Introduction
Chronic obstructive pulmonary disease (COPD) has
become a global public health problem due to the increasing
prevalence and mortality associated with this condition (1). Chronic inflammation,
oxidant/antioxidant imbalance and pulmonary cell apoptosis are
involved in the pathogenesis of COPD (2–4).
Patients with COPD suffer from an increased load on the aspiratory
muscles, particularly the diaphragm, due to airflow obstruction
(5,6). Repetitive stimulation of the diaphragm
results in overexpression of reactive oxygen species (ROS) in
muscle fibers, oxidative stress and accelerated development of
fatigue (7). Cytokines [e.g., tumor
necrosis factor-α (TNF-α) and interleukin-8 (IL-8)] have important
roles in the pathogenesis of COPD (8). AKT is an intracellular serine/threonine
protein kinase activated by different cytokines (e.g., TNF-α). AKT
is a central regulator of molecular pathways implicated in the
pathogenesis of COPD. Compounds targeting AKT are used for the
treatment of COPD (9).
Astragaloside IV (AS-IV) is extracted from
Astragalus membranaceus, which is a herb used in Traditional
Chinese Medicine as a therapeutic. It is used for the treatment of
a variety of diseases due to its anti-oxidant, anti-apoptotic,
anti-cancer and neuro-protective functions (10–16).
Attenuated unilateral ureteral obstruction results from
transforming growth factor-β (TGF-β)-induced cell apoptosis in
renal tubules, which may be inhibited by AS-IV (17). AS-IV has been reported to protect
neuronal cells from the neurotoxic effects of the
1-methyl-4-phenylpyridnium ion via the inhibition of reactive
oxygen species (ROS) production and the pro-apoptotic pathway
mediated by B-cell lymphoma 2-assocated X protein (18). AS-IV was also demonstrated to
activate the AKT pathway in human umbilical vein endothelial cells
and cardiomyocytes (19,20).
The protective functions of AS-IV in patients with
COPD have remained to be demonstrated. The aim of the present study
was to investigate the anti-apoptotic, antioxidant and
anti-inflammatory activities of AS-IV in IL-8-treated rat
diaphragmatic muscle cells and to examine the possible mechanisms
underlying the responses.
Materials and methods
Preparation of diaphragmatic muscle
cells
Primary diaphragmatic muscle cell were prepared as
previously described (21). The
study was approved by the ethics committee of Nanjing Medical
University (Nanjing, China). Diaphragm muscle strips were excised
from 2-week-old rats, minced and digested with 0.2% collagenase IV
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C for 60 min.
After washing with Dulbecco's Modified Eagle's medium (DMEM;
Hyclone; GE Healthcare, Little Chalfont, UK), the cells were
collected by centrifugation at 800 × g for 5 min at room
temperature. They were then incubated in DMEM containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C in a humidified atmosphere containing 5%
CO2.
Cell Counting Kit-8 (CCK-8) assay
Trypsinized diaphragmatic muscle cells were diluted
to 3×104 cells/ml. Of this cell suspension, 100 µl were
then seeded into each well of 96-well plates, which were incubated
at 37°C for 12 h. To determine the appropriate dose of IL-8, the
cells were treated with one of four concentrations of IL-8 (10, 20,
50 or 100 ng/ml; Sigma-Aldrich; Merck KGaA), while untreated cells
served as controls.
To investigate the effects of AS-IV, cells were
treated with 50 ng/ml IL-8 plus DMSO (the final concentration of
which did not exceed 0.1% v/v; Sigma-Aldrich; Merck KGaA) or AS-IV
(2.5, 5, 10, 20, 40 or 80 mg/l; Aladdin, Shanghai, China). At 0, 24
or 48 h after treatment, the medium in each well was replaced with
100 µl DMEM containing 10% CCK-8 reagent (Dojindo Biochemicals,
Kumamoto, Japan). The optical density (OD) was measured at a
wavelength of 450 nm on a microplate reader and used to evaluate
cell proliferation.
Cell apoptosis assay
Cultured cells were treated with 50 ng/ml IL-8 plus
DMSO, AS-IV (5, 10 or 20 mg/l) or 10 µM GSK690693 (Selleck,
Houston, TX, USA) for 48 h and then collected for annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining
with an apoptosis detection kit (BD Biosciences, San Jose, CA,
USA). The stained cells were detected using a flow cytometer (BD
Biosciences).
Measurement of caspase-3 and caspase-9
activities
The activities of caspase-3 and caspase-9 were
measured using a caspase colorimetric assay kit (Kengen
Biotechnologies, Nanjing, China) following the manufacturer's
protocol. In brief, treated cells were lysed using 100 µl
pre-cooled lysis buffer provided with the kit. The supernatant was
collected by centrifugation at 14,000 × g for 15 min at 4°C. The
protein concentration of the collected supernatant was determined
using the Bradford method. Each 50 µl of supernatant was mixed with
50 µl 2X reaction buffer containing 5 µl caspase substrate and 0.5
µl dithiothreitol, followed by incubation at 37°C for 4 h. Caspase
activity was detected using a microplate reader with measurement of
the OD at a wavelength of 400 nm. The values for the Control group
(cells without any treatment) were set as 1.0, and results were
expressed as the ratio of the treatment vs. the Control group
values.
Measurement of ROS
Treated cells were digested and resuspended with 10
mM dichlorofluorescein diacetate (Beyotime Institute of
Biotechnology, Shanghai, China) in DMEM for 20 min in the dark at
37°C. Flow cytometry was used to detect ROS.
ELISA
Concentrations of IL-6 (cat. no. HM10205), IL-8
(cat. no. HM10222) and TNF-α (cat. no. HM10001) in treated
diaphragmatic muscle cells were measured using ELISA kits
(Bio-swap, Shanghai, China) following the manufacturer's protocol.
The absorbance was detected at a wavelength of 450 nm and the
concentrations of IL-6, IL-8, and TNF-α were calculated based on a
standard curve.
Western blot analysis
Treated cells were lysed using
radioimmunoprecipitation assay lysis buffer (Solarbio, Shanghai,
China) and a protease inhibitor cocktail (Sigma-Aldrich; Merck
KGaA). They were centrifuged at 12,000 × g at 4°C for 15 min. The
supernatant was collected and the proteins were quantified using a
bicinchoninic acid protein quantification kit (Thermo Fisher
Scientific, Inc.). Equal amounts of total protein (30 mg) were
separated by 10% SDS-PAGE and transferred to nitrocellulose
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked using 5% skimmed milk at room temperature for 1 h and were
then incubated with antibodies against AKT (cat. no. 9272; 1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA), p-AKT (cat. no.
9271; 1:1,000; Cell Signaling Technology, Inc.), and GAPDH (cat.
no. 5174; 1:2,000; Cell Signaling Technology, Inc.) followed by
incubation with goat anti-mouse (cat. no. A0216, 1:1,000) or
anti-rabbit (cat. no. A0208; 1:1,000) secondary antibodies
(Beyotime Institute of Biotechnology). Blots were visualized using
enhanced chemiluminescence (Thermo Fisher Scientific, Inc.) and
using the Tanon-5200 Image Analyzer (Tanon Science and Technology,
Co., Ltd., Shanghai, China). The protein bands were semi-quantified
using Image J software (version 1.71; National Institutes of
Health, Bethesda, MD, USA).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Differences between groups were analyzed using one-way
analysis of variance followed by Tukey's post-hoc test. GraphPad
Prism 6.0 software (GraphPad, Inc., La Jolla, CA, USA) was used to
summarize and analyze the data. P<0.05 was considered to
indicate a statistically significant difference.
Results
AS-IV attenuates the
anti-proliferative effects of IL-8 on primary diaphragmatic muscle
cells
To determine the appropriate dose of IL-8, primary
diaphragmatic muscle cells were incubated with 0 (Control), 10, 20,
50 or 100 ng/ml IL-8 for 0, 24 and 48 h. The results of the CCK-8
assay indicated that IL-8 significantly decreased the cell
proliferation after 24 and 48 h of treatment in a dose-dependent
manner (Fig. 1A). The 50 ng/ml
concentration had effects similar to the 100 ng/ml concentration.
Thus, 50 ng/ml was selected for the subsequent experiments.
 | Figure 1.Cell proliferation was evaluated using
a CCK-8. (A) Diaphragmatic muscle cells extracted from 2-week-old
rats were treated with one of four concentrations of IL-8. At 0, 24
and 48 h after treatment, cell proliferation was measured using a
CCK-8. (B) Diaphragmatic muscle cells were treated with 50 ng/ml
IL-8 plus vehicle or various concentrations of AS-IV (2.5, 5, 10,
20, 40 or 80 mg/l). Cell proliferation was analyzed using a CCK-8
assay. *P<0.05, **P<0.01, ***P<0.001, vs. Control;
##P<0.01, ###P<0.001, vs. 10 ng/ml
IL-8; +P<0.05, ++P<0.01, vs. 20 ng/ml
IL-8. CCK, Cell Counting Kit; IL, interleukin; OD, optical density;
AS-IV, astragaloside IV. |
The effects of AS-IV on diaphragmatic muscle cell
proliferation were then assessed. AS-IV (10, 20, 40 and 80 mg/ml)
suppressed the anti-proliferative effects of IL-8 (50 ng/ml) on
primary diaphragmatic muscle cells after 24 and 48 h of treatment
(Fig. 1B). AS-IV (5 mg/l) also
attenuated the anti-proliferative effects of IL-8 after 48 h of
treatment. The 20 mg/l concentration had similar effects to those
of the 40 and 80 mg/l concentrations. Thus, three concentrations of
AS-IV (5, 10 and 20 mg/l) were selected for the subsequent
experiments.
AS-IV blocks the AKT signaling
pathway
AKT is a central regulator of molecular pathways
implicated in COPD pathogenesis (9).
To explore the effects of AS-IV on AKT signaling, primary
diaphragmatic muscle cells were incubated with a series of AS-IV
concentrations (5, 10 or 20 mg/l) and IL-8 (50 ng/ml) for 48 h.
Cells treated with IL-8 plus 10 µM GSK690693 (an AKT inhibitor)
were used as a positive control, while cells treated with IL-8 plus
DMSO were used as a negative control. The total and phosphorylated
AKT were detected using western blot analysis. The results
indicated that phosphorylation of AKT was induced by IL-8 (Fig. 2). AS-IV and GSK significantly
suppressed the induction effects of IL-8 on AKT phosphorylation.
However, the levels of AKT were not affected by the different
experimental conditions.
 | Figure 2.AS-IV blocked the AKT signaling
pathway. Diaphragmatic muscle cells were treated with 50 ng/ml IL-8
plus vehicle (DMSO), AS-IV (5, 10 or 20 mg/l), or 10 µM GSK690693
for 48 h. The concentrations of p-AKT and AKT were detected using a
western blot assay. $$$P<0.001 vs. Control;
**P<0.01, ***P<0.001, vs. IL-8 plus DMSO;
###P<0.001, vs. IL-8 plus 5 mg/l AS-IV;
++P<0.01, vs. IL-8 plus 10 mg/l AS-IV. IL,
interleukin; p-AKT, phosphorylated AKT; AS-IV, astragaloside IV;
DMSO, dimethyl sulfoxide; GSK, GSK690693. |
AS-IV attenuates cell apoptosis
induced by IL-8
To investigate the effects of AS-IV on cell
apoptosis, cells were double-labelled with annexin V-FITC/PI and
subjected to flow cytometric analysis [Fig. 3A; lower right quadrant (Annexin
V+ PI−) indicates apoptotic cells]. A
significant increase in the apoptotic rate compared with that in
the Control group was observed in the IL-8 plus DMSO-treated group.
The results indicated that AS-IV attenuated IL-8-induced cell
apoptosis in a dose-dependent manner. GSK690693 had similar effects
to those of AS-IV. The caspase-3 and caspase-9 activities measured
using biochemical analysis were consistent with the results of the
flow cytometric analysis (Fig. 3B).
Overall, it was indicated that AS-IV had an anti-apoptotic effect
on IL-8-treated diaphragmatic muscle cells.
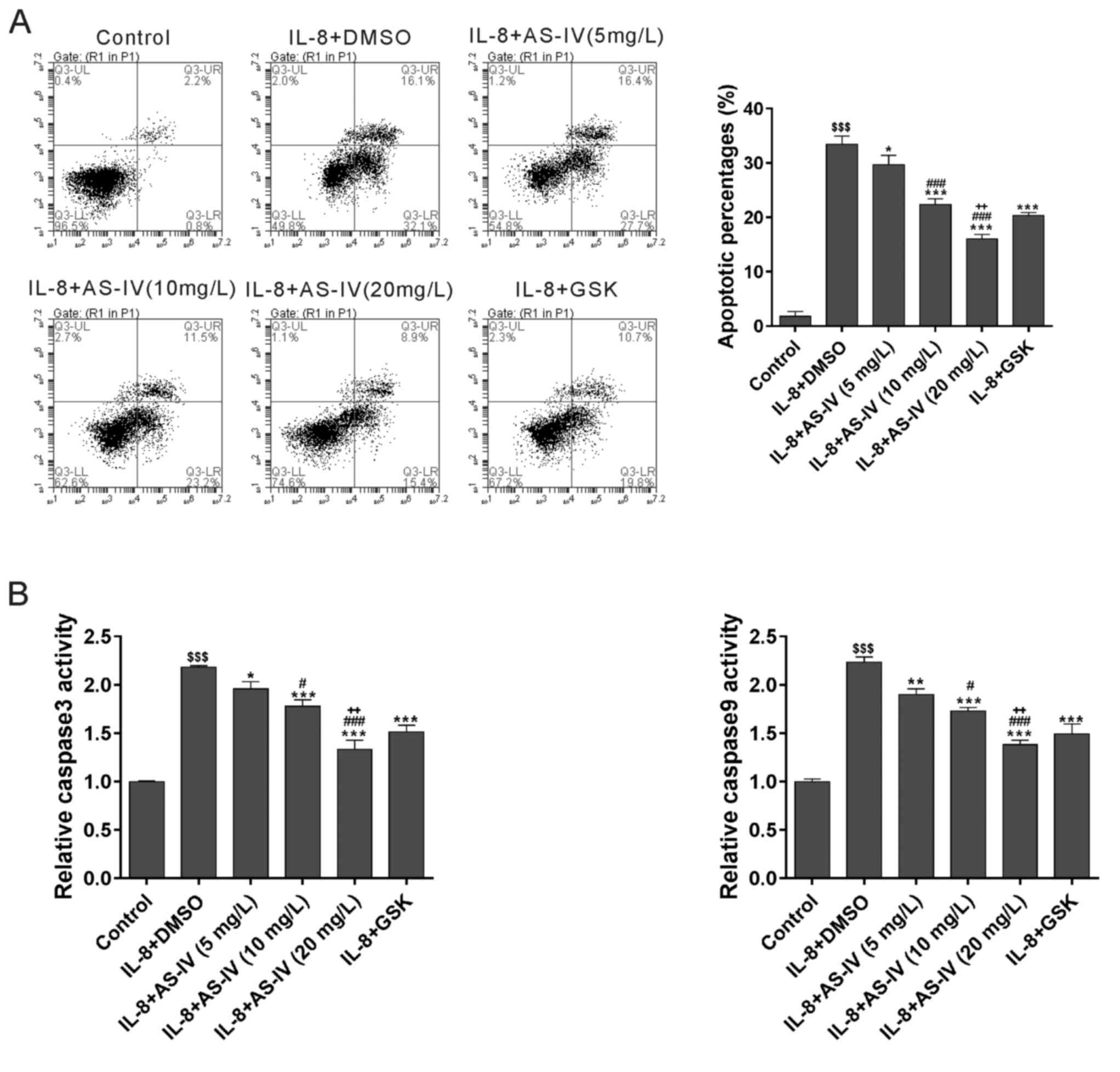 | Figure 3.AS-IV attenuates cell apoptosis
induced by IL-8. (A) Diaphragmatic muscle cells were treated as
indicated for 48 h and then collected for annexin V/propidium
iodide staining and flow cytometric analysis. Cells in the lower
right quadrant and the upper right quadrant underwent early and
late apoptosis, respectively. (B) The caspase 3 and caspase 9
activities were detected using a caspase colorimetric assay kit.
$$$P<0.001 vs. Control; *P<0.05, **P<0.01,
***P<0.001, vs. IL-8 plus DMSO; #P<0.05,
###P<0.001, vs. IL-8 plus 5 mg/l AS-IV;
++P<0.01, vs. IL-8 plus 10 mg/l AS-IV. IL,
interleukin; AS-IV, astragaloside IV; DMSO, dimethyl sulfoxide;
GSK, GSK690693; UR, upper right; LR, lower right; UL, upper left;
LL, lower left; Q, quadrant. |
Effect of AS-IV on IL-8-induced ROS
production
ROS production was determined after 48 h of
treatment with IL-8. IL-8 induced ROS production, which was
significantly suppressed by GSK690693. Furthermore, AS-IV had a
dose-dependent inhibitory effect on IL-8-induced ROS production
(Fig. 4).
Effect of AS-IV on IL-8-induced
pro-inflammatory factor production
The concentrations of the pro-inflammatory factors
TNF-α, IL-6 and IL-8 in the culture medium were determined using
ELISA (Fig. 5). IL-8 treatment
significantly increased the concentrations of these 3
pro-inflammatory factors. The concentrations were significantly
suppressed by additional treatment with GSK690693 and AS-IV,
respectively.
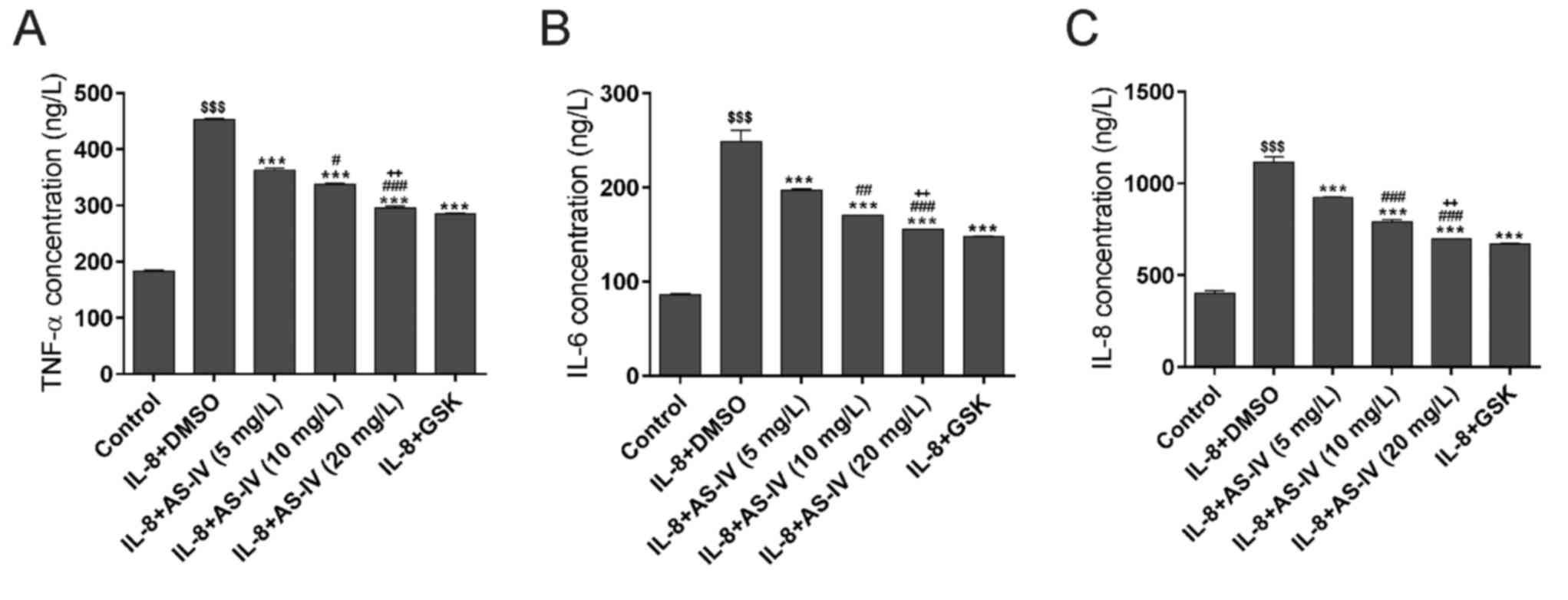 | Figure 5.AS-IV suppresses the IL-8-induced
inflammatory response. Reduced concentrations of the
proinflammatory factors (A) TNF-α, (B) IL-6 and (C) IL-8 were
detected using ELISA. $$$P<0.001 vs. Control;
***P<0.001, vs. IL-8 plus DMSO; #P<0.05,
##P<0.01, ###P<0.001, vs. IL-8 plus 5
mg/l AS-IV; ++P<0.01, vs. IL-8 plus 10 mg/l AS-IV.
IL, interleukin; AS-IV, astragaloside IV; DMSO, dimethyl sulfoxide;
GSK, GSK690693; TNF, tumor necrosis factor. |
Discussion
COPD is a progressive and inflammatory disease that
includes the sequela of airflow obstruction. COPD is usually
accompanied by breathlessness and fatigue, and eventual respiratory
failure from the effects of repeated infections and oxygen deficits
(1). As the most important
respiratory muscle, the diaphragm is responsible for ~75% of the
mechanical function that occurs during respiration. Damage of the
function of the diaphragm has a key role in the induction of
respiratory failure (2).
Overproduction of ROS induces apoptosis of diaphragmatic muscle
cells, which results in diaphragm fatigue (17). In the present study, rat
diaphragmatic muscle cells treated with IL-8 were used as a model
of inflammation. The significantly increased ROS production
accompanied by an increased apoptotic rate indicated that ROS
overproduction and diaphragmatic fatigue were associated with the
pathogenesis of respiratory failure.
The pharmacological properties of AS-IV have
recently gained the attention of researchers (9). Studies have revealed the anti-oxidant
and anti-apoptotic functions of AS-IV in various tissue and cell
types (13). Inhibition of ROS
production was reported to ameliorate monocyte apoptosis (8,18). The
inhibitory effect of AS-IV on oxidative stress was demonstrated to
contribute to the prevention of acute kidney damage (19). A previous study revealed the critical
roles of oxidative stress and apoptosis in the pathogenesis of
diaphragmatic fatigue (6). Based on
the above results, the present study was designed to investigate
the protective effects of AS-IV on diaphragmatic muscle cells and
examine the associated mechanisms. The results indicated that AS-IV
and anti-oxidants including N-acetyl-l-cysteine (NAC) and catalase
(results not shown) attenuated the anti-proliferative effects of
IL-8 on diaphragmatic muscle cells. Flow cytometric analysis
revealed that treatment with AS-IV suppressed the apoptosis and ROS
generation induced by IL-8 in diaphragmatic muscle cells.
Caspase-9, an initiator caspase, and caspase-3, an effector
caspase, have direct roles in the apoptotic pathway (22). The changes in caspase-3 and caspase-9
activities determined in the study were consistent with the flow
cytometric results. Treatment with NAC and catalase also suppressed
cell apoptosis and ROS generation (results not shown). In the IL-8
plus AS-IV-treated cells, reductions in the pro-inflammatory
cytokines IL-6, IL-8 and TNF-α were observed compared with those in
the IL-8-treated cells. This result indicated that the inflammatory
response in the diaphragmatic muscle cells was inhibited. Taken
together, these results indicated that AS-IV had anti-oxidant,
anti-apoptotic and anti-inflammatory effects. These results were
consistent with those of other studies (10–14).
The AKT pathway contributes to key biological
processes, including cell proliferation, apoptosis and inflammation
(9). The effects of AS-IV on the AKT
pathway have been investigated in human umbilical vein endothelial
cells and cardiomyocytes (19,20). The
results of the present study indicated that the level of
phosphorylation of AKT was increased in IL-8-treated diaphragmatic
muscle cells; this response was markedly attenuated by AS-IV and
the AKT inhibitor GSK690693, respectively. The present results
suggested that the AKT pathway is involved in the protective role
of AS-IV in diaphragmatic muscle cells.
In conclusion, the present study indicated that the
inflammatory diaphragmatic muscle cells induced by IL-8 had
increased rates of AKT phosphorylation, reduced cell proliferation,
and increased apoptosis and ROS production. AS-IV exerted
anti-oxidant, anti-apoptotic and anti-inflammatory effects to
attenuate the effects of IL-8. Additional animal experiments should
be performed to further explore this, as the present study suggests
that AS-IV may be used for the treatment of diaphragmatic
fatigue.
Acknowledgements
This study was supported by the Science and
Technology Projects of Nanjing (grant no. 201605029).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ni Y, Shi G, Yu Y, Hao J, Chen T and Song
H: Clinical characteristics of patients with chronic obstructive
pulmonary disease with comorbid bronchiectasis: A systemic review
and meta-analysis. Int J Chron Obstruct Pulmon Dis. 28:1465–1475.
2015. View Article : Google Scholar
|
|
2
|
Smith-Blair N: Mechanisms of diaphragm
fatigue. AACN Clin Issues. 13:307–319. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Demedts IK, Demoor T, Bracke KR, Joos GF
and Brusselle GG: Role of apoptosis in the pathogenesis of COPD and
pulmonary emphysema. Respir Res. 7:532006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kirkham PA and Barnes PJ: Oxidative stress
in COPD. Chest. 144:266–273. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kyroussis D, Polkey MI, Keilty SE, Mills
GH, Hamnegard CH, Moxham J and Green M: Exhaustive exercise slows
inspiratory muscle relaxation rate in chronic obstructive pulmonary
disease. Am J Respir Crit Care Med. 153:787–793. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mador MJ, Kufel TJ, Pineda LA and Sharma
GK: Diaphragmatic fatigue and high-intensity exercise in patients
with chronic obstructive pulmonary disease. Am J Respir Crit Care
Med. 161:118–123. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reid MB: Free radicals and muscle fatigue:
Of ROS, canaries, and the IOC. Free Radic Biol Med. 44:169–179.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keatings VM, Collins PD, Scott DM and
Barnes PJ: Differences in interleukin-8 and tumor necrosis
factor-alpha in induced sputum from patients with chronic
obstructive pulmonary disease or asthma. Am J Respir Crit Care Med.
153:530–534. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bozinovski S, Vlahos R, Hansen M, Liu K
and Anderson GP: Akt in the pathogenesis of COPD. Int J Chron
Obstruct Pulmon Dis. 1:31–38. 2006.PubMed/NCBI
|
|
10
|
Cao LL, Li WZ, Si XL, Sun L and Li WP:
Protective effect and mechanism of astragaloside IV on oxidative
stress injury of mesangial cells. Zhongguo Zhong Yao Za Zhi.
38:725–730. 2013.(In Chinese). PubMed/NCBI
|
|
11
|
Ji KT, Tang JF and Chai JD: Effect of
astragaloside against the oxidative damage on endothelial cells.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 31:807–810. 2011.(In Chinese).
PubMed/NCBI
|
|
12
|
Sun L, Li W, Li W, Xiong L, Li G and Ma R:
Astragaloside IV prevents damage to human mesangial cells through
the inhibition of the NADPH oxidase/ROS/Akt/NFkappaB pathway under
high glucose conditions. Int J Mol Med. 34:167–176. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang A, Zheng Y, Que Z, Zhang L, Lin S,
Le V, Liu J and Tian J: Astragaloside IV inhibits progression of
lung cancer by mediating immune function of Tregs and CTLs by
interfering with IDO. J Cancer Res Clin Oncol. 140:1883–1890. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qiu YY, Zhu JX, Bian T, Gao F, Qian XF, Du
Q, Yuan MY, Sun H, Shi LZ and Yu MH: Protective effects of
astragaloside IV against ovalbumin-induced lung inflammation are
regulated/mediated by T-bet/GATA-3. Pharmacology. 94:51–59. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shang L, Qu Z, Sun L, Wang Y, Liu F, Wang
S, Gao H and Jiang F: Astragaloside IV inhibits adenovirus
replication and apoptosis in A549 cells in vitro. J Pharm
Pharmacol. 63:688–694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun J, Chen XL, Zheng JY, Zhou JW and Ma
ZL: Astragaloside IV protects new born rats from anesthesia-induced
apoptosis in the developing brain. Exp Ther Med. 12:1829–1835.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hu JY, Han J, Chu ZG, Song HP, Zhang DX,
Zhang Q and Huang YS: Astragaloside IV attenuates hypoxia-induced
cardiomyocyte damage in rats by upregulating superoxide dismutase-1
levels. Clin Exp Pharmacol Physiol. 36:351–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang ZG, Wu L, Wang JL, Yang JD, Zhang J,
Zhang J, Li LH, Xia Y, Yao LB, Qin HZ and Gao GD: Astragaloside IV
prevents MPP+-induced SH-SY5Y cell death via the
inhibition of Bax-mediated pathways and ROS production. Mol Cell
Biochem. 364:209–216. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang L, Liu Q, Lu L, Zhao X, Gao X and
Wang Y: Astragaloside IV stimulates angiogenesis and increases
hypoxia-inducible factor-1α accumulation via phosphatidylinositol
3-kinase/Akt pathway. J Pharmacol Exp Ther. 338:485–491. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia Y, Zuo D, Li Z, Liu H, Dai Z, Cai J,
Pang L and Wu Y: Astragaloside IV inhibits doxorubicin-induced
cardiomyocyte apoptosis mediated by mitochondrial apoptotic pathway
via activating the PI3K/Akt pathway. Chem Pharm Bull (Tokyo).
62:45–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Demoule A, Divangahi M, Danialou G,
Gvozdic D, Larkin G, Bao W and Petrof BJ: Expression and regulation
of CC class chemokines in the dystrophic (mdx) diaphragm. Am J
Respir Cell Mol Biol. 33:178–185. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicologic pathology. 35:495–516. 2007.
View Article : Google Scholar : PubMed/NCBI
|



















