Introduction
Inflammatory bowel disease (IBD), including
ulcerative colitis (UC) and Crohn's disease, is an autoimmune
disease of the intestine (1). IBD
is influenced by a number of factors, such as eating habits,
disorders of the intestinal flora (2) and the immune system (3). There is a lack of specific therapeutic
target in UC; therefore, the strategy of UC treatment involves
immune regulation and inhibition of inflammation (4,5). There
have been previous precedents of successful prevention and cure of
inflammatory diseases using traditional Chinese herbal medicines
(6,7). Ding's herbal enema (DHEP) was created
by Ding Zemin, an eighth-generation descendant of the Ding family.
DHEP has been used in The Third Affiliated Hospital of Nanjing
University of Chinese Medicine (Nanjing, China) for >50 years
and has an excellent curative effect in IBD treatment (8). DHEP contains Lonicerae
japonicae flos (Jinyinhua), Sanguisorba officinalis L.
(Diyu), Bletilla striata (Thunb.) Rchb.F. (Baiji),
Phellodendron chinense Schneid. (Huangbo), Coptis
chinensis Franch (Huanglian) and Portulaca oleracea L.
(Machixian). All of the constituents of the DHEP solution that are
from traditional Chinese medicine.
Studies have demonstrated that Jinyinhua, Diyu and
Baiji contain β-sitosterol (9,10).
β-sitosterol has immunomodulatory and anti-inflammatory activity,
and is present in numerous plants (11). A number of studies have revealed
that β-sitosterol can inhibit inflammation through the NF-κB
pathway (12-16).
Animal experiments suggest that β-sitosterol can significantly
reduce colonic shortening, disease activity index (DAI) and fetal
hemoglobin content in mice (17).
Additionally, β-sitosterol specifically increases the activity of T
helper (Th) cells, and increases the activity of T cells and
natural killer cells (18).
The present study investigated the mechanism of
action of DHEP and its possible active ingredient, β-sitosterol, in
the treatment of UC. C57BL/6J mice were treated with 3.5% (w/v)
dextran sulfate sodium (DSS) for 7 days to generate an animal model
of colitis. DHEP and β-sitosterol were administered for 7 days, and
the results indicated that DHEP and β-sitosterol inhibited the
inflammatory response and restored the Th17/regulatory T cell
(Treg) balance by regulating intestinal microbiota and short-chain
fatty acids (SCFAs).
Materials and methods
Animals
A total of 50 adult female C57BL/6 mice (6-8 weeks
old), weighing 20-24 g, were obtained from Yangzhou University
Comparative Medical Center (Yangzhou, China). Mice were fed with
free access to food and drinking water and housed in a
temperature-controlled room (22±4˚C) under a 12-h dark-light cycle
with a relative humidity of 50%. Animal welfare and experimental
procedures were carried out in accordance with the guidelines and
the associated ethical regulations of The Experimental Animal
Ethics Committee of the Nanjing Hospital of Chinese Medicine
Affiliated to Nanjing University of Chinese Medicine (Nanjing,
China), which approved the animal experiments (approval no.
2020-10-002).
Reagents
DHEP was obtained from The Third Affiliated Hospital
of Nanjing University of Chinese Medicine (Nanjing, China).
β-sitosterol was purchased from Beijing Solarbio Science &
Technology Co., Ltd. (cat. no. IS0690). Mesalazine (5-ASA) was
purchased from Ethypharm SAS (cat. no. 160306). DSS (36-50 kDa) was
purchased from MP Biomedicals, LLC (cat. no. 160110). IL-6 (cat.
no. 12912), TNF-α (cat. no. 3707) and p65 (cat. no. 6956)
antibodies were obtained from Cell Signaling Technology, Inc.
Cyclooxygenase (COX)-2 (cat. no. BM4419) antibodies were obtained
from Wuhan Boster Biological Technology, Ltd. IL-17A (cat. no.
ml037864) and IL-10 (cat. no. ml037873) enzyme-linked immunosorbent
assay (ELISA) kits were purchased from Shanghai Enzyme-Linked
Biotechnology Co., Ltd.
Determination of β-sitosterol in
DHEP
DHEP powder (20 g) was extracted using methanol (100
ml x 3) at room temperature. After removal of methanol under
reduced pressure (~-0.1 Mpa), the aqueous brownish syrup (120 ml)
was suspended in H2O (100 ml) and then partitioned with
petroleum ether (50 ml x 3) to obtain 500 mg petroleum
ether-soluble fraction. The fraction was further fractionated on a
silica gel column eluted with petroleum ether-acetone (from 1:0 to
0:1) to obtain eleven fractions (1-10)
according to thin-layer chromatography analysis. Fraction three (65
mg) was loaded onto a silica gel column and eluted with
methanol-CHCl3 (20:80, 30:70 and 40:60; 50 ml of each mixture) to
yield four subfractions (fractions 3-1 to 3-4). Fraction 3-2 was
analyzed by gas chromatography. The analytes (1 µl) were separated
using an Agilent 6890N-5975B GC system (Agilent Technologies, Inc.)
equipped with a flame ionization detector and an HP-5 column (30 m
x 250 µm x 0.25 µm) using 99.99% nitrogen as a carrier gas and
99.99% hydrogen as an auxiliary gas at a flow rate of 1.2 ml/min
with a split ratio of 10:1. The temperatures of injector and
detector were 250˚C and 280˚C, respectively. The oven temperature
was gradually increased from 100˚C to 250˚C at a rate of 50˚C/min
and from 250˚C to 280˚C at a rate of 10˚C/min and was held for 4
min.
Animal models of DSS-induced acute
colitis and treatments
Mice were treated with 3.5% (w/v) DSS in their
drinking water for 7 days, followed by switching to regular
drinking water for 7 days. Test compounds were administered via an
enema per day for 7 days following DSS treatment. To investigate
the effect of DHEP, all mice were randomly divided into 5 groups:
Control (no DSS treatment, orally administered with sterile water;
n=12), vehicle (DSS treated for 7 days, orally administered with
sterile water; n=12), DHEP (administered by enema at a dose of 7.2
g/kg; n=12), 5-ASA (orally administered at a dose of 0.2 g/kg; N =
12) and β-sitosterol (administered by enema at dose of 0.1 g/kg;
n=12). At 24 h after the last enema, mice that had been fasting for
12 h were sacrificed.
Evaluation of DAI
Body weight, stool consistency and rectal bleeding
of the mice were recorded daily and scored according to Cooper's
scoring criteria (19). The mean of
the three scores was calculated and recorded as the DAI. The body
weight loss was scored as 0 when there was no loss in weight; 1 for
a loss of 1-5%; 2 for a loss of 5-10%; 3 for a loss of 10-15%; or 4
for a loss of >15%. Regarding stool consistency, 0 points
indicated normal pellets, 2 points indicated loose stools that did
not stick to the anus and 4 points indicated diarrhea. For rectal
bleeding, 0 points were given for negative (-) results in the
Hemoccult test (20); 1 point for
positive (+) results; 2 points for positive (++) results; 3 points
for positive (+++) results; and 4 points for gross rectal bleeding
(++++) results.
Histopathology
A representative sample from the middle region of
the colon was fixed in 4% paraformaldehyde at 4˚C for 24 h,
embedded in paraffin, sectioned (5-µm), stained with hematoxylin
(at room temperature for 5 min) and eosin (at room temperature for
2 min) and then observed using an IX 51 epifluorescence Olympus
microscope (Olympus Corporation) equipped with a DP-26 digital
camera at x40 magnification.
16S DNA high-throughput
sequencing
The construction of a high-throughput sequencing
library and sequencing based on the Illumina MiSeq platform was
performed by Genewiz, Inc. A Qubit 2.0 Fluorometer (Invitrogen;
Thermo Fisher Scientific, Inc.) was used to detect the DNA
concentration of the samples and to construct a sequencing library
using the MetaVxTM Library Construction kit (Genewiz, Inc.). Using
10 nmol of DNA as a template, a series of PCR primers designed by
Genewiz, Inc. were used to amplify the prokaryotic 16S ribosomal
(r)DNA, including two highly variable regions of V3 and V4. The V3
and V4 regions were amplified using an upstream primer containing
the 5'-CCTACGGRRBGCASCAGKVRVGAAT-3' sequence and a downstream
primer containing the 5'-GGA CTACNVGGGTWTCTAATCC-3' sequence. In
addition, a primer with an index was added to the end of the PCR
product of the 16S rDNA by PCR for next-generation sequencing. The
library quality was determined using an Agilent 2100 Bioanalyzer
(Agilent Technologies, Inc.) and library concentrations were
determined using a Qubit 2.0 fluorometer (Invitrogen; Thermo Fisher
Scientific, Inc.). After the DNA library was mixed, the Illumina
MiSeq (Illumina, Inc.) instrument was used in accordance with the
manufacturer's instructions. The instrument performed 2x300 bp
double-end sequencing (paired end) reactions, and the sequence
information was read by the MiSeq Control Software (v4.0) that
comes with MiSeq.
SCFAs measurements
Fresh feces (0.2 g) were suspended in 1 ml of
deionized water and homogenized by vortexing for 1 min before
centrifugation (138,00 x g at 4˚C for 30 min). The supernatant from
the colon and cecum (630 and 720 µl, respectively) was mixed with
25% metaphosphoric acid at a ratio of 9:1 (v:v) and was shaken at
37˚C for 4 h. The supernatant was collected and stored at -20˚C
before gas chromatography analysis. Standard curves were
constructed according to a previously described method (15). Specifically, 1 µl of the analytes
was separated using an Agilent 6890N-5975B GC system equipped with
an flame ionization detector and a DB-FFAP column (30 m x 250 µm x
0.25 µm; Agilent Technologies Inc.) (21) using 99.99% nitrogen as a carrier gas
and 99.99% hydrogen as an auxiliary gas at a flow rate of 0.8
ml/min with a split ratio of 50:1. The temperatures of injector and
detector were 250˚C and 280˚C, respectively. The oven temperature
was gradually increased from 60˚C to 220˚C at a rate of 20˚C/min
and held for 1 min (22).
Flow cytometry
The Treg and Th17 cells in the mouse spleen and
mesenteric lymph nodes (MLNs) were isolated using a Mouse
Lymphocyte Separation Solution kit (cat. no. DKW33-R0100; Dakewe
Biotech Co., Ltd.) according to the manufacturer's instructions.
The differentiation of Treg and Th17 cells in the spleen and MLNs
were analyzed with extracellular staining, membrane
permeabilization, fixation, intracellular staining were performed
using BD Pharmingen™ Transcription Factor Buffer Set kit (cat. no.
562574; BD Biosciences) according to the manufacturer's
instructions, and flow analysis was conducted. To analyse the Treg
cells, lymphocyte cells were stained with FITC-conjugated anti-CD4
(1:500; cat. no. 557307; BD Pharmingen; BD Biosciences) and
P-phycoerythrin (PE)-conjugated anti-Foxp3 (1:500; cat. no.
12-4771-82; eBioscience; Thermo Fisher Scientific, Inc.). For Th17
cells, lymphocyte cells were stained with
allophycocyanin-conjugated anti-CD4 (1:400; cat. no. 100411;
BioLegend, Inc.) and PE-conjugated anti-IL-17A (1:500; cat. no.
506903; BioLegend, Inc.). The samples were analysed by BD Accuri C6
flow cytometry (BD Biosciences), and the data was analysed by
FlowJo v10 software (FlowJo LLC).
Enzyme-linked immunosorbent assay
(ELISA)
The analyses of the fecal calprotectin (cat. no.
ml037105, Shanghai Enzyme-Linked Biotechnology Co., Ltd.) (23), IL-10 (from colon tissues) and IL-17A
(from colon tissues) were performed using the corresponding ELISA
kit. The experimental procedure was based on the manufacturer's
instructions. The fluorescence was measured using a microplate
reader (BioTek Instruments, Inc.) with excitation at 450 nm.
Determination of myeloperoxidase (MPO)
activity
The MPO activity in colon tissues and serum was
determined using an MPO detection kit (cat no. A044-1-1; Nanjing
Jiancheng Bioengineering Institute) according to the to the
manufacturer's protocols.
Western blotting
Mouse colon tissue was collected and lysed in RIPA
lysis buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology). The protein content was determined by the BCA
method. Equal quantities (50 µg) of protein lysate were loaded into
each lane of a 10% SDS-polyacrylamide gel and wet-transferred onto
a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.)
using a Bio-Rad electro transfer apparatus. After blocking with 5%
skimmed milk powder in Tris-buffered saline and 0.1% Tween-20 at
room temperature for 2 h, the membranes were incubated with the
primary antibodies. The following antibodies were used: p65
(1:500), COX-2 (1:500), IL-6 (1:1,000), TNF-α (1:100) and actin
(cat. no. ab6276, 1:5,000; Epitomics; Abcam). Horseradish
peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (cat. no.
D110058, 1:10,000; Sangon Biotech Co., Ltd.) was used as the
secondary antibody. Immunoblotted bands were visualized with the
Immobilon Western Chemilum HRP Substrate (cat. no. WBKLS0100;
MilliporeSigma) using a JS-680 B Gel Documentation and Analysis
System (Bio-Rad Laboratories, Inc.) and quantified using Image Lab
v3.0 (Bio-Rad Laboratories, Inc.) and Quantity One v4.62 (Bio-Rad
Laboratories, Inc.) software. All the proteins were normalized to
the corresponding actin level.
Statistical analysis
Statistical analysis was performed using one-way
ANOVA, followed by Tukey's post hoc test. Ordinal data were
analyzed using Kruskal-Wallis with Dunn's post hoc test.
Quantitative data are presented as mean ± standard deviation
(unless otherwise shown). P<0.05 was considered to indicate a
statistically significant difference. The data presented represent
at least three independent experiments. The 16S rDNA data was
analysed by QIIME data analysis package (v1.9.1; http://qiime.org/); the α diversity was represented by
the Shannon indexes, and the β diversity represented by principal
coordinate analysis (PCA). Sequences were grouped into operational
taxonomic units (OTUs) using the clustering program VSEARCH
(v1.9.6; https://github.com/torognes/vsearch) against the Silva
119 database (https://www.arb-silva.de/) pre-clustered at 97%
sequence identity. Linear discriminant analysis effect size (LEfSe;
http://huttenhower.sph.harvard.edu/galaxy/) to obtain
the OTU for each group. Differences in relative abundances of OTUs
were calculated using Tukey's honest significant difference test.
Data were analysed by the GraphPad Prism 7.0 software (GraphPad
Software, Inc.).
Results
HEP and β-sitosterol attenuates
DSS-induced UC in mice
C57BL/6 mice were treated with 3.5% (w/v) DSS for 7
days to induce UC (Fig. 1A)
(24). On day 7, the bleeding score
(Fig. 1C), diarrhea score (Fig. 1D), DAI (Fig. 1E) and calprotectin content (an
indicator of colitis (Fig. 1G)
(24-26)
were increased in mice treated with DSS compared with the vehicle,
whereas the body weight (Fig. 1B)
was decreased. In addition, compared with the control group, the
mice body weight was significantly shortened when DSS treated with
DSS for 7 days (Fig. 1F). These
results indicated that the mouse model was successfully established
(27,28). DHEP, compared with the vehicle
group, was administered by enema for 7 days and attenuated the
symptoms of colitis (loss of body weight, diarrhea, rectal bleeding
and DAI) (Fig. 1B-E). In addition,
for β-sitosterol treatment, the loss of body weight, rectal
bleeding and DAI score were relieved remarkably compared with the
vehicle group (Fig. 1B-E). These
results suggested that DHEP and β-sitosterol might be a positive
effect on the treatment of DSS-induced UC mice model.
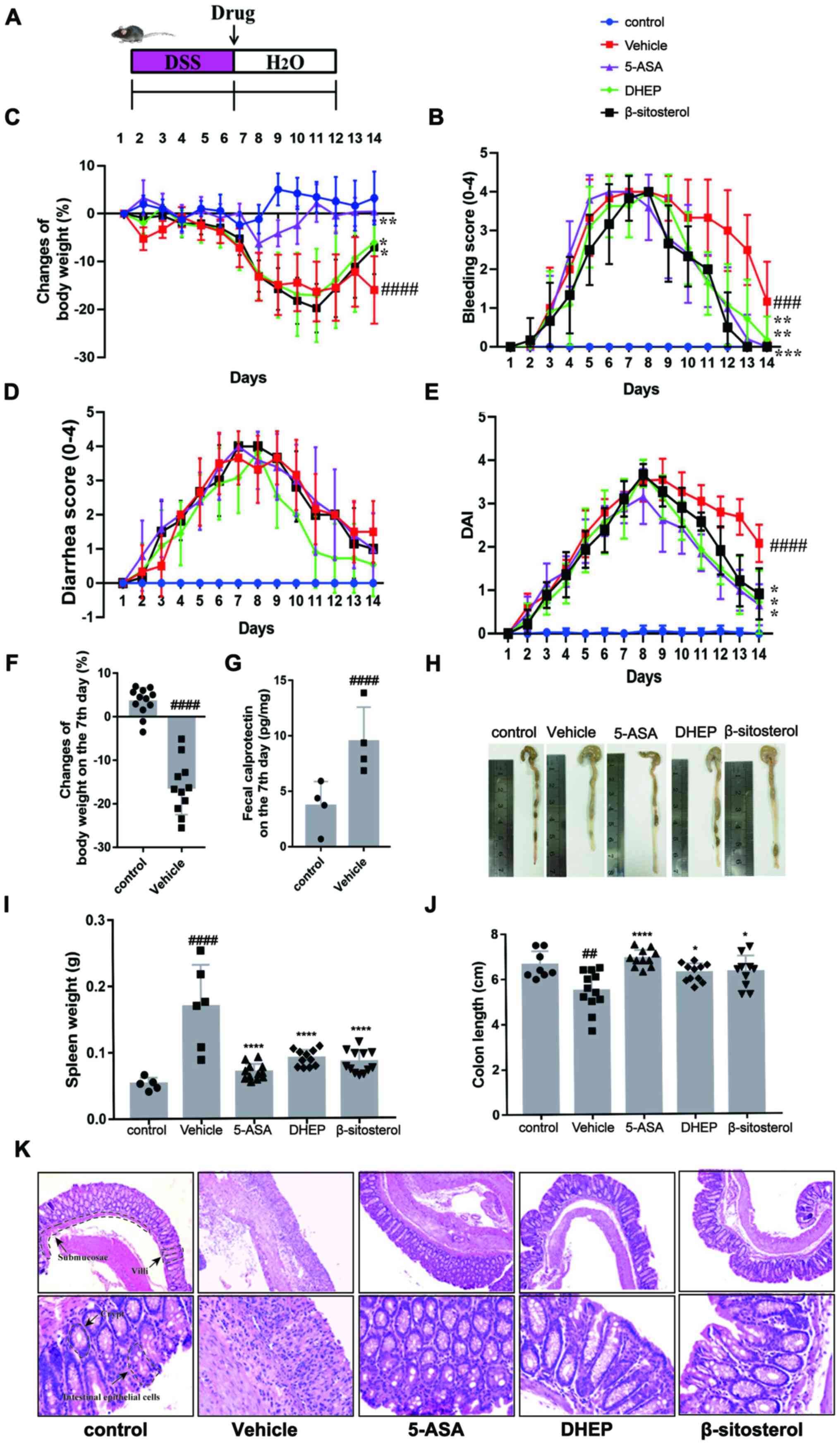 | Figure 1DHEP and β-sitosterol treatment of
acute colitis in vivo. (A) Schematic overview of the
experimental design. Changes in (B) body weights, (C) clinical
bleeding scores, (D) clinical diarrhea scores and (E) DAI are
presented, and all statistical values are compared on day 14. (F)
Weight loss and (G) fecal calprotectin content in feces after
colitis induction. (H) Representative images of whole colons and
colon length. (I) Spleen mass of mice after the end of the
experiment. (J) Quantification of whole colons and colon length.
(K) Colon sections were counterstained with hematoxylin and eosin,
and high-magnification images are presented in the bottom row (top
row magnification, x100; bottom row, x400). n=4-12.
##P<0.01, ###P<0.005 and
####P<0.001 vs. control group; *P<0.05,
**P<0.01, ***P<0.005 and
****P<0.001 vs. vehicle group. DHEP, Ding's herbal
enema; DAI, disease activity index; 5-ASA, mesalazine; DSS, dextran
sulfate sodium. |
As presented in Fig.
1H and J, the colon was
edematous and significantly shortened in the vehicle group of mice
compared with that in the control group. A reduction in colon
length in DSS-treated mice was significantly attenuated by
treatment with 5-ASA, DHEP and β-sitosterol. Additionally, 5-ASA,
DHEP and β-sitosterol significantly reduced the weight of the
spleen compared with the vehicle, which was the largest immune
organ (Fig. 1I). Comparison with
the control group mice identified certain pathological changes in
the DSS-treated mice (Fig. 1K),
including damaged intestinal epithelial cells, submucosal edema,
disappearance of villi and crypts and a large number of
infiltrating inflammatory cells (Fig.
1K). However, these pathological changes were relieved after
administration of 5-ASA, DHEP and β-sitosterol (Fig. 1K).
DHEP and β-sitosterol remodels the
distribution of intestinal microbiota in mice
To determine whether intestinal microbiota were
affected by treatment with 5-ASA, DHEP and β-sitosterol, 16S rDNA
sequencing was used to evaluate the abundance of intestinal flora
in mice. After the sequencing data were analyzed by QIIME, the α
diversity for all groups represented by the Shannon indexes is
presented in Fig. 2A. Notably, the
diversity in the DSS-treated groups were similar to that in the
control group. By contrast, the diversity in the 5-ASA group was
significantly decreased compared with that in the vehicle group.
However, these differences were not significant in the DHEP- and
β-sitosterol-treated groups (Fig.
2A). β diversity represented by PCA is presented in Fig. 2B. The distributions in the DHEP and
5-ASA groups were relatively concentrated, and the distribution
between the groups was relatively dispersed. There was no overlap
between the DHEP and 5-ASA groups. There was a partial overlap
between the vehicle and β-sitosterol groups and the control group;
however, the overall distribution was relatively dispersed, which
may be due to individual differences and intervention effects in
mice. The bacterial composition at the phylum level is presented in
Fig. 2C. The abundance at this
level was variable, including Firmicutes, Bacteroidetes and
Proteobacteria. The abundance of Bacteroidetes and Proteobacteria
were increased in DHEP group compared with vehicle group, but the
abundance of Firmicutes was decreased. These results indicated that
DHEP might be able to regulate the ratios of
Bacteroidetes/Firmicutes and Proteobacteria/Firmicutes. In
addition, in the β-sitosterol group, the ratios of Bacteroidetes/
Firmicutes and Proteobacteria/ Firmicutes were upregulated, but not
to the same level as the DHEP group.
To identify the bacteria possibly regulated by DHEP
and β-sitosterol, LEfSe analysis was used to obtain the OTUs for
each group. As a result, 42 significant OTUs were identified, along
with their linear discriminant analysis score (Fig. 2D), and a heatmap was generated
(Fig. 2E). Notably, 39 OTUs were
significantly altered in the control, 5-ASA, DHEP and β-sitosterol
groups (Fig. 2F). Comparison with
the vehicle group indicated that 5-ASA altered 21 OTUs. The 12
reduced OTUs in the 5-ASA group (OTU 42, 321, 8, 7, 43, 182, 100,
203, 183, 48, 125 and 286) are highlighted in red, and 9 increased
OTUs (OTU 73, 16, 13, 60, 77, 39, 41, 136 and 71) are highlighted
in green in Fig. 2F. In the
DHEP-treated group, 19 OTUs were altered compared with those in the
vehicle group. As presented in Fig.
2F, 11 OTUs (OTU 321, 12, 120, 182, 239, 203, 83, 233, 183, 93
and 196) were reduced and are highlighted in red, which belonged to
the genera of Erysipelatoclostridium,
Bifidobacterium, Anaerotruncus, Defluviitaleaceae
UCG-011, Family XIII AD3011 group, Tyzzerella,
Eubacterium coprostanoligenes, Proteus and three
uncultured genera; and a total of seven OTUs (OTU 73, 3, 13, 29,
41, 136 and 1) were increased, which belonged to the genera of
Bacteroides, Lactobacillus, Turicibacter,
Parabacteroides, Eubacterium xylanophilum group and
one uncultured genus. In the β-sitosterol-treated group, six OTUs
(OTU 22, 182, 100, 203, 93 and 286) were reduced (Fig. 2F). The six reduced OTU belonged to
Akkermansia, Defluviitaleaceae UCG-011,
Ruminococcaceae UCG-005, Tyzzerella and
Oligella Only four OTUs were increased (OTU 270, 13, 29 and
41), which belonged to Lactobacillus, Parabacteroides
and two uncultured genera. Thus, 34 OTUs were altered by 5-ASA,
DHEP and β-sitosterol, and only four OTUs (OTU 182, 203, 13 and 41)
were modulated in the same direction as in the control group for
all three treatment groups. These four OTUs belonged to the genera
Anaerotruncus, Ruminococcaceae UCG-005 and
Turicibacter and uncultured bacterium, which belonged to the
Firmicutes, Firmicutes, Bacteroidetes and Firmicutes phyla,
respectively (Fig. 2G).
DHEP and β-sitosterol treatment
increases the profiles of SCFAs in the colon of mice with
DSS-induced UC
SCFAs are the end-products generated from food with
a high dietary content by gut microbiota and can alleviate IBD in
animal models (29). To evaluate
whether DHEP and β-sitosterol increased the release of SCFAs, the
SCFA content in the colon of mice was evaluated (Fig. 3). The levels of SCFAs, including
acetic acid (Fig. 3A and B), propionic acid (Fig. 3C and D) and butyric acid (Fig. 3E and F) were significantly reduced in the colon
of DSS-treated mice compared with the control group, and the levels
of SCFAs were increased in the 5-ASA, DHEP and β-sitosterol groups
compared with the vehicle group. These results indicated that the
beneficial effects of DHEP in colitis involved an increase in the
SCFA content in the colon.
DHEP and β-sitosterol regulates the
Th17/Treg balance in mice with DSS-induced UC
The Th17/Treg transformation balance is important
for the maintenance of intestinal homeostasis (30). To investigate whether DHEP and
β-sitosterol had a positive effect on the balance of Th17/Treg
cells, the expression levels of IL-17A and IL-10, which are
specifically expressed in Th17 cells and Treg cells, respectively,
were determined. The results demonstrated that the numbers of Treg
cells in the MLNs (Fig. 4A) and
spleen (Fig. 4B) of mice with
DSS-induced UC were significantly lower compared with those in the
control mice. After administration with 5-ASA or β-sitosterol for 7
days, the number of Treg cells was significantly increased in MLNs
(Fig. 4A) but markedly decreased in
spleen (Fig. 4B) compared with the
vehicle. In the DHEP-treated mice group, the number of Treg cells
was significantly increased in both the MLNs (Fig. 4A) and spleen (Fig. 4B) compared with the vehicle. As
presented in Fig. 4C, treatment
with 5-ASA and β-sitosterol significantly decreased the number of
Th17 cells in the spleen compared with that in the vehicle group;
however, DHEP did not demonstrate this effect. Unexpectedly, the
IL-17A level was significantly decreased in the control group
compared with the vehicle (Fig.
4E). Additionally, 5-ASA, DHEP and β-sitosterol-treated mice
manifested an opposite trend in their IL-17A and IL-10 expression
levels. IL-10 was significantly increased compared with the vehicle
(Fig. 4D), and the IL-17A
expression level was significantly reduced compared with the
vehicle (Fig. 4E).
DHEP and β-sitosterol reduces
inflammation in the DSS-induced colitis
Fecal calprotectin is an acute inflammatory marker
that was significantly increased in the DSS-treated vehicle group
of mice compared with the control; in addition, it was
significantly reduced in 5-ASA-, DHEP- and β-sitosterol-treated
mice (Fig. 5A). High levels of MPO
enzyme are expressed in neutrophils, reflect the infiltration of
neutrophils and indirectly correspond to inflammation in certain
tissues (31). MPO levels in the
tissue and serum were significantly upregulated in the vehicle
group of mice compared with the control, and significantly
downregulated in the 5-ASA-, DHEP- and β-sitosterol-treated mice
compared with the vehicle (Fig. 5B
and C). p65 (Fig. 5D), IL-6 (Fig. 5F) and TNF-α (Fig. 5G) were secreted in the colon of
DSS-treated mice; the expression levels of p65 and IL-6 were
significantly increased in the vehicle group compared with the
control, and significantly reduced by treatment with 5-ASA and
β-sitosterol compared with the vehicle. TNF-α followed this same
pattern; however, the changes in expression level were not
significant. However, DHEP only lowered the secretion of p65
compared with the vehicle (Fig.
5D). 5-ASA and DHEP treatment also reduced the protein
expression level of COX-2 in DSS-treated mice compared with the
vehicle, which was detected in the pathological state of
inflammation; β-sitosterol had no effect on the COX-2 level
(Fig. 5E and H).
 | Figure 5Expression levels of various
inflammatory factors in mice. (A) Fecal calprotectin content in
feces after sacrifice of the mice. MPO content in (B) colon tissue
and (C) serum of mice. Quantification of western blotting showing
(D) p65, (E) COX-2, (F) IL-6 and (G) TNF-α. (H) Western blot
analysis of actin, p65, COX-2, IL-6 and TNF-α. N=4-6.
###P<0.001 vs. control group; *P<0.05,
**P<0.01, ***P<0.001 and
****P<0.0001 vs. vehicle group. MPO, myeloperoxidase;
COX-2, cyclooxygenase-2; 5-ASA, mesalazine; DHEP, Ding's herbal
enema. |
Discussion
DHEP is a traditional Chinese medicine used as an
enema treatment, which was developed by Professor Xie Jinyu and
Professor Ding Zemin (8). DHEP is
effective in the clinical treatment of UC (8). The present study determined a
preliminary mechanism of action of DHEP treatment in DSS-induced UC
and analyzed the content of β-sitosterol in DHEP by gas
chromatography. The results presented in Fig. S1 indicated that the concentration
of β-sitosterol in DHEP was ~13.9 mg/g. The DHEP dose in mice was
determined according to the clinical dose. The clinical dose of
DHEP in Nanjing Hospital of Chinese Medicine Affiliated to Nanjing
University of Chinese Medicine is 0.58 g/kg/day; according to the
dose conversion between human and mice (mouse dose = human dose x
12.33) (32), the theoretical dose
for mouse administration was 7.15 g/kg/day. Hence, a dose of 7.2
g/kg/day was used in the present study. Thus, the concentration of
β-sitosterol in DHEP was ~13.9 mg/g, and the dose of 100 mg/kg/day
of β-sitosterol was selected as the therapeutic dose.
Intestinal homeostasis depends on the dynamic
interaction between intestinal flora, intestinal epithelial cells
and immune cells (33). These
microorganisms coexist with humans and play a notable role in the
regulation of the mammalian immune system. The homeostasis of the
ecosystem formed by the gut microbiota and the host is important
for human health (34,35). Intestinal flora plays a role in the
pathogenesis of IBD. The present study analyzed the gut microbiota
via next-generation sequencing. The results of LEfSe analysis at
the OTU level indicated differences in the abundance between five
groups. In the DSS-treated vehicle group, 15 OTUs were
significantly increased and 11 OTUs were considerably decreased.
Increased OTUs were assigned to the Akkermansia,
Bacteroides and Anaerotruncus genera that were the
most abundant and extensively studied members of the commensal
microbiota. These bacteria benefit the host by fermentation of
complex dietary carbohydrates and modulation of mucosal
glycosylation (36). Additionally,
genera with increased abundance included
Erysipelatoclostridium, Ruminococcaceae,
Ruminococcaceae UCG-014, Defluviitaleaceae UCG-011
and Ruminococcaceae UCG-005 of the Firmicutes phylum. The
abundance of the Firmicutes phylum in the colons of patients with
IBD was increased (36). Comparison
with the vehicle group indicated that 11 OTUs were reduced and
seven OTUs were increased. Reduced OTUs belonged to the Firmicutes
phylum, except OTU 196, which belonged to the Proteobacteria
phylum. Additionally, four out of the seven reduced OTUs (OTU 73,
3,13 and 29) belonged to the Bacteroidetes phylum and three OTUs
(OTU 41, 136 and 1) belonged to the Firmicutes phylum. These
results suggested that DHEP may rebalance the flora that was
altered by DSS treatment.
SCFAs, such as acetate, n-propionate and n-butyrate,
are derived by microbial fermentation of dietary fiber in the
intestine (37). An increasing
number of studies have revealed that SCFAs are critical for the
regulation of the immune system, especially butyric acid (38), and that SCFAs can promote the
development of Treg cells and inhibit the development of Th17 cells
(39,40). The balance between these two cell
types is needed for immune regulation and can regulate the immune
function of the body (41). Th17
cells are mainly present on the intestinal mucosal barrier surface
to protect the host from microbial invasion through the epithelium
(42). Treg cells are mainly
present in the intestinal mucosa and control the excessive immune
response caused by T cells that may damage the host tissue. Mature
Th17 cells can express a variety of effectors molecules, including
IL-17, TNF-α, IL-17A and other cytokines (42,43).
IL-17A plays an important role in the recruitment, activation and
migration of granulocytes (44).
These processes accelerate the polarization of Th17 cells, leading
to intestinal inflammation (45).
The present study demonstrated that β-sitosterol and DHEP could
significantly increase the SCFA content in the intestine of mice,
particularly the amount of butyric acid. DHEP and β-sitosterol
significantly increased the proportion of Tregs in the MLNs, and
there were no significant differences in the ratio of Tregs between
DHEP and β-sitosterol treatments.
β-sitosterol has immunomodulatory and
anti-inflammatory activity, and is found in numerous plants
(11). A large number of studies
have indicated that β-sitosterol can inhibit inflammation through
the NF-κB pathway (12-16).
However, the present study suggested that β-sitosterol can
significantly reduce colonic shortening, DAI and fecal hemoglobin
content in mice. In addition, β-sitosterol specifically increases
the activity of Th cells and increases the activity of T cells
(18). In the present study,
β-sitosterol significantly reduced body weight loss, diarrhea
score, bleeding score, colon index and spleen index. In addition,
both DHEP and β-sitosterol significantly increased the proportion
of Tregs in the MLNs, and there was no significant difference in
the ratio of Tregs between DHEP and β-sitosterol treatments.
β-sitosterol could significantly reduce Th17 cells in the spleen,
but DHEP had no significant effect. These results suggested that
β-sitosterol could be used as a potential drug for the treatment of
UC.
Thus, the current study demonstrated that
β-sitosterol and the clinically effective DHEP enema solution
protected mice from DSS-induced colitis. The present study
identified four therapeutic mechanisms of both treatments,
including effective inhibition of inflammatory responses, and
regulation of intestinal microbiota and SCFAs to restore the
Th17/Treg balance. However, additional comprehensive studies may be
focused on the following three points: i) Investigation of the
notable role of the gut microbiota in the mechanism of action; ii)
identification of other active substances in DHEP that are
effective in the treatment of UC in addition to β-sitosterol; and
iii) identification of DHEP-induced changes in other intestinal
flora metabolites in addition to SFCAs, which are important for the
treatment of UC. Furthermore, the efficacy of the active ingredient
β-sitosterol is clinically validated, thus simplifying the
convenient administration of the preparation.
Supplementary Material
Determination of the concentration of
β-sitosterol in DHEP. Gas chromatogram of (A) β-sitosterol
standardand (B) DHEP. DHEP, Ding's herbal enema.
Acknowledgements
Not applicable.
Funding
This work was supported by Nanjing Medical Science and Technique
Development Foundation (grant no. QRX17090), Nanjing Famous
Traditional Chinese Medicine Studio (grant no. ZSM-2017-NJ),
Multi-Disciplinary Integrated Diagnosis and Treatment Platform for
Inflammatory Bowel Disease (grant no. 00302010524), Natural Science
Foundation of Jiangsu Province (grant no. SBK20180140), Young
Talent Cultivation Program of the Key Subject of ‘Chinese Medicine
Anorectal Diseases’ of the State Administration of Chinese Medicine
(grant nos. GCPY201701 and GCPY201902) and The Third Chinese
Medicine Experts' Academic Experience Succession Work Project of
Jiangsu Provincial (grant no. 2019-SSPSC-DK).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the BioProject repository
(https://www.ncbi.nlm.nih.gov/bioproject/PRJNA753609).
Authors' contributions
YYT and YD acquired and analyzed the data, and
drafted and revised the manuscript. XZ, SMZ and GJD performed the
animal experiments. XY, DCX and PC obtained and analyzed the 16s
rDNA sequencing data. JMZ, JZM and ML obtained and analyzed the
SCFAs measurement data. SCH and YL performed the flow cytometry
experiment and data analysis. YTZ and HX performed the western
blotting experiment and data analysis. KD and YJD designed the
study, provided final approval of the version to be published and
agreed to be accountable for all aspects of the work in ensuring
that questions related to the accuracy or integrity of any part of
the work are appropriately investigated and resolved. YJD and KD
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The Experimental Animal Ethics Committee of the
Nanjing Hospital of Chinese Medicine Affiliated to Nanjing
University of Chinese Medicine approved the animal experiments
(approval no. 2020-10-002).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guan Q: A Comprehensive review and update
on the pathogenesis of inflammatory bowel disease. J Immunol Res:
Dec 1, 2019 (Epub ahead of print). doi: 10.1155/2019/7247238.
|
|
2
|
Desreumaux P and Colombel JF:
Modifications and roles of intestinal flora in inflammatory bowel
diseases. Gastroenterol Clin Biol. 25:C89–C93. 2001.
|
|
3
|
Huang Y and Chen Z: Inflammatory bowel
disease related innate immunity and adaptive immunity. Am J Transl
Res. 8:2490–2497. 2016.PubMed/NCBI
|
|
4
|
Coskun M, Vermeire S and Nielsen OH: Novel
targeted therapies for inflammatory bowel disease. Trends Pharmacol
Sci. 38:127–142. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rogler G: Where are we heading to in
pharmacological IBD therapy? Pharmacol Res. 100:220–227.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zheng K, Shen H, Jia J, Lu Y, Zhu L, Zhang
L and Shen Z: Traditional Chinese medicine combination therapy for
patients with steroid-dependent ulcerative colitis: Study protocol
for a randomized controlled trial. Trials. 18(8)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sałaga M, Zatorski H, Sobczak M, Chen C
and Fichna J: Chinese herbal medicines in the treatment of IBD and
colorectal cancer: A review. Curr Treat Options Oncol. 15:405–420.
2014.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shicai H, Kang D and Xu Y: Clinical
efficacy of Kuijie enema liquid by air- ressured herb enema
combined with Zhuling Xianglian decoction in the treatment of
ulcerative colitis and its effect on the level of inflammatory
factors of patients. Hebei J Tradit Chin Med. 41:367–371. 2019.(In
Chinese).
|
|
9
|
Shen T, He YL, Sun GP, Liu WX and Zheng
SZ: Studies on chemical constituents of Sanguisorba longifolia
Bertol. Indian J Chem B. 47:1600–1604. 2008.
|
|
10
|
He X, Wang X, Fang J, Zhao Z, Huang L, Guo
H and Zheng X: Bletilla striata: Medicinal uses, phytochemistry and
pharmacological activities. J Ethnopharmacol. 195:20–38.
2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Feng S, Dai Z, Liu A, Wang H, Chen J, Luo
Z and Yang CS: β-Sitosterol and stigmasterol ameliorate dextran
sulfate sodium-induced colitis in mice fed a high fat Western-style
diet. Food Funct. 8:4179–4186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Paniagua-Pérez R, Flores-Mondragón G,
Reyes-Legorreta C, Herrera-López B, Cervantes-Hernández I,
Madrigal-Santillán O, Morales-González JA, Álvarez-González I and
Madrigal-Bujaidar E: Evaluation of the anti-inflammatory capacity
of beta-sitosterol in rodent assays. Afr J Tradit Complement Altern
Med. 14:123–130. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liz R, Zanatta L, dos Reis GO, Horst H,
Pizzolatti MG, Silva FR and Fröde TS: Acute effect of β-sitosterol
on calcium uptake mediates anti-inflammatory effect in murine
activated neutrophils. J Pharm Pharmacol. 65:115–122.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim KA, Lee IA, Gu W, Hyam SR and Kim DH:
β-Sitosterol attenuates high-fat diet-induced intestinal
inflammation in mice by inhibiting the binding of
lipopolysaccharide to toll-like receptor 4 in the NF-κB pathway.
Mol Nutr Food Res. 58:963–972. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bin Sayeed MS, Karim SMR, Sharmin T and
Morshed MM: Critical analysis on characterization, systemic effect,
and therapeutic potential of beta-sitosterol: A plant-derived
orphan phytosterol. Medicines (Basel). 3(3)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yin Y, Liu X, Liu J, Cai E, Zhu H, Li H,
Zhang L, Li P and Zhao Y: Beta-sitosterol and its derivatives
repress lipopolysaccharide/d-galactosamine-induced acute hepatic
injury by inhibiting the oxidation and inflammation in mice. Bioorg
Med Chem Lett. 28:1525–1533. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Simin F, Ke N, Ping S, Guoping R, Peilong
S and Zisheng L: Research on the β-sitosterol and stigmasterol
therapeutic effect of acute colitis in mice. J Chin Cereals Oils
Assoc. 33:80–86, 94. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fraile L, Crisci E, Córdoba L, Navarro MA,
Osada J and Montoya M: Immunomodulatory properties of
beta-sitosterol in pig immune responses. Int Immunopharmacol.
13:316–321. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wirtz S, Neufert C, Weigmann B and Neurath
MF: Chemically induced mouse models of intestinal inflammation. Nat
Protoc. 2:541–546. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ramadass SK, Jabaris SL, Perumal RK,
HairulIslam VI, Gopinath A and Madhan B: Type I collagen and its
daughter peptides for targeting mucosal healing in ulcerative
colitis: A new treatment strategy. Eur J Pharm Sci. 91:216–224.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xia Z, Han Y, Wang K, Guo S, Wu D, Huang
X, Li Z and Zhu L: Oral administration of propionic acid during
lactation enhances the colonic barrier function. Lipids Health Dis.
16(62)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tao JH, Duan JA, Jiang S, Guo JM, Qian YY
and Qian DW: Simultaneous determination of six short-chain fatty
acids in colonic contents of colitis mice after oral administration
of polysaccharides from Chrysanthemum morifolium Ramat by
gas chromatography with flame ionization detector. J Chromatogr B
Analyt Technol Biomed Life Sci. 1029-1030:88–94. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Patel A, Panchal H and Dubinsky MC: Fecal
calprotectin levels predict histological healing in ulcerative
colitis. Inflamm Bowel Dis. 23:1600–1604. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Okayasu I, Hatakeyama S, Yamada M, Ohkusa
T, Inagaki Y and Nakaya R: A novel method in the induction of
reliable experimental acute and chronic ulcerative colitis in mice.
Gastroenterology. 98:694–702. 1990.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gisbert JP and McNicholl AG: Questions and
answers on the role of faecal calprotectin as a biological marker
in inflammatory bowel disease. Dig Liver Dis. 41:56–66.
2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Erbayrak M, Turkay C, Eraslan E, Cetinkaya
H, Kasapoglu B and Bektas M: The role of fecal calprotectin in
investigating inflammatory bowel diseases. Clinics (São Paulo).
64:421–425. 2009.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zha Z, Lv Y, Tang H, Li T, Miao Y, Cheng
J, Wang G, Tan Y, Zhu Y, Xing X, et al: An orally administered
butyrate-releasing xylan derivative reduces inflammation in dextran
sulphate sodium-induced murine colitis. Int J Biol Macromol.
156:1217–1233. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gao X, Cao Q, Cheng Y, Zhao D, Wang Z,
Yang H, Wu Q, You L, Wang Y, Lin Y, et al: Chronic stress promotes
colitis by disturbing the gut microbiota and triggering immune
system response. Proc Natl Acad Sci USA. 115:E2960–E2969.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chang PV, Hao L, Offermanns S and
Medzhitov R: The microbial metabolite butyrate regulates intestinal
macrophage function via histone deacetylase inhibition. Proc Natl
Acad Sci USA. 111:2247–2252. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yan JB, Luo MM, Chen ZY and He BH: The
function and role of the Th17/Treg cell balance in inflammatory
bowel disease. J Immunol Res: Dec 15, 2020 (Epub ahead of print).
doi: 10.1155/2020/8813558.
|
|
31
|
Hansberry DR, Shah K, Agarwal P and
Agarwal N: Fecal myeloperoxidase as a biomarker for inflammatory
bowel disease. Cureus. 9:e1004. 2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huang JH, Huang XH, Chen ZY and Zheng QS:
Dose conversion among different animals and healthy volunteers in
pharmacological study. Chinese J Pharmacol Toxicol. 9:1069–1072.
2004.
|
|
33
|
Wlodarska M, Kostic AD and Xavier RJ: An
integrative view of microbiome-host interactions in inflammatory
bowel diseases. Cell Host Microbe. 17:577–591. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhu ZH, Wang QY and Wu Q: On the
examination of the Darcy permeability of soft fibrous porous media;
new correlations. Chem Eng Sci. 173:525–536. 2017.
|
|
35
|
Bamias G, Pizarro TT and Cominelli F:
Pathway-based approaches to the treatment of inflammatory bowel
disease. Transl Res. 167:104–115. 2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Eck A, Zintgraf LM, de Groot EF, de Meij
TG, Cohen TS, Savelkoul PH, Welling M and Budding AE:
Interpretation of microbiota-based diagnostics by explaining
individual classifier decisions. BMC Bioinformatics.
18(441)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Feng Y, Wang Y, Wang P, Huang Y and Wang
F: short-chain fatty acids manifest stimulative and protective
effects on intestinal barrier function through the inhibition of
NLRP3 inflammasome and autophagy. Cell Physiol Biochem. 49:190–205.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tian Y, Xu Q, Sun L, Ye Y and Ji G:
Short-chain fatty acids administration is protective in
colitis-associated colorectal cancer development. J Nutr Biochem.
57:103–109. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Levy M, Thaiss CA, Zeevi D, Dohnalová L,
Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig
Y, et al: Microbiota-modulated metabolites shape the intestinal
microenvironment by regulating NLRP6 inflammasome signaling. Cell.
163:1428–1443. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhang SL, Wang SN and Miao CY: Influence
of microbiota on intestinal immune system in ulcerative colitis and
its intervention. Front Immunol. 8(1674)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ahmadi M, Yousefi M, Abbaspour-Aghdam S,
Dolati S, Aghebati-Maleki L, Eghbal-Fard S, Khabbazi A, Rostamzadeh
D, Alipour S, Shabani M, et al: Disturbed Th17/Treg balance,
cytokines, and miRNAs in peripheral blood of patients with Behcet's
disease. J Cell Physiol. 234:3985–3994. 2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gálvez J: Role of Th17 cells in the
pathogenesis of human IBD. ISRN Inflamm.
2014(928461)2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Miossec P and Kolls JK: Targeting IL-17
and TH17 cells in chronic inflammation. Nat Rev Drug Discov.
11:763–776. 2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hus I, Maciag E and Roliński J: The role
of Th17 cells in anti-cancer immunity. Postepy Hig Med Dosw.
64:244–250. 2010.PubMed/NCBI(In Polish).
|
|
45
|
Bettelli E, Carrier Y, Gao W, Korn T,
Strom TB, Oukka M, Weiner HL and Kuchroo VK: Reciprocal
developmental pathways for the generation of pathogenic effector
TH17 and regulatory T cells. Nature. 441:235–238. 2006.PubMed/NCBI View Article : Google Scholar
|



















