Introduction
Pirarubicin (THP) is an antitumor drug commonly used
for treatment of several types of cancer, with fewer toxic
cardiovascular effects compared with its analogue doxorubicin
(1,2). THP intercalates into DNA and interacts
with topoisomerase II to inhibit DNA replication and promote cancer
cell apoptosis (3). A previous study
indicated that treatment with a low dose of THP induces MG-63 cell
cycle arrest and apoptosis by decreasing the expression of
proliferating cell nuclear antigen, cyclin D1, cyclin E and
apoptosis regulator Bcl-2, and increasing the expression of
apoptosis regulator Bax (4).
Furthermore, THP inhibits the expression of cyclin B1 and
phosphorylation of cyclin-dependent kinase 1 (Cdc2) in
multidrug-resistant osteosarcoma cells (5). In addition, THP induces autophagy in
bladder cancer cells (6). However,
the molecular mechanisms underlying the effect of THP on cervical
cancer cell apoptosis remain to be elucidated.
Ubiquitin-specific peptidase 22 (USP22) is a
ubiquitin hydrolase containing a zinc-finger domain at the
N-terminus and a ubiquitin-specific peptidase domain at the C
terminus (7). USP22 acts as a
subunit of the SAGA transcriptional complex and deubiquitylates
histones H2A and H2B to promote gene transcription (8,9). USP22
may co-activate the Myc proto-oncogene protein or cellular tumor
antigen p53-driven target gene transcription (10). Furthermore, USP22 interacts with
non-histone substrates and its deubiquitinase activity leads to the
stabilization of cyclooxygenase-2, sirtuin 1,
fructose-bisphosphatase 1 and cyclin B1 (11–14).
Therefore, USP22 serves a number of roles in the regulation of cell
proliferation, cell cycle and apoptosis and may promote
tumorigenesis. Upregulated expression of USP22 was previously
detected in several types of cancer, including lung and colon
cancer, and is associated with tumor recurrence, metastasis and
poor survival of patients with cancer (15,16). By
contrast, the knockdown of USP22 leads to cell cycle arrest and
reduces cell viability (10).
Increased expression of USP22 among patients with cancer is
associated with decreased survival rates and this gene may serve as
a target for cancer therapy (17).
In our previous study, the 3.0-kb USP22 promoter was cloned to
identify the basic activity region containing motifs for the
binding of cyclic AMP-responsive element-binding protein (CREB),
transcriptional activator MYB and E3 ubiquitin-protein ligase SP1
(SP1), regulating the USP22 promoter activity (18). Previous studies indicated that
chemotherapeutic drug cisplatin (19) or histone deacetylase inhibitor
trichostatin A (TSA) (20) induce
cancer cell apoptosis by inhibiting the expression of USP22. THP
induces cancer cell apoptosis by modulating the expression of
several regulators of proliferation and apoptosis (5). The current study hypothesized that THP
may directly or indirectly downregulate the expression of USP22
through modulating the expression or activity of transcription
factors and promote cancer cell apoptosis.
Human cervical cancer HeLa cells were used in the
present study to determine the effect of THP on apoptosis and the
expression levels of USP22, and to elucidate the underlying
mechanisms. The results indicated that THP induced HeLa cell
apoptosis and decreased the transcription of USP22 by inhibiting
CREB-1 phosphorylation and binding to the USP22 promoter. These
results may provide novel insights into the molecular mechanisms
underlying the pharmacological action of THP in inducing cancer
cell apoptosis.
Materials and methods
Cell culture
Human cervical cancer HeLa cells were obtained from
the Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai,
China) and cultured in Dulbecco's modified Eagle's medium
supplemented with 10% fetal bovine serum (both Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100
µg/ml streptomycin at 37°C in a humidified atmosphere of 5%
CO2. THP (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was initially dissolved in phosphate buffered saline (PBS) and
subsequently diluted in the culture medium to the desired
concentrations.
Flow cytometry
The effect of THP on apoptosis of HeLa cells was
determined by flow cytometry using the Annexin V-FITC Apoptosis
Detection kit, according to the manufacturer's protocol (Beyotime
Institute of Biotechnology, Haimen, China). Briefly, HeLa cells
(1×105 cells/well) were cultured in 6-well plates
overnight and treated in triplicate with vehicle PBS or THP
(100–1,000 ng/ml) for 24 h at 37°C. The cells were washed and
stained with Annexin V-FITC/propidium iodide and the percentages of
apoptotic cells were examined by flow cytometry using the
FACSCalibur platform (BD Biosciences, Franklin Lakes, NJ, USA). The
results were analyzed using the CellQuest software, version 5.1 (BD
Biosciences).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
HeLa cells were treated in triplicate with vehicle
or 100–1,000 ng/ml THP for 24 h or with vehicle or 500 ng/ml THP
for 3–24 h at 37°C. Total RNA from cells treated with vehicle or
THP was extracted using TRIzol reagent (Thermo Fisher Scientific,
Inc.), and reverse transcribed into cDNA using the TIANScript RT
kit (Tiangen Biotech Co., Ltd., Beijing, China). The relative
levels of USP22 mRNA to GAPDH mRNA transcripts in individual groups
of cells were determined by the qPCR using the SYBR Green PCR
master mix (Tiangen Biotech Co., Ltd.) and ABI 7500 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
PCR thermocycling conditions were as follows: Initial denaturation
at 95°C for 5 min, 40 cycles of denaturation at 95°C for 30 sec,
annealing at 60°C for 30 sec and elongation at 72°C for 30 sec, and
the final elongation at 72°C for 1 min. The following primers were
used: Forward, 5′-GTGTCTTCTTCGGCTGTTTA-3′ and reverse,
5′-CTCCTCCTTGGCGATTATTT-3′; USP22 (158 bp) forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse, 5′-AGGGGCCATCCACAGTCTTC-3′
for GAPDH (258 bp). Data were analyzed using the 2−ΔΔCq
method (21).
Western blot analysis
HeLa cells were treated in triplicate with vehicle
or THP (100–1,000 ng/ml) for 24 h. Cells were washed and lysed in
radioimmunoprecipitation assay solution (Beyotime Institute of
Biotechnology) containing a cocktail of protease inhibitors
(Sigma-Aldrich; Merck KGaA), followed by centrifugation at 12,000 ×
g and 4°C for 10 min. Protein concentrations in individual lysates
were determined using the bicinchoninic acid method. Samples of 30
µg protein/lane were separated by SDS-PAGE on 10% gels and
electrophoretically transferred onto polyvinylidene difluoride
membranes. The membranes were blocked with 5% dry skim milk in
tris-buffered saline with Tween-20 and incubated with primary
antibodies against CREB-1 (1:1,000 dilution; cat. no. sc-374227),
phosphorylated CREB-1 (Ser133; 1:500 dilution; cat. no. sc-101663),
USP22 (1:1,000 dilution; cat. no. sc-69082) and GAPDH (1:5,000
dilution; cat. no. sc-20358) at 4°C overnight (all Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). The membranes were
subsequently washed and the bound antibodies were detected with
horseradish peroxidase-conjugated secondary mouse anti-goat (cat.
no. sc-2354) and anti-rabbit (cat. no. sc-2357; both 1:8,000; Santa
Cruz Biotechnology, Inc.) antibodies. ECL kit (Beyotime
Biotechnology) was used for visualization. The levels of target
proteins relative to control GAPDH were determined by densitometric
analysis using ImageJ software (version 1.38; National Institutes
of Health, Bethesda, MD, USA).
Transfection and dual luciferase
assays
The USP22 promoter and its mutant constructs were
generated as previously described (18,19).
HeLa cells were cultured in 24-well plates and transfected with 0.8
µg pGL3-basic construct, P-2828 (−2828/+52), P-595 (−595/+52), or
P-210 (−21/+52) promoter regions together with 0.2 µg pRL-TK
(Promega Corporation, Madison, WI, USA) using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. A
total of 24 h after transfection, cells were treated with 500 ng/ml
THP and cultured for another 24 h at 37°C. Subsequently, cells were
lysed and used for dual luciferase assays using
Dual-Luciferase® Reporter Assay System (Promega
Corporation) according to the manufacturer's protocols. In
addition, cells were transfected with plasmids: P-210, P-210/MYB
mutant (mut), P-210/CREB mut, P-210/SP1 mut (0.5 µg for each), with
or without 0.5 µg plasmid for CREB-1 expression [pCMV-CREB
(Beyotime Institute of Biotechnology)] as previously described
(19). The empty vector pCMV-control
(0.5 µg) was used as the blank group. Following treatment with THP,
the cells were lysed and used for dual luciferase assays as
described above.
Chromatin immunoprecipitation
(ChIP)
The impact of treatment with THP on the binding of
CREB-1 to the USP22 promoter in HeLa cells was determined using
ChIP, as previously described (18).
Briefly, HeLa cells (1×107/group) were treated with or
without (the black group) 500 ng/ml THP for 24 h. Subsequently, the
THP-treated or untreated cells were fixed with 1% formaldehyde and
lysed in SDS lysis buffer, followed by sonication and
centrifugation at 12,000 × g and 4°C for 10 min. In the THP-treated
group, a total of 10 ml supernatant was removed and used as the
input sample. Subsequently, cell lysates were incubated with 1 µg
anti-CREB-1 (cat. no. sc-374227) or negative control immunoglobulin
G (cat. no. sc-2025; both Santa Cruz Biotechnology, Inc.)
antibodies overnight at 4°C and the resulting immunocomplex was
purified by magnetic protein-G beads. The immunocomplex was washed,
eluted and crosslinked with formaldehyde, followed by treatment
with proteinase K at 67°C overnight. The remaining DNA was purified
using spin columns and analyzed by PCR using the following primers:
forward, CREB 5′-GTCTACCCAGAGCCTAACGG-3′ and reverse,
5′-GCGGAGGCCGGACAAAGATGGG-3′. A PCR analysis was conducted to
analyze DNA using 2×Taq PCR Mastermix (Tiangen, Inc., China). The
procedure used for PCR was as follows: 95°C for 5 min, followed by
32 cycles of 95°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec,
and the final elongation at 72°C for 1 min. The PCR products were
resolved by agarose gel electrophoresis on 2% gel with ethidium
bromide and semi-quantitatively analyzed by ImageJ software
(version 1.38).
Statistical analysis
Data are presented as the mean ± standard deviation.
The differences between groups were analyzed by one-way analysis of
variance followed by Tukey's HSD post-hoc test/Tukey-Kramer method.
P<0.05 was considered to indicate a statistically significant
difference.
Results
THP induces apoptosis in HeLa
cells
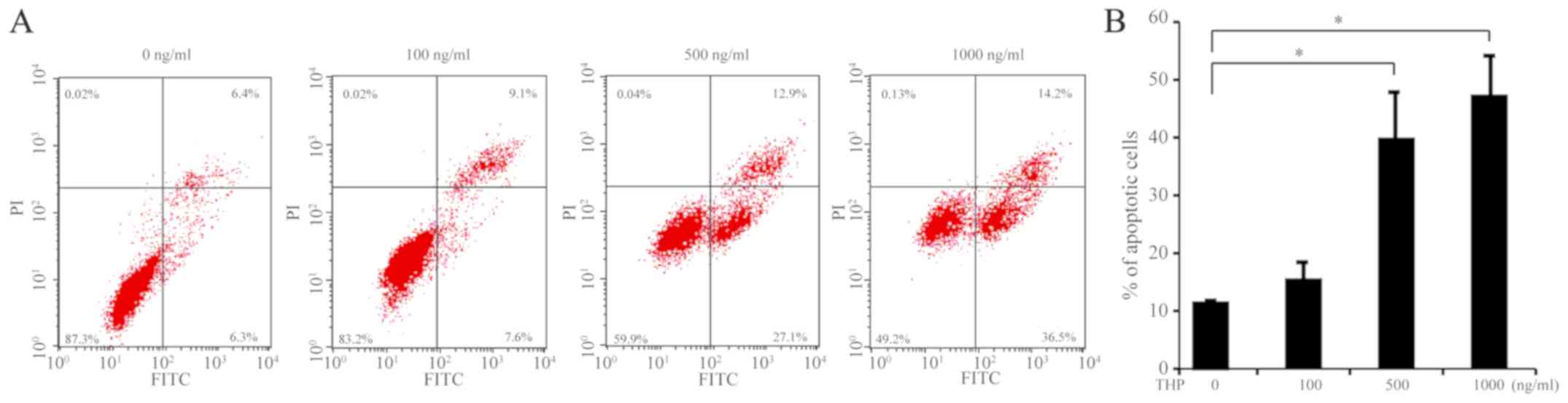
HeLa cells were treated with 0, 100, 500 or 1,000
ng/ml of THP for 24 h, and the percentages of apoptotic cells were
determined by flow cytometry. Compared with the vehicle group,
treatment with THP at 100 ng/ml did not significantly alter the
percentages of apoptotic HeLa cells (Fig. 1). However, treatment with THP at a
dose of 500 and 1,000 ng/ml significantly increased the percentages
of apoptotic cells compared with the vehicle group (both P<0.05)
and the effect of THP on apoptosis exhibited a dose-dependent
trend. The above results indicate that treatment with THP induced
HeLa cell apoptosis in vitro.
THP suppresses endogenous USP22
expression
Previous studies indicated that USP22 was associated
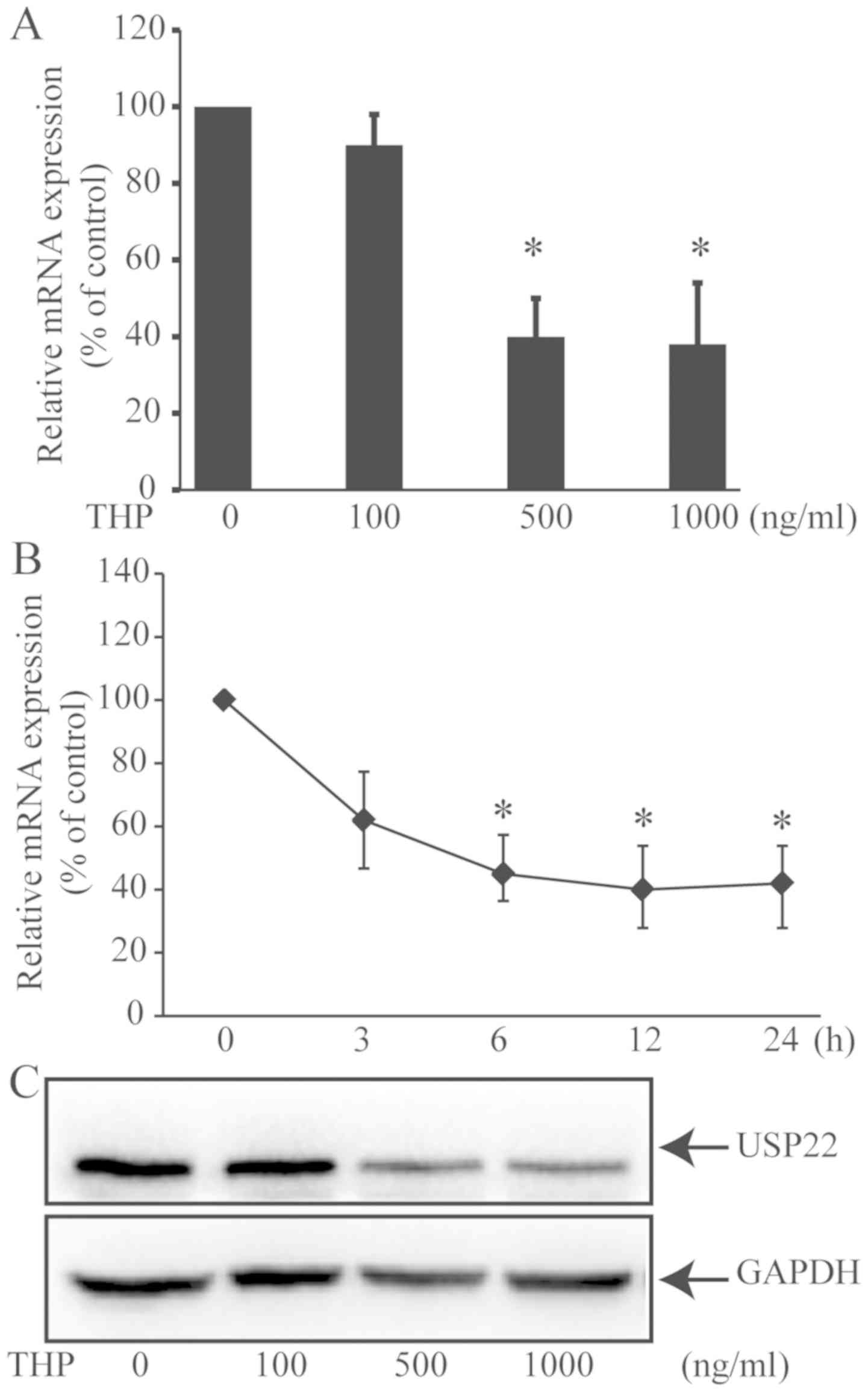
with antitumor chemotherapy-induced apoptosis (22). The current study tested whether
treatment with THP could alter the expression of USP22. HeLa cells
were treated with different doses of THP for 24 h and the relative
levels of USP22 mRNA transcripts were determined by RT-qPCR
(Fig. 2A). Treatment with 100 ng/ml
THP did not significantly alter the expression levels of USP22 mRNA
transcripts; however, treatment with 500 or 1,000 ng/ml THP
significantly decreased the relative levels of USP22 mRNA
transcripts in HeLa cells (both P<0.05). Furthermore, treatment
with THP at 500 ng/ml for 3–12 h significantly decreased the
relative levels of USP22 mRNA transcripts compared with the vehicle
group in what appeared to be a time-dependent manner (Fig. 2B). In addition, treatment with THP at
500 or 1,000 ng/ml markedly reduced the relative protein expression
level of USP22 in HeLa cells (Fig.
2C). These results indicate that THP may effectively decrease
the expression of USP22 in HeLa cells.
THP decreases the USP22 promoter
activity dependent on the CREB motif
To investigate the effect of THP on the USP22
promoter activity, HeLa cells were transfected with the control
plasmid pGL3-basic or plasmids with human wild-type USP22 promoter
regions P-2828, P-595, or P-210, followed by treatment with vehicle
or 500 ng/ml THP for 24 h. Luciferase activities of individual
groups of cells are presented in Fig.
3A. Compared with cells treated with vehicle, treatment with
THP significantly reduced the USP22 promoter-driven luciferase
activity in all groups (all P<0.05; Fig. 3A). These results indicated that THP
inhibited the USP22 promoter activity. Subsequently, HeLa cells
were transfected with P-210, P-210/MYB mut, P-210/CREB mut or
P-210/SP1 and treated with THP, followed by the luciferase activity
measurement in individual groups of cells (Fig. 3B). The results indicated that
treatment with THP significantly decreased the P-210-controlled
luciferase activity, regardless of the mutation in MYB or SP1
binding sequences in HeLa cells. However, treatment with THP did
not alter the P-210/CREB mut-controlled luciferase activity in HeLa
cells. Therefore, inhibition of USP22 expression by THP may be
dependent on the CREB binding in HeLa cells. The current study
further investigated whether CREB-1 over-expression could attenuate
the THP-decreased USP22 promoter activity. HeLa cells were
co-transfected with P-210 and plasmid for CREB-1 expression for 24
h, and treated with THP. Overexpression of exogenous CREB did not
alter P-210 WT promoter activity and the THP-decreased USP22
promoter activity in HeLa cells (Fig. 3C
and D).
 | Figure 3.THP inhibits the USP22 promoter
activity partially dependent on the binding sequence of CREB in
HeLa cells. (A) HeLa cells were transfected with the indicated
plasmids for the USP22 promoter-controlled luciferase expression
and treated with, or without, 500 ng/ml of THP. The luciferase
activity in individual groups of cells was determined. (B) HeLa
cells were transfected with P-210/MYB mut, P-210/CREB mut and
P-210/SP1 mut, and treated with, or without, 500 ng/ml of THP. The
luciferase activity in individual groups of cells was determined.
*P<0.05 vs. the control group. (C) HeLa cells were transfected
with CREB-1 overexpression plasmids and pCMV plasmids, which were
used as the blank control. (D) CREB-1 overexpression did not rescue
the THP-decreased USP22 promoter activity. HeLa and CREB-1
overexpressing HeLa cells were treated with, or without, THP and
the luciferase activity of individual groups of cells was
determined. Representative images or data presented as the mean ±
standard deviation of each group from three separate experiments
are included. USP22, ubiquitin specific peptidase 22; CREB, cyclic
AMP-responsive element-binding protein-1; THP, pirarubicin; MYB,
transcriptional activator MYB; SP1, E3 ubiquitin-protein ligase
SP1; mut, mutant. |
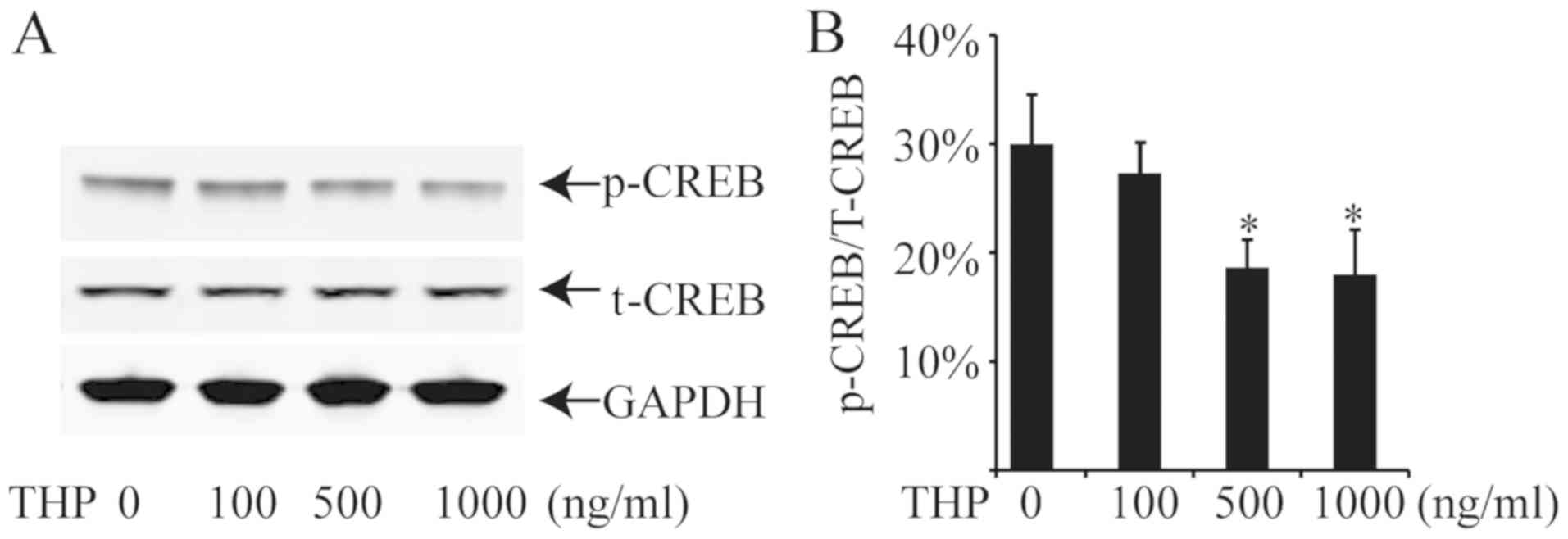
Treatment with THP decreases CREB
phosphorylation
Phosphorylation of CREB is required for its
transcriptional activity (23). The
present study used western blotting to investigate whether THP
could alter the phosphorylation of CREB to decrease the expression
of USP22 in HeLa cells. Treatment with 100, 500 or 1,000 ng/ml THP
for 12 h did not significantly alter total CREB-1 protein
expression in HeLa cells (Fig. 4).
However, treatment with 500 or 1,000 ng/ml THP significantly
decreased the levels of phosphorylated CREB-1 (Ser133) in HeLa
cells. These results indicate that THP decreased the USP22
expression by inhibiting the CREB-1 phosphorylation in HeLa
cells.
THP inhibits CREB binding to the USP22
promoter
Our previous study indicated that CREB-1 directly
binds to the CREB binding sequence of the basic promoter region of
USP22 (24). The present study used
ChIP assays with anti-CREB-1 antibody to investigate whether THP
could modulate CREB-1 binding to the USP22 promoter. Treatment with
THP significantly decreased the level of USP22 promoter DNA bound
by anti-CREB-1 antibody in HeLa cells (Fig. 5). These results demonstrated that
treatment with THP may decrease the binding of CREB-1 to the
promoter region of USP22 and inhibit the USP22 promoter
activity.
Discussion
Previous studies indicated that treatment with THP
can trigger apoptosis of human osteosarcoma (5) and hepatocellular carcinoma (25) cells, and induce autophagy of human
cervical cancer cells (6). In the
current study, treatment with THP induced cervical cancer cell
apoptosis in vitro. The results of the current study support
previous observations and indicate that THP may exhibit potent
toxicity against numerous types of malignancies.
Upregulated USP22 expression is associated with
tumor progression and oncogenesis, while USP22 silencing can induce
cell cycle arrest to inhibit growth in several types of tumors
(26,27). Furthermore, extracellular stimuli can
activate T and B lymphocytes and upregulate the expression of USP22
(28). Our previous study revealed
that transcription factors SP1 and CREB-1 bound to the USP22
promoter and regulated the expression of USP22 (18). In addition, certain chemotherapeutic
drugs, including cisplatin and TSA, downregulate the expression of
USP22 and trigger apoptosis in HeLa cells (19,20). In
the current study, treatment with THP decreased the expression of
USP22 in HeLa cells in a dose- and time-dependent manner. These
results support previous observations and indicate that treatment
with THP may downregulated the expression of oncogenic and
proliferation-associated factors in tumor cells (4,5).
THP is an anthracycline antineoplastic drug
inhibiting DNA synthesis in tumor cells (3). THP decreases microRNA-21 expression,
stabilizes autophagy related 4B cysteine peptidase mRNA and
modulates the phosphorylation of serine/threonine-protein kinase
mTOR, ribosomal protein S6 kinase beta-1, eukaryotic translation
initiation factor 4E-binding protein 1 and Cdc2 in different types
of tumor cells (6,25,29). In
the current study, treatment with THP reduced the USP22 promoter
activity in HeLa cells regardless of the presence or absence of the
binding sequence of MYB or SP1. However, the inhibitory effect of
THP on USP22 expression was dependent on the presence of the CREB
binding sequence in the USP22 promoter. Therefore, binding of
CREB-1 to the USP22 promoter may be required for THP-mediated
inhibition of USP22 expression in HeLa cells. Overexpression of
CREB in the present study did not to rescue the THP-mediated
inhibition of USP22 promoter-controlled luciferase activity in HeLa
cells. The present study demonstrated that treatment with THP
significantly reduced the phosphorylation of CREB-1 at Ser 133 and
the binding of CREB-1 to the USP22 promoter in HeLa cells. Previous
studies suggested that the protein kinase A (PKA), protein kinase B
or mitogen activated kinase signaling is necessary for the
activation of CREB and its binding to the USP22 promoter (30–32). THP
may decrease PKA activity and phosphorylation of CREB-1 to reduce
CREB-1 binding to the USP22 promoter, leading to a decreased
expression level of USP22 in HeLa cells. Consequently, the
downregulated expression of USP22 promoted the apoptosis of HeLa
cells. Therefore, the novel results of the present study may be
used to elucidate the underlying mechanism of THP-mediated
inhibition of USP22 expression in HeLa cells.
In conclusion, the present study indicated that THP
induced apoptosis of HeLa cells and decreased the expression of
USP22 in dose- and time-dependent manner. THP significantly reduced
the USP22 promoter activity, dependent on the binding sequence of
CREB, which was not affected by CREB-1 over-expression.
Furthermore, THP significantly inhibited the phosphorylation of
CREB-1 at ser133 and its binding to the USP22 promoter. Therefore,
the novel results of the current study may be used to elucidate the
molecular mechanisms underlying the pharmacological pro-apoptotic
effect of THP in tumor cells.
Acknowledgements
Not applicable.
Funding
The present study was supported by the grants from
the National Nature Science Foundation of China (grant nos.
81460172 and 81860165), Natural Science Foundation of Jiangxi,
China (grant no. 20151BAB205056) and the Visiting Scholar Special
Funding of Jiangxi Association for Science and Technology (grant
no. 2016).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JX and TW designed the study and analyzed the data.
XZ and LG performed the western blotting and reverse
transcription-quantitative polymerase chain reaction. JL performed
cell culture. XX performed the chromatin immunoprecipitation. All
authors interpreted the results, and produced and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shinozaki T, Watanabe H, Yanagawa T,
Shirakura K and Takagishi K: Pirarubicin-based versus
doxorubicin-based osteosarcoma chemotherapy. Ann Pharmacother.
36:996–999. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li JJ, Di GH, Tang LC, Yu KD, Hu Z, Liu
GY, Lu JS, Wu J, Han QX, Shen ZZ and Shao ZM: Adjuvant therapy of
breast cancer with pirarubicin versus epirubicin in combination
with cyclophosphamide and 5-fluorouracil. Breast J. 17:657–660.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zou HY, Wu HL, Zhang Y, Li SF, Nie JF, Fu
HY and Yu RQ: Studying the interaction of pirarubicin with DNA and
determining pirarubicin in human urine samples: Combining
excitation-emission fluorescence matrices with second-order
calibration methods. J Fluoresc. 19:955–966. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu SY, Song SX, Lin L and Liu X:
Molecular mechanism of cell apoptosis by paclitaxel and pirarubicin
in a human osteosarcoma cell line. Chemotherapy. 56:101–107. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng SE, Xiong S, Lin F, Qiao GL, Feng T,
Shen Z, Min DL, Zhang CL and Yao Y: Pirarubicin inhibits
multidrug-resistant osteosarcoma cell proliferation through
induction of G2/M phase cell cycle arrest. Acta Pharmacol Sin.
33:832–838. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu Y, Ni Z, Yan X, Dai X, Hu C, Zheng Y,
He F and Lian J: Targeting the MIR34C-5p-ATG4B-autophagy axis
enhances the sensitivity of cervical cancer cells to pirarubicin.
Autophagy. 12:1105–1117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee HJ, Kim MS, Shin JM, Park TJ, Chung HM
and Baek KH: The expression patterns of deubiquitinating enzymes,
USP22 and Usp22. Gene Expr Patterns. 6:277–284. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Y, Lang G, Ito S, Bonnet J, Metzger
E, Sawatsubashi S, Suzuki E, Le Guezennec X, Stunnenberg HG,
Krasnov A, et al: A TFTC/STAGA module mediates histone H2A and H2B
deubiquitination, coactivates nuclear receptors, and counteracts
heterochromatin silencing. Mol Cell. 29:92–101. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang XY, Pfeiffer HK, Thorne AW and
McMahon SB: USP22, an hSAGA subunit and potential cancer stem cell
marker, reverses the polycomb-catalyzed ubiquitylation of histone
H2A. Cell Cycle. 7:1522–1524. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang XY, Varthi M, Sykes SM, Phillips C,
Warzecha C, Zhu W, Wyce A, Thorne AW, Berger SL and McMahon SB: The
putative cancer stem cell marker USP22 is a subunit of the human
SAGA complex required for activated transcription and cell-cycle
progression. Mol Cell. 29:102–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao H, Tian Y, Yang Y, Hu F, Xie X, Mei J
and Ding F: USP22 acts as an oncogene by regulating the stability
of cyclooxygenase-2 in non-small cell lung cancer. Biochem Biophys
Res Commun. 460:703–708. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin Z, Yang H, Kong Q, Li J, Lee SM, Gao
B, Dong H, Wei J, Song J, Zhang DD and Fang D: USP22 antagonizes
p53 transcriptional activation by deubiquitinating Sirt1 to
suppress cell apoptosis and is required for mouse embryonic
development. Mol Cell. 46:484–494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Atanassov BS and Dent SY: USP22 regulates
cell proliferation by deubiquitinating the transcriptional
regulator FBP1. EMBO Rep. 12:924–930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin Z, Tan C, Qiu Q, Kong S, Yang H, Zhao
F, Liu Z, Li J, Kong Q, Gao B, et al: Ubiquitin-specific protease
22 is a deubiquitinase of CCNB1. Cell Discov. 1(pii): 150282015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu J, Yang D, Zhang H, Liu W, Zhao Y, Lu
H, Meng Q, Pang H, Chen X, Liu Y and Cai L: USP22 promotes tumor
progression and induces epithelial-mesenchymal transition in lung
adenocarcinoma. Lung Cancer. 88:239–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Y, Yang Y, Li J, Liu H, Chen F, Li B,
Cui B and Liu Y: USP22 drives colorectal cancer invasion and
metastasis via epithelial-mesenchymal transition by activating AP4.
Oncotarget. 8:32683–32695. 2017.PubMed/NCBI
|
|
17
|
Glinsky GV: Death-from-cancer signatures
and stem cell contribution to metastatic cancer. Cell Cycle.
4:1171–1175. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiong J, Che X, Li X, Yu H, Gong Z and Li
W: Cloning and characterization of the human USP22 gene promoter.
PLoS One. 7:e527162012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xiong J, Gong Z, Zhou X, Liu J, Jiang HE,
Wu P and Li W: p38 mitogen-activated protein kinase inhibits USP22
transcription in HeLa cells. Biomed Rep. 3:461–467. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiong J, Xu X, Zhou X, Liu J, Gong Z, Wu P
and Li W: USP22 transcriptional activity is negatively regulated by
the histone deacetylase inhibitor trichostatin A. Mol Med Rep.
10:3343–3347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ling S, Li J, Shan Q, Dai H, Lu D, Wen X,
Song P, Xie H, Zhou L, Liu J, et al: USP22 mediates the multidrug
resistance of hepatocellular carcinoma via the SIRT1/AKT/MRP1
signaling pathway. Mol Oncol. 11:682–695. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xie Z, Liu D, Liu S, Calderon L, Zhao G,
Turk J and Guo Z: Identification of a cAMP-response element in the
regulator of G-protein signaling-2 (RGS2) promoter as a key
cis-regulatory element for RGS2 transcriptional regulation by
angiotensin II in cultured vascular smooth muscles. J Biol Chem.
286:44646–44658. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiong J, Zhou X, Gong Z, Wang T, Zhang C,
Xu X, Liu J and Li W: PKA/CREB regulates the constitutive promoter
activity of the USP22 gene. Oncol Rep. 33:1505–1511. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He X, Li J, Guo W, Liu W, Yu J, Song W,
Dong L, Wang F, Yu S, Zheng Y, et al: Targeting the microRNA-21/AP1
axis by 5-fluorouracil and pirarubicin in human hepatocellular
carcinoma. Oncotarget. 6:2302–2314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou D, Liu P, Sun DW, Chen ZJ, Hu J, Peng
SM and Liu YL: USP22 down-regulation facilitates human
retinoblastoma cell aging and apoptosis via inhibiting TERT/P53
pathway. Eur Rev Med Pharmacol Sci. 21:2785–2792. 2017.PubMed/NCBI
|
|
27
|
Tang B, Tang F, Li B, Yuan S, Xu Q,
Tomlinson S, Jin J, Hu W and He S: High USP22 expression indicates
poor prognosis in hepatocellular carcinoma. Oncotarget.
6:12654–12667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ovaa H, Kessler BM, Rolén U, Galardy PJ,
Ploegh HL and Masucci MG: Activity-based ubiquitin-specific
protease (USP) profiling of virus-infected and malignant human
cells. Proc Natl Acad Sci USA. 101:2253–2258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li K, Chen X, Liu C, Gu P, Li Z, Wu S, Xu
K, Lin T and Huang J: Pirarubicin induces an autophagic
cytoprotective response through suppression of the mammalian target
of rapamycin signaling pathway in human bladder cancer cells.
Biochem Biophys Res Commun. 460:380–385. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Montminy MR, Sevarino KA, Wagner JA,
Mandel G and Goodman RH: Identification of a cyclic-AMP-responsive
element within the rat somatostatin gene. Proc Natl Acad Sci USA.
83:6682–6686. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vo N and Goodman RH: CREB-binding protein
and p300 in transcriptional regulation. J Biol Chem.
276:13505–13508. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Delghandi MP, Johannessen M and Moens U:
The cAMP signalling pathway activates CREB through PKA, p38 and
MSK1 in NIH 3T3 cells. Cell Signal. 17:1343–1351. 2005. View Article : Google Scholar : PubMed/NCBI
|