pairwise
comparisons; P<0.05 was considered to indicate a statistically
significant difference.
Results
Micro-CT scanning
The Micro-CT examination revealed, as shown in
Fig. 1A, that the skull surface in
the vehicle group underwent severe destruction. By contrast, in the
sham group of mice, the skull surface was smooth, without
significant osteolysis. In the vehicle group and the low- and
high-dose neomangiferin groups, osteolysis occurred to varying
degrees. Specifically, the surface area of the skull samples was
characterized in terms of the depth of bone resorption fossa. The
above data showed that the UHMWPE particles induced osteolysis.
Neomangiferin significantly inhibited bone destruction, increased
BMD (Fig. 1B) and BV/TV (Fig. 1C), and decreased the number of bone
resorption pits and total porosity within the skull region of
interest (Fig. 1D and E). This
analysis confirmed that neomangiferin effectively inhibited the
calvarial osteolysis induced by UHMWPE particles. Following
neomangiferin treatment, skull destruction was mild, with less
damage observed in the high-dose neomangiferin group. The analysis
of bone status (Fig. 1B-E) confirmed
that the effect of high-dose neomangiferin on bone resorption was
significantly greater than that of low-dose neomangiferin.
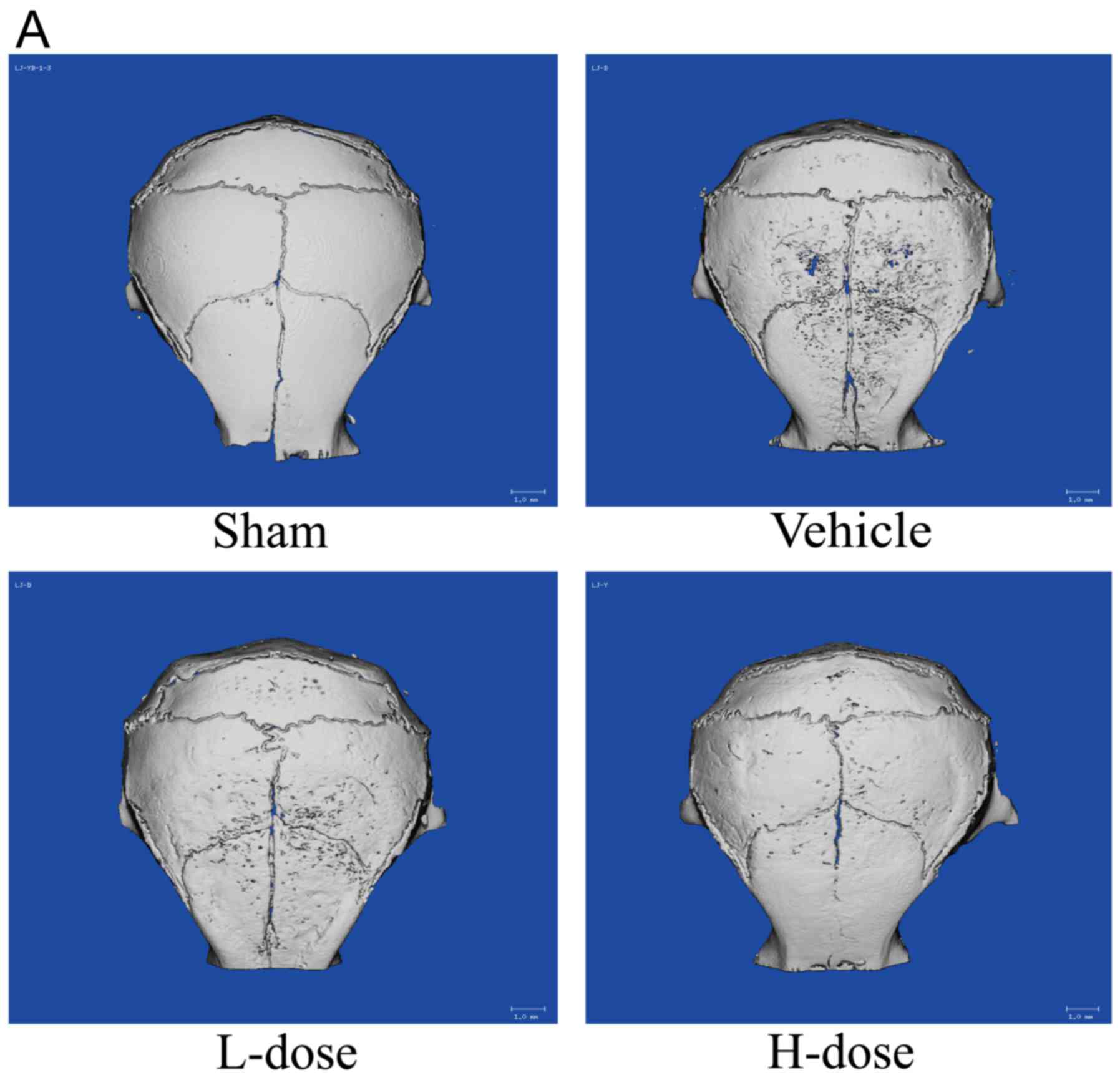 | Figure 1.Neomangiferin inhibits UHMWPE
particle-induced calvarial osteolysis in mice. (A) Micro-computed
tomography scans of the chondral callus induced by UHMWPE particles
in each group. (B) BMD, (C) BV/TV, (D) pit number, (E) porosity
percentage of each experimental specimen (within ROI, 6×6 mm); n=6.
Following one-way analysis of variance, an SNK-q test was performed
to determine statistical significance. Results are expressed as the
mean ± standard deviation (*P<0.05, **P<0.01 and
***P<0.001, compared with the positive control group). UHMWPE,
ultra-high-molecular-weight polyethylene; ns, no statistical
significance; BMD, bone mineral density; BV/TV, bone volume/tissue
volume ratio; L-dose, low dose (2.5 mg/kg neomangiferin); H-dose,
high dose (5 mg/kg neomangiferin). |
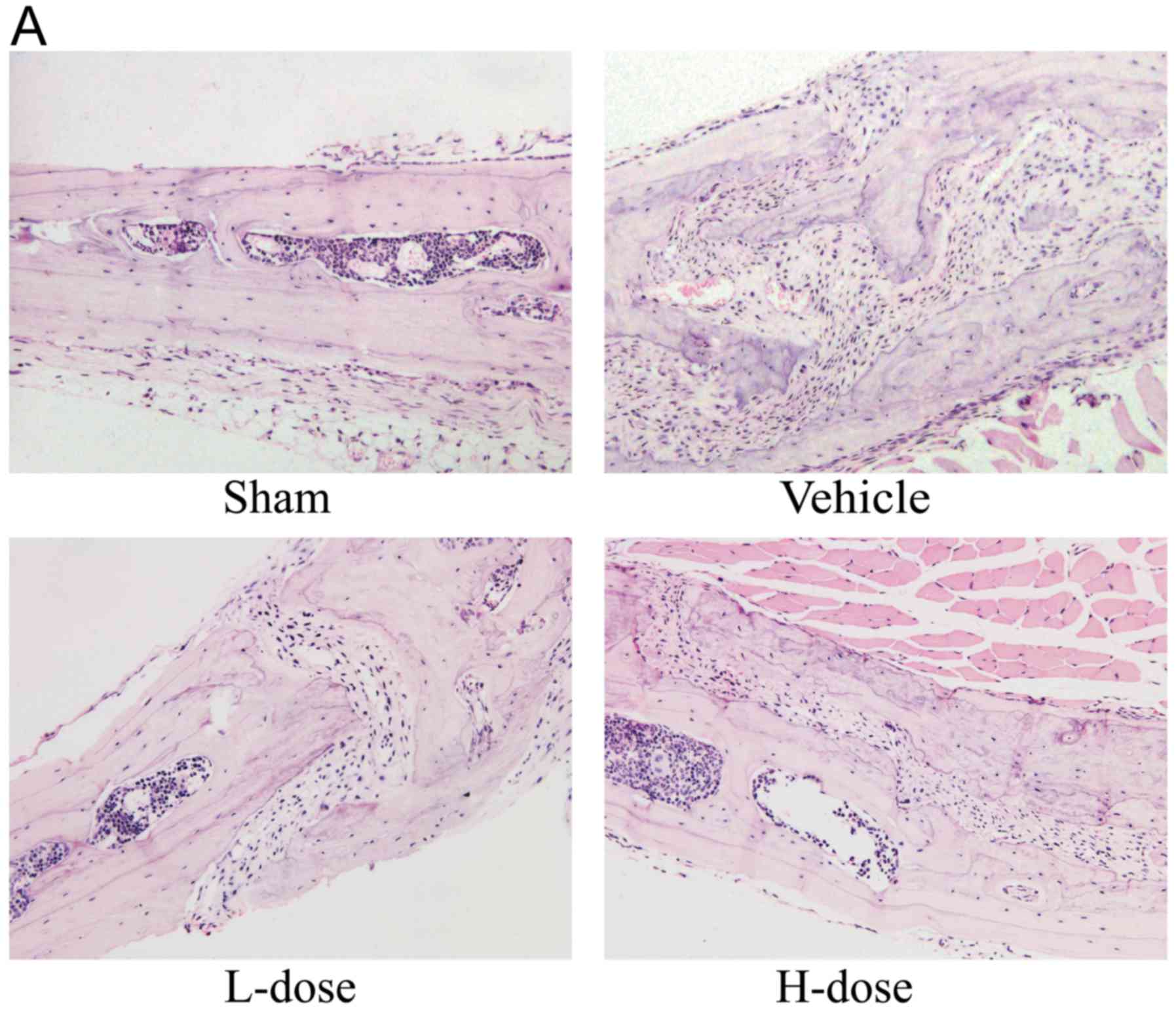
Histopathological analysis via H&E
staining and TRAP staining
Inflammatory cells, macrophages, and multinucleated
osteoclasts were detected in the skulls of mice that received
UHMWPE particles (Fig. 2A). In
agreement with the micro-CT examination results, TRAP staining
showed that a greater number of positively stained cells were
observed on the cranial surface of the vehicle group (Fig. 2B). The quantity of TRAP-positive
cells was decreased as the neomangiferin dose increased (Fig. 2C). In addition, the
histomorphological observations revealed that the area of bone
erosion was markedly decreased following neomangiferin application
(Fig. 2D). Therefore, these results
indicated that neomangiferin inhibited UHMWPE particle-induced
osteolysis.
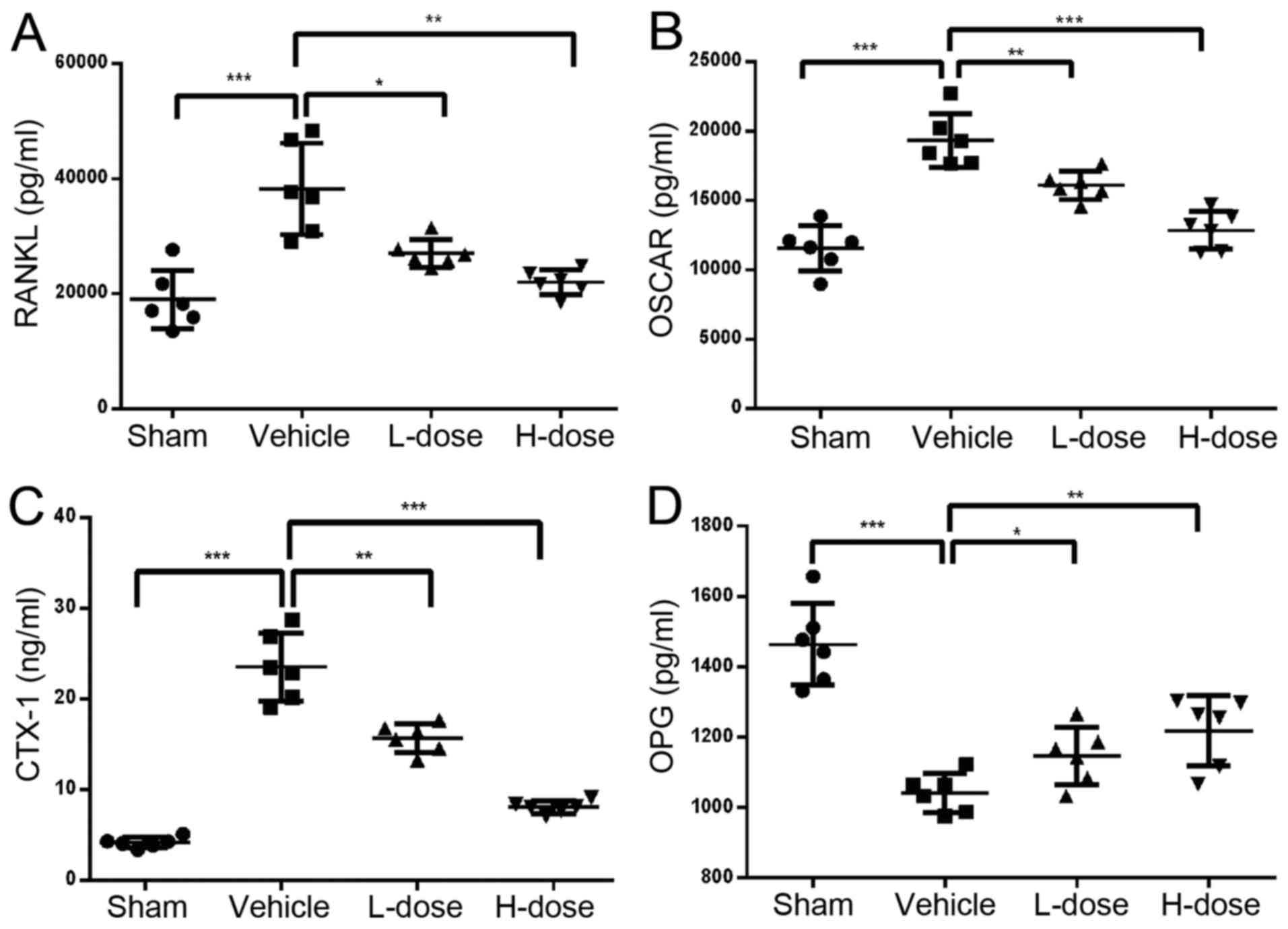
Expression of RANKL, OSCAR, OPG,
CTX-1, TNF-α and IL-1β
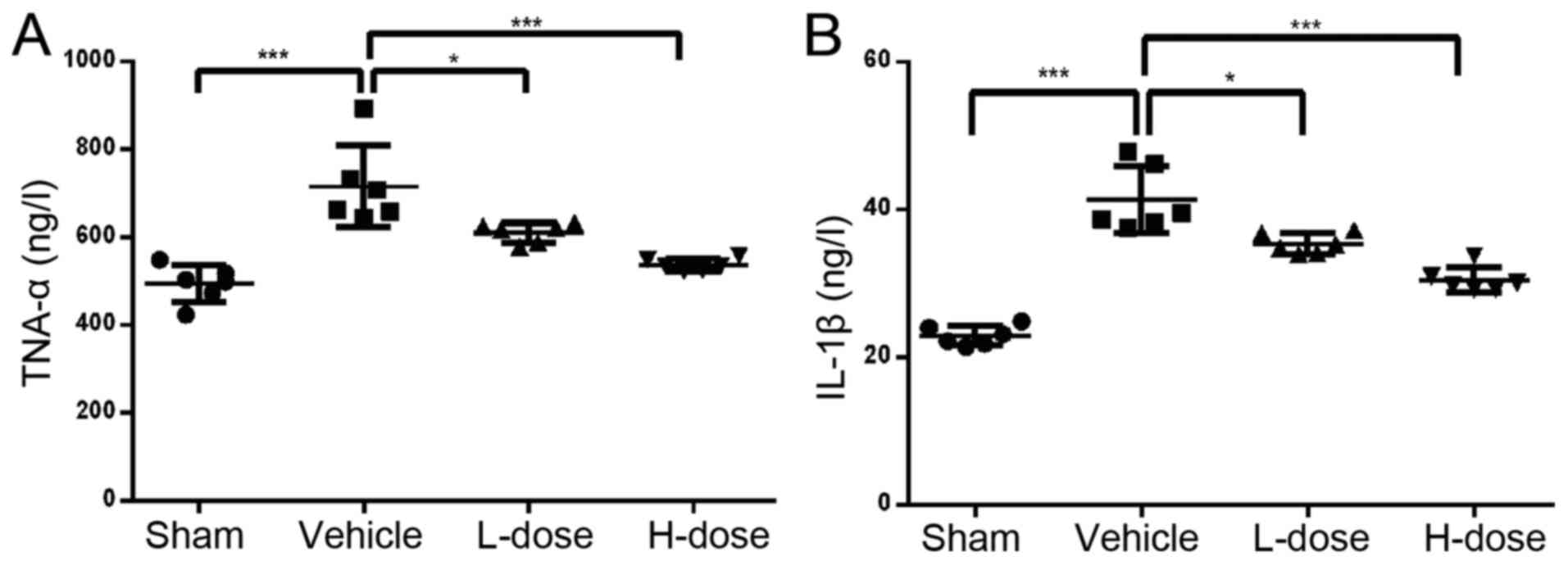
The ELISA results of celiac arterial blood showed
that the expression levels of TNF-α and IL-1β in the drug treatment
groups were lower those in the positive control group, and the
greater the dose, the lower the concentration (Fig. 3A and B). The expression levels of
RANKL, OSCAR and CTX-1 were highest in the positive control group
compared with those in the other groups (P<0.05; Fig. 4A-C). In addition, the expression of
CTX-1, RANKL and OSCAR decreased with the increase in neomangiferin
dose. The expression of OPG was the lowest in the positive control
group. In the high-dose neomangiferin group, the expression of OPG
was significantly increased (Fig.
4D). Neomangiferin reduced the expression of RANKL, OSCAR and
CTX-1, increased the expression of OPG, and inhibited the
expression of proinflammatory factors TNF-α and IL-1β. These
results were consistent with the previous ELISA analysis, micro-CT
examination, and pathological observations. Therefore, these
findings suggested that neomangiferin inhibited osteoclast
proliferation and differentiation during UHMWPE particle-induced
osteolysis, and thereby protected and promoted osteoblast
differentiation.
Discussion
AL is one of the main reasons for the failure of
artificial joint replacements, and prosthesis loosening is
considered to be associated with several factors. Studies have
shown that the AL of prostheses is associated with extensive
infiltration of wear particles. Prosthetic wear particle-induced
osteolysis at the bone interface (prosthesis-osteoclastic
interface) is a leading cause of loosening of prostheses (21). The prosthesis wear particles can
induce an inflammatory reaction involving macrophages in the tissue
surrounding the prosthesis, osteoclast activation, and inhibition
of osteogenic gene expression (22–24).
Inhibition of an early inflammatory response can guarantee implant
bone interface stability at the early stage, thereby reducing the
influence of wear particles and the physical transfer of the
inflammatory media (25). Therefore,
inhibiting the inflammatory reaction caused by wear particles and
inhibiting osteoclast differentiation are some of the primary means
to reduce or prevent the prosthetic loosening. However, there
remains a lack of modalities and drugs for the treatment of these
problems, and side effects are difficult to manage (26–30). The
extract of a Chinese herbal medicine has shown efficacy and mild
side effects, thus providing an innovative idea for the treatment
of bone destruction-related diseases (31–34).
Neomangiferin is a compound from a Chinese medicine substance,
which has antioxidative, anti-inflammatory, antiviral,
immunoregulatory and antitumor effects (11,35).
Neomangiferin is more bioactive than previous forms of mangiferin
in order to have a more marked effect on osteolysis (36,37). The
traditional Chinese medicine Erxian decoction can promote bone
tissue formation, suppress bone resorption and increase bone
mineral density, and neomangiferin is one of the main active
ingredients. In our previous study, it was found that neomangiferin
inhibited osteoclast differentiation in vitro, whereas in
vivo neomangiferin inhibited the inflammatory osteolytic
effect; however, the possible mechanism remained to be fully
elucidated. The mouse model of calvarial inflammatory osteolysis
induced by UHMWPE particles used in the present study can simulate
joint aseptic osteolysis. Neomangiferin treatment of this tissue
environment inhibited osteoclast formation; therefore, it was
analyzed for possible curative effects on osteolysis. Specifically,
the possible mechanisms underlying the inhibition of UHMWPE
particle-induced inflammatory osteolysis were examined.
The results of the micro-CT and histological
morphological analyses showed that neomangiferin attenuated UHMWPE
particle-induced osteolysis of the skull. In addition, with
increased drug concentration, neomangiferin reduced the number of
osteoclasts and bone damage. In terms of the mechanism underlying
the neomangiferin-driven suppression of osteolysis, the following
two assumptions can be made: First, the animal model with
neomangiferin injection demonstrated inhibition of osteoclast
activity. Prosthesis wear particles stimulate monocyte-derived
precursor cell macrophages to differentiate into osteoclasts and
enhance their activity, thereby disrupting the dynamic balance of
bone resorption and bone formation (38,39).
Therefore, the inhibition of osteoclast proliferation and
differentiation is a key treatment aim for osteolysis induced by
wear particles. In the present study, compared with the negative
control group, the number of TRAP-positive cells in the UHMWPE
particle group increased significantly. By contrast, the number of
TRAP-positive cells in the animal model treated with neomangiferin
was significantly lower than in the UHMWPE particle group. In the
present study, following neomangiferin treatment, the extent of
skull erosion and severity of damage were significantly decreased.
Combined with results of the previous experiment, the present data
suggested that neomangiferin may inhibit osteoclast formation and
differentiation, thus inhibiting osteolysis. Second, neomangiferin
may inhibit bone destruction by disrupting the dynamic balance
between OPG and RANKL. The combination of RANKL and RANK can
regulate the phosphorylation of several downstream signal pathways,
including NF-κB, nuclear factor of activated T-cells, and
extracellular signal-regulated kinase, in addition to promoting
osteoclast proliferation and differentiation, and activating
osteoclast maturation to induce bone resorption (40). OPG is expressed in bone marrow
stromal cells and osteoblasts. By binding to RANKL, OPG inhibits
the binding of RANKL to RANK and prevents the overproduction of
osteoclasts (41). Wear
particle-induced inflammatory osteolysis can upregulate the
expression level of RANKL and inhibit the expression of OPG,
thereby inducing osteoclast formation and promoting bone resorption
(42–44). Therefore, the dynamic balance of OPG
and RANKL affects the wear particle-induced level of osteolysis
(45). Compared with the negative
control group, the expression levels of OSCAR, RANKL and CTX-1 were
increased in the positive control group, whereas that of OPG was
decreased. Following 3 weeks of neomangiferin treatment, the
expression level of OPG was increased, whereas the expression
levels of RANKL, OSCAR and CTX-1 were decreased. Based on the above
results, it was hypothesized that neomangiferin suppresses the
osteolysis induced by UHMWPE particles by regulating the expression
of RANKL and OPG.
Wear particles can induce monocytes to produce
cytokines, including IL-1β and TNF-α, within the OPG-RANKL-RANK
signaling pathway and induce the activation of monocyte-derived
macrophage precursor cells (46,47).
Additionally, wear particles can transform them into activated
osteoclasts, induce fibroblast release of collagenase and
prostaglandin E2 associated with bone resorption, and inhibit type
I collagen synthesis and osteocalcin in osteoblasts (8), and thus accelerate bone destruction and
osteolysis. In the present study, the results of micro-CT, H&E
staining, TRAP staining, and serological ELISA showed that the
number of osteoclasts and bone resorption pits in the neomangiferin
group decreased with increasing drug concentration. Consequently,
the extent of damage decreased in the region of mouse calvarial
bone resorption. The experiments showed that UHMWPE particles
significantly upregulated the expression of inflammatory cytokines,
however, neomangiferin significantly inhibited the expression of
IL-1β and TNF-α and osteoclastogenesis. TNF-α and IL-1β are the
major inflammatory cytokines found in the surrounding tissues of
loose prostheses. They are considered as an effective medium for
bone resorption. The expression and activity of RANKL are regulated
by these proinflammatory cytokines, further supporting their key
role in wear particle-induced osteolysis (41,48,49).
Furthermore, the inhibitory effect was dose-dependent. Therefore,
neomangiferin may inhibit the inflammatory bone resorption induced
by UHMWPE particles by reducing the secretion of proinflammatory
cytokines and by reducing osteoclastogenesis.
In conclusion, the in vivo experiments showed
that neomangiferin inhibited the activation of osteoclasts and
thereby influenced the UHMWPE particle-induced osteolystic process.
However, the possible mechanism is to be determined in future
experiments. In this process, the anti-inflammatory effects of
neomangiferin and its ability to modulate the expression of RANKL
and OPG may be important. These results show that neomangiferin may
be a promising treatment of wear particle-induced inflammatory
osteolysis.
Acknowledgements
Not applicable.
Funding
This study was supported by the Guangxi Natural
Science Foundation (grant no. 2017GXNSFAA198258).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
J-MZ and JY designed the current study; H-TW, JL,
S-TM, W-YF, QW and H-YZ performed the experiments, analyzed the
data and prepared the figures; H-TW wrote the manuscript. All
authors reviewed the article.
Ethics approval and consent to
participate
The animal protocol was approved by the Animal
Ethics Committee of Guangxi Medical University (approval no.
201707006), and the Guidelines for Care and Use of Laboratory
Animals were strictly followed.
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AL
|
aseptic loosening
|
|
BMD
|
bone mineral density
|
|
BV/TV
|
bone volume/tissue volume ratio
|
|
ELISA
|
enzyme-linked immunosorbent assay
|
|
H&E
|
hematoxylin and eosin
|
|
micro-CT
|
micro-computed tomography
|
|
PBS
|
phosphate-buffered saline
|
|
TRAP
|
tartrate-resistant acid
phosphatase
|
|
UHMWPE
|
ultra-high-molecular-weight
polyethylene
|
|
RANKL
|
receptor activator of nuclear
factor-κB ligand
|
|
OSCAR
|
osteoclast-related receptor
|
|
CTX-1
|
cross-linked C-telopeptide of type I
collagen
|
|
OPG
|
osteoprotegerin
|
References
|
1
|
Sabokbar A, Kudo O and Athanasou NA: Two
distinct cellular mechanisms of osteoclast formation and bone
resorption in periprosthetic osteolysis. J Orthop Res. 21:73–80.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shin DK, Kim MH, Lee SH, Kim TH and Kim
SY: Inhibitory effects of luteolin on titanium particle-induced
osteolysis in a mouse model. Acta Biomater. 8:3524–3531. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goodman SB, Gibon E, Pajarinen J, Lin TH,
Keeney M, Ren PG, Nich C, Yao Z, Egashira K, Yang F and Konttinen
YT: Novel biological strategies for treatment of wear
particle-induced periprosthetic osteolysis of orthopaedic implants
for joint replacement. J R Soc Interface. 11:201309622014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goodman SB, Gibon E and Yao Z: The basic
science of periprosthetic osteolysis. Instr Course Lect.
62:201–206. 2013.PubMed/NCBI
|
|
5
|
Warme BA, Epstein NJ, Trindade MC,
Miyanishi K, Ma T, Saket RR, Regula D, Goodman SB and Smith RL:
Proinflammatory mediator expression in a novel murine model of
titanium-particle-induced intramedullary inflammation. J Biomed
Mater Res B Appl Biomater. 71:360–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang SY, Wu B, Mayton L, Mukherjee P,
Robbins PD, Evans CH and Wooley PH: Protective effects of IL-1Ra or
vIL-10 gene transfer on a murine model of wear debris-induced
osteolysis. Gene Ther. 11:483–491. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pacifici R: Role of T cells in ovariectomy
induced bone loss-revisited. J Bone Miner Res. 27:231–239. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jonitz-Heincke A, Lochner K, Schulze C,
Pohle D, Pustlauk W, Hansmann D and Bader R: Contribution of human
osteoblasts and macrophages to bone matrix degradation and
proinflammatory cytokine release after exposure to abrasive
endoprosthetic wear particles. Mol Med Rep. 14:1491–1500. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yagil-Kelmer E, Kazmier P, Rahaman MN, Bal
BS, Tessman RK and Estes DM: Comparison of the response of primary
human blood monocytes and the U937 human monocytic cell line to two
different sizes of alumina ceramic particles. J Orthop Res.
22:832–838. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Petit A, Mwale F, Antoniou J, Zukor DJ and
Huk OL: Effect of bisphosphonates on the stimulation of macrophages
by alumina ceramic particles: A comparison with
ultra-high-molecular-weight polyethylene. J Mater Sci Mater Med.
17:667–673. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lim SM, Kang GD, Jeong JJ, Choi HS and Kim
DH: Neomangiferin modulates the Th17/Treg balance and ameliorates
colitis in mice. Phytomedicine. 23:131–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li J, Chen L, Wu H, Lu Y, Hu Z, Lu B,
Zhang L, Chai Y and Zhang J: The mixture of salvianolic acids from
salvia miltiorrhiza and total flavonoids from anemarrhena
asphodeloides attenuate sulfur mustard-induced injury. Int J Mol
Sci. 16:24555–24573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seo CS, Ha H, Kim YJ and Jungb JY:
HPLC-pDA simultaneous determination and protective effect of
Anemarrhena asphodeloides against acute renal failure. Nat Prod
Commun. 9:829–832. 2014.PubMed/NCBI
|
|
14
|
Zhou C, Zhou J, Han N, Liu Z, Xiao B and
Yin J: Beneficial effects of neomangiferin on high fat diet-induced
nonalcoholic fatty liver disease in rats. Int Immunopharmacol.
25:218–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu X, Gao JJ, Landao-Bassonga E, Pavlos
NJ, Qin A, Steer JH, Zheng MH, Dong Y and Cheng TS: Thonzonium
bromide inhibits RANKL-induced osteoclast formation and bone
resorption in vitro and prevents LPS-induced bone loss in vivo.
Biochem Pharmacol. 104:118–130. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu X, Gao J, Ng PY, Qin A, Steer JH,
Pavlos NJ, Zheng MH, Dong Y and Cheng TS: Alexidine dihydrochloride
attenuates osteoclast formation and bone resorption and protects
against LPS-induced osteolysis. J Bone Miner Res. 31:560–572. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen H, Guo T, Wang D and Qin R: Vaccaria
hypaphorine impairs RANKL-induced osteoclastogenesis by inhibition
of ERK, p38, JNK and NF-κB pathway and prevents inflammatory bone
loss in mice. Biomed Pharmacother. 97:1155–1163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song F, Wei C, Zhou L, Qin A, Yang M,
Tickner J, Huang Y, Zhao J and Xu J: Luteoloside prevents
lipopolysaccharide-induced osteolysis and suppresses RANKL-induced
osteoclastogenesis through attenuating RANKL signaling cascades. J
Cell Physiol. 233:1723–1735. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Al-Quhali AM, Sun Y, Bai X, Jin Z and Yu
G: Surgical modification of the murine calvaria osteolysis model.
Biomed Res Int. 2015:8026972015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wedemeyer C, Xu J, Neuerburg C,
Landgraeber S, Malyar NM, von Knoch F, Gosheger G, von Knoch M,
Löer F and Saxler G: Particle-induced osteolysis in
three-dimensional micro-computed tomography. Calcif Tissue Int.
81:394–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun Q, Fu Y, Sun A, Shou Y, Zheng M, Li X
and Fan D: Correlation of E-selectin gene polymorphisms with risk
of ischemic stroke A meta-analysis. Neural Regenerat Res.
6:1731–1735. 2011.
|
|
22
|
Chiu R, Ma T, Smith RL and Goodman SB:
Ultrahigh molecular weight polyethylene wear debris inhibits
osteoprogenitor proliferation and differentiation in vitro. J
Biomed Mater Res A. 89:242–247. 2009.PubMed/NCBI
|
|
23
|
Chiu R, Ma T, Smith RL and Goodman SB:
Polymethylmethacry late particles inhibit osteoblastic
differentiation of bone marrow osteoprogenitor cells. J Biomed
Mater Res A. 77:850–856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kadoya Y, Revell PA, al-Saffar N,
Kobayashi A, Scott G and Freeman MA: Bone formation and bone
resorption in failed total joint arthroplasties: Histomorphometric
analysis with histochemical and immunohistochemical technique. J
Orthop Res. 14:473–482. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmalzried TP, Kwong LM, Jasty M,
Sedlacek RC, Haire TC, O'Connor DO, Bragdon CR, Kabo JM, Malcolm AJ
and Harris WH: The mechanism of loosening of cemented acetabular
components in total hip arthroplasty. Analysis of specimens
retrieved at autopsy. Clin Orthop Relat Res. 274:60–78. 1992.
|
|
26
|
Kotian P, Boloor A and Sreenivasan S:
Study of adverse effect profile of parenteral zoledronic acid in
female patients with osteoporosis. J Clin Diagn Res. 10:OC04–OC06.
2016.PubMed/NCBI
|
|
27
|
Beaudoin C, Jean S, Bessette L, Ste-Marie
LG, Moore L and Brown JP: Denosumab compared to other treatments to
prevent or treat osteoporosis in individuals at risk of fracture: A
systematic review and meta-analysis. Osteoporos Int. 27:2835–2844.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsubaki M, Komai M, Itoh T, Imano M,
Sakamoto K, Shimaoka H, Takeda T, Ogawa N, Mashimo K, Fujiwara D,
et al: Nitrogen-containing bisphosphonates inhibit RANKL- and
M-CSF-induced osteoclast formation through the inhibition of ERK1/2
and Akt activation. J Biomed Sci. 21:102014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
He LG, Li XL, Zeng XZ, Duan H, Wang S, Lei
LS, Li XJ and Liu SW: Sinomenine induces apoptosis in RAW 264.7
cell-derived osteoclasts in vitro via caspase-3 activation. Acta
Pharmacol Sin. 35:203–210. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng M, Ge Y, Li H, Yan M, Zhou J and
Zhang Y: Bergapten prevents lipopolysaccharide mediated osteoclast
formation, bone resorption and osteoclast survival. Int Orthop.
38:627–634. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs from 1981 to 2014. J Nat Prod. 79:629–661.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang J, Fu B, Lu F, Hu X, Tang J and Huang
L: Inhibitory activity of linarin on osteoclastogenesis through
receptor activator of nuclear factor κB ligand-induced NF-κB
pathway. Biochem Biophys Res Commun. 495:2133–2138. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu G, Ma C, Wang P, Zhang P, Qu X, Liu S,
Zhai Z, Yu D, Gao J, Liang J, et al: Pilose antler peptide
potentiates osteoblast differentiation and inhibits
osteoclastogenesis via manipulating the NF-κB pathway. Biochem
Biophys Res Commun. 491:388–395. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nie S, Xu J, Zhang C, Xu C, Liu M and Yu
D: Salicortin inhibits osteoclast differentiation and bone
resorption by down-regulating JNK and NF-κB/NFATc1 signaling
pathways. Biochem Biophys Res Commun. 470:61–67. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Henc I, Kokotkiewicz A, Łuczkiewicz P,
Bryl E, Łuczkiewicz M and Witkowski JM: Naturally occurring
xanthone and benzophenone derivatives exert significant
anti-proliferative and proapoptotic effects in vitro on synovial
fibroblasts and macrophages from rheumatoid arthritis patients. Int
Immunopharmacol. 49:148–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liao HL, Wu QY, HU HG, Zang ZH, Song L and
Yang Q: Structure modification of mangiferin. West China J Pharma
Sci. 23:385–387. 2008.
|
|
37
|
Hong YF, Han GY and Guo XM: Isolation and
structure determination of xanthone glycosides of Anemarrhena
asphodeloides. Yao Xue Xue Bao. 32:473–475. 1997.(In Chinese).
PubMed/NCBI
|
|
38
|
Broadhead ML, Clark JC, Dass CR, Choong PF
and Myers DE: Therapeutic targeting of osteoclast function and
pathways. Expert Opin Ther Targets. 15:169–181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Landgraeber S, Quint U, Classen T and
Totsch M: Senescence in cells in aseptic loosening after total hip
replacement. Acta Biomater. 7:1364–1368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boyle WJ, Simonet WS and Lacey DL:
Osteoclast differentiation and activation. Nature. 423:337–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhai Z, Qu X, Li H, Yang K, Wan P, Tan L,
Ouyang Z, Liu X, Tian B, Xiao F, et al: The effect of metallic
magnesium degradation products on osteoclast-induced osteolysis and
attenuation of NF-κB and NFATc1 signaling. Biomaterials.
35:6299–6310. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cadosch D, Gautschi OP, Chan E, Simmen HP
and Filgueira L: Titanium induced production of chemokines
CCL17/TARC and CCL22/MDC in human osteoclasts and osteoblasts. J
Biomed Mater Res A. 92:475–483. 2010.PubMed/NCBI
|
|
43
|
Jämsen E, Kouri VP, Olkkonen J, Cör A,
Goodman SB, Konttinen YT and Pajarinen J: Characterization of
macrophage polarizing cytokines in the aseptic loosening of total
hip replacements. J Orthop Res. 32:1241–1246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jablonski H, Rekasi H and Jäger M: The
influence of calcitonin gene-related peptide on markers of bone
metabolism in MG-63 osteoblast-like cells co-cultured with THP-1
macrophage-like cells under virtually osteolytic conditions. BMC
Musculoskelet Disord. 17:1992016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park SJ, Lee EJ, Kim YH, Shin JE and Kang
YH: Inhibitory effects of gossypin on RANKL-induced osteoclast
differentiation and bone resorption in murine macrophages (LB364).
FASEB J 28. 2014.
|
|
46
|
Nich C, Takakubo Y, Pajarinen J, Ainola M,
Salem A, Sillat T, Rao AJ, Raska M, Tamaki Y, Takagi M, et al:
Macrophages-Key cells in the response to wear debris from joint
replacements. J Biomed Mater Res A. 101:3033–3045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ingham E and Fisher J: The role of
macrophages in osteolysis of total joint replacement. Biomaterials.
26:1271–1286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Lin TH, Yao Z, Sato T, Keeney M, Li C,
Pajarinen J, Yang F, Egashira K and Goodman SB: Suppression of
wear-particle-induced pro-inflammatory cytokine and chemokine
production in macrophages via NF-κB decoy oligodeoxynucleotide: A
preliminary report. Acta Biomater. 10:3747–3755. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu X, Zhu S, Cui J, Shao H, Zhang W, Yang
H, Xu Y, Geng D and Yu L: Strontium ranelate inhibits
titanium-particle-induced osteolysis by restraining inflammatory
osteoclastogenesis in vivo. Acta Biomater. 10:4912–4918. 2014.
View Article : Google Scholar : PubMed/NCBI
|