Introduction
Pancreatic cancer is a malignancy of the pancreas,
which is associated with a poor prognosis (1,2), and the
morbidity and mortality rates associated with pancreatic cancer
have increased globally in recent years (3-5).
Currently, surgery is the only possible cure for pancreatic cancer,
however, due to the high degree of malignancy, surgical resection
rates are low, with unsatisfactory treatment efficacies (6,7).
Therefore, the identification of a novel means for early diagnosis
and effective treatment for pancreatic cancer is urgently required.
A current lack of knowledge regarding the exact molecular
mechanism(s) of pancreatic cancer progression reduces the
possibility of early diagnosis and timely treatment (8,9).
Therefore, the identification of effective biomarkers to improve
understanding of the possible pathogenesis of pancreatic cancer is
an urgent requirement.
It is well established that microRNAs (miRNAs) are
key components of the non-coding RNA family that are capable of
extensively regulating gene expression (10-12).
The mature miRNAs are derived from their precursors, either
generated from spliceosomes or transcribed from the genome
(13). Previously published studies
have confirmed that miRNAs are abnormally expressed in a variety of
malignancies, and function either as oncogenes or as tumor
suppressors (14-16).
miRNAs exhibit the characteristics of high tissue specificity and
stability, with altered expression patterns during tumor
development (17-19).
Therefore, the potential of miRNAs in the diagnosis of a variety of
cancer types may provide researchers with a means to identify and
characterize potential biomarkers.
In the present study, data were accessed from The
Cancer Genome Atlas (TCGA) and Gene Expression Omnibus (GEO)
databases to explore key miRNAs in pancreatic cancer. Comprehensive
bioinformatics analysis was used to identify differentially
expressed miRNAs (DE-miRNAs). The target genes of prognostic
DE-miRNAs were predicted. Gene Ontology (GO) and Kyoto Encyclopedia
of Genes and Genomes (KEGG) analyses were subsequently performed to
analyze the functional enrichment of the gene set, and to identify
significant pathways that are associated with the target genes. The
present study aimed to investigate the involvement of miRNAs
critical to pancreatic cancer progression, and to seek a means of
improved disease diagnosis.
Materials and methods
Data processing and identification of
DE-miRNAs
The miRNA sequencing data were downloaded from
FireBrowse (version 0.4.13; http://firebrowse.org/), which is a data integration
database associated with the TCGA database. The data were processed
using R language (version 3.4.4; https://www.r-project.org/) and normalized by
log2 transformation. miRNAs that were differentially
expressed between pancreatic cancer and healthy tissues were
analyzed using the Limma package in R language with the criteria of
|log2FC (fold change)|>1.0 and P<0.05. Results are presented
in volcano plots, which were plotted using GraphPad prism.v.5
(GraphPad Software, Inc.), and in heat maps using the open source
markup language, HEML (20). The
gene expression profile (GSE62452) and miRNA expression profile
(GSE43796) (21,22) were downloaded from the GEO database
(https://www.ncbi.nlm.nih.gov/geo/). A
total of 31 samples were identified in GSE43796, including 26
pancreatic cancer samples and 5 healthy samples. The GSE43796 data
were collected based on the GPL15159 platform Agilent-031181
Unrestricted_Human_miRNA_V16.0_Microarray 030840 (probe name
version). The GSE62452 dataset containing 130 samples, including 69
tumor tissues and 61 adjacent tissues, were collected based on the
GPL6244 platform (HuGene-1_0-st) Affymetrix Human Gene 1.0 ST Array
[transcript (gene) version]. Profiles were analyzed using the GEO2R
online tool (http://www.ncbi.nlm.nih.gov/geo/geo2r/).
Overall survival (OS) analysis and
target gene prediction
OS analyses were evaluated using the online tool,
OncoLnc (23), which links TCGA
survival data to the expression of mRNA, miRNA or long non-coding
RNA (lncRNA). miRNAs or genes were identified if they were
significantly associated with OS. Target genes were predicted using
the online analysis tools, TargetScan (http://www.targetscan.org/) (24) and DIANA-mT (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=mrmicrot/index)
(25,26). To further enhance the accuracy of the
bioinformatics analysis, Venn diagrams were used to identify
overlapping target genes.
Functional enrichment analyses
KEGG pathway and GO enrichment analyses were
performed for the functional annotation and pathway analyses, to
identify the biological processes, cellular components, molecular
functions and biological pathways that the genes were significantly
enriched in. The results were visualized using ClueGO, which is one
of the apps in Cytoscape (27). A
gene count ≥3 was set as the cut-off criterion.
Gene expression comparison
The Human Protein Atlas (version 15.0; https://www.proteinatlas.org) (28), an open platform to provide large
amounts of transcriptomics and proteomics data of all 24,000 human
proteins, is composed of a Tissue Atlas, Cell Atlas, and Pathology
Atlas. Immunohistochemistry results (https://v15.proteinatlas.org/ENSG00000105810-CDK6/tissue/pancreas;
https://v15.proteinatlas.org/ENSG00000105810-CDK6/cancer/tissue/pancreatic+cancer;
https://v15.proteinatlas.org/ENSG00000171848-RRM2/tissue/pancreas;
and https://v15.proteinatlas.org/ENSG00000171848-RRM2/cancer/tissue/pancreatic+cancer)
were obtained from this database to make comparisons between
healthy pancreatic tissues and pancreatic tumor tissues.
Results
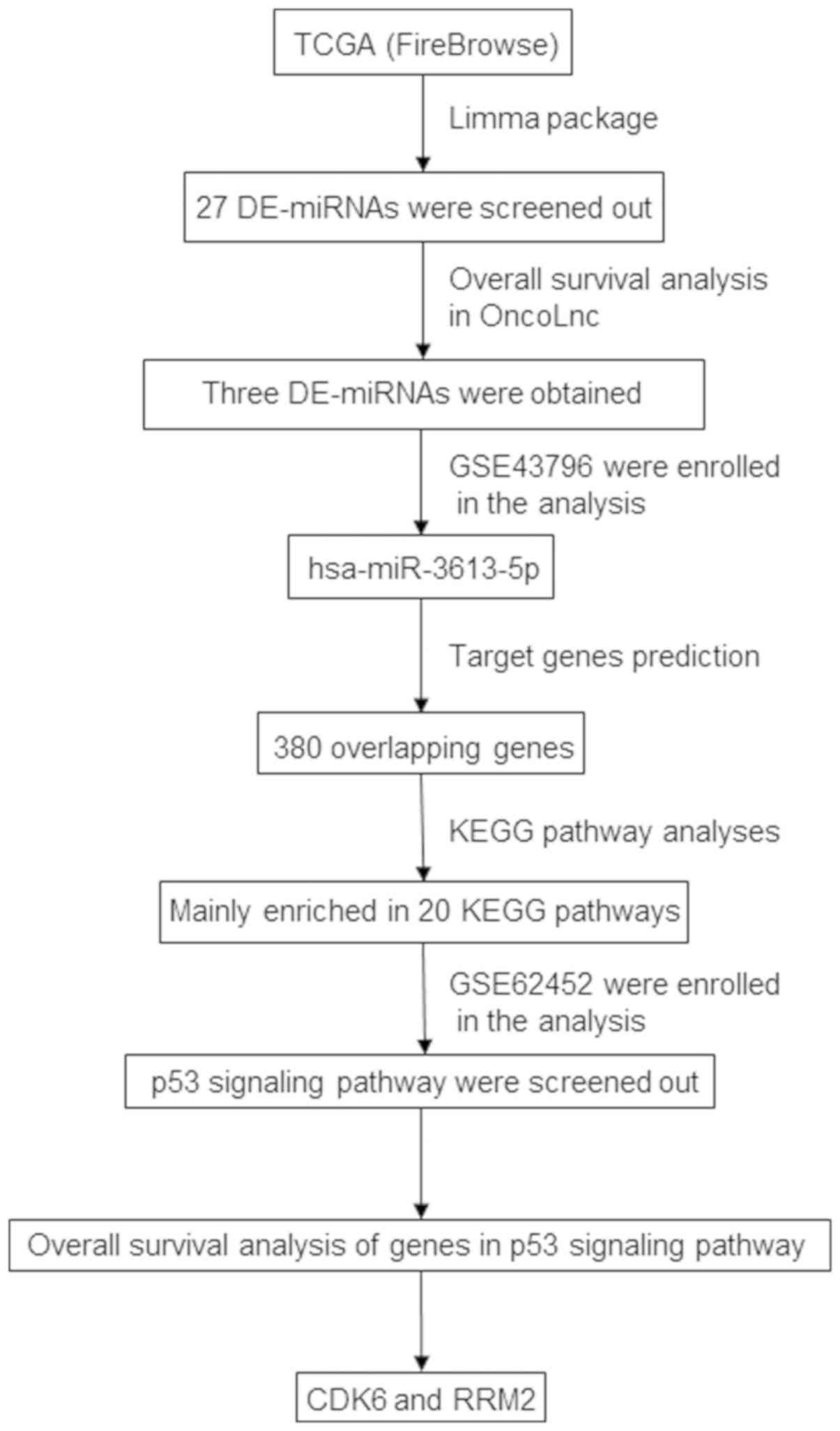
Overall presentation of the entire
analytical process
The Limma package in R language was used to analyze
the miRNA sequencing data from the TCGA database to identify the
DE-miRNAs. To increase the accuracy of the results, two microarrays
were downloaded from the GEO database during the analysis. These
results demonstrated that hsa-miR-3613-5p may be the most reliable
prognostic DE-miRNA. The target genes of hsa-miR-3613-5p were
predominantly enriched in 20 KEGG pathways, and the p53 signaling
pathway was the main focus in the final analysis. Furthermore,
survival analysis of genes in the p53 signaling pathway was also
performed, and only the genes for cyclin-dependent kinase 6 (CDK6)
and ribonucleoside-diphosphate reductase subunit M2 (RRM2)
exhibited prognostic value. A flow-chart of the entire analysis is
presented in Fig. 1.
Identification of DE-miRNAs in miRNA
sequencing data and OS analysis
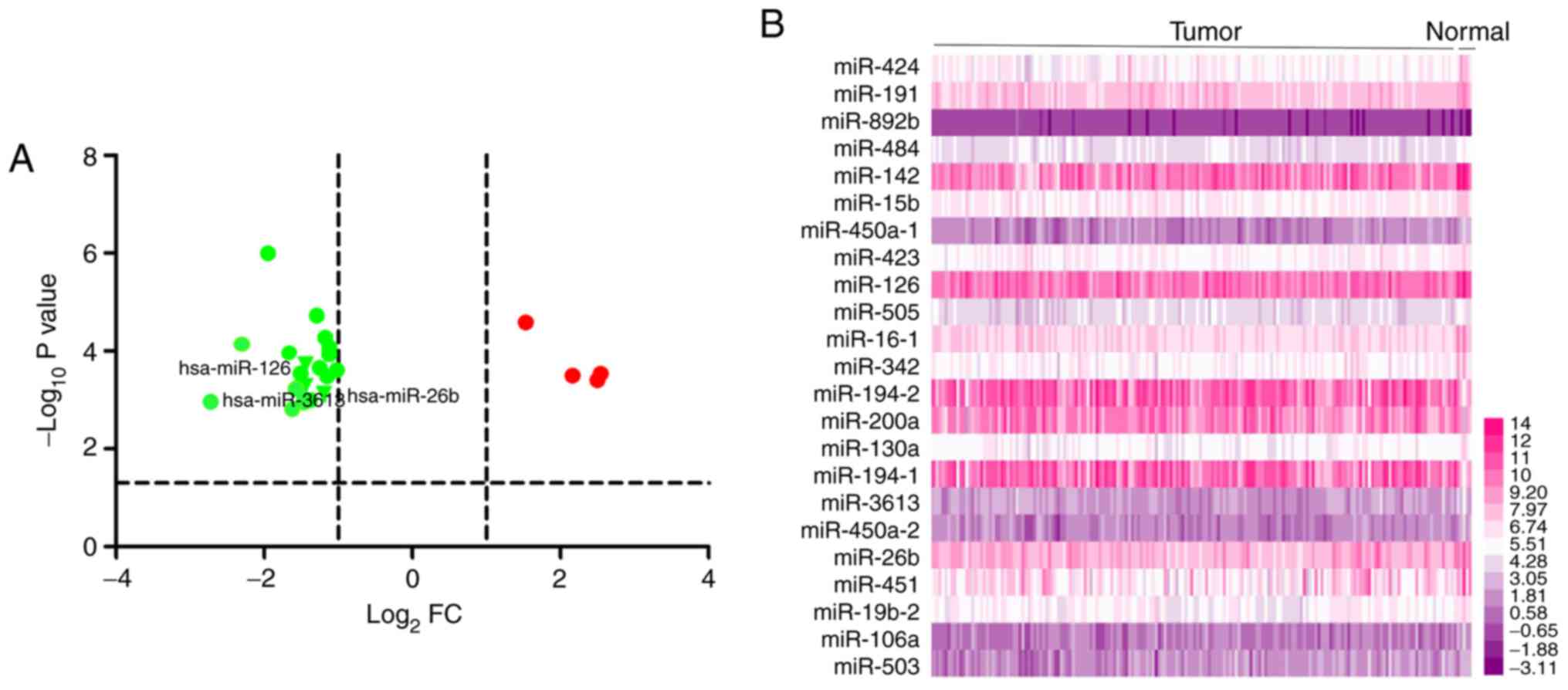
According to the criteria of |log2FC|>1.0 and
P<0.05, a total of 23 DE-miRNAs in miRNA sequencing data were
identified, including four upregulated (hsa-miR-892b,
hsa-miR-194-2, hsa-miR-200a and hsa-miR-194-1) and 19 downregulated
(hsa-miR-424, hsa-miR-191, hsa-miR-484, hsa-miR-142, hsa-miR-15b,
hsa-miR-450a-1, hsa-miR-423, hsa-miR-126, hsa-miR-505,
hsa-miR-16-1, hsa-miR-342, hsa-miR-130a, hsa-miR-3613,
hsa-miR-450a-2, hsa-miR-26b, hsa-miR-451, hsa-miR-19b-2,
hsa-miR-106a and hsa-miR-503) miRNAs (Table I). The results are also presented as
a volcano plot and heat-map (Fig.
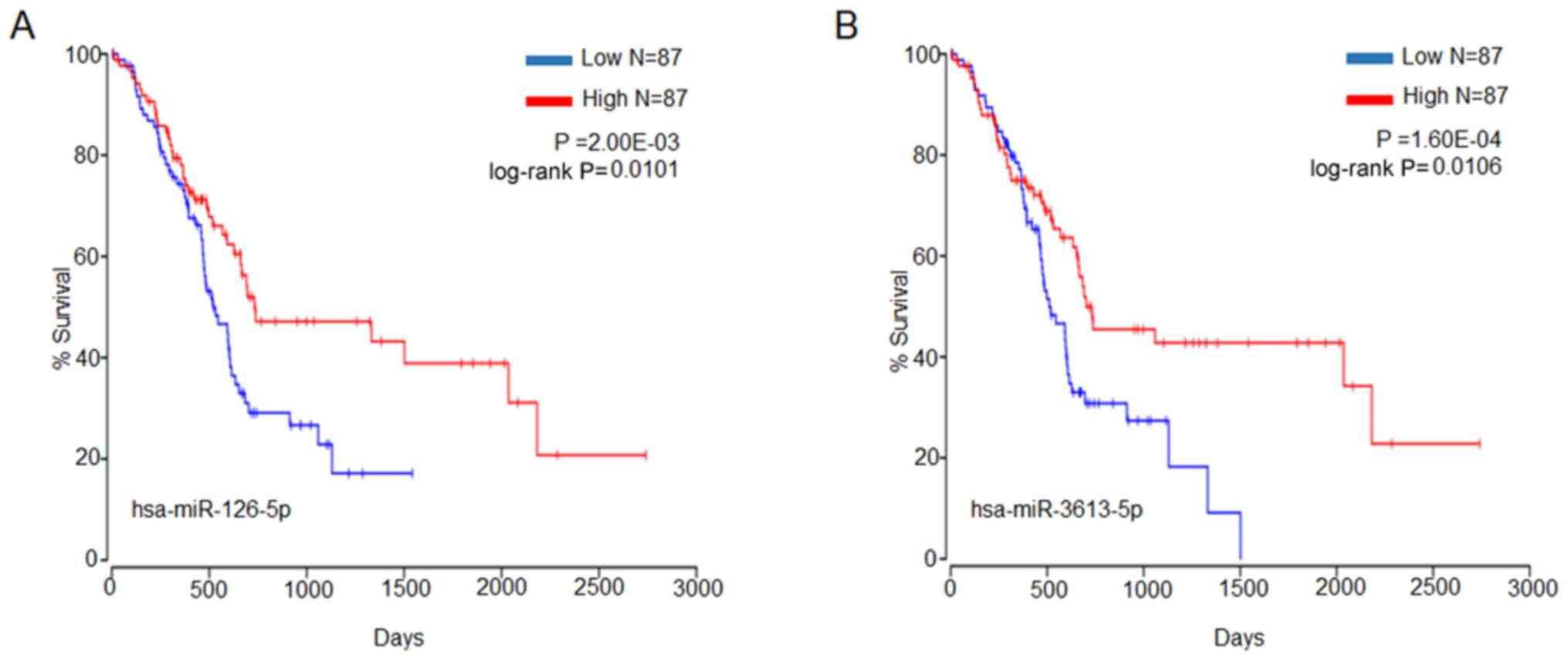
2). To identify the miRNAs that could potentially be associated
with OS of patients with pancreatic cancer, the online tool OncoLnc
was used to access TCGA survival data to evaluate the association
between miRNA expression and patient survival. Two miRNAs were
identified that may be associated with OS, with P-values and
log-rank values of P<0.05 (Fig.
3). These were hsa-miR-126-5p and hsa-miR-3613-5p, which are
highlighted in the volcano plot (Fig.
2).
 | Table IDifferentially expressed miRNAs in
TCGA. |
Table I
Differentially expressed miRNAs in
TCGA.
| A, Upregulated |
|---|
| miRNA_ID |
log2FC | P-value | adj.P.Val |
|---|
| hsa-miR-892b | 1.530606 |
2.62x10-5 |
5.48x10-3 |
| hsa-miR-194-2 | 2.544289 |
2.89x10-4 |
1.89x10-2 |
| hsa-miR-200a | 2.161983 |
3.18x10-4 |
1.92x10-2 |
| hsa-miR-194-1 | 2.497915 |
3.98x10-4 |
2.19x10-2 |
| B,
Downregulated |
| miRNA_ID |
log2FC | P-value | adj.P.Val |
| hsa-miR-424 | -1.94731 |
1.00x10-6 |
5.24x10-4 |
| hsa-miR-191 | -1.29315 |
1.88x10-5 |
4.91x10-3 |
| hsa-miR-484 | -1.17521 |
5.37x10-5 |
9.356x10-3 |
| hsa-miR-142 | -2.36313 |
6.35x10-5 |
9.49x10-3 |
| hsa-miR-15b | -1.12279 |
8.34x10-5 |
1.09x10-2 |
| hsa-miR-450a-1 | -1.66695 |
1.05x10-4 |
1.21x10-2 |
| hsa-miR-423 | -1.12274 |
1.15x10-4 |
1.21x10-2 |
| hsa-miR-126 | -1.44387 |
1.90x10-4 |
1.72x10-2 |
| hsa-miR-505 | -1.24908 |
2.19x10-4 |
1.76x10-2 |
| hsa-miR-16-1 | -1.01975 |
2.46x10-4 |
1.84x10-2 |
| hsa-miR-342 | -1.50755 |
2.86x10-4 |
1.89x10-2 |
| hsa-miR-130a | -1.1491 |
3.30x10-4 |
1.92x10-2 |
| hsa-miR-3613 | -1.44412 |
5.13x10-4 |
2.55x10-2 |
| hsa-miR-450a-2 | -1.55496 |
5.65x10-4 |
2.69x10-2 |
| hsa-miR-26b | -1.1955 |
7.51x10-4 |
3.41x10-2 |
| hsa-miR-451 | -2.71252 |
8.47x10-4 |
3.60x10-2 |
| hsa-miR-19b-2 | -1.38624 |
1.01x10-3 |
4.04x10-2 |
| hsa-miR-106a | -1.4397 |
1.07x10-3 |
4.15x10-2 |
| hsa-miR-503 | -1.6143 |
1.36x10-3 |
4.91x10-2 |
Target gene prediction
Subsequently, datasets associated with miRNA and
pancreatic cancer were searched in the GEO database, and the
following data were selected: i) >30 samples; ii) non-coding RNA
profiles; and iii) the period between January 1, 2013 and December
31, 2018. GSE43796 from the GEO database was accessed, and the
GEO2R online tool was used to obtain the DE-miRNAs. The top 250
miRNAs were thereby identified (Table
II), and the two above-mentioned prognostic miRNAs intersected,
leaving only hsa-miR-3613-5p. The association between hsa-miR-3613
and clinical features was evaluated in patients with breast cancer
(Table SI). The results indicated
that hsa-miR-3613 was significantly associated with clinical stage
(P=0.034) and histologic type (P=0.002). Target genes of
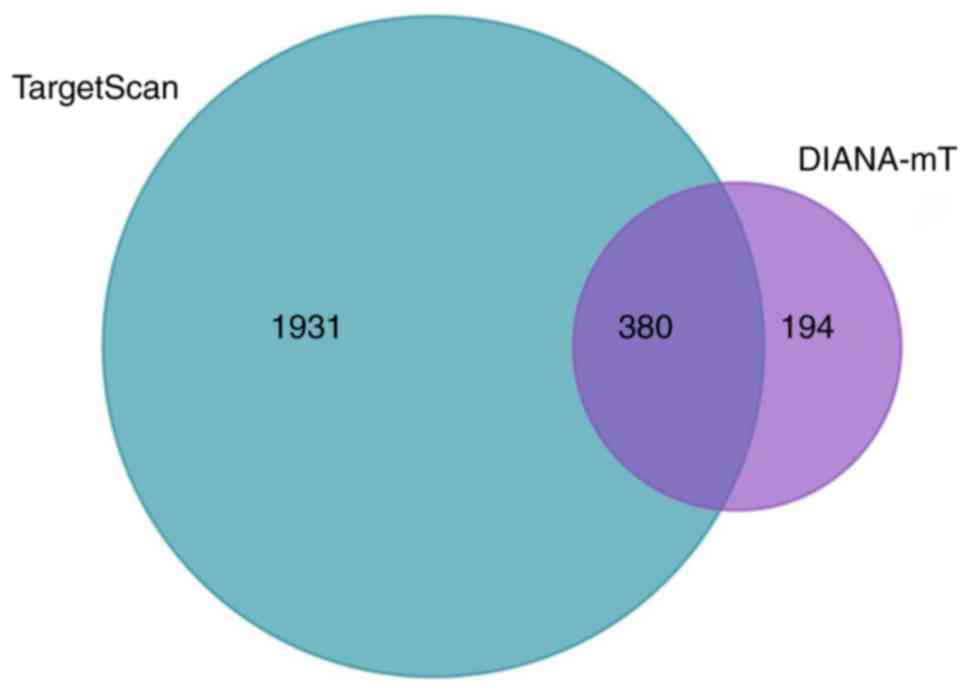
hsa-miR-3613-5p were predicted using the TargetScan and DIANA-MT
online analysis tools. As a result of this analysis, a total of 380
overlapping genes of hsa-miR-3613-5p were identified (Fig. 4).
 | Table IITop 250 miRNAs in the GSE43796
dataset. |
Table II
Top 250 miRNAs in the GSE43796
dataset.
| miRNA_ID |
log2FC | P-value | adj.P.Val |
|---|
| hsa-miR-216b | -4.8039 |
4.65x10-10 |
1.3x10-6 |
| hsa-miR-216a | -5.4037 |
8.97x10-10 |
1.3x10-6 |
| hsa-miR-216b | -3.716 |
1.10x10-9 |
1.3x10-6 |
| hsa-miR-216a | -4.8889 |
1.64x10-9 |
1.44x10-6 |
| hsa-miR-217 | -4.4452 |
2.34x10-9 |
1.44x10-6 |
| hsa-miR-217 | -3.8 |
2.46x10-9 |
1.44x10-6 |
| hsa-miR-216b | -3.169 |
3.30x10-9 |
1.47x10-6 |
| hsa-miR-216a | -4.0446 |
3.34x10-9 |
1.47x10-6 |
| hsa-miR-217 | -3.1523 |
1.26x10-8 |
4.94x10-6 |
| hsa-miR-216a | -1.6859 |
2.2x10-7 |
7.75x10-5 |
| hsa-miR-216b | -0.4224 |
8.8x10-7 | 0.000282 |
| hsa-miR-4269 | -1.3437 |
1.64x10-6 | 0.000483 |
| hsa-miR-217 | -1.2283 |
2.28x10-6 | 0.000618 |
| hsa-miR-4269 | -1.4259 |
2.49x10-6 | 0.000626 |
| kshv-miR-K12-8 | -1.568 |
3.17x10-6 | 0.000744 |
| hsa-miR-564 | -1.1939 |
4.87x10-6 | 0.000963 |
| hsa-miR-564 | -1.2361 |
5.06x10-6 | 0.000963 |
|
hsa-miR-3616-3p | -0.4127 |
5.14x10-6 | 0.000963 |
|
hsa-miR-130ba | -0.3289 |
5.46x10-6 | 0.000963 |
| hsa-miR-564 | -1.1964 |
5.72x10-6 | 0.000963 |
| kshv-miR-K12-8 | -1.4459 |
5.74x10-6 | 0.000963 |
| hsa-miR-1307 | 0.5635 |
6.74x10-6 | 0.001079 |
| hsa-miR-3916 | -0.1009 |
8.93x10-6 | 0.001368 |
| hsa-miR-320e | 0.8174 |
9.66x10-6 | 0.001419 |
| hsa-miR-944 | -0.1126 |
1.15x10-5 | 0.001617 |
| hsa-miR-106b | 0.9524 |
1.39x10-5 | 0.001887 |
|
hsa-miR-148aa | -0.4605 |
1.6x10-5 | 0.002029 |
| hsa-miR-130b | -1.9221 |
1.61x10-5 | 0.002029 |
|
hsa-miR-99ba | -0.1775 |
1.88x10-5 | 0.002286 |
| hsa-miR-498 | 0.4483 |
1.97x10-5 | 0.002315 |
|
hsa-miR-148aa | -1.2935 |
2.04x10-5 | 0.002316 |
| hsa-miR-671-3p | 0.1226 |
2.17x10-5 | 0.002391 |
| hsa-miR-3164 | -0.1302 |
2.84x10-5 | 0.002998 |
|
hsa-miR-548x_v16.0 | 0.1154 |
2.94x10-5 | 0.002998 |
| hsa-miR-106b | 1.1226 |
3.02x10-5 | 0.002998 |
| hsa-miR-320d | 0.7308 |
3.06x10-5 | 0.002998 |
| hsa-miR-4299 | -1.905 |
3.8x10-5 | 0.003576 |
| hsa-miR-548o | -0.118 |
3.86x10-5 | 0.003576 |
| hsa-miR-93 | 1.2234 |
4x10-5 | 0.003611 |
| hsa-miR-331-3p | 1.1039 |
4.39x10-5 | 0.003864 |
| hsa-miR-3164 | -0.0978 |
4.61x10-5 | 0.003957 |
| hsa-miR-130b | -1.6642 |
4.72x10-5 | 0.003958 |
|
hsa-miR-125a-5p | 1.7078 |
5.11x10-5 | 0.00419 |
|
hsa-miR-29aa | 0.9139 |
5.37x10-5 | 0.004299 |
| hsa-miR-93a | 0.2658 |
5.75x10-5 | 0.004505 |
| hsa-miR-299-3p | -0.7055 |
6.01x10-5 | 0.004604 |
| hsa-miR-4299 | -1.8101 |
6.2x10-5 | 0.004646 |
|
hsa-miR-195a | -0.1081 |
8.79x10-5 | 0.00645 |
| hsa-miR-210 | 2.4038 |
8.99x10-5 | 0.006465 |
| hsa-miR-151-3p | 0.6122 | 0.000104 | 0.007232 |
|
hsa-miR-3616-3p | -0.4232 | 0.000105 | 0.007232 |
| hsa-miR-498 | 0.2281 | 0.000109 | 0.007391 |
| hsa-miR-4307 | -0.1549 | 0.000113 | 0.007447 |
| hsa-miR-613 | -0.1262 | 0.000114 | 0.007447 |
|
hsa-miR-148ba | -0.1138 | 0.00013 | 0.008259 |
| hsa-miR-148a | -2.5367 | 0.000131 | 0.008259 |
|
hsa-miR-3145-3p | -0.0763 | 0.000145 | 0.008957 |
| hsa-miR-186 | 0.713 | 0.000164 | 0.009947 |
| hsa-miR-302b | -0.1691 | 0.000185 | 0.011055 |
| hsa-miR-942 | -0.1017 | 0.000189 | 0.011107 |
|
hsa-miR-99ba | -0.1313 | 0.000207 | 0.011853 |
| hsa-miR-548l | -0.0957 | 0.000212 | 0.011853 |
| hsa-miR-3681 | -0.0908 | 0.000212 | 0.011853 |
| hsa-miR-93a | 0.1248 | 0.00022 | 0.011853 |
| hsa-miR-361-5p | 0.9421 | 0.000225 | 0.011853 |
| hsa-miR-582-5p | 1.6334 | 0.000227 | 0.011853 |
|
hsa-miR-3613-5p | -0.1532 | 0.000231 | 0.011853 |
| hsa-miR-3132 | -1.3333 | 0.000231 | 0.011853 |
| hsa-miR-553 | -0.1416 | 0.000232 | 0.011853 |
| hsa-miR-302b | -0.1588 | 0.000239 | 0.011976 |
| hsa-miR-423-3p | 0.3666 | 0.000241 | 0.011976 |
|
hcmv-miR-US25-1a | -0.1146 | 0.000247 | 0.012062 |
| hsa-miR-548t | -0.1388 | 0.000262 | 0.012307 |
| hsa-miR-521 | -0.082 | 0.000262 | 0.012307 |
|
hsa-miR-3129-5p | -0.1044 | 0.000262 | 0.012307 |
| hsa-miR-99b | 1.5986 | 0.000267 | 0.012366 |
|
hsa-miR-105a | -0.1008 | 0.000271 | 0.012399 |
| hsa-miR-3179 | -0.0771 | 0.000283 | 0.0128 |
| hsa-miR-186 | 0.6795 | 0.000309 | 0.013787 |
| hsa-miR-1306 | 0.234 | 0.000317 | 0.01397 |
| hsa-miR-1289 | -0.0883 | 0.000328 | 0.014269 |
| hsa-miR-579 | -0.1067 | 0.00034 | 0.014628 |
| hsa-miR-301a | 1.8527 | 0.00036 | 0.015276 |
| hsa-miR-148a | -2.337 | 0.00037 | 0.015288 |
| hsa-miR-103a | 1.124 | 0.000371 | 0.015288 |
| ebv-miR-BART13 | -0.582 | 0.000373 | 0.015288 |
| hsa-miR-448 | -0.1176 | 0.000381 | 0.015434 |
| hsa-miR-299-5p | -1.0644 | 0.000401 | 0.015917 |
| hsa-miR-582-5p | 1.5887 | 0.000402 | 0.015917 |
| hsa-miR-634 | 0.1183 | 0.000431 | 0.016744 |
| hsa-miR-3175 | -0.054 | 0.000433 | 0.016744 |
|
hsa-miR-148aa | -0.1302 | 0.00044 | 0.016859 |
| hsa-miR-548m | -0.1191 | 0.000484 | 0.018165 |
| hsa-miR-3935 | 0.1933 | 0.000485 | 0.018165 |
| hsa-miR-591 | -0.0909 | 0.000497 | 0.018364 |
| hsa-miR-210 | 2.3178 | 0.0005 | 0.018364 |
| hsa-miR-556-3p | -0.1154 | 0.000527 | 0.019151 |
| hsa-miR-548j | -0.0797 | 0.000551 | 0.01949 |
| hsa-miR-103a | 1.0183 | 0.000553 | 0.01949 |
| hsa-miR-4304 | 0.1412 | 0.000553 | 0.01949 |
| hsa-miR-30c-2a | -0.4667 | 0.000577 | 0.020116 |
|
hsa-miR-2116a | 0.1207 | 0.000583 | 0.020146 |
| hsa-miR-361-3p | 0.7409 | 0.00061 | 0.020859 |
| hsa-miR-1537 | -0.1087 | 0.000616 | 0.020877 |
| hsa-miR-573 | -0.1069 | 0.00064 | 0.02121 |
| hsa-miR-3198 | -0.7624 | 0.000641 | 0.02121 |
| hsa-miR-1324 | -0.073 | 0.00065 | 0.02121 |
| hsa-miR-99b | 1.4301 | 0.000653 | 0.02121 |
| hsa-miR-107 | 1.058 | 0.000656 | 0.02121 |
| hsa-miR-587 | -0.0948 | 0.000663 | 0.021248 |
| hsa-miR-586 | -0.0974 | 0.000686 | 0.021727 |
| hsa-miR-325 | -0.1108 | 0.000691 | 0.021727 |
| hsa-miR-24 | 0.9059 | 0.000705 | 0.021812 |
| hsa-miR-128 | 1.1449 | 0.000706 | 0.021812 |
| hsa-miR-892a | -0.1117 | 0.000713 | 0.021833 |
| hsa-miR-573 | -0.109 | 0.000719 | 0.021833 |
| hsa-miR-1265 | -0.0783 | 0.000727 | 0.021835 |
|
hsa-miR-30c-1a | -0.3181 | 0.000731 | 0.021835 |
| hsa-miR-183 | 2.7044 | 0.000747 | 0.022129 |
| hsa-miR-301a | 1.8224 | 0.000771 | 0.022642 |
|
hsa-miR-193a-3p | -1.5641 | 0.000797 | 0.0232 |
|
hsa-miR-125a-5p | 1.3715 | 0.000809 | 0.023366 |
| hsa-miR-331-3p | 0.8881 | 0.000839 | 0.023999 |
| hsa-miR-3115 | -0.0798 | 0.000845 | 0.023999 |
|
hsa-let-7da | 0.3463 | 0.000856 | 0.024136 |
| hsa-miR-183 | 2.6632 | 0.000864 | 0.024164 |
|
hsa-miR-3180-5p | 0.0999 | 0.000887 | 0.024611 |
| hsa-miR-1539 | 0.0936 | 0.000898 | 0.024707 |
| hsa-miR-1306 | 0.1827 | 0.000945 | 0.025729 |
| hsa-miR-3142 | -0.0824 | 0.000949 | 0.025729 |
|
hsa-miR-548d-5p | -0.1196 | 0.000977 | 0.026272 |
| hsa-miR-183 | 2.5675 | 0.001 | 0.026774 |
| hsa-miR-582-3p | 0.5736 | 0.00102 | 0.027098 |
| hsa-miR-569 | -0.0898 | 0.00104 | 0.027306 |
| hsa-miR-411 | -0.813 | 0.00106 | 0.027402 |
| hsa-miR-3668 | -0.0768 | 0.00107 | 0.027402 |
| hsa-miR-320b | 0.7204 | 0.00107 | 0.027402 |
| hsa-miR-302f | -0.0959 | 0.00107 | 0.027402 |
|
hsa-miR-183a | 1.5187 | 0.00111 | 0.02783 |
| hsa-miR-93 | 0.9869 | 0.00112 | 0.02783 |
| hsa-miR-182 | 2.0297 | 0.00113 | 0.02783 |
| hsa-miR-4317 | 0.7724 | 0.00113 | 0.02783 |
| hsa-miR-544 | -0.1508 | 0.00114 | 0.02783 |
| hsa-miR-1179 | -0.074 | 0.00114 | 0.02783 |
| hsa-miR-1243 | -0.0774 | 0.00116 | 0.028001 |
|
hcmv-miR-UL36a | -0.0762 | 0.00116 | 0.028001 |
|
hsa-miR-30aa | -1.209 | 0.00117 | 0.028137 |
| hsa-miR-1179 | -0.073 | 0.00123 | 0.029223 |
|
hsa-miR-3140-3p | -0.1157 | 0.00124 | 0.029223 |
| hsa-miR-1286 | 0.0726 | 0.00124 | 0.029223 |
| hsa-miR-563 | -0.1156 | 0.00125 | 0.029223 |
| hsa-miR-30e | -0.7409 | 0.00127 | 0.029223 |
| ebv-miR-BART8 | -0.0971 | 0.00127 | 0.029223 |
| hsa-miR-4307 | -0.1001 | 0.00128 | 0.029223 |
|
hsa-miR-3158-3p | -0.054 | 0.00141 | 0.031927 |
| hsa-miR-520h | -0.1088 | 0.00142 | 0.031927 |
| hsa-miR-619 | -0.0715 | 0.00142 | 0.031927 |
|
hsa-miR-30aa | -0.9917 | 0.00144 | 0.032039 |
|
hsa-miR-138-2a | 0.1095 | 0.00148 | 0.032687 |
| hsa-miR-182 | 2.0004 | 0.00152 | 0.03353 |
| hsa-miR-484 | 0.9217 | 0.00154 | 0.033733 |
| hsa-miR-425 | 0.8842 | 0.00157 | 0.03375 |
| hsa-miR-4310 | 0.1418 | 0.00158 | 0.03375 |
| hsa-miR-302c | -0.106 | 0.00158 | 0.03375 |
|
hsa-miR-15ba | -0.1306 | 0.00159 | 0.03375 |
| hsa-miR-302f | -0.0859 | 0.0016 | 0.03375 |
| hsa-miR-2053 | -0.1115 | 0.0016 | 0.03375 |
| hsa-miR-4291 | 0.8359 | 0.00167 | 0.035042 |
| hsa-miR-421 | 0.5925 | 0.00169 | 0.035259 |
| hsa-miR-381 | -1.9681 | 0.00171 | 0.035396 |
|
hsa-miR-3647-3p | -0.1454 | 0.00173 | 0.035724 |
| hsa-miR-328 | 0.803 | 0.00178 | 0.036488 |
| hsa-miR-3620 | 0.1262 | 0.00185 | 0.037581 |
| hsa-miR-3927 | -0.0686 | 0.0019 | 0.03843 |
|
hsa-miR-92a-2a | -0.0791 | 0.00192 | 0.038565 |
| hsa-miR-4317 | 0.3214 | 0.00193 | 0.038645 |
| hsa-miR-149 | 1.1225 | 0.00196 | 0.038946 |
|
hsa-miR-183a | 1.0302 | 0.00197 | 0.038946 |
| hsa-miR-484 | 1.0961 | 0.00206 | 0.040621 |
| hsa-miR-3198 | -0.6895 | 0.00215 | 0.041996 |
| hsa-miR-190b | -0.1413 | 0.00216 | 0.041996 |
| hsa-miR-553 | -0.086 | 0.0022 | 0.0425 |
| hsa-miR-324-5p | 1.5161 | 0.00222 | 0.042666 |
| hsa-miR-182 | 2.3243 | 0.00223 | 0.042666 |
|
hsa-miR-624a | 0.0748 | 0.00227 | 0.04329 |
| hsa-let-7e | 1.2111 | 0.00233 | 0.044225 |
| hsa-miR-30a | -1.6194 | 0.00241 | 0.045407 |
| hsa-miR-181b | 1.2205 | 0.00244 | 0.045689 |
| hsa-miR-1294 | -0.0642 | 0.00248 | 0.046233 |
| hsa-miR-320a | 0.4892 | 0.00251 | 0.046444 |
| hsa-miR-491-5p | 0.5918 | 0.00252 | 0.046444 |
| hsa-miR-4293 | 0.081 | 0.00253 | 0.046485 |
| hsa-miR-345 | 0.2807 | 0.00259 | 0.047307 |
| hsa-miR-563 | 0.0935 | 0.00268 | 0.048439 |
|
hsa-miR-219-1-3p | -0.0921 | 0.00268 | 0.048439 |
| hsa-miR-3924 | -0.0974 | 0.0027 | 0.048517 |
|
hsa-miR-367a | -0.0887 | 0.0028 | 0.050028 |
|
ebv-miR-BART10a | -0.1116 | 0.00282 | 0.050028 |
| hsa-miR-1284 | -0.0713 | 0.00283 | 0.050028 |
| hsa-miR-1291 | 0.1029 | 0.00286 | 0.05032 |
| hsa-miR-608 | -0.057 | 0.00298 | 0.052183 |
| hsa-miR-425 | 0.8292 | 0.003 | 0.052407 |
| hsa-miR-4291 | 0.9583 | 0.00315 | 0.054597 |
|
hsa-miR-19b-2a | -0.1085 | 0.00316 | 0.054597 |
|
hsa-miR-19b-1a | 0.1263 | 0.00319 | 0.054855 |
| hsa-miR-548u | -0.0593 | 0.00328 | 0.056055 |
| hsv2-miR-H22 | -1.2156 | 0.0033 | 0.056055 |
| hsa-miR-374c | 0.1584 | 0.00331 | 0.056055 |
| hsa-miR-219-5p | -0.5987 | 0.00333 | 0.056055 |
|
hsa-miR-29ca | 1.0172 | 0.00338 | 0.056676 |
|
hsa-miR-493a | -0.7182 | 0.00339 | 0.056676 |
|
hsa-miR-302aa | 0.0797 | 0.0035 | 0.057987 |
|
hsa-miR-130ba | -0.097 | 0.00351 | 0.057987 |
| hsa-miR-651 | -0.079 | 0.00352 | 0.057987 |
|
hiv1-miR-TAR-3p | 0.361 | 0.00357 | 0.058286 |
| hsa-miR-24 | 0.6936 | 0.00357 | 0.058286 |
|
hsa-let-7da | 0.2023 | 0.00363 | 0.058837 |
| hsa-miR-140-5p | 1.0041 | 0.00364 | 0.058837 |
|
hsa-miR-548b-3p | -0.0828 | 0.00368 | 0.059172 |
| hsa-miR-3154 | 0.31 | 0.00371 | 0.059172 |
| hsa-miR-324-5p | 1.3843 | 0.00372 | 0.059172 |
| hsa-miR-33a | 0.5461 | 0.00374 | 0.059172 |
| hsa-miR-154 | -1.5917 | 0.00375 | 0.059172 |
| hsa-miR-107 | 0.966 | 0.00381 | 0.059939 |
| hsv2-miR-H22 | -1.1749 | 0.00393 | 0.061263 |
| hsa-miR-3649 | -0.068 | 0.00395 | 0.061263 |
|
hsa-miR-182a | 0.8636 | 0.00396 | 0.061263 |
| hsa-miR-501-3p | 0.3569 | 0.00396 | 0.061263 |
| hsa-miR-449b | -0.0656 | 0.0041 | 0.062902 |
|
hsa-miR-3942-5p | -0.0792 | 0.00412 | 0.062902 |
| hsa-let-7e | 1.2761 | 0.00412 | 0.062902 |
|
hcmv-miR-UL22Aa | -0.0787 | 0.00421 | 0.063705 |
| hsa-miR-3684 | -0.0467 | 0.00426 | 0.063705 |
| hsa-miR-585 | -0.0906 | 0.00427 | 0.063705 |
| hsa-miR-3606 | -0.0616 | 0.00427 | 0.063705 |
| hsa-miR-191 | 0.2472 | 0.00428 | 0.063705 |
|
hsa-miR-18ba | -0.0939 | 0.0043 | 0.063705 |
| hsa-miR-4309 | -0.056 | 0.00432 | 0.063705 |
| hcmv-miR-UL22A | 0.0758 | 0.00432 | 0.063705 |
| hsa-miR-320c | 0.6075 | 0.00444 | 0.06516 |
| hsa-miR-942 | -0.1076 | 0.00455 | 0.066499 |
| hsa-miR-3190 | 0.1172 | 0.00458 | 0.066689 |
|
hsa-miR-374aa | -0.0927 | 0.00461 | 0.066754 |
|
hsa-miR-3675-3p | 0.0965 | 0.00462 | 0.066754 |
| hsa-miR-374c | 0.6226 | 0.00467 | 0.067088 |
|
hsa-miR-3152-3p | -0.0574 | 0.00474 | 0.067654 |
|
hsa-miR-3189-3p | 0.1598 | 0.00474 | 0.067654 |
| hsa-miR-29a | 0.6544 | 0.00482 | 0.067818 |
| hsa-miR-892a | -0.0808 | 0.00482 | 0.067818 |
|
hsa-miR-2355-5p | 0.358 | 0.00483 | 0.067818 |
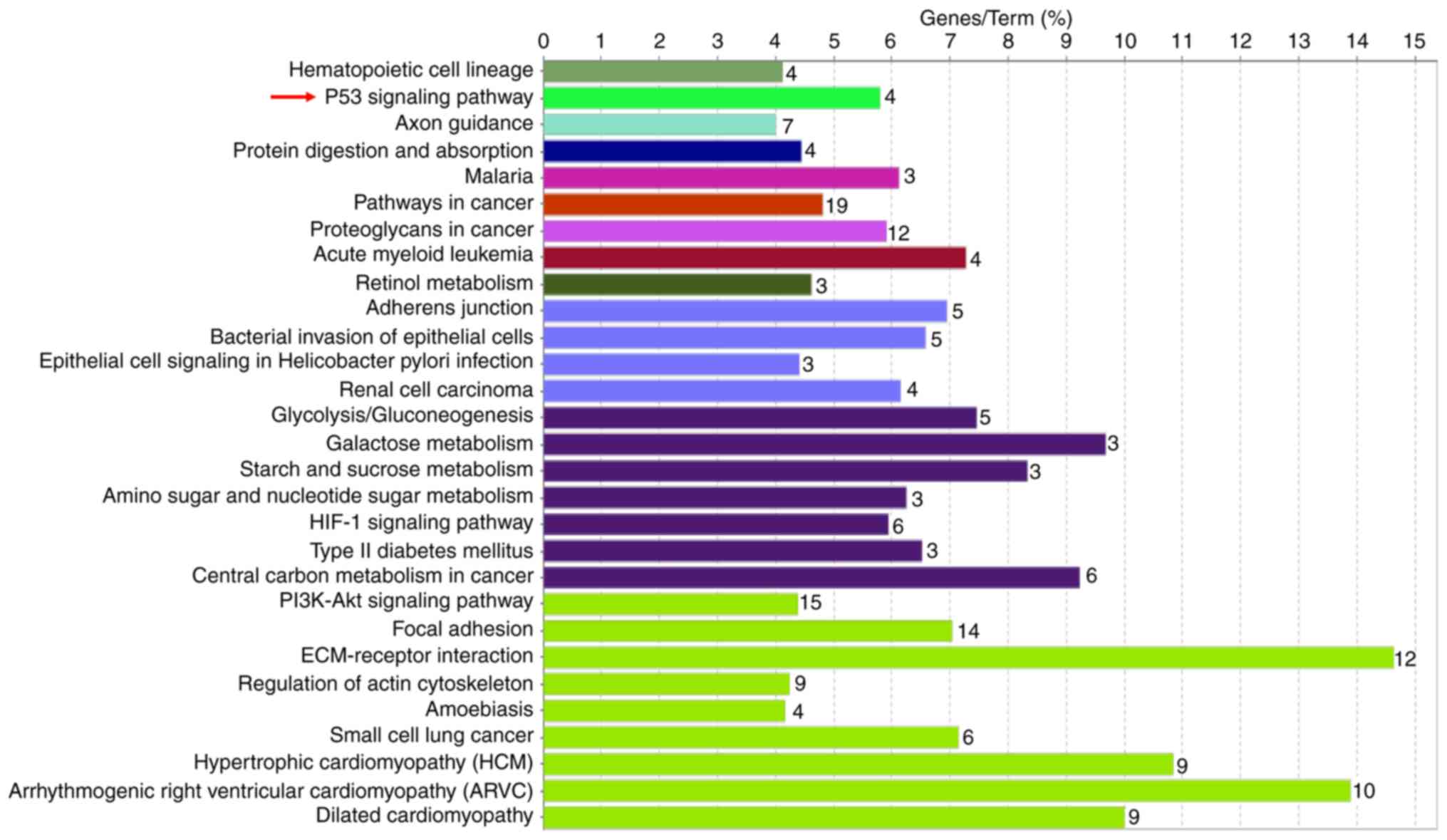
KEGG pathway and GO enrichment
analyses of target genes
To elucidate the biological function of overlapping
genes, enrichment analyses were performed using ClueGO. The results
obtained indicated that genes were predominantly enriched in 20
KEGG pathways, including the tissue growth factor-β, p53 and glioma
signaling pathways (Fig. 5A). The GO
biological process terms were predominantly enriched in ‘central
nervous system development’ (Fig.
5B). To increase the accuracy, one further microarray
(GSE62452) from the GEO database was also accessed. The following
screens were selected: i) >30 samples; ii) expression profiles;
and iii) the period between January 1, 2013 and December 31, 2018.
The GEO2R online tool was applied to explore the differentially
expressed genes, and functional analyses of the top 250 genes
(Table SII) were performed. The p53
signaling pathway was also identified in the KEGG pathway (Fig. 6).
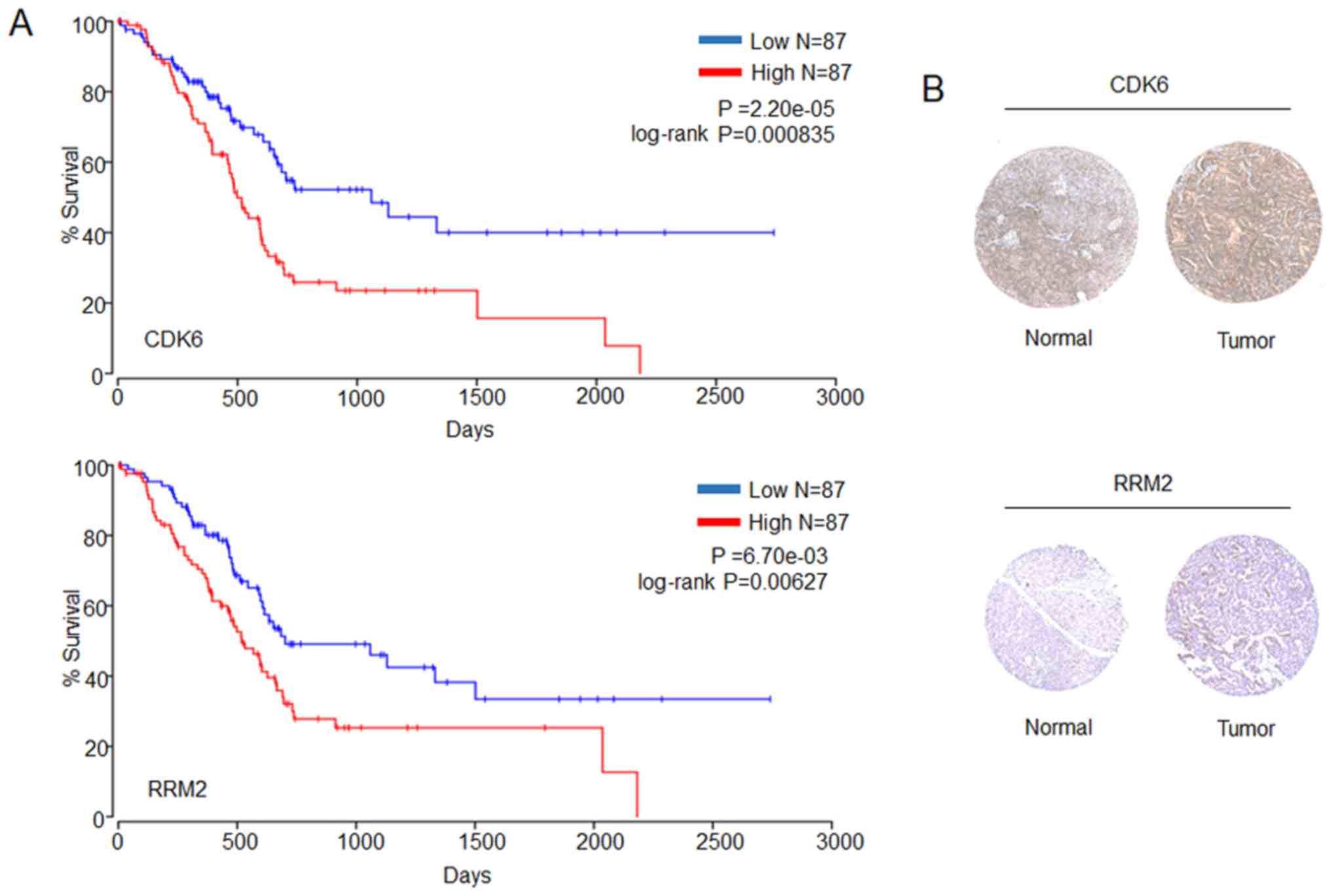
Survival analysis and prognostic gene
expression of the p53 signaling pathway
An OS analysis of the genes in the p53 signaling
pathway was performed. The results obtained indicated that only the
CDK6 and RRM2 genes were associated with OS (Fig. 7A). Subsequently, the Human Protein
Atlas was queried to obtain the immunohistochemistry results of
CDK6 and RRM2 gene expression in healthy and cancer tissues
(Fig. 7B). All the results obtained
were in agreement that the two genes are highly expressed in
tumors, with poor prognosis.
Discussion
Pancreatic cancer is an aggressively malignant
digestive tract tumor with a poor prognosis (29-31).
The high mortality rate is due to the lack of early detection
methods, and an inability to successfully treat patients once they
are diagnosed (32). Understanding
the molecular mechanism of pancreatic cancer progression is of
critical importance to improve the diagnosis and treatment of this
disease (8,9,33).
Currently, microarrays and high-throughput sequencing analyses have
provided us with the expression of thousands of genes in the human
genome, and numerous studies have already enabled certain
predictions to be made using these data (34-36).
The aim is to identify novel targets through such techniques, in
order to guide the treatment of pancreatic cancer and improve the
efficiency of early diagnosis.
The advent of miRNA research has opened up novel
avenues for understanding the post-transcriptional level of gene
regulation. The discovery of dysregulated miRNAs in a variety of
cancer types has led numerous researchers to study the use of
miRNAs as potential biomarkers for early detection, as well as
therapeutic agents for the treatment of cancer (11-12). miRNAs are short
(19-24 nucleotides) non-coding RNA molecules, which have emerged as
promising prognostic, diagnostic and therapeutic tools that may be
targeted in the fight against pancreatic cancer (37-40).
In the present study, the miRNA sequencing profiles
of 185 pancreatic tumor samples from the TCGA database have been
presented. GEO datasets were obtained through a reading of the
literature to explore which one would be of the greatest benefit
for analysis. The most recent datasets were selected and contained
>30 samples. In the majority of recently published studies, the
TCGA and GEO databases have usually been selected to expand the
sample range at the beginning of the process of screening data, and
other data to accurately quantify the results in subsequent
analyses have not subsequently been introduced. However, the
present study factored in a novel approach: The continuous
introduction of GEO datasets to narrow the scope of the filter, in
an attempt to render the results more accurate.
By retrieving literature in PubMed, a total of 14
articles were identified that related of hsa-miR-3613-5p, 6 of
which exhibited meaningful results. hsa-miR-3613 was a newly
identified miRNA, which has been found to be related to gastric
cancer (41). Chen et al
(42) demonstrated that hsa-miR-3613
is upregulated in drug-resistant breast cancer cell lines through
Affymetrix GeneChip miRNA 4.0 array. However, the experiments
suggested that it was not able to serve as a potential source for
biomarker detection or as an optimal chemotherapeutic choice for
patients with breast cancer. Hsa-miR-3613 was revealed in a study
by Chong et al (43) to be
one of the most markedly downregulated miRNAs in recurrent
epithelial ovarian cancer (EOC), and may be regarded as a biomarker
for the prediction of recurrence in EOC. Castro-Magdonel et
al (44) demonstrated that the
presence of hsa-miR-3613 is critical for tumor suppression in
retinoblastoma. In addition, a further study in the published
literature has revealed that hsa-miR-3613 may be associated with
leukemia/small lymphocytic lymphoma (45). Hsa-miR-3613 was also demonstrated to
be highly expressed in gluteofemoral compared with subcutaneous
adipocytes (46). To the best of our
knowledge, however, no articles have been published that have
explored the association between hsa-miR-3613 and pancreatic
cancer. Therefore, the present study aimed to investigate this
association via high-throughput sequencing analyses.
In addition, genes located downstream of
hsa-miR-3613 were also analyzed. The two most recently updated
target prediction databases were selected to predict the target(s)
of hsa-miR-3613, and KEGG and GO analyses of the target genes were
performed to probe for points of intersection.
Previously published studies have revealed the p53
signaling pathway is an important pathway that is closely
associated with cancer development (47-50),
and mutations in tumor protein 53 (TP53) elicited changes in the
expression of numerous other genes (51-53).
Two reviews related to pancreatic cancer attracted attention.
Makohon-Moore and Iacobuzio-Donahue (54) reported that genomic features are
closely associated with the pathogenesis of pancreatic cancer, and
TP53 undergoes somatic mutations in up to 85% of pancreatic
cancers. Another review, written by Rachagani et al
(37), summarized the relationship
between miRNAs and tumor suppressor genes (including p53, p16 and
SMAD4) in the pathogenesis, diagnosis and therapy of pancreatic
cancer. This study concluded that p53 not only has the potential to
regulate miRNA expression, but in turn miRNA can also regulate p53
expression. Additionally, the prognostic value of genes in the p53
signaling pathway were evaluated during the current analysis,
revealing that the genes for CDK6 and RRM2 are closely associated
with prognostic value.
Taken together, through comprehensive bioinformatics
analysis, the results presented in the current study identified a
prognostic miRNA involved in the progression of pancreatic cancer.
These findings may lead to the identification of a number of key
miRNAs, genes and pathways for future investigation into the
mechanisms and biomarkers of pancreatic cancer. However, the
present study did have certain limitations. The main limitation of
the present study was due to the retrospective nature of the
analysis. At the same time, the small sample size reduced the
statistical power, and the limited number of samples expressing
hsa-miR-3613 should also be considered as a limiting factor in data
analysis. In addition, the data presented in the current study
could not fully explain why hsa-miR-3613 was expressed at a high
level in pancreatic cancer and resulted in an improved overall
prognosis. Further studies using cancer cell lines and animal
models should be undertaken in the future to gain additional
mechanistic insight into the implications of hsa-miR-3613
expression in disease progression.
Supplementary Material
Association of hsa-miR-3613 with
clinical features.
Top 250 genes in GSE62452.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the
National Natural Science Foundation of China (grant no.
81872156).
Availability of data and materials
The miRNA sequencing data of pancreatic cancer are
available in FireBrowse (version 0.4.13; http://firebrowse.org/). The datasets of miRNA
expression profiles for pancreatic cancer (GSE43796) and gene
expression profiles for pancreatic cancer (GSE62452) are available
in the GEO on the NCBI website (http://www.ncbi.nlm.nih.gov/geo).
Authors' contributions
JM and SS carried out the design of this study,
performed the statistical analysis and drafted the manuscript. CS,
NiL, NaL and LX promoted the development of this project, and
interpreted the data regarding the functional and pathway
enrichment and PPI network construction. TY and YL helped to edit
the manuscript, and downloaded datasets from the GEO and
interpreted the primary data regarding pancreatic cancer. ML
participated in the study design and coordination and also helped
to edit the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shimura T, Shibata M, Gonda K, Kofunato Y,
Okada R, Ishigame T, Kimura T, Kenjo A, Kono K and Marubashi S:
Significance of circulating galectin-3 in patients with
pancreatobiliary cancer. Anticancer Res. 37:4979–4986.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ohya A, Yamanoi K, Shimojo H, Fujii C and
Nakayama J: Gastric gland mucin-specific o-glycan expression
decreases with tumor progression from precursor lesions to
pancreatic cancer. Cancer Sci. 108:1897–1902. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Park J, Choi Y, Namkung J, Yi SG, Kim H,
Yu J, Kim Y, Kwon MS, Kwon W, Oh DY, et al: Diagnostic performance
enhancement of pancreatic cancer using proteomic multimarker panel.
Oncotarget. 8:93117–93130. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kwon HM, Kang EJ, Kang K, Kim SD, Yang K
and Yi JM: Combinatorial effects of an epigenetic inhibitor and
ionizing radiation contribute to targeted elimination of pancreatic
cancer stem cell. Oncotarget. 8:89005–89020. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Han T, Zhuo M, Hu H, Jiao F and Wang LW:
Synergistic effects of the combination of 5-Aza-CdR and
suberoylanilide hydroxamic acid on the anticancer property of
pancreatic cancer. Oncol Rep. 39:264–270. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brooks J, Fleischmann-Mundt B, Woller N,
Niemann J, Ribback S, Peters K, Demir IE, Armbrecht N, Ceyhan GO,
Manns MP, et al: Perioperative, spatiotemporally coordinated
activation of T and NK cells prevents recurrence of pancreatic
cancer. Cancer Res. 78:475–488. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mirkin KA, Hollenbeak CS and Wong J:
Greater lymph node retrieval and lymph node ratio impacts survival
in resected pancreatic cancer. J Surg Res. 220:12–24.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Khan MA, Azim S, Zubair H, Bhardwaj A,
Patel GK, Khushman M, Singh S and Singh AP: Molecular drivers of
pancreatic cancer pathogenesis: Looking inward to move forward. Int
J Mol Sci. 18(E779)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Du YX, Liu ZW, You L, Wu WM and Zhao YP:
Advances in understanding the molecular mechanism of pancreatic
cancer metastasis. Hepatobiliary Pancreat Dis Int. 15:361–370.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen L and Kang C: miRNA interventions
serve as ‘magic bullets’ in the reversal of glioblastoma hallmarks.
Oncotarget. 6:38628–38642. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu W, Ma R and Yuan Y:
Post-transcriptional regulation of genes related to biological
behaviors of gastric cancer by long noncoding RNAs and microRNAs. J
Cancer. 8:4141–4154. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shirafkan N, Mansoori B, Mohammadi A,
Shomali N, Ghasbi M and Baradaran B: MicroRNAs as novel biomarkers
for colorectal cancer: New outlooks. Biomed Pharmacother.
97:1319–1330. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kim VN, Han J and Siomi MC: Biogenesis of
small RNAs in animals. Nat Rev Mol Cell Biol. 10:126–139.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Huang L, Cai JL, Huang PZ, Kang L, Huang
MJ, Wang L and Wang JP: miR19b-3p promotes the growth and
metastasis of colorectal cancer via directly targeting ITGB8. Am J
Cancer Res. 7:1996–2008. 2017.PubMed/NCBI
|
|
15
|
Long M, Zhan M, Xu S, Yang R, Chen W,
Zhang S, Shi Y, He Q, Mohan M, Liu Q and Wang J: miR-92b-3p acts as
a tumor suppressor by targeting Gabra3 in pancreatic cancer. Mol
Cancer. 16(167)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bayraktar R, Van Roosbroeck K and Calin
GA: Cell-to-cell communication: Micrornas as hormones. Mol Oncol.
11:1673–1686. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Fujiwara T, Uotani K, Yoshida A, Morita T,
Nezu Y, Kobayashi E, Yoshida A, Uehara T, Omori T, Sugiu K, et al:
Clinical significance of circulating miR-25-3p as a novel
diagnostic and prognostic biomarker in osteosarcoma. Oncotarget.
8:33375–33392. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu Y, Zuo J, Tan Q, Zar Thin K, Li P, Zhu
M, Yu M, Fu Z, Liang C and Tu J: Plasma miR-92a-2 as a biomarker
for small cell lung cancer. Cancer Biomark. 18:319–327.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Schwarzenbach H: Clinical relevance of
circulating, cell-free and exosomal microRNAs in plasma and serum
of breast cancer patients. Oncol Res Treat. 40:423–429.
2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Deng W, Wang Y, Liu Z, Cheng H and Xue Y:
HemI: A toolkit for illustrating heatmaps. PLoS One.
9(e111988)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang S, He P, Wang J, Schetter A, Tang W,
Funamizu N, Yanaga K, Uwagawa T, Satoskar AR, Gaedcke J, et al: A
novel MIF signaling pathway drives the malignant character of
pancreatic cancer by targeting NR3C2. Cancer Res. 76:3838–3850.
2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Park M, Kim M, Hwang D, Park M, Kim WK,
Kim SK, Shin J, Park ES, Kang CM, Paik YK and Kim H:
Characterization of gene expression and activated signaling
pathways in solid-pseudopapillary neoplasm of pancreas. Mod Pathol.
27:580–593. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Anaya J: OncoLnc: Linking TCGA survival
data to mRNAs, miRNAs, and lncRNAs. PeerJ Computer Science.
2(e67)2016.
|
|
24
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4(7554)2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Paraskevopoulou MD, Georgakilas G,
Kostoulas N, Vlachos IS, Vergoulis T, Reczko M, Filippidis C,
Dalamagas T and Hatzigeorgiou AG: DIANA-microT web server v5.0:
Service integration into miRNA functional analysis workflows.
Nucleic Acids Res. 41:W169–W173. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Reczko M, Maragkakis M, Alexiou P, Grosse
I and Hatzigeorgiou AG: Functional microRNA targets in protein
coding sequences. Bioinformatics. 28:771–776. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shannon P, Ozier O, Baliga NS, Wang JT,
Ramage D, Amin N, Schwikowski B, Ideker T and Markiel A: Cytoscape:
A software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pontén F, Jirström K and Uhlen M: The
human protein atlas-a tool for pathology. J Pathol. 216:387–393.
2008.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Karmakar S, Kaushik G, Nimmakayala R,
Rachagani S, Ponnusamy MP and Batra SK: MicroRNA regulation of
K-ras in pancreatic cancer and opportunities for therapeutic
intervention. Semin Cancer Biol. 54:63–71. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lu Z, Lai ZQ, Leung AWN, Leung PS, Li ZS
and Lin ZX: Exploring brusatol as a new anti-pancreatic cancer
adjuvant: Biological evaluation and mechanistic studies.
Oncotarget. 8:84974–84985. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Choi M, Bien H, Mofunanya A and Powers S:
Challenges in ras therapeutics in pancreatic cancer. Semin Cancer
Biol. 54:101–108. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Halbrook CJ and Lyssiotis CA: Employing
metabolism to improve the diagnosis and treatment of pancreatic
cancer. Cancer Cell. 31:5–19. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang L and Xie K: Nitric oxide and
pancreatic cancer pathogenesis, prevention, and treatment. Curr
Pharm Des. 16:421–427. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen WJ, Tang RX, He RQ, Li DY, Liang L,
Zeng JH, Hu XH, Ma J, Li SK and Chen G: Clinical roles of the
aberrantly expressed lncRNAs in lung squamous cell carcinoma: A
study based on RNA-sequencing and microarray data mining.
Oncotarget. 8:61282–61304. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen Y, Teng L, Liu W, Cao Y, Ding D, Wang
W, Chen H, Li C and An R: Identification of biological targets of
therapeutic intervention for clear cell renal cell carcinoma based
on bioinformatics approach. Cancer Cell Int. 16(16)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liang B, Li Y and Wang T: A three miRNAs
signature predicts survival in cervical cancer using bioinformatics
analysis. Sci Rep. 7(5624)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rachagani S, Macha MA, Heimann N,
Seshacharyulu P, Haridas D, Chugh S and Batra SK: Clinical
implications of miRNAs in the pathogenesis, diagnosis and therapy
of pancreatic cancer. Adv Drug Delivy Rev. 81:16–33.
2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chitkara D, Mittal A and Mahato RI: miRNAs
in pancreatic cancer: Therapeutic potential, delivery challenges
and strategies. Adv Drug Deliv Rev. 81:34–52. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li L, Li Z, Kong X, Xie D, Jia Z, Jiang W,
Cui J, Du Y, Wei D, Huang S and Xie K: Down-regulation of
microRNA-494 via loss of SMAD4 increases FOXM1 and β-catenin
signaling in pancreatic ductal adenocarcinoma cells.
Gastroenterology. 147:485–497. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Schultz NA, Dehlendorff C, Jensen BV,
Bjerregaard JK, Nielsen KR, Bojesen SE, Calatayud D, Nielsen SE,
Yilmaz M, Holländer NH, et al: MicroRNA biomarkers in whole blood
for detection of pancreatic cancer. JAMA. 311:392–404.
2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bibi F, Naseer MI, Alvi SA, Yasir M,
Jiman-Fatani AA, Sawan A, Abuzenadah AM, Al-Qahtani MH and Azhar
EI: MicroRNA analysis of gastric cancer patients from Saudi Arabian
population. BMC Genomics. 17(751)2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen X, Lu P, Wang DD, Yang SJ, Wu Y, Shen
HY, Zhong SL, Zhao JH and Tang JH: The role of miRNAs in drug
resistance and prognosis of breast cancer formalin-fixed
paraffin-embedded tissues. Gene. 595:221–226. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chong GO, Jeon HS, Han HS, Son JW, Lee YH,
Hong DG, Lee YS and Cho YL: Differential microrna expression
profiles in primary and recurrent epithelial ovarian cancer.
Anticancer Res. 35:2611–2617. 2015.PubMed/NCBI
|
|
44
|
Castro-Magdonel BE, Orjuela M, Camacho J,
García-Chéquer AJ, Cabrera-Muñoz L, Sadowinski-Pine S,
Durán-Figueroa N, Orozco-Romero MJ, Velázquez-Wong AC,
Hernández-Ángeles A, et al: miRNome landscape analysis reveals a 30
miRNA core in retinoblastoma. BMC Cancer. 17(458)2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Grygalewicz B, Woroniecka R, Rygier J,
Borkowska K, Rzepecka I, Łukasik M, Budziłowska A, Rymkiewicz G,
Błachnio K, Nowakowska B, et al: Monoallelic and biallelic
deletions of 13q14 in a group of CLL/SLL patients investigated by
CGH haematological cancer and SNP array (8x60K). Mol Cytogenet.
9(1)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tsiloulis T, Pike J, Powell D, Rossello
FJ, Canny BJ, Meex RC and Watt MJ: Impact of endurance exercise
training on adipocyte microRNA expression in overweight men. FASEB
J. 31:161–171. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu K, Zhao X, Gu J, Wu J, Zhang H and Li
Y: Effects of 12C6+ heavy ion beam irradiation on the p53 signaling
pathway in HepG2 liver cancer cells. Acta Biochim Biophys Sin
(Shanghai). 49:989–998. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Song D, Zhao J, Su C, Jiang Y and Hou J:
Etoposide induced NMI promotes cell apoptosis by activating the
ARF-p53 signaling pathway in lung carcinoma. Biochem Biophys Res
Commun. 495:368–374. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wu Z, Zhu X, Xu W, Zhang Y, Chen L, Qiu F,
Zhang B, Wu L, Peng Z and Tang H: Up-regulation of CIT promotes the
growth of colon cancer cells. Oncotarget. 8:71954–71964.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Mello SS, Valente LJ, Raj N, Seoane JA,
Flowers BM, McClendon J, Bieging-Rolett KT, Lee J, Ivanochko D,
Kozak MM, et al: A p53 super-tumor suppressor reveals a tumor
suppressive p53-Ptpn14-Yap axis in pancreatic cancer. Cancer Cell.
32:460–473. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Morita K, Noura M, Tokushige C, Maeda S,
Kiyose H, Kashiwazaki G, Taniguchi J, Bando T, Yoshida K, Ozaki T,
et al: Autonomous feedback loop of RUNX1-p53-CBFB in acute myeloid
leukemia cells. Sci Rep. 7(16604)2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Chen H, Qian J, Werner S, Cuk K, Knebel P
and Brenner H: Development and validation of a panel of five
proteins as blood biomarkers for early detection of colorectal
cancer. Clin Epidemiol. 9:517–526. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Masetti M, Acquaviva G, Visani M, Tallini
G, Fornelli A, Ragazzi M, Vasuri F, Grifoni D, Di Giacomo S,
Fiorino S, et al: Long-term survivors of pancreatic adenocarcinoma
show low rates of genetic alterations in KRAS, TP53 and SMAD4.
Cancer Biomark. 6:323–334. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Makohon-Moore A and Iacobuzio-Donahue CA:
Pancreatic cancer biology and genetics from an evolutionary
perspective. Nat Rev Cancer. 16:553–565. 2016.PubMed/NCBI View Article : Google Scholar
|