Introduction
Endothelial cells regulate vascular homeostasis and
form a biologically active blood-tissue barrier, thus serving key
roles in regulation of vascular tone, control of inflammatory
responses and vascular permeability, and the balance between the
coagulation and fibrinolysis systems (1,2).
Alterations in the functional and morphological architecture of the
endothelium impair normal physiological processes. This is known as
endothelial dysfunction and results in disruptions in the barrier
function, increased prothrombotic/procoagulant activity and
impaired vasomotion due to increased or decreased synthesis of
vasoactive substances (3-5).
Disturbed vascular homeostasis results in vasoconstriction,
leukocyte adhesion, platelet activation, predisposition to
thrombosis, coagulation disorders, pro-oxidative changes and
vascular inflammation (4). Deep vein
thrombosis (DVT) is a clinical condition characterized by
coagulation in the veins of the upper and often lower extremities.
The pathogenesis of DVT involves endothelial dysfunction
(endothelial damage-dysfunction), hypercoagulability and stasis
(slow blood flow). These mechanisms may lead to venous obstruction
and thromboembolism, resulting in serious consequences ranging from
post-thrombotic syndrome to pulmonary thromboembolism (6,7).
Oxidative stress is a key factor that contributes to the
pathogenesis of numerous cardiovascular diseases, including
atherosclerosis, hypertension, heart failure, ischemia/reperfusion
injury and thrombosis (8-11).
Oxidative stress is important for reactive oxygen species (ROS)
production, inflammatory responses, cell growth, proliferation,
modulation of extracellular matrix production, maintenance of
endothelial-vascular smooth muscle cells, apoptosis and
angiogenesis control, particularly in the vascular system (2,12,13).
The nuclear factor erythroid-2-like 2
(Nrf2)/Kelch-like ECH-associated protein 1 (Keap1) signaling
pathway protects numerous cell types, tissues, organs and systems
from the pathogenic effects of oxidative stress. Therefore, Nrf2 is
referred to as a ‘multi-organ protector’ (14). The Nrf2/Keap1 signaling pathway is
involved in antioxidant defense mechanisms in cardiovascular
diseases, such as atherosclerosis and hypertension (15,16).
This pathway controls the cellular response that prevents injury to
redox-sensitive cellular components, where Nrf2 has been identified
as the main regulator of oxidant/antioxidant balance (16). Nrf2 is a transcription factor that
regulates transcription of numerous genes involved in
tumorigenesis, inflammation, radiation, detoxification of
xenobiotics and inhibition of ROS (17). Keap1 is a suppressor of Nrf2 when
cells are not under stress (15,17-19).
Upon exposure to oxidative stress, cells release Nrf2, which
activates cytoprotective genes and forms heterodimers with Maf
family proteins, mediated via transcription factors (20). A nuclear localization sequence
mediates binding of the Nrf2 heterodimer to the antioxidant
response element (ARE) sequence following translocation to the
nucleus. Thus, the transcriptional activation of cytoprotective
genes, such as NADPH quinone dehydrogenase 1 (NQO-1), superoxide
dismutase 1, heme hxygenase 1 (HO-1) and glutathione S-transferase
A2 (GSTA2), begins (21-23).
Previous studies have demonstrated that activation of the
Nrf2/Keap1 signaling pathway may serve a key role in
cardioprotection, and disordered activation of the pathway may
increasse susceptibility to endothelial dysfunction (24-28).
Further investigations are required to better understand the
interaction between the cardiovascular system and the Nrf2/Keap1
signaling pathway, which may be an initiator of pathogenesis in
cardiovascular diseases.
To the best of our knowledge, there is currently
little research regarding the association between the Nrf2/Keap1
signaling pathway (which acts as a cellular defence against
oxidative stress) and DVT, a cardiovascular disease that involves
pathogenic endothelial damage. The present study aimed to
investigate pathogenic mutations in the Nrf2/Keap1 signaling
pathway and to determine whether there is an imbalance in Nrf2 and
Keap1 levels in patients with DVT in order to identify the
underlying mechanism of pathogenesis in DVT. The objectives of the
study were to identify pathogenic mutations and expression levels
of genes involved in the Nrf2/Keap1 signaling pathway in patients
with DVT; to measure total antioxidant values in blood samples from
patients with DVT; and to characterize the association between
expression levels of Nrf2 and Keap1 pathogenic mutations, levels of
antioxidants and DVT.
Materials and methods
Study population
The study population are of comprised 27 patients
(20 men and seven women), aged 20-74 years, who were admitted to
Faculty of Medicine, Nigde Omer Halisdemir University (Nigde,
Turkey) with the diagnosis of primary (spontaneous) DVT. In
addition, a healthy control group consisting of 10 people (six men
and four women), aged 20-74 years was included in the present
study. The present study was performed in accordance with the Code
of Ethics of the World Medical Association (Declaration of
Helsinki) for experiments involving humans. The study protocol was
approved by Erciyes University School of Medicine Ethics Committee
(approval no. 2018/533; Kayseri, Turkey). All patients agreed to
participate in the study, which was conducted between March and
April 2019. Written informed consent was provided by all patients.
Demographic, clinical and genetic data regarding the patient group
are summarized in Table I.
 | Table IDemographic, clinical and genetic
data of patients with DVT. |
Table I
Demographic, clinical and genetic
data of patients with DVT.
| Characteristic | Patient data |
|---|
|
Sex | |
|
Male/Female | 20/7 |
| Mean age,
years | 42 (range,
24-70) |
| Site of
thrombosis | |
|
Lower
extremity | 25 |
|
Upper
extremity | 1 |
|
Pulmonary
embolism | 1 |
| Oral anticoagulant
use | |
|
Warfarin | 24 |
|
Rivaroxaban | 3 |
| Frequency of
Kelch-like ECH-associated protein 1 mutations | 24 (88%) |
| Frequency of
nuclear factor erythroid-2-like 2 mutations | 14 (52%) |
| Frequency of FV
G/A | 7 (26%) |
| Frequency of FV
A/A | 6 (22%) |
| Frequency of PT
G/A | 4 (15%) |
| Frequency of PT
A/A | 0 |
| Hypertension | 6 (22%) |
| White blood cells,
x109/l | 8.22
(5.80-14.40) |
| Red blood cells,
x109/l | 5.22
(4.15-6.98) |
| Partial
thromboplastin time | 40.27±9.05 |
| Thromboplastin
time | 22.66±6.66 |
| International
normalized ratio | 1.98±0.57 |
| Hemoglobin,
g/dl | 15.01±1.58 |
| Hematocrit, % | 44.68 ±4.52 |
| Mean corpuscular
volume, fl | 86.38±8.51 |
| Mean corpuscular
hemoglobin, pg | 29.05±3.26 |
| Platelet count,
x109/l | 239.8
(139.0-355.0) |
| Red blood cell
distribution width, % | 13.86±1.11 |
| Procalcitonin,
% | 0.22±0.06 |
| Platelet
distribution width, % | 16.01±0.43 |
| Mean platelet
volume, fl | 9.11±1.15 |
Color Doppler ultrasonography, venography, magnetic
resonance imaging and computed tomography methods can be used to
diagnose DVT. In the present study, the diagnosis of DVT was based
on physical examination and venous color Doppler ultrasonography.
The age range of the patients with primary DVT was 24-70 years.
Subjects were excluded if they had previously undergone surgical
interventions, received or ongoing treatment for cancer within the
previous 6 months, had given birth within the previous 6 weeks,
were receiving hormonal therapy or using oral contraceptives, had a
history of acute myocardial infarction, or were immobile.
The age range of the healthy control group is
similar to that of the patient group. The healthy control group was
selected from the individuals who came to the hospital for checkup
purposes and the group is composed of individuals who do not have
any disease in the coagulation system or acute and/or chronic
disease, including hypertension, diabetes, obesity and endocrine
diseases.
Sample collection
Blood samples (5 ml) were collected from subjects in
sterile EDTA tubes for DNA and RNA isolation.
Mutation screening
DNA was isolated from whole blood using the GeneJET
Genomic DNA Purification kit (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. DNA was then stored
at -20̊C until use. DNA concentration was quantified using a Genova
Nano 3 spectrophotometer (Jenway; Bibby Scientific Ltd.). The
coding regions of Nrf2 and Keap1 genes were amplified via PCR. A
total of ~200 ng DNA was used as template for PCR amplification.
Primer sequences are listed in Table
II. The total volume of the reaction mix was 25 µl, comprising
16.75 µl sterile water, 2.5 µl reaction buffer [200 mM
(NH4)2SO4], 1 µl dNTP mix (A, C,
G, T; 200 mM), 1.25 µl MgCl2 (25 mM), 1 µl forward and
reverse primers (10 pmol), 0.5 µl Taq polymerase (500 units;
Fermentas; Thermo Fisher Scientific, Inc.) and 1 µl template DNA
(>500 ng/µl for each sample). Amplification reactions were
performed using a Veriti VR 96-Well Thermal Cycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Thermocycling
conditions were as follows: Initial denaturation at 94̊C for 5 min,
followed by 40 cycles of denaturation for 45 sec at 94̊C, primer
annealing for 90 sec at 58̊C, extension for 90 sec at 72̊C, and
final extension for 10 min at 72̊C. Following amplification, 10 µl
PCR products were separated by electrophoresis on a 2% agarose gel
containing GelRed in 1X TAE buffer for 40 min at 90 V. A GeneRuler
100 bp DNA Ladder (Thermo Fisher Scientific, Inc.) was used as a
marker. Subsequently, gels were visualized using a Gel Logic 100
system (Kodak). PCR products were then sequenced using a 3500 XL
DNA Sequencer (Applied Biosystems; Thermo Fisher Scientific, Inc.).
Data were analyzed using data collection GeneMapper™ ID-X Software
(v1.6; Applied Biosystems; Thermo Fisher Scientific, Inc.) and
Chromas (v2.6.5; Technelysium Pty Ltd.) to determine sequence
changes compared with reference Nrf2 and Keap1 sequences obtained
from the Ensembl genome browser (https://www.ensembl.org/index.html) (29).
 | Table IIPrimer sequences and amplicon lengths
for Nrf2 and Keap1. |
Table II
Primer sequences and amplicon lengths
for Nrf2 and Keap1.
| Gene/exon | Forward primer
(5'à3') | Reverse primer
(5'à3') | Amplicon, bp |
|---|
| Keap1/2-2 |
ATACCAAGCAGGCCTTTGG |
ATGGAGATGGAGGCCGTGT | 265 |
| Keap1/2-3 |
ATGGAGCGCCTCATTGAA |
AGCCCCACTTCCCCGCT | 263 |
| Keap1/3-1 |
CCGTCCCACTGTCGCCCTC |
TGCAGGATCTCGCACTTC | 260 |
| Keap1/3-2 |
AGCTGCAGAAGTGCGAG |
GTTGTTCCTGCCGCCCACG | 296 |
| Keap1/4 |
CATTTTTCTTACGCCCTTGC |
GTTTCACCCCAGGATGGTAG | 290 |
| Nrf2/1 |
CGTGTAGCCGATTACCGAGT |
CAGACCTTCCCGCAACTTC | 454 |
| Nrf2/2 |
CCACCATCAACAGTGGCATA |
CCTGCCATAACTTTCCCAAG | 400 |
| Nrf2/2-2 |
CCTGAGTTCAGTACCCAACCTAT |
TGAGCTTCAGTTGCTCATCG | 281 |
| Nrf2/3 |
GCTTACTCATCCCCTGTTGG |
TAGCACCCTCCAATCCTTCC | 355 |
| Keap1 (mRNA) |
GTGTCCATTGAGGGTATCCACC |
GCTCAGCGAAGTTGGCGAT | 211 |
| Nrf2 (mRNA) |
TCAGCGACGGAAAGAGTATGA |
CCACTGGTTTCTGACTGGATT | 174 |
| RPLP0 (mRNA) |
CAGATTGGCTACCCAACTGTT |
GGAAGGTGTAATCCGTCTCCC | 97 |
| FV G1691A |
TGCCCAGTGCTTAACAAGACCA |
CTTGAAGGAAATGCCCCATTA | 110 |
| PT G20210A |
CCGCTGGTATCAAATGGGG |
CCAGTAGTATTACTGGCTCTTCCTG | 106 |
| FV G1691A
Hybridization probes |
5'-LC-Red705-TGTCCTTGAAGTAACCTTTCAGAAATTCTG-3'-PHO |
5'-GGCGAGGAATACAGGTAT-3'-Flu | N/A |
| PT G20210A
Hybridization probes |
5'-LC-Red640-TCCCAGTGCTATTCATGGGC-3'-PHO |
5'-CTCAGCGAGCCTCAATG-3'-Flu | N/A |
Genotyping of factor V (FV) 1691 G-A and prothrombin
(PT/Factor II) 20210 G-A mutations was performed via Real-Time
Polymerase Chain Reaction (RT-PCR), with fluorescence melting curve
detection analysis via a Light Cycler system (Roche Diagnostics
GmbH). The primers and probes used in the present study were
summarized in Table II. Melting
point analysis was performed using LightMix®
in-vitro diagnostics Factor V (Leiden; cat.no. 40-0594-64)
and Factor II G20210A mutation (cat. no. 40-0593-64) detection kits
according to the manufacturer's protocols (TIB Molbiol;
Syntheselabor GmbH).
In silico analysis of Keap1/Nrf2
pathway mutations
Polymorphism Phenotyping v2 (PolyPhen-2; http://genetics.bwh.harvard.edu/pph2)
can predict the potential impact of amino acid substitutions on the
stability and function of human proteins using structural and
comparative evolutionary considerations. The prediction is based on
sequence, phylogenetic and structural features that characterize
the substitution. PolyPhen-2 performs functional annotation of
single-nucleotide polymorphisms (SNPs), maps coding SNPs to gene
transcripts, extracts protein sequence annotations and structural
attributes, and builds conservation profiles. The program estimates
the probability of the missense mutation being damaging and
provides both a qualitative prediction (probably damaging, possibly
damaging, benign or unknown) and a quantitative score (30). It includes a high-quality multiple
protein sequence alignment pipeline and a prediction method that
uses machine-learning classification. In addition, to determine the
possible pathogenicity of the identified mutations, the scores were
provided by the Catalog of Somatic Mutations in Cancer (COSMIC)
databases (https://cancer.sanger.ac.uk/cosmic) (31).
Evolutionary conservation of the detected mutant
amino acids was estimated among different species (Homo sapiens,
Rattus norvegicus, Mus musculus, Pan troglodytes, Felis domesticus,
Canis lupus familiaris, Bos taurus, Sus scrofa and Xenopus
tropicalis) using the Homologous Gene-Multiple Seguence
Alignment Viewer 1.12.0 tool in the National Center for
Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/genbank/) .
Except for novel mutations, the registration number
of the ones detected before was obtained from Human Gene Mutation
Database 2020.1 (HGMD; http://www.hgmd.cf.ac.uk/ac/index.php) (32).
Nrf2/Keap1 pathway gene expression
analysis
RNA isolation was isolated from whole blood using
PureZOLTM solution, according to the manufacturer's instructions
(Bio-Rad Laboratories, Inc.). The Genova Nano 3 nanodrop UV
spectrophotometer was used to determine the purity and
concentration of RNA samples, which were stored at -80̊C until use.
Protein contamination was evaluated via A260/A280 ratio; samples
with a ratio of 1.8-2.2 were used for subsequent experimentation.
RNA samples were stored in a final volume of 500 ng RNA for use in
cDNA synthesis.
Reverse transcription (RT)-qPCR was used to detect
the expression levels of Nrf2 and Keap1 target genes. Primer
sequences are (Table II) were
designed using the Primer3 Input (v0.4.0) program (http://primer3.ut.ee/) (33). cDNA synthesis was performed using a
First Strand Transducer c-DNA synthesis kit (Roche Diagnostics
GmbH) according to the manufacturer's instructions. The RPLP0 gene
was used as the reference gene. mRNA expression levels were
detected using a SYBR Green I Master mix (Bio-Rad Laboratories,
Inc.) in a Rotor-GeneQ thermocycler (Qiagen, GmbH), thermocycling
conditions were as follows: Initial denaturation at 95̊C for 5 min,
followed by 40 cycles of denaturation for 15 sec at 95̊C, primer
annealing for 60 sec at 60˚C, extension for 30 sec at 72˚C, and 1
cycle of melting curve for 30 sec at 55-95˚C, according to the
manufacturer's instructions. Each experiment was repeated three
times. mRNA expression levels were calculated according to Pfaffl's
method (34). Relative
quantification of gene expression levels was performed using the
comparative quantification cycle (Cq) method, in which the amounts
of the target genes are expressed as 2exp-ΔCq (35).
Analysis of total antioxidant capacity
(TAC)
The water-soluble analogue of vitamin E (Trolox;
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid),
2,2'-azino-bis (3-ethylbenz-thiazoline-6-sulfonic acid) (ABTS),
potassium persulfate and potassium phosphate were purchased from
Sigma-Aldrich (Merck KGaA). ABTS, potassium persulfate and
potassium phosphate and ultra-pure grade were commercially
available. Peripheral venous blood from all patients was collected
for the measurement of TAC levels. All blood samples were collected
in vacutainer tubes without anticoagulant and centrifuged at 1,800
x g for 10 min at room temperature. The serum samples obtained were
stored at -20˚C until use.
TAC is based on the bleaching of the dark
blue-green-colored ABTS radical when it is reduced in the presence
of antioxidant molecules. The bleaching rate of the reaction is
determined using a spectrophotometer and is associated with the TAC
of the sample. The reaction rate is calibrated using Trolox, which
is widely used as the antioxidant standard agent for TAC assays,
and results are expressed in µmol Trolox equivalent/l (µmol
TE/l).
ABTS (7 mmol/l) was dissolved in PBS, then potassium
persulfate (2.45 mmol/l) was added and the solution was mixed at
room temperature. The ABTS.+ stock solution was kept in
the dark at room temperature for 12-16 h before use. The stock
solution of ABTS.+ was diluted with PBS (pH 7.4) to
obtain an absorbance of 0.70 at 734 nm, measured using a UV-2700
UV-Vis spectrophotometer (Shimadzu Corporation). The diluted
ABTS.+ was then mixed with the aforementioned serum
samples and left to stand for 10 min at room temperature. The
change in absorbance was monitored at 734 nm using the UV-Vis
spectrophotometer. Trolox was used as a control. Results are
expressed as µmol TE/L.
Statistical analysis
Data are presented as the mean ± SD. The analysis of
TAC and gene expression were repeated for three times. Statistical
analysis of gene expression levels was performed by one-way ANOVA
followed by Dunnett's test and Student's t-test using SPSS
Statistics software (v22.0; IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Demographic and clinical analysis of
patients with DVT
The age distribution of the patient population was
24-70 years, with a mean age of 42 years. The patient population
comprised 20 men and seven women (74 and 26% respectively).
Thrombosis was detected in the lower extremity veins of 25
patients, the upper extremity veins of one patient and the
pulmonary artery of one patient. A total of eight patients had a
history of hypertension. Warfarin was used to treat 24 patients and
Rivaroxaban was used to treat three patients. Demographic, clinical
and genetic data of the patient group are summarized in Table I.
Results of mutation screening
Mutation analysis was performed using Sanger DNA
sequencing of DNA isolated from blood samples of patients with DVT.
The mutation analysis identified 23 changes in the Nrf2 and Keap1
genes (11 missense mutations, five synonymous nucleotide changes,
two splice site mutations and five non-coding region nucleotide
changes). Of these changes, 16 had previously been recorded in the
HGMD and seven were novel changes. The detected mutations exhibited
heterozygous genotypes. Changes were detected in 24 (89%) patients
with DVT (Fig. 1). The mutations
detected in the present study (those with accesion number) had not
been associated with DVT disease previously.
The changes detected in the Keap1 gene were located
on the functional intervening region (IVR) and broad-complex,
tramtrack and bric-a-brac (BTB) domains of the protein encoded by
the Keap1 gene. A total of 10 missense mutations, five synonymous
nucleotide substitutions and one splice site mutation was detected
in the Keap1 gene. Of these, three were novel nucleotide
substitutions. Nucleotide substitutions in the Nrf2/Keap1 pathway
are presented in Fig. 1. A schematic
representation of domain architecture of the identified proteins
and mutations is presented in Fig.
2.
 | Figure 2Schematic representation of domain
architecture of the Keap1 and Nrf2 proteins and mutations detected
in patients with deep vein thrombosis. (A) Human Keap1 is a
polypeptide comprising 624 amino acids. There are two different
mutation points (c.313/314 C>T) that encode the amino acid at
105 codon. (B) Human Nrf2 is a polypeptide comprising 605 amino
acids, which contains seven Neh domains.;Keap1, Kelch-like
ECH-associated protein 1; Nrf2, nuclear factor erythroid-2-like 2;
Neh, Nrf2-ECH homology; NTR, N-terminal region; BTB, broad-complex,
tramtrack and bric-a-brac; IVR, intervening region; KELC/DGR,
Kelch/double glycine repeat; CTR, C-terminal region; RXRα, retinoid
X receptor α; β-TrCP, β-transducin repeats-containing protein. |
It was determined that 12 changes (p.R116Q, p.E121K,
p.P105L, p.S146S, p.I145N, p.L145I, p.P130T, p.P105L, p.E244Q,
p.E117D, p.M120I and p.G114W) found in Keap1 may be pathogenic, as
PolyPhen-2 analysis resulted in scores close to 1. These mutations
are listed in the COSMIC database as somatic mutations. To the best
of our knowledge, in the present study, p.I145N, p.P105L and
p.M120I (missense) changes were identified for the first time. An
intronic substitution, c.639 +8 G>A, has previously been
identified as a splice site mutation in on the NCBI database
(https://www.ncbi.nlm.nih.gov/snp/rs1466903180#clinical_significance).
n the present study, seven changes [one missense, one splice site
region, one intronic region and four 3' untranslated regions (3'
UTRs)] were detected in the Nfr2 gene. Of these detected changes,
three had previously been recorded in the HGMD. The present study
identified four novel mutations in the 3'UTR (Fig. 2).
The mutations detected in patients with DVT, as well
as genotype frequencies of FV 1691 G-A and PT 20210 G-A mutations,
are characterized in Table III.
Changes in Nrf2 were detected in 14 (52%) patients. The
subsititution p.P154S detected in the Nrf2 gene was located on the
Nrf2-ECH homology (Neh) 5 transactivation domain. The subsititution
c.46-698 C>A is registered in the COSMIC database (reference no.
COSM6971525); it was located on the splice site region and detected
in 10 (42%) patients. When the Nrf2 genes of different species of
organisms were compared, seven functional Neh domains with high
evolutionary conservation were detected and named Neh1-7. The
c.46-698 C>A and c.46-36 T>A variants detected in the Nrf2
gene were located on the ETGE-Neh2 domain, which is a Keap1 binding
site. The Neh2 domain is a large regulatory domain that contains
seven lysine residue sequences responsible for two binding sites
(ETGE and DLG motifs) that are associated with the regulation of
Nrf2 stability and ubiquitin conjugation (18). The missense mutation p.E244Q was
detected on the Keap1 IVR domain. This domain contains a conserved
nuclear export signal sequence, which is important for localization
of Keap1 in the cytoplasm (17,18). The
splice site mutations c.639+8 G>A and c. 46-698 C>A were
detected in the Keap1 and Nrf2 genes, respectively. As the c.46-698
C>A mutation detected in the Nrf2 gene is located on the first
base of the completely conserved splice site across the species in
the evolutionary process, this mutation may cause the absence of
Nrf2 expression levels. A total of 15 patients carried both Keap1
and Nrf2 substitutions.
 | Table IIIMutations of the Nrf2/Keap1 pathway
in patients with DVT. |
Table III
Mutations of the Nrf2/Keap1 pathway
in patients with DVT.
| Case | Age | Sex | Genotype and
location | Gene name | Type of
mutation | Amino acid
change | Clinical
significance | Accession no. | Thrombosis | FV G1691A | FII G20210A | Onset age |
|---|
| 1 | 23 | M | c.438 C>T | Keap1-
BTB-Domain | Synonymous | p.S146S | 0,83-
Pathogenic | Cosm5609243 | Upper
Extremity | G/G | G/G | 20 |
| | | c.460 C>T | Nrf2-Neh5 | Missense | p.P154S | Benign | rs763327009 | | | | |
| 2 | 34 | F | c.438 C>T | Keap1-
BTB-Domain | Synonymous | p.S146S | 0,83-
Pathogenic | Cosm5609243 | Lower
Extremity | G/G | G/A | 29 |
| 3 | 44 | M | c.639+8 G>A | Keap1-Splice
site | Intronic
variant | - | NA | rs1466903180 | Lower
Extremity | A/A | G/G | 37 |
| | | c.46-698C>A | Nrf2 | Splice acceptor
variant | - | | Cosm6971525 | | | | |
| 4 | 23 | M | c.474 T>C | Keap1- BTB-
Domain | Synonymous | p.G158G | Neutral | Cosm4131169 | Lower
Extremity | G/G | G/G | 19 |
| | |
c.594+76C>Aa | Nrf2-3'UTR | Intronic
variant | - | | Novel | | | | |
| 5 | 27 | M | c.474 T>C | Keap1-
BTB-Domain | Synonymous | p.G158G | Neutral | Cosm4131169 | Lower
Extremity | G/A | G/G | 27 |
| | | c.46-698C>A | Nrf2 | Splice acceptor
variant | - | | Cosm6971525 | | | | |
| 6 | 42 | M | c.348G>A | Keap1-
BTB-Domain | Synonymous | p.R116R | Neutral | rs759047759 | Lower
Extremity | A/A | G/G | 42 |
| | | c.347 G>A | | Missense | p.R116Q |
0,85-Pathogenic | Cosm3796565 | | | | |
| | | c.46-698C>A | Nrf2 | Splice acceptor
variant | - | NA | Cosm6971525 | | | | |
| 7 | 55 | M | - | - | - | - | - | - | Lower
Extremity | A/A | G/G | 47 |
| 8 | 44 | M | c.313/314
C>Ta | Keap1- BTB
Domain | Missense | p.P105L | 0,99-
Pathogenic | Novel | Lower
Extremity | G/A | G/A | 38 |
| | | c.348G>A | | Missense | p.R116Q |
0,85-Pathogenic | Cosm3796565 | | | | |
| | | c. 347G>A | | Synonymous | p.R116R | Neutral | rs759047759 | | | | |
| | | c.388 C>T | | Missense | p.P130T | 1,00-
Pathogenic | Cosm6970908 | | | | |
| | | c.340 G>T | | Missense | p.G114W |
0,94-Pathogenic | Cosm1725916 | | | | |
| | | c.361 G>A | | Missense | p.E121K | 0,68-
Pathogenic | Cosm69679929 | | | | |
| | | c.240 A>G | | Missense | p.T80T | Neutral | rs1215286054 | | | | |
| | | c.46-698C>A | | Splice acceptor
variant | | | Cosm6971525 | | | | |
| 9 | 56 | F | c.388 C>T | Keap1-
BTB-Domain | Missense | p.P130T | 1,00-
Pathogenic | Cosm6970908 | Lower
Extremity | G/G | G/G | 52 |
| | | c.347 G>A | | | p.R116Q |
0,85-Pathogenic | Cosm3796565 | | | | |
| | | c.348G>A | | Synonymous | p.R116R | Neutral | rs759047759 | | | | |
| 10 | 20 | M | c.438 C>T | Keap1-
BTB-Domain | Synonymous | p.S146S | 0,83-
Pathogenic | Cosm5609243 | Lower
Extremity | A/A | G/G | 20 |
| | | c.730 G>C | Keap1-
IVR-Domain | Missense | p.E244Q | 0,92-
Pathogenic | Cosm6986830 | | | | |
| | | c.351 G>T | Keap1-
BTB-Domain | Missense | p.E117D | 0,97-
Pathogenic | Cosm6917923 | | | | |
| | | c.347 G>A | | Synonymous | p.R116R | Neutral | rs759047759 | | | | |
| | | c.348G>A | | Missense | p.R116Q |
0,85-Pathogenic | Cosm3796565 | | | | |
| | | c.361 G>A | | Missense | p.E121K | 0,68-
Pathogenic | Cosm69679929 | | | | |
| | |
c.594+24T>Aa | Nrf2-3'UTR | Intronic
variant | - | - | Novel | | | | |
| 11 | 37 | F | c.438 C>T | Keap1-
BTB-Domain | Synonymous | p.S146S | 0,83-
Pathogenic | Cosm5609243 | Lower
Extremity | G/G | G/G | 28 |
| | | c.388 C>T | | Missense | p.P130T | 1,00-
Pathogenic | Cosm6970908 | | | | |
| | | c.348G>A | | Synonymous | p.R116R | Neutral | rs759047759 | | | | |
| 12 | 54 | M | c.474 T>C | Keap1- BTB
Domain- | Synonymous | p.G158G | Neutral | Cosm4131169 | Pulmonary
Artery | G/G | G/G | 54 |
| | | c.348G>A | | Synonymous | p.R116R | Neutral | rs759047759 | | | | |
| | | c.347 G>A | | Missense | p.R116Q |
0,85-Pathogenic | Cosm3796565 | | | | |
| | | c.46-698C>A | Nrf2 | Splice acceptor
variant | | | Cosm6971525 | | | | |
| 13 | 57 | M | c.348G>A | Keap1-
BTB-Domain | Synonymous | p.R116R | Neutral | rs759047759 | Lower
Extremity | G/G | G/G | 47 |
| | | c.347 G>A | | Missense | p.R116Q |
0,85-Pathogenic | Cosm3796565 | | | | |
| | | c.46-698C>A | Nrf2 | Splice acceptor
variant | - | - | Cosm6971525 | | | | |
| | | c.594+76C>A | Nrf2-3'UTR | Intronic
variant | - | - | Novel | | | | |
| 14 | 22 | M | c.348G>A | Keap1- BTB-Domain
- | Synonymous | p.R116R | Neutral | rs759047759 | Lower
Extremity | A/A | G/G | 19 |
| | | c.347 G>A | | Missense | p.R116Q |
0,85-Pathogenic | Cosm3796565 | | | | |
| | | c.438 C>T | | Synonymous | p.S146S | 0,83-
Pathogenic | Cosm5609243 | | | | |
| | | c.46-698C>A | Nrf2 | Splice acceptor
variant | | | Cosm6971525 | | | | |
| 15 | 27 | M | - | - | - | - | - | - | Lower
Extremity | G/G | G/G | 22 |
| 16 | 60 | M | c.347 G>A | Keap1-
BTB-Domain | Missense | p.R116Q |
0,85-Pathogenic | Cosm3796565 | Lower
Extremity | G/G | G/G | 54 |
| | | c.438 C>T | | Synonymous | | 0,83-
Pathogenic | Cosm5609243 | | | | |
| | | c.46-698C>A | Nrf2 | Splice acceptor
variant | - | - | Cosm6971525 | | | | |
| 17 | 28 | M | c.348G>A | Keap1-
BTB-Domain | Synonymous | p.R116R | Neutral | rs759047759 | Lower
Extremity | G/G | G/A | 23 |
| | | c.347 G>A | | Missense | p.R116Q |
0,85-Pathogenic | Cosm3796565 | | | | |
| | | c.438 C>T | | Synonymous | p.S146S | 0,83-
Pathogenic | Cosm5609243 | | | | |
| | | c.434 T>Aa | | Missense | p.I145N | 0,99--
Pathogenic | Novel | | | | |
| 18 | 48 | F | c.347 G>A | Keap1- BTB-Domain
- | Missense | p.R116Q |
0,85-Pathogenic | Cosm3796565 | Lower
Extremity | A/A | G/G | 43 |
| | | c.438 C>T | | Synonymous | p.S146S | 0,83-
Pathogenic | Cosm5609243 | | | | |
| | | c.434 T>A | | Missense | p.I145N |
0,99-Pathogenic | Novel | | | | |
| | | c.435 C>A | | Missense | p.I145L | 0,90-
Pathogenic | Cosm6492287 | | | | |
| | | c.388 C>T | | Missense | p.P130T | 1,00-
Pathogenic | Cosm6970908 | | | | |
| | | c.46-36T>A | Nrf2 | Intronic
variant | | | rs751650669 | | | | |
| 19 | 41 | M | c.360 G>Ta | Keap1-
BTB-Domain | Missense | p.M120I | 0,81-
Pathogenic | Novel | Lower
Extremity | G/G | G/G | 33 |
| | | c.474 T>C | | Synonymous | p.G158G | Neutral | Cosm4131169 | | | | |
| | | c.46-698C>A | Nrf2 | Splice acceptor
variant | | - | Cosm6971525 | | | | |
| 20 | 74 | F | c.348G>A | Keap1-
BTBDomain | Synonymous | p.R116R | Neutral | rs759047759 | Lower
Extremity | | | 62 |
| | | c.347 G>A | | Missense | p.R116Q |
0,85-Pathogenic | Cosm3796565 | | | | |
| | | c.474 T>C | | Synonymous | p.G158G | Neutral | Cosm4131169 | | | | |
| 21 | 44 | M | c.347 G>A | Keap1-
BTB-Domain | Missense | p.R116Q |
0,85-Pathogenic | Cosm3796565 | Lower
Extremity | G/A | G/G | 37 |
| | | c.360 G>T | | | p.M120I | 0,81-
Pathogenic | Novel | | | | |
| | | c.438 C>T | | Synonymous | p.S146S | 0,83-
Pathogenic | Cosm5609243 | | | | |
| 22 | 69 | F | c.360 G>T | Keap1- BTB-
Domain | Missense | p.M120I | 0,81-
Pathogenic | Novel | Lower
Extremity | G/G | G/G | 64 |
| | | c.438C>T | | Synonymous | p.S146S | 0,83-
Pathogenic | Cosm5609243 | | | | |
| | | c.474 T>C | | Synonymous | p.G158G | Neutral | Cosm4131169 | | | | |
| | |
c.594+79C>Aa | Nrf2-3'UTR | Intronic
variant | - | | Novel | | | | |
| 23 | 23 | M | c.360 G>T | Keap1- BTB | Missense | p.M120I | 0,81-
Pathogenic | Novel | Lower
Extremity | G/G | G/G | 17 |
| | | c.474 T>C | | Synonymous | p.G158G | Neutral | Cosm4131169 | | | | |
| | |
c.40310delAa | Nrf2-3'UTR | Intronic
variant | - | - | Novel | | | | |
| 24 | 64 | M | c.438 C>T | Keap1-
BTB-Domain | Synonymous | p.S146S | 0,83-
Pathogenic | Cosm5609243 | Lower
Extremity | G/G | G/A | 57 |
| | | c.46-698C>A | Nrf2 | Splice acceptor
variant | - | - | Cosm6971525 | | | | |
| 25 | 44 | F | c.438 C>T | Keap1-
BTB-Domain | Synonymous | p.S146S | 0,83-
Pathogenic | Cosm5609243 | Lower
Extremity | G/A | G/G | 36 |
| 26 | 60 | M | - | - | - | - | - | - | Lower
Extremity | G/G | G/G | 53 |
| 27 | 23 | M | c.360 G>T | Keap1-
BTB-Domain | Missense | p.M120I | 0,81-
Pathogenic | Novel | Lower
Extremity | G/A | G/G | 21 |
Results of in silico analysis of
Keap1/Nrf2 pathway mutations
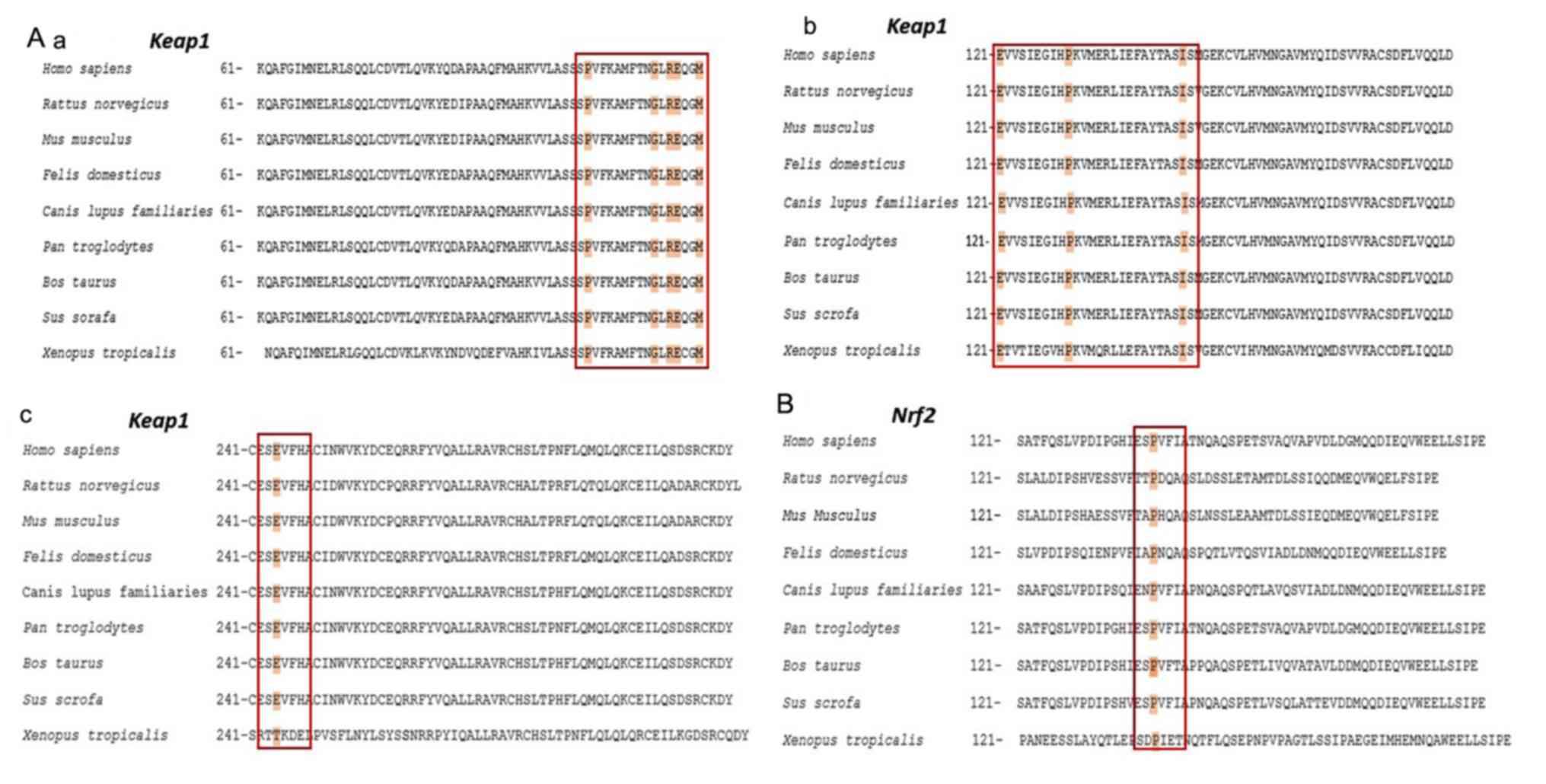
The comparison of amino acid sequences affected by
the mutations detected in the present study demonstrated that the
missense mutations p.P105L, p.G114W, p.R116Q, p.G114W, p.E117D,
p.M120I, p.P130T, p.I145N and p.E244Q of Keap1 exhibited amino acid
substitutions at a key point that is conserved throughout the
evolutionary process across a number of species, including H.
sapiens and X. tropicalis (Fig. 3). The missense mutation p.P154S
detected in Nrf2 was also located at this point conserved.
According to the results of PolyPhen-2 analysis, the majority of
missense mutations detected in Keap1 and Nrf2 may be pathogenic as
their pathogenicity scores were close to 1. These missense
mutations are detailed in Table
III.
Results of Nrf2/Keap1 pathway gene
expression level analysis
For gene expression analysis, RT-qPCR was used to
determine the expression levels of Nrf2 and Keap1 and the reference
gene RPLP0 in 27 patients with DVT and 10 healthy controls. The
relative mRNA expression levels of Keap1 were significantly higher
than that of Nrf2 in patients with DVT (P=0.02; Fig. 4A). Furthermore, Keap1 mRNA expression
levels were higher in the patient group compared with those in the
control group (P<0.05; Fig.
4B).
TAC level analysis
TAC levels differed between patients with DVT, with
a range of 2.99-1.09 mmol TE/l (Fig.
5). Certain patients (Cases 13, 8, 16, 24 and 12) with splice
site mutations demonstrated high TAC values (2.94, 2.66, 2.66, 2.61
and 2.59 mmol TE/l, respectively). However, patients with no
mutational change (Cases 15 and 26) exhibited lower TAC levels than
the other patients.
Discussion
Cardiovascular diseases are among the leading causes
of global mortality, according to the World Health Organization:
Cardiovascular diseases were reported to be responsible for 31%
(17.9 million) of global deaths in 2018, and cardiovascular
disease-associated mortality is estimated to reach 22.2 million in
2030 (28,36).Venous thromboembolism, including DVT,
is the third leading cause of death worldwide associated with
cardiovascular disease after coronary artery disease and stroke in
2018(37). In the cardiovascular
system, ROS production, vascular tone control, inflammatory
responses, cell growth and proliferation, modulation of
extracellular matrix production, apoptosis and angiogenesis are
important for maintaining the function of endothelial and vascular
smooth muscle cells (1,12). Numerous studies have demonstrated
that endothelial dysfunction is the first step in the pathogenesis
of several cardiovascular diseases (3,28,38).
Cellular damage caused by oxidative stress serves a key role in
most pathophysiological conditions that occur due to endothelial
dysfunction (1,24-26,38).
Numerous antioxidant pathways, including the
Nrf2/Keap1 signaling pathway, are involved in cellular redox
homeostasis (4,27). Nrf2/Keap1 regulates the expression
levels of antioxidants and phase II detoxification enzymes
(39-41).
Nrf2 is highly expressed in tissues where detoxification reactions
take place and is a key regulator of the cellular defense mechanism
in several organs, including the brain, lung, bladder, kidney,
liver and ovary, as well as macrophages and erythrocytes (4,42). The
Nrf2/Keap1 signaling pathway maintains the healthy endothelial
phenotype under normal physiological conditions. The endothelium is
a type of tissue located at the border between blood and tissues,
and is primarily involved in the regulation of vascular tone,
thromboresistivity, inflammation of the vascular wall and cellular
adhesion (43). Increased ROS
production in the endothelium activates Nrf2, which causes
increased expression levels of intracellular HO-1, glutathione
peroxidase (GPx), GSH, glutamate-cysteine ligase modifier subunit,
sulfiredoxin-1, NQO1, protease-activated receptor 4 and oxidative
stress-induced growth inhibitor 1 genes in arterial endothelial
cells (25).
Studies using animal models have concluded that
insufficient levels of Nrf2 in various types of tissue concurrently
are associated with susceptibility to a number of diseases,
including cardiovascular disease (10,26,42,43).
Studies investigating the role of the Nrf2/Keap1 signaling pathway
in atherosclerosis, hypertension, myocardial infarction and
ischemia have been conducted; however, to the best of our
knowledge, there are currently no studies regarding Nrf2/Keap1
signaling in thromboembolism, which is the third leading cause of
cardiovascular disease-associated mortality, after coronary artery
disease and stroke (25,26,44). In
the present study, Sanger DNA sequencing analysis was used to
detect mutations/SNPs in the Nrf2/Keap1 signaling pathway genes in
27 patients with DVT. Of 23 mutations detected in the Nrf2/Keap1
signaling pathway in 24 (89%) patients, 11 were missense mutations,
five were synonymous nucleotide changes, two were splice site
mutations and five were non-coding region nucleotide changes. The
present study identified seven novel mutations; 16 mutations had
been previously reported. Gene expression level analysis was
performed using RT-qPCR. The Keap1 BTB domain is the binding site
for the Cullin 3 (Cul3)-E3-dependent ubiquitin ligase complex,
which suppresses Nrf2 by promoting Nrf2 ubiquitination and
subsequent proteosomal degradation (45,46).
Keap1 is known to dimerize via its BTB domain; deletion of the BTB
domain from Keap1 prevents binding with Cul3, demonstrating that
the BTB domain is required for Keap1-Cul3 binding (46). In the present study, the missense
mutations detected in the Keap1 BTB domain may lead to continuous
suppression of gene expression levels of Nrf2. Mutations in this
domain are associated with ovarian and pancreatic cancer, as well
as malignant melanoma (46-48).
To the best of our knowledge, however, the association between DVT
and mutations in this domain has not yet been investigated. Further
studies using larger populations of patients with DVT are required
to clarify the association between emerging relapses and Keap1 BTB
domain mutations.
In the present study, c.639+8 G>A and c.46-698
C>A splice site mutations were detected in Keap1 and Nrf2 genes,
respectively. c.46-698 C>A may cause the absence of Nrf2
expression levels as this mutation is located in the first base of
the splice site, which is conserved among species in the
evolutionary process. Nrf2 overexpression has been reported to
increase the expression levels of ARE-dependent antioxidant
enzymes, such as NQO1, GST, HO-1 and GPx, which serve key roles in
cellular defense mechanisms against ROS (15,27).
Studies using human and animal models have demonstrated that lack
of Nrf2 expression levels may be a risk factor in the pathogenesis
of numerous disorders, including cardiovascular diseases (10,12,15,47).
Nrf2 expression levels in patients with DVT were demonstrated to be
lower compared with those of Keap1 in the present study. This may
be due to splice site mutations in the Neh2 domain, which is the
Keap1 binding site located on Nrf2. This may also be caused by
missense mutations on the BTB domain, which allow the formation of
the Nrf2 inhibitor complex by binding to the homodimerizable RING
box protein 1-bound Cul3 protein on Keap1 (46,48).
In the present study, two patients (Cases 9 and 26)
exhibited low Nrf2 expression levels. Case 9 exhibited two missense
changes and one synonymous change (p.P130T, p.R116Q and p.R116R,
respectively), whereas no changes were detected in Case 26.
Furthermore, changes were detected in seven of the eight patients
with a history of hypertension. To the best of our knowledge,
previous studies have not yet identified the association between
blood pressure and the expression of genes involved in the
Nrf2/Keap1 signaling pathway; however, Nrf2 may serve a key role in
regulating blood pressure (10,12,49).
Missense mutations were detected in the Nrf2/Keap1 genes in Cases
27 and 12, who were diagnosed with thrombosis in the upper
extremity veins and pulmonary embolism, respectively. A Nrf2 splice
site mutation was detected in Case 12. The Neh3, Neh4 and Neh5
domains of Nrf2 are transactivation sites of the Nrf2 molecule.
These sites are associated with histone acetyltransferases and bind
to the coactivator cAMP-response element binding protein to
facilitate Nrf2 transcription (24,50). In
the present study, the p.P154S missense mutation was detected on
the Neh5 domain of the Nrf2 gene and was classified as benign
following functional pathogenicity analysis. Animal models using
Nrf2-/- mammals have demonstrated
susceptibility to oxidative damage, as Nrf2 serves a key role in
the adaptive response of the cell to oxidative stress. A previous
study using Nrf2-/- mice
demonstrated increased tissue damage and tumorigenesis, severe
inflammation and high levels of DNA, lipid and protein oxidation
products in experimental animals exposed to chemical or
xenobiotic-induced oxidative damage (51). Studies have demonstrated that
Nrf2-/- mice are more susceptible
not only to cancer but also to asthma, emphysema, pulmonary
fibrosis and acute lung injury (46,52,53). In
addition, it has been demonstrated that decreased expression levels
of Nrf2 are associated with risk of acute lung injury, pulmonary
edema and inflammation (20).
Nrf2 expression levels may be controlled by KLF2,
which is involved with a different pathway. Krüppel-like factor 2
(KLF2) is a key transcription factor in laminar flow-mediated
cytoprotective responses in endothelial cells. Studies have
demonstrated that KLF2 transcription is stimulated in endothelium
under atheroprotective laminar flow via the MAPK/ERK kinase
5-extracellular signal-regulated protein kinase 5 (ERK5)-MEF2
signaling pathway. ERK5 activation induced by flow may exert
cytoprotective effects via Nrf2-ARE-dependent expression of
antioxidant genes independently of KLF2(54).
Human Keap1 contains 27 cysteine residues, which
convert the protein into a redox sensor for endogenous and
environmental oxidative signals and electrophilic reactions
(55-57).
Under normal physiological conditions, critical cysteine residues
(Cys 151, 273 and 288) are present on Keap1; however, in the
presence of increased levels of ROS or electrophiles they are
oxidized, resulting in the separation of the Keap1 tertiary
structure from Nrf2 (19,22,25). The
cysteine residues 273, 288 and 297 on the Keap1 IVR domain are
important for Nrf2 activation and inhibition (17,19).
These cysteine residues serve a key role in altering the
conformation of Keap1, which leads to the transcriptional
activation of Nrf2 and its targets. In the present study, p.E244Q
missense mutation located near these cysteine residues on the IVR
domain was identified, with a pathogenic effect score of 0.95. Case
10, the youngest patient in the present study, carried this
mutation with the highest number of changes in the Nrf2/Keap1
signaling pathway.
Nrf2 and Keap1 are highly conserved in the
evolutionary process. However, cysteine substitutes the amino acid
tyrosine located at codon 212 of the Keap1 IVR domain in the sperm
whale (P. microcephalus) and in the blind mole rat, which
can live >20 years in hypoxic conditions (>3% O2)
and whose resistance to cancer has been previously demonstrated
(57). This codon change may
contribute to the redox sensor property of the Keap1 protein in
electrophilic reactions. The cysteine present in this location
functions via binding and degrading of Nrf2(58). The presence of this variant in
long-lived organisms may affect cellular Nrf2 levels, thus
contributing to the emergence of the specific adaptive phenotype by
altering gene expression levels of the antioxidant pathway.
DVT arises from complex interactions between genetic
and environmental factors. Expression levels of certain genes
regulate cellular environmental phenomena, such as redox
homeostasis. The Nrf2/Keap1 signaling pathway controls the
expression levels of antioxidant enzymes. However, to the best of
our knowledge, previous studies have not yet identified an
association between Nrf2/Keap1 signaling, antioxidant levels and
DVT.
In the present study, antioxidant values of patients
with DVT were measured as an indicator of total antioxidant levels.
Antioxidant levels were notably higher in patients with splice site
mutations, compared with patients that exhibited no mutational
change. Patients with splice site mutations exhibited a notable
antioxidant defense mechanism, which may be an evolutionary
response to avoid the harmful effects of increased levels of free
radicals that are generated following oxidative stress. In
addition, decreased Nrf2 expression levels were associated with low
antioxidant values in the present study. DVT may be associated with
oxidative imbalance as the Nrf2 region regulates levels of
antioxidant enzymes. Determination of Nrf2/Keap1 and antioxidant
levels may present a precursor of DVT that can be used for clinical
diagnosis.
The results of the present study indicated that
Keap1 missense mutations may be involved in the development of DVT.
The amino acid residues affected by these mutations are conserved
across species. Furthermore, these mutations may alter the
expression levels of genes involved in antioxidant processes, and
may also exhibit pathogenic properties, as demonstrated by the
functional pathogenic effect analysis.
In summary, 23 changes (11 missense mutations, five
synonymous nucleotide changes, two splice site mutations and five
non-coding region nucleotide changes) were detected, seven of which
were identified for the first time in the present study. However,
the present study used a limited sample group. Future
investigations using larger samples size are required to clarify
the epidemiology of genetic mutations responsible for DVT. The
present study identified the frequency and molecular
characteristics of mutations in the Nrf2/Keap1 signaling pathway in
patients with DVT. In conclusion, as the Nrf2/Keap1 complex serves
a key role in preventing oxidative stress-induced injuries,
pharmacological agents that stimulate Nrf2 activity may provide a
novel approach for the treatment of oxidative stress-associated
diseases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed in the present study
are included in the published article.
Authors' contributions
DFAB designed the study. DFAB and SI drafted the
manuscript. DFAB, TE, SI and YE performed the experiments. DFAB, SI
and TK analyzed the data. All authors participated in the writing
and revision of the manuscript. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Erciyes University (Kayseri, Turkey). All participants
provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chistiakov DA, Orekhov AN and Bobryshev
YV: Endothelial Barrier and Its Abnormalities in Cardiovascular
Disease. Front Physiol. 6(365)2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Förstermann U: Oxidative stress in
vascular disease: Causes, defense mechanisms and potential
therapies. Nat Clin Pract Cardiovasc Med. 5:338–349.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Loscalzo J: Oxidative stress in
endothelial cell dysfunction and thrombosis. Pathophysiol Haemost
Thromb. 32:359–360. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen B, Lu Y, Chen Y and Cheng J: The role
of Nrf2 in oxidative stress-induced endothelial injuries. J
Endocrinol. 225:R83–R99. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Konukoglu D and Uzun H: Endothelial
dysfunction and hypertension. Adv Exp Med Biol. 956:511–540.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bengisun U: Basic treatment principles of
deep vein thrombosis and pulmonary embolism. TOTBID Derg.
18:505–513. 2019.
|
|
7
|
Kruger PC, Eikelboom JW, Douketis JD and
Hankey GJ: Deep vein thrombosis: Update on diagnosis and
management. Med J Aust. 210:516–524. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Harrison D, Griendling KK, Landmesser U,
Hornig B and Drexler H: Role of oxidative stress in
atherosclerosis. Am J Cardiol. 91 (3A):7A–11A. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Harrison DG, Gongora MC, Guzik TJ and
Widder J: Oxidative stress and hypertension. J Am Soc Hypertens.
1:30–44. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Howden R: Nrf2 and cardiovascular defense.
Oxid Med Cell Longev. 2013(104308)2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Park JG and Oh GT: The role of peroxidases
in the pathogenesis of atherosclerosis. BMB Rep. 44:497–505.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
da Costa RM, Rodrigues D, Pereira CA,
Silva JF, Alves JV, Lobato NS and Tostes RC: Nrf2 as a Potential
Mediator of Cardiovascular Risk in Metabolic Diseases. Front
Pharmacol. 10(382)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Galley JC and Straub AC: Redox control of
vascular function. Arterioscler Thromb Vasc Biol. 37:e178–e184.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lee JM, Li J, Johnson DA, Stein TD, Kraft
AD, Calkins MJ, Jakel RJ and Johnson JA: Nrf2, a multi-organ
protector? FASEB J. 19:1061–1066. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Smith RE, Tran K, Smith CC, McDonald M,
Shejwalkar P and Hara K: The Role of the Nrf2/ARE antioxidant
system in preventing cardiovascular diseases. Diseases.
4(34)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kim J, Cha YN and Surh YJ: A protective
role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in
inflammatory disorders. Mutat Res. 690:12–23. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kansanen E, Kuosmanen SM, Leinonen H and
Levonen AL: The Keap1-Nrf2 pathway: Mechanisms of activation and
dysregulation in cancer. Redox Biol. 1:45–49. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Baird L and Dinkova-Kostova AT: The
cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol.
85:241–272. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hua CC, Chang LC, Tseng JC, Chu CM, Liu YC
and Shieh WB: Functional haplotypes in the promoter region of
transcription factor Nrf2 in chronic obstructive pulmonary disease.
Dis Markers. 28:185–193. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kensler TW, Wakabayashi N and Biswal S:
Cell survival responses to environmental stresses via the
Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 47:89–116.
2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Robledinos-Antón N, Fernández-Ginés R,
Manda G and Cuadrado A: Activators and Inhibitors of NRF2: A Review
of Their Potential for Clinical Development. Oxid Med Cell Longev.
2019(9372182)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tebay LE, Robertson H, Durant ST, Vitale
SR, Penning TM, Dinkova-Kostova AT and Hayes JD: Mechanisms of
activation of the transcription factor Nrf2 by redox stressors,
nutrient cues, and energy status and the pathways through which it
attenuates degenerative disease. Free Radic Biol Med. 88 (Pt
B):108–146. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tu W, Wang H, Li S, Liu Q and Sha H: The
anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE
signaling pathway in chronic diseases. Aging Dis. 10:637–651.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Satta S, Mahmoud AM, Wilkinson FL, Yvonne
Alexander M and White SJ: The role of Nrf2 in cardiovascular
function and disease. Oxid Med Cell Longev.
2017(9237263)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sarutipaiboon I, Settasatian N, Komanasin
N, Kukongwiriyapan U, Sawanyawisuth K, Intharaphet P, Senthong V
and Settasatian C: Association of Genetic Variations in NRF2, NQO1,
HMOX1, and MT with severity of coronary artery disease and related
risk factors. Cardiovasc Toxicol. 20:176–189. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Korytina GF, Akhmadishina LZ, Aznabaeva
YG, Kochetova OV, Zagidullin NS, Kzhyshkowska JG, Zagidullin SZ and
Viktorova TV: Associations of the NRF2/KEAP1 pathway and
antioxidant defense gene polymorphisms with chronic obstructive
pulmonary disease. Gene. 692:102–112. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Barančík M, Grešová L, Barteková M and
Dovinová I: Nrf2 as a key player of redox regulation in
cardiovascular diseases. Physiol Res. 65 (Suppl 1):S1–S10.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cunningham F, Achuthan P, Akanni W, Allen
J, Amode MR, Armean IM, Bennett R, Bhai J, Billis K, Boddu S, et
al: Ensembl 2019. Nucleic Acids Res. 47 (D1):D745–D751.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Adzhubei I, Jordan DM and Sunyaev SR:
Predicting functional effect of human missense mutations using
PolyPhen-2. Curr Protoc Hum Genet. 7:7–20. 2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tate JG, Bamford S, Jubb HC, Sondka Z,
Beare DM, Bindal N, Boutselakis H, Cole CG, Creatore C, Dawson E,
et al: COSMIC: The catalogue of somatic mutations in cancer.
Nucleic Acids Res. 47 (D1):D941–D947. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Stenson PD, Mort M, Ball EV, Evans K,
Hayden M, Heywood S, Hussain M, Phillips AD and Cooper DN: The
Human Gene Mutation Database: Towards a comprehensive repository of
inherited mutation data for medical research, genetic diagnosis and
next-generation sequencing studies. Hum Genet. 136:665–677.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Untergasser A, Cutcutache I, Koressaar T,
Ye J, Faircloth BC, Remm M and Rozen SG: Primer3--new capabilities
and interfaces. Nucleic Acids Res. 40(e115)2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29(e45)2001.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
36
|
World Health Organization (WHO):
Cardiovascular diseases (CVDs). WHO, Geneva, 2017. https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds).
Accessed May 17, 2017.
|
|
37
|
Raskob GE, Angchaisuksiri P, Blanco AN,
Buller H, Gallus A, Hunt BJ, Hylek EM, Kakkar A, Konstantinides SV,
McCumber M, et al: ISTH Steering Committee for World Thrombosis
Day: Thrombosis: A major contributor to global disease burden.
Thromb Res. 134:931–938. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Heitzer T, Schlinzig T, Krohn K, Meinertz
T and Münzel T: Endothelial dysfunction, oxidative stress, and risk
of cardiovascular events in patients with coronary artery disease.
Circulation. 104:2673–2678. 2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Suzuki T and Yamamoto M: Molecular basis
of the Keap1-Nrf2 system. Free Radic Biol Med. 88 (Pt B):93–100.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Barrera-Rodríguez R: Importance of the
Keap1-Nrf2 pathway in NSCLC: Is it a possible biomarker? Biomed
Rep. 9:375–382. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Li C, Cheng L, Wu H, He P, Zhang Y, Yang
Y, Chen J and Chen M: Activation of the KEAP1-NRF2-ARE signaling
pathway reduces oxidative stress in Hep2 cells. Mol Med Rep.
18:2541–2550. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Surh YJ, Kundu JK and Na HK: Nrf2 as a
master redox switch in turning on the cellular signaling involved
in the induction of cytoprotective genes by some chemopreventive
phytochemicals. Planta Med. 74:1526–1539. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Vanhoutte PM, Shimokawa H, Feletou M and
Tang EH: Endothelial dysfunction and vascular disease - a 30th
anniversary update. Acta Physiol (Oxf). 219:22–96. 2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rangasamy T, Guo J, Mitzner WA, Roman J,
Singh A, Fryer AD, Yamamoto M, Kensler TW, Tuder RM, Georas SN, et
al: Disruption of Nrf2 enhances susceptibility to severe airway
inflammation and asthma in mice. J Exp Med. 202:47–59.
2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kobayashi E, Suzuki T and Yamamoto M:
Roles nrf2 plays in myeloid cells and related disorders. Oxid Med
Cell Longev. 2013(529219)2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Pandey P, Singh AK, Singh M, Tewari M,
Shukla HS and Gambhir IS: The see-saw of Keap1-Nrf2 pathway in
cancer. Crit Rev Oncol Hematol. 116:89–98. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Konstantinopoulos PA, Spentzos D,
Fountzilas E, Francoeur N, Sanisetty S, Grammatikos AP, Hecht JL
and Cannistra SA: Keap1 mutations and Nrf2 pathway activation in
epithelial ovarian cancer. Cancer Res. 71:5081–5089.
2011.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Qin JJ, Cheng XD, Zhang J and Zhang WD:
Dual roles and therapeutic potential of Keap1-Nrf2 pathway in
pancreatic cancer: A systematic review. Cell Commun Signal.
17(121)2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Silva-Palacios A, Königsberg M and Zazueta
C: Nrf2 signaling and redox homeostasis in the aging heart: A
potential target to prevent cardiovascular diseases? Ageing Res
Rev. 26:81–95. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Nioi P, Nguyen T, Sherratt PJ and Pickett
CB: The carboxy-terminal Neh3 domain of Nrf2 is required for
transcriptional activation. Mol Cell Biol. 25:10895–10906.
2005.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K,
Yamamoto M, Talalay P and Kensler TW: Sensitivity to carcinogenesis
is increased and chemoprotective efficacy of enzyme inducers is
lost in nrf2 transcription factor-deficient mice. Proc Natl Acad
Sci USA. 98:3410–3415. 2001.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Aoki Y, Sato H, Nishimura N, Takahashi S,
Itoh K and Yamamoto M: Accelerated DNA adduct formation in the lung
of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol Appl
Pharmacol. 173:154–160. 2001.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Enomoto A, Itoh K, Nagayoshi E, Haruta J,
Kimura T, O'Connor T, Harada T and Yamamoto M: High sensitivity of
Nrf2 knockout mice to acetaminophen hepatotoxicity associated with
decreased expression of ARE-regulated drug metabolizing enzymes and
antioxidant genes. Toxicol Sci. 59:169–177. 2001.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Kim M, Kim S, Lim JH, Lee C, Choi HC and
Woo CH: Laminar flow activation of ERK5 protein in vascular
endothelium leads to atheroprotective effect via NF-E2-related
factor 2 (Nrf2) activation. J Biol Chem. 287:40722–40731.
2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sihvola V and Levonen AL: Keap1 as the
redox sensor of the antioxidant response. Arch Biochem Biophys.
617:94–100. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Dinkova-Kostova AT, Holtzclaw WD, Cole RN,
Itoh K, Wakabayashi N, Katoh Y, Yamamoto M and Talalay P: Direct
evidence that sulfhydryl groups of Keap1 are the sensors regulating
induction of phase 2 enzymes that protect against carcinogens and
oxidants. Proc Natl Acad Sci USA. 99:11908–11913. 2002.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Schmidt H, Hangmann J, Shams I, Avivi A
and Hankeln T: Molecular evolution of antioxidant and hypoxia
response in long-lived, cancer-resistant blind mole rats: The
Nrf2-Keap1 pathway. Gene. 577:293–298. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Taguchi K, Motohashi H and Yamamoto M:
Molecular mechanisms of the Keap1–Nrf2 pathway in stress response
and cancer evolution. Genes Cells. 16:123–140. 2011.PubMed/NCBI View Article : Google Scholar
|