Introduction
Hydroxyapatite [HA;
Ca10(PO4)6(OH)2] has a
superior biocompatibility to human tissue, slow biodegradability
in situ, and excellent osteoconductivity and
osteoinductivity. Therefore, it has become one of the most
favorable biomaterials in many tissue-engineering applications,
such as scaffolds for bone repair and bioactive coating on metallic
prosthetic implants (1–4). Recently, nano-sized HA (nHA) particles
have also been tested as a potential biomaterial for drug delivery.
An arginine-modified nHA delivery system has previously been
developed and demonstrated to successfully deliver intracellular
DNAzymes in both in vitro cell lines and in vivo
mouse xenograft (5–7).
In addition to being used for drug delivery, nHA
particles have been demonstrated to have an anti-cancer effect on
multiple types of cancer cell lines and animal models of cancer. In
a rabbit model of hepatic VX2 tumor implant, Hu et al
(8) demonstrated that intravenous
injection of 20 mg/kg nHA collosol significantly reduced tumor
growth. In vitro studies have demonstrated that nHA
particles significantly inhibited the proliferation of human colon
carcinoma HCT116 cells, lymphatic leukemia P388 cells, gastric
cancer SGC-7901 cells, osteoblast-like MG-63 cells, hepatoma HepG2
cells and breast cancer MCF-7 cells (9–14). The
molecular and cellular mechanism underlying the anti-cancer effect
appears to be associated with induction of apoptosis and cell cycle
arrest, downregulation of oncogenes and upregulation of tumor
suppressor genes (9–15). Chen et al (9) demonstrated that nHA induced
mitochondria-dependent apoptosis in human gastric cancer cells by
upregulating the expression of pro-apoptotic protein B cell
lymphoma (Bcl)-2-associated X protein and reducing mitochondrial
membrane potential and the release of cytochrome C. The expression
of oncogene c-Myc in tumor tissue of a rabbit model of implanted
hepatic VX2 tumors was significantly reduced by nHA (8), and the expression of the tumor
suppressor p53 in human breast cancer MCF-7 cells was increased by
nHA (15).
The effect of nHA on glioma cells has also been
investigated. Chu et al (16)
demonstrated that nHA significantly inhibited the proliferation of
human glioma U251 and SHG44 cells in a dose- and time-dependent
manner, and tail vein injection of nHA collosol significantly
reduced the tumor volume in nude mice transplanted with the human
glioma cells. Consistent with the findings from other human cell
lines, the inhibitory effect of nHA on glioma cell growth was
associated with the stimulation of apoptosis. It has been
demonstrated that genetic and morphological characteristics of
different glioma cell lines are varied (17). For instance, U87MG ATCC cells express
wild type p53, whereas p53 in U251 cells is mutated (18). In addition, the behaviors of U251 and
U87MG ATCC cells in mouse xenograft model are also different
(17). Therefore, the intracellular
signaling pathways regulating key cellular processes, such as
proliferation and cell cycle, may vary in these cell lines,
resulting in different response to anti-cancer reagents. Therefore,
to further investigate the role of nHA in the regulation of glioma
development and the underlying molecular mechanism, the effect of
nHA was examined on the behavior human glioma U87MG ATCC cells and
rat glioma C6 cells.
Materials and methods
Cell culture
Rat glioma cell line C6 and human glioma cell line
U87MG ATCC were purchased from American Type Culture Collection
(ATCC; Manassas, VA, USA). The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Thermo Fisher Scientific, Inc.), 100 µg/ml penicillin and 100 µg/ml
streptomycin and incubated at 37°C with 5% CO2. The
U87MG ATCC cell line is known to be misidentified, but a previous
study has indicated that this cell line is likely to be derived
from a glioblastoma (19).
Preparation of nHA suspension
nHA was prepared using the electrode position
method. A titanium sheet was used as the cathode, and Pt was used
as the anode. The electrolyte solution contained 0.04 M
Ca(NO3)2 and 0.01 M
NH4H2PO4 with a pH at 5.0–6.0. The
preparation temperature was 80°C and the density was 10
mA/cm2. The deposition time was 10 min. The
characteristics of the nHA are presented in Fig. 1. Autoclaved nHA (1 mg/ml) was
suspended in DMEM media containing 1.5 mg/ml dispersant agent
polyethylene glycol-200 (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and mixed by ultrasound sonication. The nHA suspension was
stored at 4°C for future use and sonicated at 24 kHz and 400 W for
5 min prior to use. Following sputter-coating with gold, the nHA
was observed using scanning electron microscopy (KYKY-1000B;
Chinese Academy of Sciences, Beijing Science Instrument Development
Center, Beijing, China) and photographed. The molecular weight of
nHA is 1004 and the standard hydroxyapatite XRD profile (file no.
074-0566) was used.
Determination of cell viability
Cell viability was determined via MTT assay
(Sigma-Aldrich; Merck KGaA). Briefly, C6 and U87MG ATCC cells in
the exponential growth phase were collected, re-suspended in DMEM,
seeded in 96-well plate at 4×103 cells/100 µl DMEM/well,
and incubated at 5% CO2 and 37°C overnight.
Subsequently, the cells were exposed to nHA at 0, 20, 40, 60, 80 or
100 µg/ml (0, 19.9, 39.8, 59.7, 79.6 and 99.6 µM, respectively) and
incubated for a further 48 h. At 4 h prior to the end of the
incubation, 20 µl MTT solution (0.5 mg/ml) was added to the cells.
At the end of the incubation, 150 µl dimethylsulfoxide
(Sigma-Aldrich; Merck KGaA) was added to terminate the MTT
reaction, and the plate was then kept at room temperature (22-25°C)
in darkness on a shaker at 80 rpm for 10 min. Absorbance at 490 nm
was determined in a plate reader (Thermo Fisher Scientific, Inc.).
Six wells were used for each treatment and the experiment was
repeated at least 3 times. Wells containing no cells were used as
blank controls.
Hoechst staining
Cover glasses were put into a 12-well plate. C6
cells in exponential growth phase were seeded at 5×104
cells/1 ml DMEM/well and incubated at 37°C for 24 h. The cells were
then exposed to nHA at 0, 20, 60 or 100 µg/ml in fresh DMEM media
and incubated for a further 24 h. Culture media were removed and
the cells were then fixed with 2.5% glutaraldehyde (0.5 ml/well) at
room temperature for 10 min or at 4°C overnight. The cells were
subsequently washed twice with PBS, and 0.5 ml Hoechst 33258 (0.5
µg/ml; Sigma-Aldrich; Merck KGaA) was added in each well. The plate
was incubated at room temperature (22-25°C) for 20–30 min and
washed 2–3 times at room temperature (22-25°C) with PBS. The cells
were observed and images were captured under fluorescent microscopy
(magnification, ×100).
Flow cytometry
C6 and U87MG ATCC cells were seeded in 6-well plates
at a density of 2×105 cells/well overnight at 37°C and
treated with nHA at 0, 20, 40, 60, 80 or 100 µg/ml for 48 h at
37°C. Floating cells in the culture media were collected by
centrifugation (1,500 × g at 4°C for 3 min). The attached cells
were harvested by trypsinization. The collected cells were washed
twice with PBS following centrifugation at 4°C (1,000 × g; 5 min),
re-suspended in 250 µl binding buffer (Becton, Dickinson and
Company, Franklin, Lakes, NJ, USA) containing 5 µl Annexin
V-fluorescein isothiocyanate and 10 µl propidium iodide (Becton,
Dickinson and Company), and incubated at room temperature (22-25°C)
for 15 min in darkness. The cell suspension was then diluted with
200 µl PBS, filtered through 300-mesh filters, and analyzed using a
BD FACSCalibur™ flow cytometer. Apoptosis and cell cycle were
analyzed using FlowJo software (Version 10, Tree Star, Inc.,
Ashland, OR, USA). The experiment was repeated at least 3
times.
Transwell invasion assay
U87MG ATCC cell invasion assay was performed using
24-well Transwell plates (8-µm pore size; Corning Inc., Corning,
NY, USA) coated with Matrigel (1 mg/ml; Becton, Dickinson and
Company). Cells (5×104/well) were seeded in the upper
chambers of the wells in 200 µl serum-free media containing HA at
0, 20, 40 or 60 µg/ml. The lower chambers contained 500 µl DMEM
supplemented with 10% FBS. The cells were incubated at 4°C for
overnight, and the cells remaining on the upper surface of the
chamber were removed using cotton swabs. The cells on the other
side of the membrane were fixed at room temperature (22-25°C) with
methanol for 10 min and stained with Giemsa solution (Beyotime
Institute of Biotechnology, Beijing, China) at room temperature
(22-25°C) for 15 min. The penetrated cells at the lower surface of
the membrane were counted under a microscope (magnification, ×100;
BX51; Olympus Corporation, Tokyo, Japan). A total of 5 fields of
view for each membrane were randomly selected and the average cell
number of the 5 fields was calculated. The experiment was repeated
at least 3 times.
Subcellular localization of nuclear
factor (NF)-κB p65
C6 cells in exponential growth phase were cultured
in 6-well plates containing cover glasses at a density of
5×104 cells/1 ml DMEM/well and incubated at 37°C for 24
h. The cells were then exposed to nHA at 0, 20, 60, or 100 µg/ml
and incubated for a further 24 h. The culture media were removed
and the plate was washed with PBS twice for 5 min each. The cells
were fixed with 2.5% glutaraldehyde (0.5 ml/well) at room
temperature for 15 min, washed with PBS three times for 5 min each,
and permeabilized with 0.5 ml 0.2% Triton X-100 at room temperature
for 15 min. After the cells were blocked with blocking buffer [10%
bovine serum albumin (Beyotime Institute of Biotechology) in PBS]
for 1 h at room temperature, the cells were incubated with rabbit
anti-rat NF-κB p65 antibody (cat. no. PA5 16545; 1:800 dilution;
Thermo Fisher Scientific, Inc.) at room temperature for 1 h. The
cells were washed with PBS three times for 5 min each and incubated
with anti-rabbit IgG conjugated with Cy3 (cat. no. D111107; 1:1,000
dilution; Boster Biological Technology, Wuhan, China) at room
temperature for 1 h in the dark. Following washing with PBS twice
for 10 min each, the cells were exposed to Hoechst 33258 (0.5
µg/ml; Sigma-Aldrich; Merck KGaA) at 37°C for 5 min in dark. The
cover glass was washed 3 times with PBS, mounted on a slide, and
observed under a confocal fluorescent microscope (magnification,
×200, Nikon Corporation, Tokyo, Japan) and images of fluorescence
staining were captured.
Western blotting
C6 and U87MG ATCC cells in exponential growth phase
were exposed to nHA at 0, 20, 60 or 100 µg/ml for 24 h at 37°C and
collected using a cell scraper and centrifugation at 4°C at 300 × g
for 5 min. The collected cells were lysed in ice-cold
radioimmunoprecipitation assay buffer containing protease inhibitor
phenylmethane sulfonyl fluoride and phosphatase inhibitor cocktail
(Sigma-Aldrich; Merck KGaA) and centrifuged at 10,000 × g at 4°C
for 10 min. The supernatant was collected and protein concentration
of the supernatant was determined by bicinchoninic acid assay. A
total of 50 µg protein from each sample was loaded in 12% SDS-PAGE
gel for electrophoresis. Proteins were then transferred onto a
polyvinylidene difluoride membrane. The membrane was blocked with
5% dry skimmed milk in PBS at room temperature for 2 h, washed, and
incubated with the following primary antibodies: Rabbit anti-rat or
human Bcl-2 (cat. no. 12789-1-AP; 1:1,000 dilution; ProteinTech
Group, Inc., Chicago, IL, USA), rabbit anti-rat or human
cyclooxygenase (Cox)-2 (cat. no. 12375-1-AP; 1:800 dilution;
ProteinTech Group, Inc.), mouse anti-rat or human survivin (cat.
no. sc-101433; 1:800 dilution; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA), rabbit anti-rat or human vascular endothelial
growth factor (VEGF; cat. no. 19003-1-AP; 1:1,000 dilution;
ProteinTech Group, Inc.), mouse anti-rat or human GAPDH (cat. no.
60004-1-Ig; 1:2,000 dilution; ProteinTech Group, Inc.), rabbit
anti-rat or human matrix metalloproteinase (MMP)-2 (cat. no. 87809;
1:800 dilution; Cell Signaling Technology, Inc., Danvers, MA, USA),
rabbit anti-rat or human MMP-9 (cat. no. 13667; 1:1,000 dilution;
Cell Signaling Technology, Inc.), rabbit anti-rat or human NF-κB
p65 (cat. no. PA5 16545; 1:1,000 dilution; Thermo Fisher
Scientific, Inc.), phospho NF-κB p65 (cat. no. 3022; 1:1,000
dilution; Cell Signaling Technology, Inc.) and mouse anti-rat or
human β-actin (cat. no. 66009-1-Ig; 1:2,000 dilution; ProteinTech
Group, Inc.) at 4°C overnight. Following thorough washing, the
membrane was incubated with horseradish peroxidase-conjugated
secondary antibodies (cat. nos. ZDR5306 and ZDR5307; 1:2,000
dilution; Beijing Golden Bridge Biotechnology Co., Ltd, Beijing,
China) at room temperature for 1 h. The membrane was then washed
thoroughly 4 times for 15 min each. The protein signal was
developed using an enhanced chemiluminescence detection kit (Thermo
Fisher Scientific, Inc.) and exposed on films. Quantity One
software (v4.6.6, Bio-Rad Laboratories, Inc., Hercules, USA) was
used to quantify the intensity of protein signals. The experiment
was repeated at least 3 times.
Statistical analysis
Data are presented as mean ± standard deviation.
Statistical analysis was performed using SPSS software (version
17.0; SPSS, Inc., Chicago, IL, USA). The statistical significance
of differences was determined using Student's two-tailed t-test in
two groups and one-way analysis of variance followed by Dunnett's
test in multiple groups. P-values were 2-sided and P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of nHA
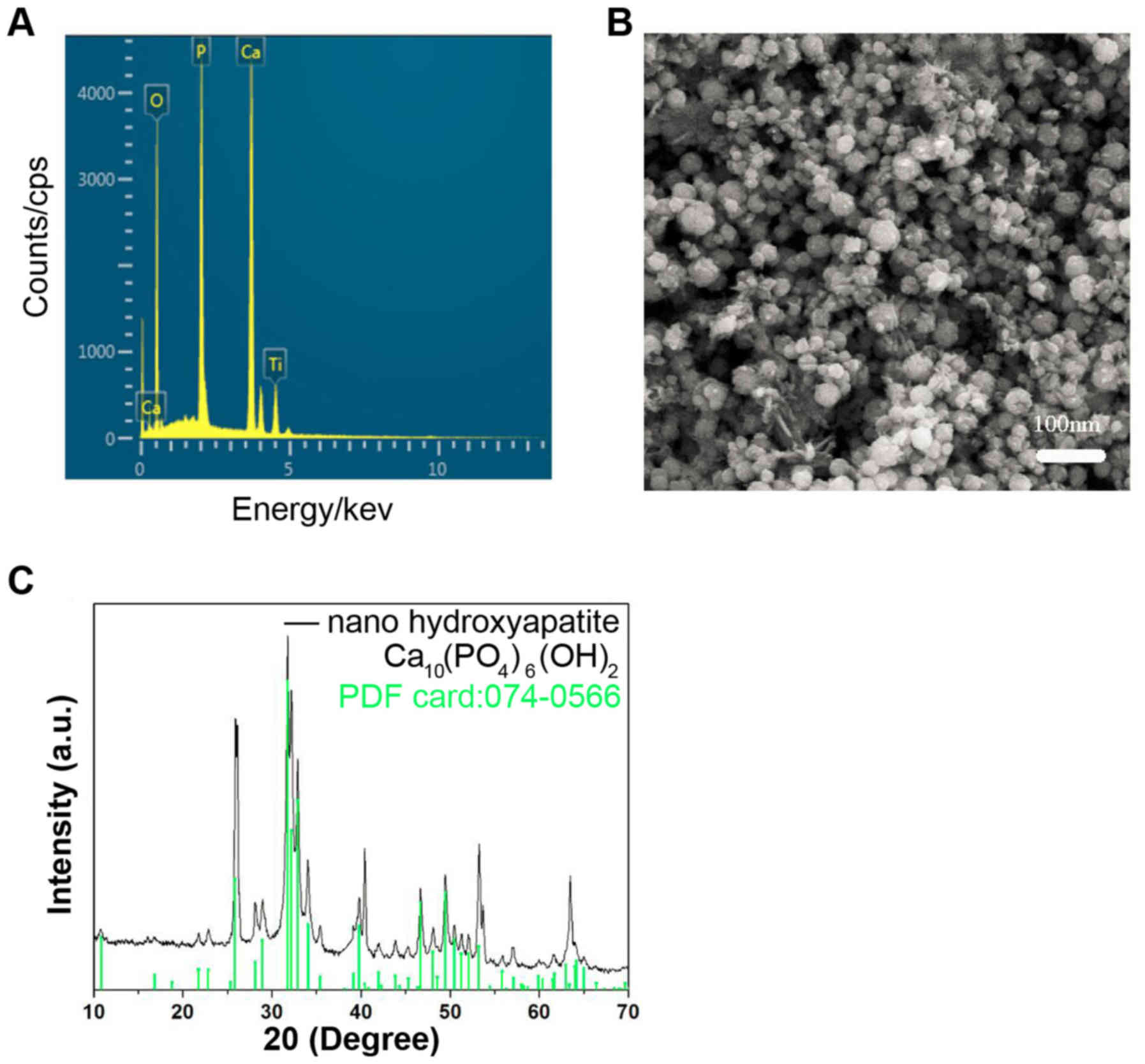
The energy dispersive X-ray spectrometry
demonstrated that the predominant components in the nHA were Ca, P
and O, and Ti in the spectrum was from the cathode substrate
(Fig. 1A). The particle size of nHA
was ~25 nm (Fig. 1B). The X-ray
diffraction (XRD) (Fig. 1C) of the
nHA revealed that the characteristic peaks of the prepared nHA
matched the standard hydroxyapatite XRD profile (standard nHA
powder XRD file: 074-0566) well. These results suggested that the
nHA was prepared successfully.
nHA significantly reduces glioma cell
growth
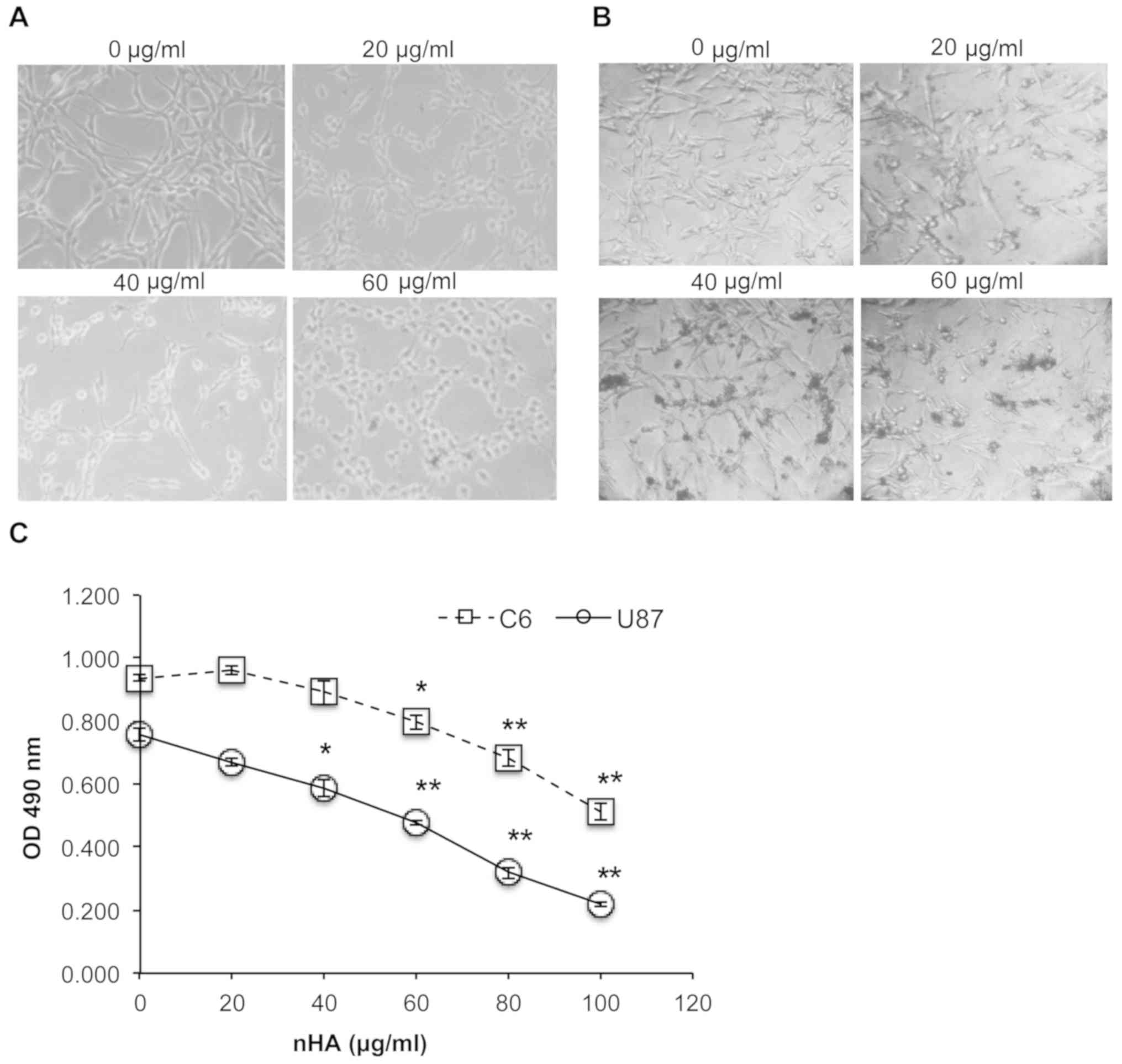
As presented in Fig. 2A
and B, the cell morphology of both C6 cells and U87MG ATCC
cells was markedly damaged by nHA at ≥20 µg/ml. Following exposure
to nHA for 24 h, cells became rounded and began to detach from the
tissue culture surface (Fig. 2A and
B). MTT assay revealed that cell viability of C6 and U87MG ATCC
cells was significantly reduced by nHA in a dose-dependent manner
(Fig. 2C). Collectively, the
anti-tumor activity of nHA was demonstrated in glioma cells.
nHA significantly regulates glioma
cell apoptosis, cell cycle and invasion
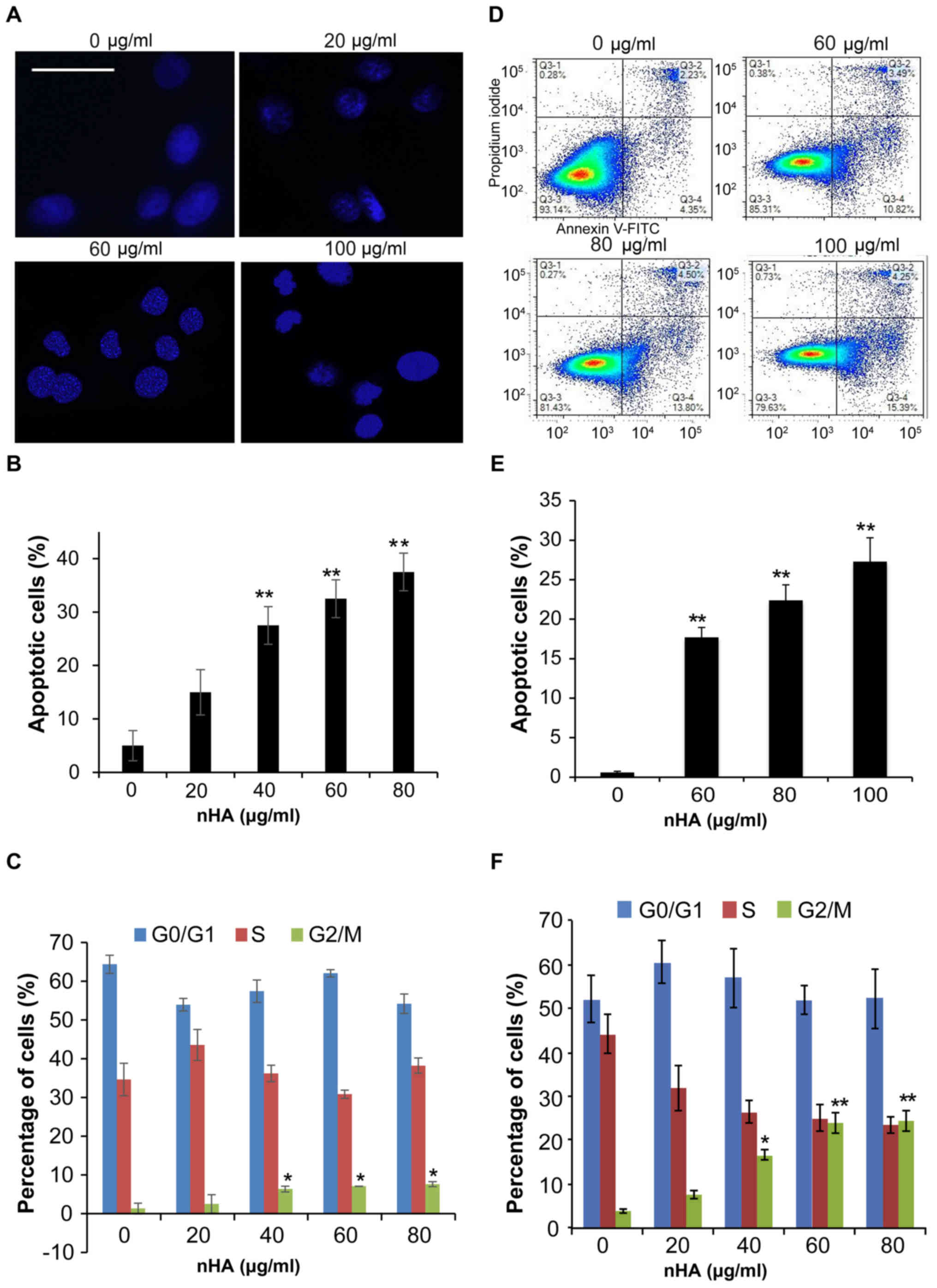
Staining of nuclei of C6 cells with Hoechst 33258
demonstrated that nuclear condensation appeared in C6 cells
following 24 h treatment with nHA ≥20 µg/ml (Fig. 3A), and flow cytometry analysis
revealed that treatment with nHA for 48 h increased the proportion
of apoptotic C6 cells in a dose-dependent manner (Fig. 3B). However, treatment with ≥40 µg/ml
nHA for 48 h significantly increased the proportion of cells in
G2/M phase, supporting that nHA induces cell cycle
G2/M arrest in C6 cells (Fig.
3C). Consistent with the results of C6 cells, exposure of U87MG
ATCC cells to nHA induced apoptosis in a dose-dependent manner
(Fig. 3D and E). In addition, nHA
also induces cell cycle G2/M arrest in U87MG cells
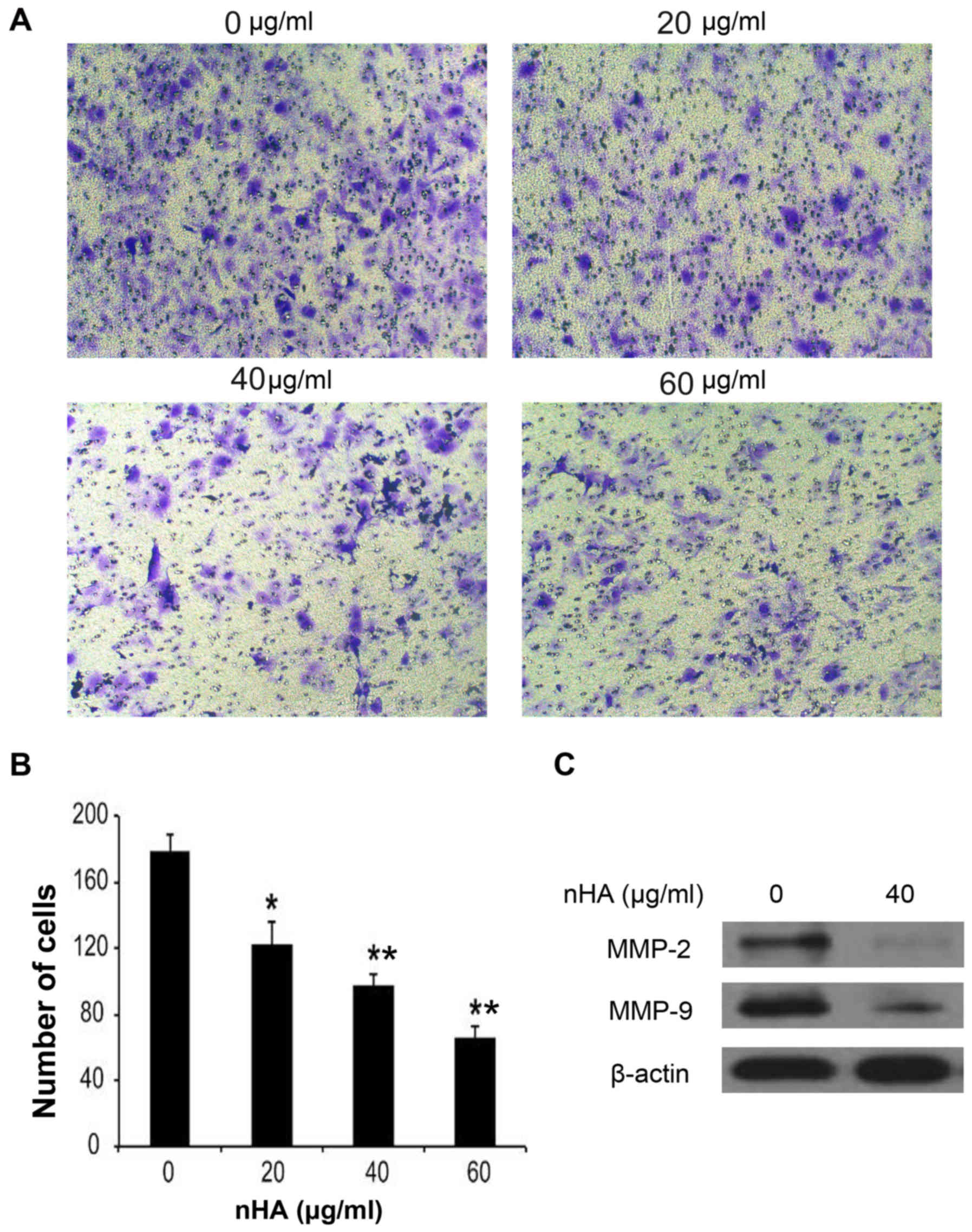
(Fig. 3F). In addition to inhibiting
cell proliferation and stimulating apoptosis, nHA significantly
inhibited the invasion of U87MG ATCC cells in a dose-dependent
manner (Fig. 4A and B). The MMP-2
and MMP-9 expression in U87MG ATCC cells were also decreased by nHA
(Fig. 4C). These results suggested
that nHA functioned as a multiple regulatory factors in glioma
cells.
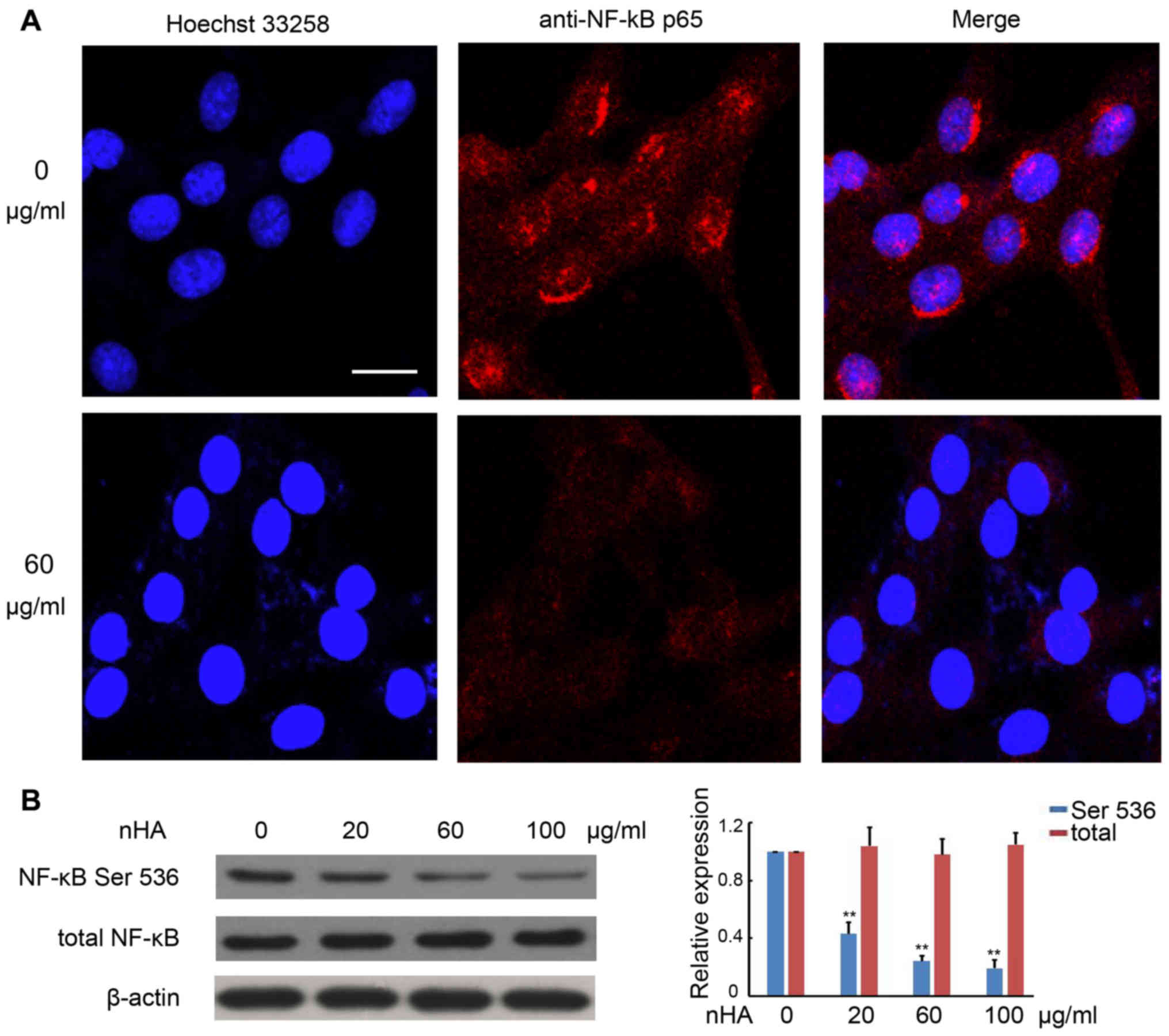
nHA inhibits NF-κB p65 nuclear
translocation
It has been demonstrated that the NF-κB signaling
pathway promotes cancer development by suppressing apoptosis
(20). Immunofluorescent staining
with anti-NF-κB p65 antibody revealed strong punctate staining
signals in the nuclei of C6 cells without exposure to nHA, whereas
cells treated with nHA exhibited weak diffused staining signals
(Fig. 5A). Western blotting further
confirmed that protein expression of phosphorylated NF-κB p65 in C6
cells was substantially reduced by nHA treatment in a
dose-dependent manner, whereas nHA had no significant effect on
total NF-κB expression in C6 cells (Fig.
5B). These results suggest that nHA may reduce NF-κB expression
and block its nuclear translocation.
nHA inhibits the protein expression of
NF-κB targeting genes
Consistently, the expressions of the downstream
NF-κB targeting molecules including Bcl-2, Cox-2, survivin, and
VEGF in C6 cells were also reduced by nHA in a dose-dependent
manner (Fig. 6A and B). Similarly,
nHA markedly reduced the protein expression of the NF-κB targeting
molecules Bcl-2, Cox-2, VEGF and survivin in C6 and U87MG ATCC
cells (Fig. 6C and D). These
findings indicate that nHA may stimulate apoptosis in the glioma
cells by inhibiting the NF-κB signaling pathway.
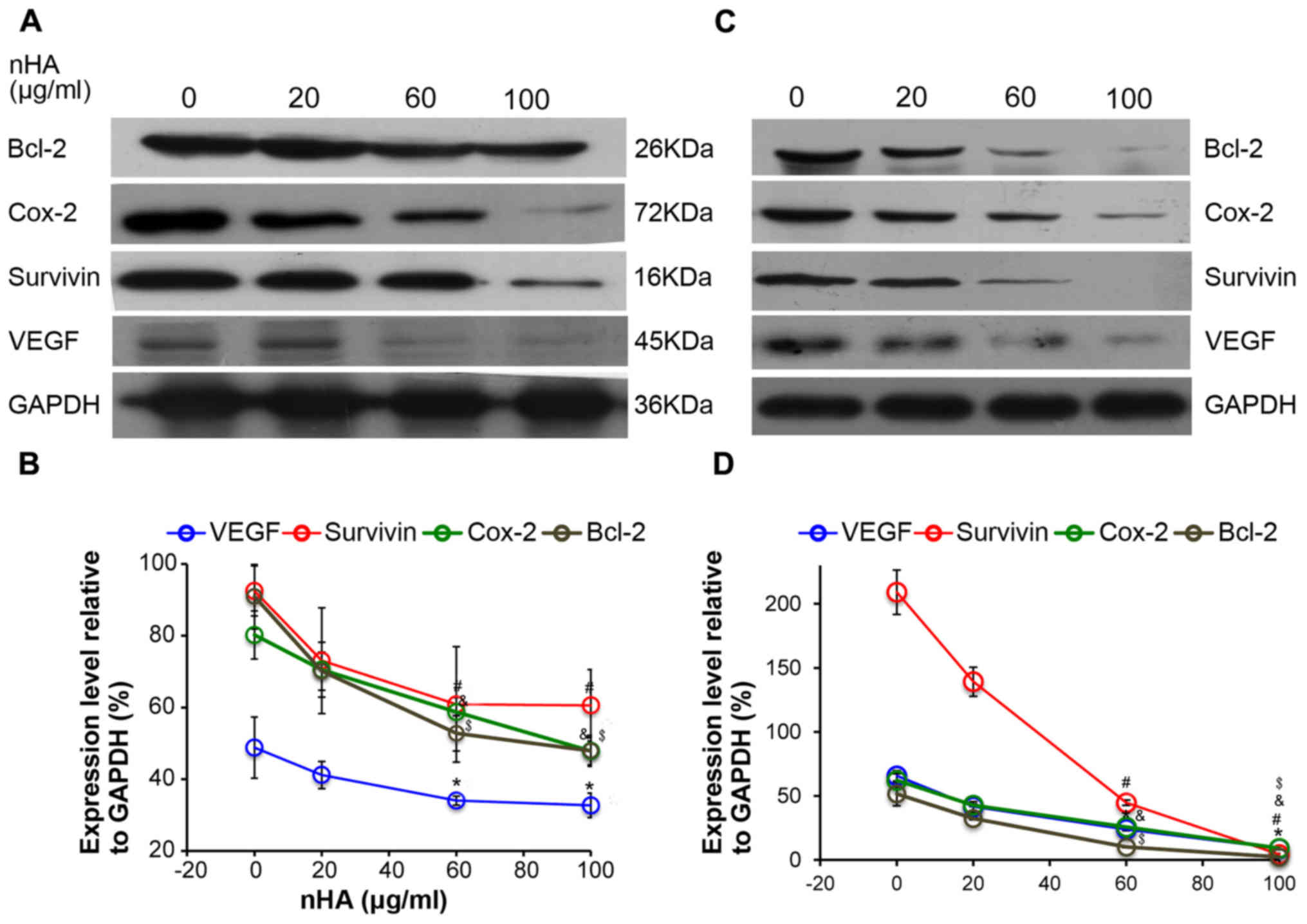 | Figure 6.nHA reduced protein expression of
nuclear factor-κB p65 target molecules. (A) Representative images
of western blotting of C6 cells. (B) Densitometry analysis of the
western blotting of C6 cells. (C) Representative images of western
blotting of U87MG ATCC cells. (D) Densitometry analysis of the
western blotting of U87MG ATCC cells. C6 and U87MG ATCC cells were
treated with 0, 20, 60 or 100 µg/ml nHA for 24 h. The experiment
was repeated 3 times. Representative images are presented.
*P<0.05, VEGF expression vs. 0 group; #P<0.05,
Survivin expression vs. 0 group; &P<0.05, Cox-2
expression vs. 0 group; $P<0.05, Bcl-2 expression vs.
with 0 group. nHA, nano-hydroxyapatite; Bcl-2, B cell lymphoma-2;
Cox-2, cyclooxygenase-2; VEGF, vascular endothelial growth
factor. |
Discussion
In the present study, it was demonstrated that nHA
significantly inhibited the proliferation and invasion of glioma
cells and induced apoptosis and cell cycle G2/M arrest. These
results are consistent with the findings of previous studies using
the same or different glioma cell lines. Chu et al (16) demonstrated that 48 h exposure to nHA
at 120 and 240 µg/ml significantly induced apoptosis of glioma U251
and SHG44 cells, respectively. Glioma U87MG ATCC and C6 cells
appeared to be more sensitive to nHA as the present study revealed
that nHA induced marked apoptotic morphologic changes in C6 cells
and U87MG ATCC cells at 20 µg/ml, and flow cytometry revealed
substantial apoptosis of C6 and U87MG ATCC cells at 20 and 60 µg/ml
nHA, respectively. Similar to the results of the present study, Xu
et al (21) also demonstrated
that exposure of C6 cells to nHA at 10 µg/ml for 48 h significantly
stimulated apoptosis and reduced cell viability. In addition to
inhibiting cell proliferation, it was demonstrated that nHA 20–60
µg/ml reduced U87MG ATCC cell invasion significantly. In an in
vitro study to investigate the effects of
poly(lactide-co-glycolide) (PLGA)/nHA microspheres as a drug
delivery system for temozolomide (TMZ) on U87MG ATCC cellular
behavior, Zhang et al (22)
demonstrated that addition of nHA in TMZ/PLGA microspheres further
reduced U87MG ATCC cell invasion, suggesting that nHA may inhibit
U87MG ATCC cell invasion either directly or indirectly by enhancing
the anti-cancer effects of TMZ in the microspheres.
A number of previous studies on cancer cell lines
have demonstrated that nHA affected the protein expression of
molecules involved in apoptosis (9,12–14). The
anti-apoptotic protein, Bcl-2, has been consistently demonstrated
to be downregulated, whereas caspase-3 and −9 were activated by nHA
in human gastric cancer cells, osteoblast-like cells and hepatoma
cells (9,12–14). In
the present study, the protein level of Bcl-2 in C6 and U87MG ATCC
cells was also significantly reduced by nHA in a dose-dependent
manner. To further investigate the molecular mechanism underlying
nHA-mediated stimulation of apoptosis of glioma cells, the
expression and activity of the nuclear factor NF-κB was examined.
It has been demonstrated that activation of NF-κB signaling can
suppress apoptosis, leading to cancer development (20). In the present study, C6 cells without
exposure to nHA presented clear NF-κB p65 activity and nHA
treatment reduced NF-κB P65 level and blocked its nuclear
translocation. One of the mechanisms by which NF-κB signaling
pathway inhibits apoptosis is to neutralize reactive oxygen species
(ROS) (23). Xu et al
(21) demonstrated that exposure of
C6 cells to nHA resulted in intracellular accumulation of ROS.
These findings suggest that nHA may induce apoptosis by
downregulating the NF-κB signaling pathway. Bcl-2, Cox-2, survivin
and VEGF are target genes of NF-κB and contribute to cancer
development (24). The present
results demonstrated that nHA significantly reduced the protein
expressions of these genes in C6 and U87MG ATCC cells. Furthermore,
as NF-κB has essential roles in inflammatory responses (20), nHA-mediated inhibition of NF-κB
suggests that nHA could have anti-inflammation effects. Future
studies are required to further evaluate this hypothesis.
The findings of the present study and previous
studies clearly demonstrate that nHA can directly induce apoptosis
and inhibit glioma cell proliferation in vitro and in animal
models (16,21). In addition, nHA has also been
demonstrated to exert therapeutic beneficial effects indirectly.
Chu et al (16) demonstrated
that combination of nHA and 1,3-bis(2-chloroethyl)-1-nitrosourea
(BCNU) significantly reduced BCNU-associated adverse reactions in a
mouse xenograft model transplanted with human glioma U251 cells.
The same research group also demonstrated that pre-exposure of U251
cells to nHA or pre-treatment of nude mice bearing U251 tumors with
nHA prior to irradiation enhanced radiosensitivity of the glioma
cells and improved irradiation efficacy (25).
The present study demonstrated that nHA
significantly inhibited glioma cell proliferation, induced
apoptosis and cell cycle G2/M arrest, and reduced invasion. The
molecular mechanism underlying the nHA-mediated anti-cancer effect
appeared to be associated with downregulation of the NF-κB
signaling pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81602186), China
Postdoctoral Science Foundation (grant no. 2017M612216) and the
Research Award Fund for Outstanding Middle-aged and Young
scientists of Shandong Province, China (grant no. BS2011SW004).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GG and AT conducted all the experiments. ZY and CW
participated in the design of the study and assisted in drafting
the manuscript. XL conducted the statistical analysis. CF
participated in the cell staining. CW designed the project and
finalized the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Billström GH, Blom AW, Larsson S and
Beswick AD: Application of scaffolds for bone regeneration
strategies: Current trends and future directions. Injury. 44 Suppl
1:S28–S33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Diez M, Kang MH, Kim SM, Kim HE and Song
J: Hydroxyapatite (HA)/poly-L-lactic acid (PLLA) dual coating on
magnesium alloy under deformation for biomedical applications. J
Mater Sci Mater Med. 27:342016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Habibovic P and de Groot K: Osteoinductive
biomaterials-properties and relevance in bone repair. J Tissue Eng
Regen Med. 1:25–32. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hutmacher DW, Schantz JT, Lam CX, Tan KC
and Lim TC: State of the art and future directions of
scaffold-based bone engineering from a biomaterials perspective. J
Tissue Eng Regen Med. 1:245–260. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yin B, Ma P, Chen J, Wang H, Wu G, Li B,
Li Q, Huang Z, Qiu G and Wu Z: Hybrid macro-porous titanium
ornamented by degradable 3D Gel/nHA micro-scaffolds for bone tissue
regeneration. Int J Mol Sci. 17:5752016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mohamadyar-Toupkanlou F,
Vasheghani-Farahani E, Hanaee-Ahvaz H, Soleimani M, Dodel M, Havasi
P, Ardeshirylajimi A and Taherzadeh ES: Osteogenic differentiation
of MSCs on fibronectin-coated and nHA-modified scaffolds. ASAIO J.
63:684–691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen Y, Yang L, Huang S, Li Z, Zhang L, He
J, Xu Z, Liu L, Cao Y and Sun L: Delivery system for DNAzymes using
arginine-modified hydroxyapatite nanoparticles for therapeutic
application in a nasopharyngeal carcinoma model. Int J
Nanomedicine. 8:3107–3118. 2013.PubMed/NCBI
|
|
8
|
Hu J, Liu ZS, Tang SL and He YM: Effect of
hydroxyapatite nanoparticles on the growth and p53/c-Myc protein
expression of implanted hepatic VX2 tumor in rabbits by intravenous
injection. World J Gastroenterol. 13:2798–2802. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen X, Deng C, Tang S and Zhang M:
Mitochondria-dependent apoptosis induced by nanoscale
hydroxyapatite in human gastric cancer SGC-7901 cells. Biol Pharm
Bull. 30:128–132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dey S, Das M and Balla VK: Effect of
hydroxyapatite particle size, morphology and crystallinity on
proliferation of colon cancer HCT116 cells. Mater Sci Eng C Mater
Biol Appl. 39:336–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li G, Huang J, Li Y, Zhang R, Deng B,
Zhang J and Aoki H: In vitro study on influence of a discrete
nano-hydroxyapatite on leukemia P388 cell behavior. Biomed Mater
Eng. 17:321–327. 2007.PubMed/NCBI
|
|
12
|
Shi Z, Huang X, Cai Y, Tang R and Yang D:
Size effect of hydroxyapatite nanoparticles on proliferation and
apoptosis of osteoblast-like cells. Acta Biomater. 5:338–345. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shi Z, Huang X, Liu B, Tao H, Cai Y and
Tang R: Biological response of osteosarcoma cells to
size-controlled nanostructured hydroxyapatite. J Biomater Appl.
25:19–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan Y, Liu C, Qian J, Wang J and Zhang Y:
Size-mediated cytotoxicity and apoptosis of hydroxyapatite
nanoparticles in human hepatoma HepG2 cells. Biomaterials.
31:730–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meena R, Kesari KK, Rani M and Paulraj R:
Effects of hydroxyapatite nanoparticles on proliferation and
apoptosis of human breast cancer cells (MCF-7). J Nanopart Res.
14:7122012. View Article : Google Scholar
|
|
16
|
Chu SH, Feng DF, Ma YB and Li ZQ:
Hydroxyapatite nanoparticles inhibit the growth of human glioma
cells in vitro and in vivo. Int J Nanomedicine. 7:3659–3666. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jacobs VL, Valdes PA, Hickey WF and De Leo
JA: Current review of in vivo GBM rodent models: Emphasis on the
CNS-1 tumour model. ASN Neuro. 3:e000632011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Radaelli E, Ceruti R, Patton V, Russo M,
Degrassi A, Croci V, Caprera F, Stortini G, Scanziani E, Pesenti E
and Alzani R: Immunohistopathological and neuroimaging
characterization of murine orthotopic xenograft models of
glioblastoma multiforme recapitulating the most salient features of
human disease. Histol Histopathol. 24:879–891. 2009.PubMed/NCBI
|
|
19
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Antwerp DJ, Martin SJ, Kafri T, Green
DR and Verma IM: Suppression of TNF-alpha-induced apoptosis by
NF-kappaB. Science. 274:787–789. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu J, Xu P, Li Z, Huang J and Yang Z:
Oxidative stress and apoptosis induced by hydroxyapatite
nanoparticles in C6 cells. J Biomed Mater Res A. 100:738–745. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang D, Tian A, Xue X, Wang M, Qiu B and
Wu A: The effect of temozolomide/poly(lactide-co-glycolide)
(PLGA)/nano-hydroxyapatite microspheres on glioma U87 cells
behavior. Int J Mol Sci. 13:1109–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Naugler WE and Karin M: NF-kappaB and
cancer-identifying targets and mechanisms. Curr Opin Genet Dev.
18:19–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chu SH, Karri S, Ma YB, Feng DF and Li ZQ:
In vitro and in vivo radiosensitization induced by hydroxyapatite
nanoparticles. Neuro Oncol. 15:880–890. 2013. View Article : Google Scholar : PubMed/NCBI
|