Introduction
Heat stroke (HS) is a life-threatening disease that
is characterized by central nervous system dysfunction,
hyperthermia and rapid progression to multiple organ failure (MOF).
Despite important developments regarding rapid cooling and
multi-organ function support, a large proportion of patients
experience permanent neurological impairment or succumb to
mortality post-HS (1). A previous
study revealed that marked levels of cytokine release following
extreme hyperthermia may lead to systemic inflammatory response
syndrome (SIRS) (2), and both the
absolute number and percentage of T helper cells are significantly
decreased during HS (3). Regulatory
T cells (Tregs) are a specialized lineage of suppressive cluster of
differentiation (CD)4 T cells. The most important characteristic of
natural and induced Tregs is the expression of
forkhead/winged-helix transcription factor (Foxp)3 that functions
as an important negative regulator of inflammation in numerous
biological contexts, including sepsis (4), which may have a similar mechanism to
HS. A previous study demonstrated that natural Tregs may attenuate
collateral tissue damage induced by vigorous antimicrobial immune
responses during sepsis (5), which
suggests that Tregs may have a potential role in the regulation of
rapidly progressing MOF induced by SIRS during early HS. However,
to the best of our knowledge, the response of Tregs to HS has not
yet been reported.
To investigate the response of Tregs to HS, the aim
of the present study was to determine the total number and function
of splenic Tregs during the early stage of HS. Considering that
Tregs suppress the activation of adaptive and innate immune cells
via association with contact-dependent or soluble mediators, the
expression levels of surface molecules, such as cytotoxic
T-lymphocyte associated protein 4 (CTLA4) and CD39/CD73, and
anti-inflammatory cytokines, such as interleukin (IL)-10,
transforming growth factor (TGF)-β and IL-35, were determined to
investigate the mechanism underlying the association between Tregs
and HS.
Materials and methods
Animals
C57/BL6 mice (age, 8–12 weeks; weight, 20–25 g;
n=20) were purchased from the Animal Center of the Chinese PLA
General Hospital (Beijing, China). Mice were housed in cages at
23°C with 55% humidity and 12-h light/dark cycles. Mice had free
access to standard food and water. All animal procedures were
approved by the Institutional Animal Care and Use Committee of
Chinese PLA General Hospital and Chinese PLA Military Medical
College.
Experimental setup
Mice were divided into an HS group (n=10) and a
negative control (NC) group (n=10). HS modeling was performed
according to previously published protocol (6). Briefly, mice were exposed to an
incubator at a temperature of 43±0.2°C and a humidity of 60±5% in
the absence of food and water, and rectal temperatures of the mice
were determined every 10 min until a maximum temperature of 42.7°C
was reached. Following this, mice were removed from the incubator
and provided with food and water ad libitum during
undisturbed recovery at 25±0.5°C for 6 h. The NC group was exposed
to an incubator with a temperature of 25±0.5°C in the absence of
food and water, and subsequently provided with food and water at
the same time as the HS group.
Cell preparation
Single-cell harvest and preparation using spleen
tissues was performed as described previously (7). Blood was flushed out using ice-cold PBS
prior to the spleen being harvested. Following this, spleen tissue
was removed, incubated in PBS with 1% bovine serum albumin (BSA;
cat. no. 16010159; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 4°C for 10 min, and then lysed with red blood cell
lysis buffer (TBD Science, Tianjin, China) at room temperature for
1–2 min, according to the manufacturer's protocol.
Flow cytometry
Non-specific binding was blocked using 5% BSA for 1
h at room temperature. Surface staining was performed using
antibodies against fluorescein isothiocyanate (FITC)-CD4 (1:10;
cat. no. 553729; BD Biosciences, Franklin Lakes, NJ, USA),
allophycocyanin (APC)-CD25 (1:10; cat. no. 557192; BD Biosciences),
phycoerythrin (PE)-CTLA-4 (1:10; cat. no. 561718; BD Biosciences),
PE-CD39 (1:80; cat. no. 12-0391-80; eBioscience; Thermo Fisher
Scientific, Inc.) and PE-CD73 (1:10; cat. no. 550741; BD
Biosciences) at room temperature for 30 min in accordance with the
manufacturers' instructions. Intracellular staining of Foxp3 using
APC-Foxp3 (1:20. cat. no. 17-5773-80; eBioscience; Thermo Fisher
Scientific, Inc.) at room temperature for 45 min was also performed
according to the manufacturer's instructions. Following three
washes with PBS, cells were observed using BD FACS Calibur flow
cytometry (BD Biosciences) and analyzed using FlowJo 7.6 software
(Tree Star, Inc., Ashland, OR, USA).
In vitro inhibition analysis
Splenic cells in NC and HS groups were harvested at
24 h post-heat stress. FITC-CD4 and APC-CD25 monoclonal fluorescent
antibodies were added to splenic cells prior to sorting using BD
FACSCalibur. Sorted cells had a purity of >95%. For Treg
functional analysis, purified Tregs were cultured at 37°C with
CD4+T cells (5×104) at ratios of 0:1, 1:1,
2:1 and 4:1 for 44 h in 96-well plates pre-coated with anti-CD3
antibodies (5 µg/ml; cat. no. 555273; BD Pharmingen; BD
Biosciences). Soluble anti-CD28 antibodies (5 µg/ml; cat. no.
553295; BD Pharmingen; BD Biosciences) were added, and cells were
subsequently incubated at 37°C for 44 h prior to proliferation
assessment using bromodeoxyuridine (BrdU) staining via flow
cytometry. Cells were then incubated at 37°C with 10 mM BrdU for a
further 4 h prior to fixing with 4% paraformaldehyde at room
temperature for 10 min. Following rinsing with PBS, cells were
treated at room temperature with 2 M HCl for 30 min. Non-specific
binding was blocked using 5% BSA for 1 h at room temperature. Cells
were then separately incubated with mouse anti-BrdU (1:200; cat.
no. MAB3222; EMD Millipore, Billerica, MA, USA) overnight at 4°C.
Following three washes with PBS, cells were incubated with
Cy5-conjugated goat anti-mouse IgG H&L pre-adsorbed secondary
antibodies (1:500; cat. no. ab6563; Abcam, Cambridge, UK) for 1 h
at room temperature. Following three washes with PBS, cells were
observed using BD FACS Calibur flow cytometry (BD Biosciences) and
analyzed using FlowJo 7.6 software (Tree Star, Inc., Ashland).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol Reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA (2 µg) was
used for cDNA synthesis, which was performed using a RevertAid
First Strand cDNA Synthesis kit (cat. no. K1622; Thermo Fisher
Scientific, Inc.). RNA was incubated with primers at 42°C for 60
min, followed by enzyme inactivation for 10 min at 95°C and storage
of the samples on ice. The IQ5 detection system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and SYBR Green Real time PCR
Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
were used for qPCR. The thermocycling conditions used for qPCR were
as follows: Denaturation for 5 min at 95°C; followed by 40 cycles
of denaturation for 30 sec at 90°C, annealing for 40 sec at 60°C
and extension for 40 sec at 72°C. Fold changes were calculated via
relative quantification using the 2−ΔΔCq method
(8). Each experiment was performed
in triplicate. qPCR primers used are listed in Table I.
 | Table I.List of nucleotides used for cDNA
amplification. |
Table I.
List of nucleotides used for cDNA
amplification.
| Gene | Direction | Sequence | Length (bp) |
|---|
| IL-10 | Forward |
5′-GCCAGAGCCACATGCTCCTA-3′ | 144 |
|
| Reverse |
5′-GATAAGGCTTGGCAACCCAAGTA-3′ |
|
| IL-35 | Forward |
5′-CTGTGCCTTGGTAGCATCTATG-3′ | 166 |
|
| Reverse |
5′-GCAGAGTCTCGCCATTATGA-3′ |
|
| TGF-β | Forward |
5′-AACAATTCCTGGCGTTACCTT-3′ | 119 |
|
| Reverse |
5′-TGTATTCCGTCTCCTTGGTTC-3′ |
|
| β-actin | Forward |
5′-TGTTACCAACTGGGACGACA-3′ | 139 |
|
| Reverse |
5′-CTGGGTCATCTTTTCACGGT-3′ |
|
Statistical analysis
Data analysis was performed using SPSS version 18.0
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard deviation. Comparisons of multiple groups of parametric
data were performed using one-way analysis of variance followed by
the Student-Newman-Keuls post-hoc test. Student's t-tests were used
for comparisons between two groups. *P<0.05 was considered to
indicate a statistically significant difference. Graphs were
produced using GraphPad Prism 5.0 software (GraphPad Software,
Inc., La Jolla, CA, USA).
Results
Numbers of splenic Tregs are
significantly decreased following HS
To observe the morphology of splenic Tregs, Foxp3
staining via FACs was performed. The results demonstrated that the
percentage of CD4+Foxp3+cells in splenic
tissues (Fig. 1A and B) was
decreased at 0, 24 and 72 h time intervals following heat stress
compared with the NC group.
Immunosuppressive capacity of splenic
Tregs is suppressed following HS
To investigate the immunosuppressive capacity of
Tregs in response to heat stress, splenic Tregs
(CD4+CD25+) and CD4+T cells were
purified at 24 h post-heat stress via flow cytometry. BrdU staining
demonstrated that the immunosuppressive capacity of splenic Tregs
in the HS group was decreased at every ratio compared with the NC
group (Fig. 2A and B).
HS downregulates CTLA4 and CD39/CD73
expression levels in splenic Tregs
Constitutive expression of CTLA-4 in Tregs is
important for the immunosuppressive function of Tregs. CD39 and
CD73 have been revealed to be highly expressed on the surface of
Tregs and are associated with the generation of extracellular
adenosine, which may also have an important role in the suppressive
function of Tregs (9,10). In the present study, the numbers of
CTLA4+Tregs (Fig. 3A and
B), CD39+Tregs (Fig. 3C
and D) and CD73+Tregs (Fig. 3E and F) detected via FACs analyses
were significantly decreased at 0, 24 and 72 h time intervals
post-heat stress, compared with the NC group.
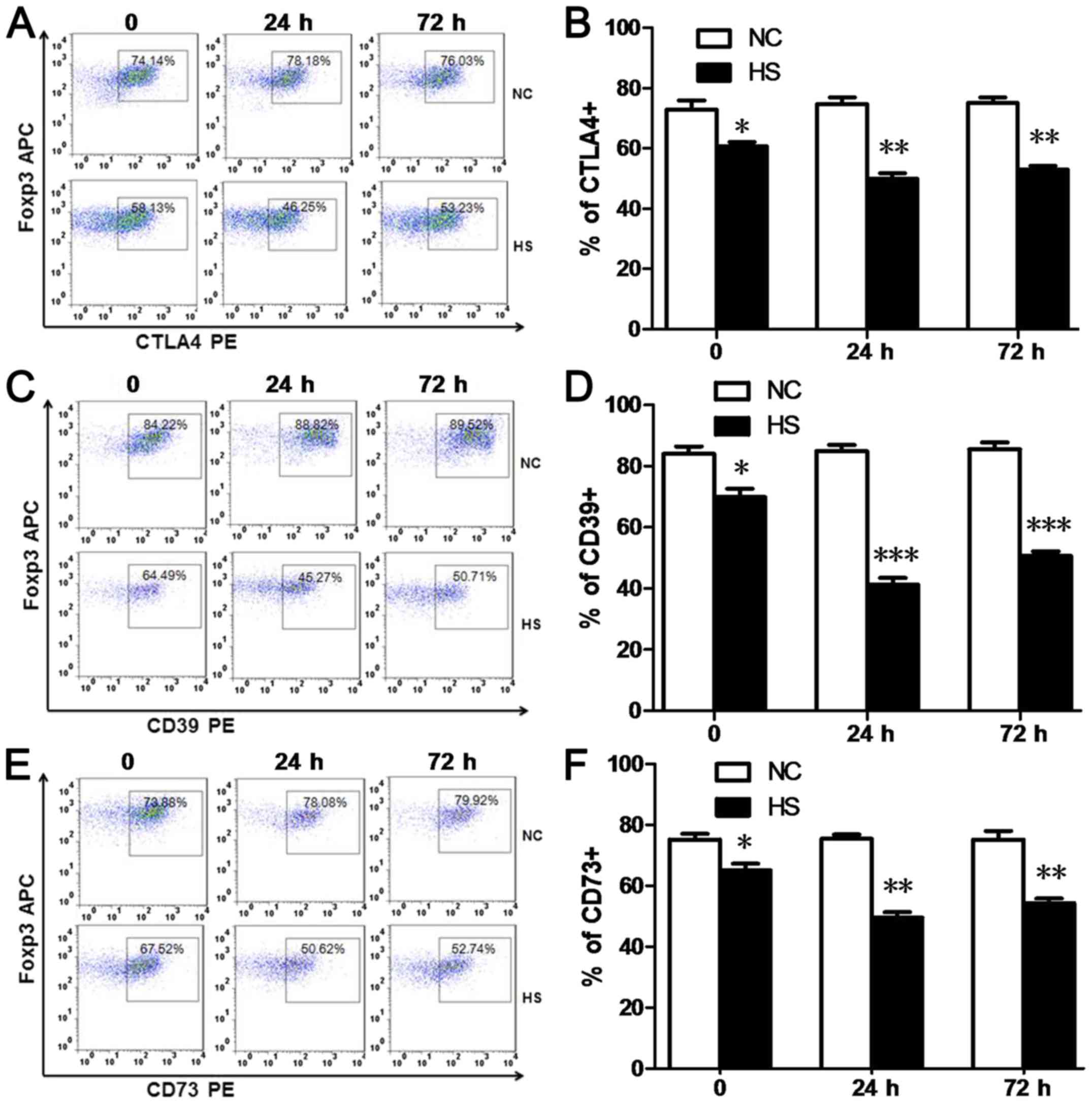 | Figure 3.Heat stroke downregulates the
expression levels of CTLA4 and CD39/CD73 in splenic Tregs. (A and
B) Splenic CTLA4+, (C and D) CD39+ and (E and
F) CD73+Tregs were defined as
CD4+Foxp3+ CTLA4+,
CD4+Foxp3+ CD39+ and
CD4+Foxp3+ CD73+splenocytes in
FACS. Percentages of CTLA4+, CD39+ and
CD73+Tregs were decreased in the HS group at 0, 24 and
72 h time intervals post-heat stress compared with the NC group.
Data are expressed as the mean ± standard deviation. *P<0.05,
**P<0.01 and ***P<0.001 vs. NC. NC, negative control; HS,
heat stroke; Foxp3, forkhead/winged-helix transcription factor; CD,
cluster of differentiation; Tregs, T regulatory cells; CTLA4,
cytotoxic T-lymphocyte associated protein 4; APC, allophycocyanin;
PE, phycoerythrin. |
HS decreases the secretion of
inhibitory cytokines by splenic Tregs
Anti-inflammatory cytokines, such as IL-10, TGF-β
and IL-35, have been suggested to be associated with the
physiological functioning of Tregs, and have been established to be
associated with Treg-mediated immunosuppression (11). In the present study, RT-qPCR was
performed to determine the expression levels of such cytokines, and
results revealed that the expression of IL-10 in splenic Tregs in
the HS group was decreased at 0, 24 and 72 h time intervals, and
the secretion levels of TGF-β and IL-35 in splenic Tregs in the HS
group were downregulated at 24 and 72 h time intervals compared
with the NC group (Fig. 4).
 | Figure 4.Heat stroke decreases the secretion
of inhibitory cytokines in splenic Tregs. Splenocytes were
harvested at 24 h post-heat stress, and splenic Tregs were sorted
by FACS analysis. Reverse transcription-quantitative polymerase
chain reaction analyses demonstrated that IL-10 production was
decreased at 0, 24 and 72 h time intervals following heat stress,
and IL-35 and TGF-β secretion levels were decreased at 24 and 72 h
following heat stress, compared with the NC group. Data are
expressed as the mean ± standard deviation. *P<0.05, **P<0.01
and ***P<0.001. NS, not significant; NC, negative control; HS,
heat stroke; IL, interleukin; TGF-β, transforming growth factor-β;
Tregs, T regulatory cells. |
Discussion
To the best of our knowledge, the present study is
the first to demonstrate that exposure to HS may deplete numbers of
Tregs. Furthermore, HS was demonstrated to decrease the expression
levels of functional surface molecules (CTLA4, CD39 and CD73) as
well as the production of inhibitory cytokines (IL-35, IL-10 and
TGF-β), which may contribute to the inhibitory effect of Tregs on
CD4+ T cell proliferation. The alterations of splenic
frequency and function may contribute to enhanced levels of SIRS,
and thus may represent a potential therapeutic target for the
treatment of HS.
Tregs are specialized immune cells that have an
important role in maintaining immune self-tolerance and preventing
autoimmune disease (4,5). Tregs are characterized by Foxp3
expression in cell nuclei (11).
During infection, Tregs may attenuate inflammation and collateral
tissue damage; however, this may also suppress the capability of
bacterial clearance (5). Recent
studies demonstrated that high numbers of circulating
CD39+ Tregs predict poor survival for patients with
sepsis (12) and levels of
Foxp3+Tregs increased in all CD4+T cells
during murine sepsis (13). However,
depletion of Tregs has been previously revealed, either via use of
anti-CD25 antibodies (14) or a
DEREG mouse model (13), to
contribute towards a negative clinical outcome in early phase
sepsis. In addition, a number of drugs may improve survival in mice
with septic shock, partially via facilitating the proliferation of
IL-10+Tregs in septic mice (15). The aforementioned studies suggested
that Tregs may attenuate collateral tissue damage induced by
vigorous antimicrobial immune responses during sepsis. Considering
that SIRS associated with HS share a similar mechanism with sepsis,
it was hypothesized that Tregs may also be associated with
HS-induced multiple organ dysfunction, which was subsequently
investigated in the present study.
In the present study, the numbers of splenic Tregs
were decreased at 0, 24 and 72 h time intervals post-heat stress.
To further investigate the Treg response in HS, splenic Tregs were
harvested at 24 h post-heat stress to determine their
immunosuppressive capacity on CD4+T cells via BrdU
staining. In vitro inhibition analysis demonstrated that the
immunosuppressive capacity of splenic Tregs in the HS group was
decreased at every ratio compared with the NC group. Therefore,
decreased levels of splenic Tregs, as well as suppressed
immunosuppressive capacity of splenic Tregs, may markedly affect
the SIRS during the early phase of HS.
Numerous surface markers have been demonstrated to
have an important role in the immunosuppressive capacity of Tregs.
CTLA4 (CD152) is a well-characterized negative regulator expressed
on T cells (16). Tregs exhibit
enhanced levels of CTLA4 expression (11) and may require CTLA4 to suppress
immune responses via regulation of the potency of
antigen-presenting cells to activate other T cells (17), as well as the inhibition of IL-2
expression, which represents an important cytokine for T-cell
expansion (16). A previous study
revealed that peripheral blood samples obtained from patients with
acute liver failure exhibited higher numbers of
CD4+CTLA4+T cells, and patients with
infections exhibited the greatest overall numbers of
CD4+CTLA4+T cells (16). Thus, in severe sepsis, suppression of
CTLA4 may improve survival in patients (18). In the present study, the number of
CTLA4+Tregs decreased following heat stress, thus
suggesting potential abatement of immunosuppressive capacity of
Tregs on CD4+T cell proliferation. CD39 and CD73 are
important components of cell surface enzymes associated with the
purinergic system, and may exert anti-inflammation effects by
decreasing the ATP/ADP ratio and increasing adenosine availability
(9). Therefore, high levels of
circulating CD39+Tregs may be used to predict poor
survival for patients with sepsis (13). However, emerging data have
demonstrated that expression levels of CD39 (9) and CD73 (10) improve the survival of patients with
microbial sepsis by attenuating systemic inflammation (9) and liver dysfunction (19). In the present study, levels of both
CD39+and CD73+Tregs were decreased in the HS
group, which may be associated with suppressed immunosuppressive
capacities exhibited by Tregs against CD4+T cell
proliferation. Based on the present study, adenosine signaling may
represent a potential therapeutic strategy for patients with
HS-induced SIRS and sequential organ dysfunction.
Tregs may also exhibit immunoregulatory effects via
the release of inhibitory cytokines, such as IL-10, TGF-β and IL-35
(20). A recent study established a
novel and effective method for the generation of human
porcine-specific Tregs exhibiting high expression levels of IL-10,
TGF-β1 and IL-35; which suggests that Tregs have an important role
in immunomodulation (21).
IL-10-producing Tregs constitute a Treg cell subset characterized
by the production of enhanced levels of IL-10, cytokine-mediated
immunosuppressive capabilities and selective migration to
peripheral tissues in order to suppress local immune responses
(20,22). Decreased production of IL-10 in Tregs
may lead to the exacerbation of tissue injury in numerous
inflammation (23,24) and autoimmune-associated diseases
(25). IL-35 exhibits strong
immunosuppressive properties and is predominantly secreted by Tregs
(20). IL-35-producing Tregs, which
predominantly produce IL-35, preferentially localize to the T cell
zone of secondary lymphoid organs and have a role in the
suppression of anti-tumor responses (20). Decreased production of IL-35 in Tregs
may attenuate autoimmune and inflammatory responses, such as
ulcerative colitis (26) and type-1
diabetes (27). TGF-β has an
important role in the suppression of immune responses, and Tregs
are its predominant source (27).
TGF-β originating from Tregs regulates immunomodulation and cell
apoptosis. Firstly, Tregs may utilize TGF-β to block cell
activation and differentiation, which subsequently suppresses the
immune response. Secondly, TGF-β may convert naïve T cells into
induced-Tregs (28). Lastly, TGF-β
produced by Tregs may protect Tregs against apoptosis and
destabilization by inducing surrounding inflammation and providing
constant stimulation (28). Thus,
peripheral Tregs and serum TGF-β reduction may induce type 1
diabetes mellitus (29), and
inhibition of TGF-β1 expression may represent a novel strategy for
the improvement of host immunosuppression therapy following sepsis
(30). In the present study, the
expression levels of IL-35, IL-10 and TGF-β in splenic Tregs were
significantly decreased compared with the NC group at 24 h
post-heat stress, which may also be attributed to the suppressed
immunosuppressive capacity of splenic Tregs. Furthermore, the
downregulation of TGF-β exhibited by splenic Tregs may have also
contributed to the reduced numbers of splenic Tregs via promotion
of Treg apoptosis during HS, which will be investigated in our
future studies.
In conclusion, to the best of our knowledge, this is
the first study to investigate the response of Tregs to HS, as well
as its potential underlying mechanisms, to determine a novel
therapeutic target of HS. The results of the present study revealed
that the total numbers and the immunosuppressive capacity of
splenic Tregs were suppressed during HS. Furthermore, it was
demonstrated that downregulation of the expression levels of
surface molecules (CTLA4, CD39 and CD73), as well as secretory
anti-inflammatory cytokines (IL10, TGF-β and IL-35), may have also
contributed to the aforementioned effects, and thus may serve as
potential therapeutic targets for the treatment of patients
suffering from HS.
Acknowledgements
Not applicable.
Funding
The present study was supported by two grants from
the National Natural Science Foundation of China (grant nos.
81501642 and 81671966).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
JH conceived and designed the study. CL, PH and M-MY
performed the experiments and analyzed the data. H-JK and F-HZ
obtained the reagents, materials and analysis tools. JH wrote the
manuscript. All authors read and approved the final study.
Ethics approval and consent to
participate
All procedures were approved by the Institutional
Animal Care and Use Committee of Chinese PLA General Hospital
(Beijing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leon LR and Helwig BG: Heat stroke: Role
of the systemic inflammatory response. J Appl Physiol (1985).
109:1980–1988. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Leon LR and Bouchama A: Heat stroke. Compr
Physiol. 5:611–647. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hammami MM, Bouchama A, Shail E,
Aboul-Enein HY and Al-Sedairy S: Lymphocyte subsets and adhesion
molecules expression in heatstroke and heat stress. J Appl Physiol
(1985). 84:1615–1621. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Veeken J, Gonzalez AJ, Cho H,
Arvey A, Hemmers S, Leslie CS and Rudensky AY: Memory of
inflammation in regulatory T cells. Cell. 166:977–990. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Belkaid Y and Rouse BT: Natural regulatory
T cells in infectious disease. Nat Immunol. 6:353–360. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leon LR, DuBose DA and Mason CW: Heat
stress induces a biphasic thermoregulatory response in mice. Am J
Physiol Regul Integr Comp Physiol. 288:R197–R204. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu J, Zhang L, Wang N, Ding R, Cui S, Zhu
F, Xie Y, Sun X, Wu D, Hong Q, et al: Mesenchymal stem cells
attenuate ischemic acute kidney injury by inducing regulatory T
cells through splenocyte interactions. Kidney Int. 84:521–531.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Csóka B, Németh ZH, Törő G, Koscsó B,
Kókai E, Robson SC, Enjyoji K, Rolandelli RH, Erdélyi K, Pacher P
and Haskó G: CD39 improves survival in microbial sepsis by
attenuating systemic inflammation. FASEB J. 29:25–36. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haskó G, Csóka B, Koscsó B, Chandra R,
Pacher P, Thompson LF, Deitch EA, Spolarics Z, Virág L, Gergely P,
et al: Ecto-5′-nucleotidase (CD73) decreases mortality and organ
injury in sepsis. J Immunol. 187:4256–4267. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmetterer KG, Neunkirchner A and Pickl
WF: Naturally occurring regulatory T cells: Markers, mechanisms,
and manipulation. FASEB J. 26:2253–2276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang H, Xu R, Lin F, Bao C, Wang S, Ji C,
Li K, Jin L, Mu J, Wang Y, et al: High circulating CD39(+)
regulatory T cells predict poor survival for sepsis patients. Int J
Infect Dis. 30:57–63. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tatura R, Zeschnigk M, Hansen W, Steinmann
J, Vidigal PG, Hutzler M, Pastille E, Westendorf AM, Buer J and
Kehrmann J: Relevance of Foxp3(+) regulatory T cells for early and
late phases of murine sepsis. Immunology. 146:144–156. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huo R, Wang L, Wang X, Zhao Y, Wang Y,
Zhao X, Chang L, Liu SL, Tong D, Zhang H and Huang Y: Removal of
regulatory T cells prevents secondary chronic infection but
increases the mortality of subsequent sub-acute infection in sepsis
mice. Oncotarget. 7:10962–10975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen X, Feng Y, Shen X, Pan G, Fan G, Gao
X, Han J and Zhu Y: Anti-sepsis protection of Xuebijing injection
is mediated by differential regulation of pro- and
anti-inflammatory Th17 and T regulatory cells in a murine model of
polymicrobial sepsis. J Ethnopharmacol. 211:358–365. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Khamri W, Abeles RD, Hou TZ, Anderson AE,
El-Masry A, Triantafyllou E, Bernsmeier C, Larsen FS, Singanayagam
A, Kudo N, et al: Increased expression of cytotoxic
T-lymphocyte-associated protein 4 by T cells, induced by B7 in
Sera, reduces adaptive immunity in patients with acute liver
failure. Gastroenterology. 153:263–276.e8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wing K, Onishi Y, Prieto-Martin P,
Yamaguchi T, Miyara M, Fehervari Z, Nomura T and Sakaguchi S:
CTLA-4 control over Foxp3+ regulatory T cell function. Science.
322:271–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang KC, Burnham CA, Compton SM, Rasche
DP, Mazuski RJ, McDonough JS, Unsinger J, Korman AJ, Green JM and
Hotchkiss RS: Blockade of the negative co-stimulatory molecules
PD-1 and CTLA-4 improves survival in primary and secondary fungal
sepsis. Crit Care. 17:R852013. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Savio LEB, de Andrade Mello P, Figliuolo
VR, de Avelar Almeida TF, Santana PT, Oliveira SDS, Silva CLM,
Feldbrügge L, Csizmadia E, Minshall RD, et al: CD39 limits P2X7
receptor inflammatory signaling and attenuates sepsis-induced liver
injury. J Hepatol. 67:716–726. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei X, Zhang J, Gu Q, Huang M, Zhang W,
Guo J and Zhou X: Reciprocal expression of IL-35 and IL-10 defines
two distinct effector Treg subsets that are required for
maintenance of immune tolerance. Cell Rep. 21:1853–1869. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li M, Eckl J, Geiger C, Schendel DJ and
Pohla H: A novel and effective method to generate human
porcine-specific regulatory T cells with high expression of IL-10,
TGF-β1 and IL-35. Sci Rep. 7:39742017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujio K, Yamamoto K and Okamura T:
Overview of LAG-3-expressing, IL-10-producing regulatory T Cells.
Curr Top Microbiol Immunol. 410:29–45. 2017.PubMed/NCBI
|
|
23
|
Toyama M, Kudo D, Aoyagi T, Miyasaka T,
Ishii K, Kanno E, Kaku M, Kushimoto S and Kawakami K: Attenuated
accumulation of T cells and reduced production of interleukin 10
lead to the exacerbation of tissue injury in a mouse model of acute
respiratory distress syndrome. Microbiol Immunol. 62:111–123. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao M, Liu LX, Wu FL, Zhang X, Li YY, Shi
T, Li DZ and Han TT: The changes of Th17/Treg and related
cytokines: IL-17, IL-23, IL-10, and TGF-β in respiratory syncytial
virus bronchiolitis rat model. Iran J Allergy Asthma Immunol.
16:386–395. 2017.PubMed/NCBI
|
|
25
|
Li F, Ji L, Wang W, Hua F, Zhan Y, Zou S,
Yuan L, Ke Y, Min Z, Song D, et al: Insufficient secretion of IL-10
by Tregs compromised its control on over-activated CD4+ T effector
cells in newly diagnosed adult immune thrombocytopenia patients.
Immunol Res. 61:269–280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mohammadnia-Afrouzi M, Hosseini AZ,
Khalili A, Abediankenari S, Amari A, Aghili B and Nataj HH: Altered
microRNA Expression and immunosuppressive cytokine production by
regulatory T cells of ulcerative colitis patients. Immunol Invest.
45:63–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh K, Kadesjö E, Lindroos J, Hjort M,
Lundberg M, Espes D, Carlsson PO, Sandler S and Thorvaldson L:
Interleukin-35 administration counteracts established murine type 1
diabetes-possible involvement of regulatory T cells. Sci Rep.
5:126332015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tran DQ: TGF-β: The sword, the wand, and
the shield of FOXP3(+) regulatory T cells. J Mol Cell Biol.
4:29–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qiao YC, Shen J, Hong XZ, Liang L, Bo CS,
Sui Y and Zhao HL: Changes of regulatory T cells, transforming
growth factor-beta and interleukin-10 in patients with type 1
diabetes mellitus: A systematic review and meta-analysis. Clin
Immunol. 170:61–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Luan YY, Yin CF, Qin QH, Dong N, Zhu XM,
Sheng ZY, Zhang QH and Yao YM: Effect of regulatory T cells on
promoting apoptosis of T lymphocyte and its regulatory mechanism in
sepsis. J Interferon Cytokine Res. 35:969–980. 2015. View Article : Google Scholar : PubMed/NCBI
|


















