Introduction
Allergic diseases are experienced worldwide, with
various risk factors and triggers that occur according to
geographical and environmental differences (1). Allergic asthma is a heterogeneous
inflammatory lung disease, characterized by lung inflammation,
airway hyperresponsiveness (AHR) and airway remodeling (2). Statistically, ~300 million
individuals worldwide suffer from asthma (3), half of which are allergic, with the
incidence continuously increasing. Inhaled corticosteroids and
long-acting bronchodilators are the main therapeutic drugs for
clinical asthma treatment (4).
However, the available treatments are often ineffective due to the
heterogeneity of asthma and the variability of patient response to
the available medications (5).
Furthermore, certain patients have poor symptom control and suffer
from recurrent exacerbations despite strictly adhering to therapy
(6). Therefore, further
mechanistic insights into the pathogenesis of asthma and targeted
treatment strategies are required.
Environmental allergens, such as house dust mites
(HDM), are major risk factors for asthma (7). HDM are commonly present in human
dwellings and are especially abundant in mattresses, sofas, carpets
and blankets (8). HDM can also be
detected in the air, and studies using volumetric samples equipped
with sizing devices have revealed that mite allergens remain
airborne for a short period of time (9). Dermatophagoides pteronyssinus
(of the Pyroglyphidae family), usually called Der p, is one of the
main components in HDM; the first identified allergen was named Der
p 1, which is a glycoprotein (10). Sensitization to HDM is a major
independent risk factor for asthma in areas where the climate is
conducive to mite growth, especially in the tropical and
subtropical regions (11). It has
been suggested that mite fecal pellets may occasionally enter the
lung and cause inflammation and bronchoconstriction (11,12).
Previous results have demonstrated that certain HDM allergens may
have a direct effect on bronchial epithelia, inducing inflammation
through IgE-independent mechanisms (12).
Ferroptosis, which was originally observed in 2003
and formally defined by Brent Stockwell, is an iron-dependent,
non-apoptotic cell-death modality characterized by the accumulation
of lipid hydroperoxides and lipid reactive oxygen species (ROS) in
cellular membranes (13,14). Ferroptosis is associated with
mitochondrial fragmentation and cristae decrease (15). Ferroptotic cells do not exhibit any
hallmarks of apoptosis or necroptosis, and are therefore identified
through the balance between iron accumulation-induced ROS
production and the antioxidant system that avoids lipid
peroxidation (16). Glutathione
peroxidase 4 (GPX4) is the major protective mechanism against
peroxidation damage (17).
Deprivation of glutathione (GSH) can inactivate GPX4, and therefore
induce ferroptosis (18). Organs
such as the kidneys, brain, liver, heart and lung are reported to
be highly susceptible to ferroptosis under pathological conditions
(15). Ferroptosis is also
implicated in multiple human diseases, including Huntington's
disease and diffuse large B cell lymphoma. However, whether
ferroptosis participates in the pathological progression of asthma
requires further elucidation. The present study used a HDM-induced
mouse asthma model to determine the effect of HDM exposure on
allergic asthma and the underlying mechanisms associated with
ferroptosis.
Materials and methods
Animals and study protocol
In total, 20 female (6-8 week old, 20±2 g),
specified-pathogen free BALB/c mice were purchased from Shanghai
SLAC Laboratory Animal Co., Ltd. Mice were kept under laboratory
conditions (22˚C; 50-60% relative humidity; air circulation; 12-h
light-dark cycle with free access to water and food). The
experimental procedures were approved by the Ethics Committee of
Animal Experiments of Fudan University (authorization no.
2020-10-HSYY-WY-01; Shanghai, China). Mice were randomized to two
groups (10 mice/group): The normal control and HDM-induced asthma
model groups. The asthma model was prepared as previously described
(19,20) and was modified according to
pre-experimental results. On day 0, HDM extract (10 µg/40 µl) was
administered intranasally after anesthetization with 2-2.5%
isoflurane in air. From days 7-11, the mice were challenged daily
by pipetting 40 µl diluted HDM extract (20 µg/40 µl per mouse)
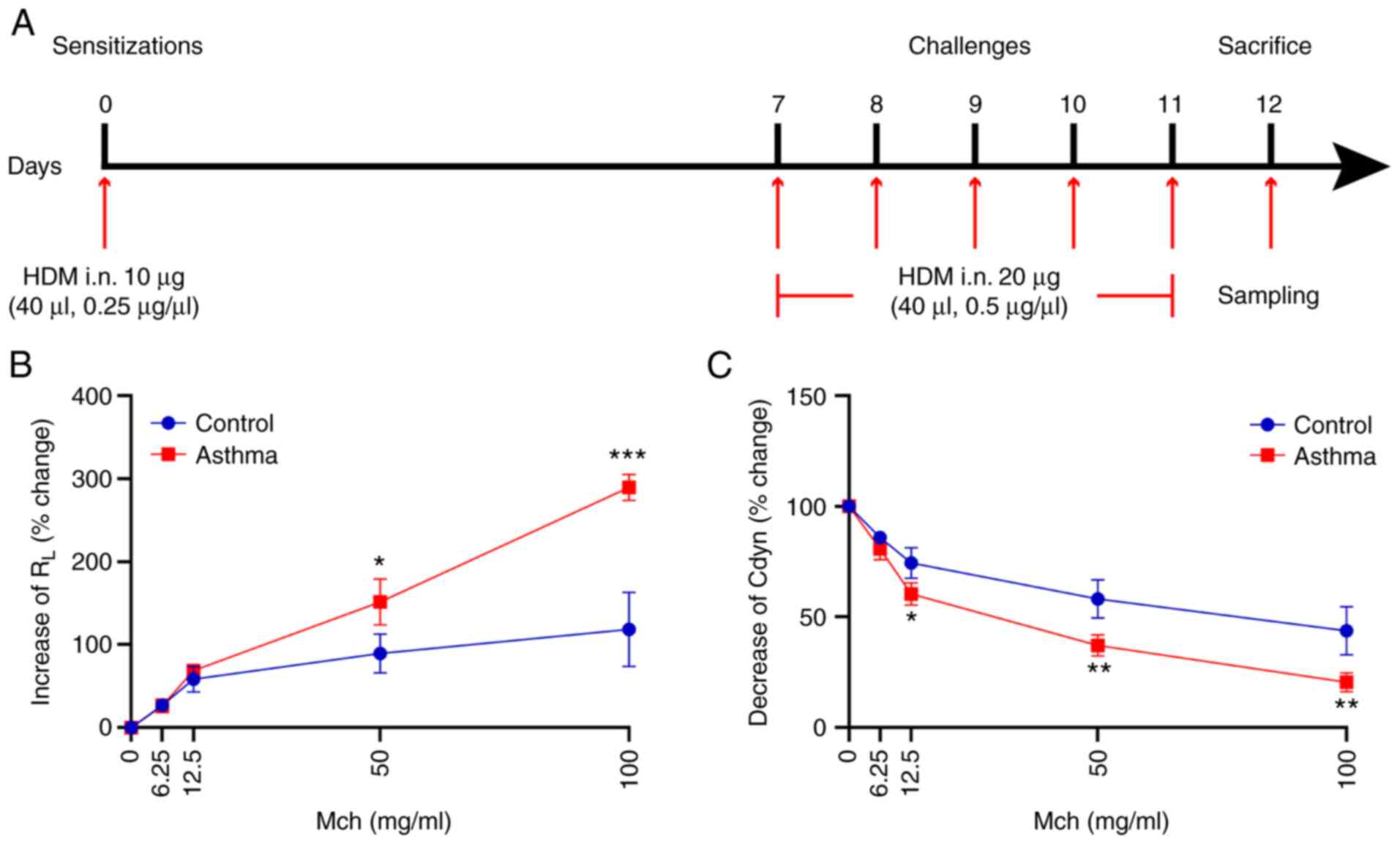
directly into the nostrils under mild anesthesia (Fig. 1A). For mice in the normal control
group, the same volume of PBS was administered intranasally at the
exact time as aforementioned.
In the present study, AHR levels, leukocyte
differential counts in bronchoalveolar lavage fluid (BALF),
inflammatory cytokines and histology were assessed to determine
whether the asthma model was successfully established. Mice with
outliers (One mouse in the control group was removed and two mice
in the model group were removed) (21,22),
including abnormal values of airway resistance (RL),
hematoxylin and eosin (H&E) staining, periodic acid Schiff
(PAS) staining and leukocyte differential counts in BALF, were
considered to have failed the modeling process, and all the values
were removed from the subsequent statistical analysis.
Reagents
Methacholine (Mch) and pentobarbital sodium were
purchased from Sigma-Aldrich (Merck KGaA). HDM extract
(Dermatophagoides pteronyssinus) was purchased from Greer
Laboratories, Inc. The mouse lung dissociation kit was purchased
from Miltenyi Biotec, Inc. Live eFluor780 (cat. no. 65-0865-14;
1:200 dilution) solution and anti-mouse CD45 eFluor 506 (cat. no.
69-0451-82; 1:100 dilution) antibodies were purchased from Thermo
Fisher Scientific, Inc. Anti-mouse CD11b BV711 (cat. no. 563168,
1:100 dilution) and anti-mouse SiglecF BV421 (cat. no. 562681,
1:100 dilution) antibodies were purchased from BD Pharmingen (BD
Biosciences). Anti-mouse Ly6C PE (cat. no. 128007, 1:200 dilution)
antibodies were purchased from BioLegend, Inc. Anti-GPX4 (cat. no.
Ab125066; 1:1,000 dilution) and anti-15 Lipoxygenase 1 (15-LO1;
cat. no. Ab244205; 1:1,000 dilution) antibodies were purchased from
Abcam, and anti-acyl-CoA synthetase long-chain family member 4
(ACSL4; cat. no. NBP2-16401; 1:1,000 dilution) and anti-catalytic
subunit solute carrier family 7 member 11 (SLC7A11; cat. no.
NB300-318; 1:1,000 dilution) antibodies were purchased from
Bio-Techne. The GSH assay kit (cat. no. A006-2) was purchased from
Nanjing Jiancheng Bioengineering Institute and ROS was measured
using dihydroethydium (DHE; Sigma-Aldrich; Merck KGaA) oxidation.
IgE [cat. no. 70-EK275-96; Multi Sciences (Lianke) Biotech, Co.,
Ltd.], IL-5 [cat. no. 70-EK205-96; Multi Sciences (Lianke) Biotech,
Co., Ltd.] and IL-13 ELISA kits [cat. no. 70-EK213/2-96; Multi
Sciences (Lianke) Biotech, Co., Ltd.] were purchased from Hangzhou
Multi Sciences (Lianke) Biotech Co., Ltd. RIPA lysis buffer was
purchased from Beyotime Institute of Biotechnology. The BCA protein
test kit was purchased from Thermo Fisher Scientific, Inc. and the
Immobilon Western Chemiluminescent HRP Substrate was purchased from
Merck KGaA.
Measurement of AHR
Within 24 h after the final HDM challenge, AHR
presented as RL and dynamic lung compliance (Cdyn) to
Mch were measured according to the manufacturer's protocols (DSI
Buxco® FinePointe Resistance and Compliance system; Data
Sciences International; Harvard Bioscience, Inc.). Mice were
weighed, intraperitoneally anesthetized with pentobarbital sodium
(50 mg/kg), incised and intubated via the trachea. Afterwards, mice
were placed into the chamber of the Resistance and Compliance
system at room temperature and the tracheal intubation was
connected to a ventilator. AHR was challenged with increasing doses
of Mch (0, 6.25, 12.5, 50 or 100 mg/ml; Each dose has a 3-min
response time and there was a 1-min interval between each dose) and
data was recorded and presented as changes in RL and
Cdyn.
Histological analysis
Anesthetized mice were sacrificed by cervical
dislocation following AHR detection. Intact left and both upper and
lower lobes of the right lung were snap frozen in liquid nitrogen
(-196˚C) and then stored at -80˚C until further use. The middle
lobe of the right lung was fixed in 4% paraformaldehyde at room
temperature for 24 h and used for subsequent histological analysis.
H&E and PAS staining were performed to determine the
inflammatory and mucus secretion changes. The middle lobe of right
lung from each mouse was removed, fixed in 4% paraformaldehyde at
room temperature for 24 h, decalcified in EDTA, dehydrated in a
graded series of ethanol solutions and embedded in paraffin.
Afterwards, lung tissue sections were obtained at a thickness of
4-µm. Lung slices were stained with H&E (hematoxylin staining
for 10 min at room temperature; eosin staining for 3 min at room
temperature) and PAS solution and imaged with light microscopy
(x100 or x200, magnifications; Nikon ECLiPSE Ni; Nikon
Corporation). A semi-quantitative grading method was used to
evaluate the degree of peribronchial inflammation as described by
Myou et al (23). The
severity of the inflammation was graded in five categories: 0,
Normal; 1, few inflammatory cells; 2, a ring of inflammatory cells
one cell layer deep; 3, a ring of inflammatory cells two to four
cells deep; and 4, a ring of inflammatory cells of four cells deep.
PAS-positive cells in each airway were counted, divided by the
circumference of the basement membrane and multiplied by
100(24). The data was collected
by three independent blinded investigators (FZT, JC, JJQ).
Transmission electron microscopy
(TEM)
Changes in mitochondrial structure were observed
using TEM. Lower lobe of right lung was cut into 1x1x3
mm3 sections and fixed in 2.5% glutaraldehyde phosphate
buffer at 4˚C for 24 h. Fixed samples were dehydrated with ethanol
and acetone, embedded (Epon812; cat. no. 45345; Sigma-Aldrich;
Merck KGaA) and dried. Afterward, the tissue was cut into 70-nm
thick sections, stained with uranyl acetate and citrate at room
temperature for 30 min and visualized using the JEM-1400 Plus
transmission electron microscope (magnification, x20,000; JEOL,
Ltd.).
Leukocyte differential counts in
BALF
BALF was collected by flushing the right lungs three
times with 1 ml PBS using a 1-ml syringe inserted into a cannula.
BALF was centrifuged for 10 min at 500 x g and 4˚C, after which the
supernatant was collected for cytokines analysis. The pellet
containing the cells was resuspend in 100 µl PBS to determine the
leukocyte differential count using the BC-5000 Vet auto hematology
analyzer (MINDRAY Medical International Co., Ltd.).
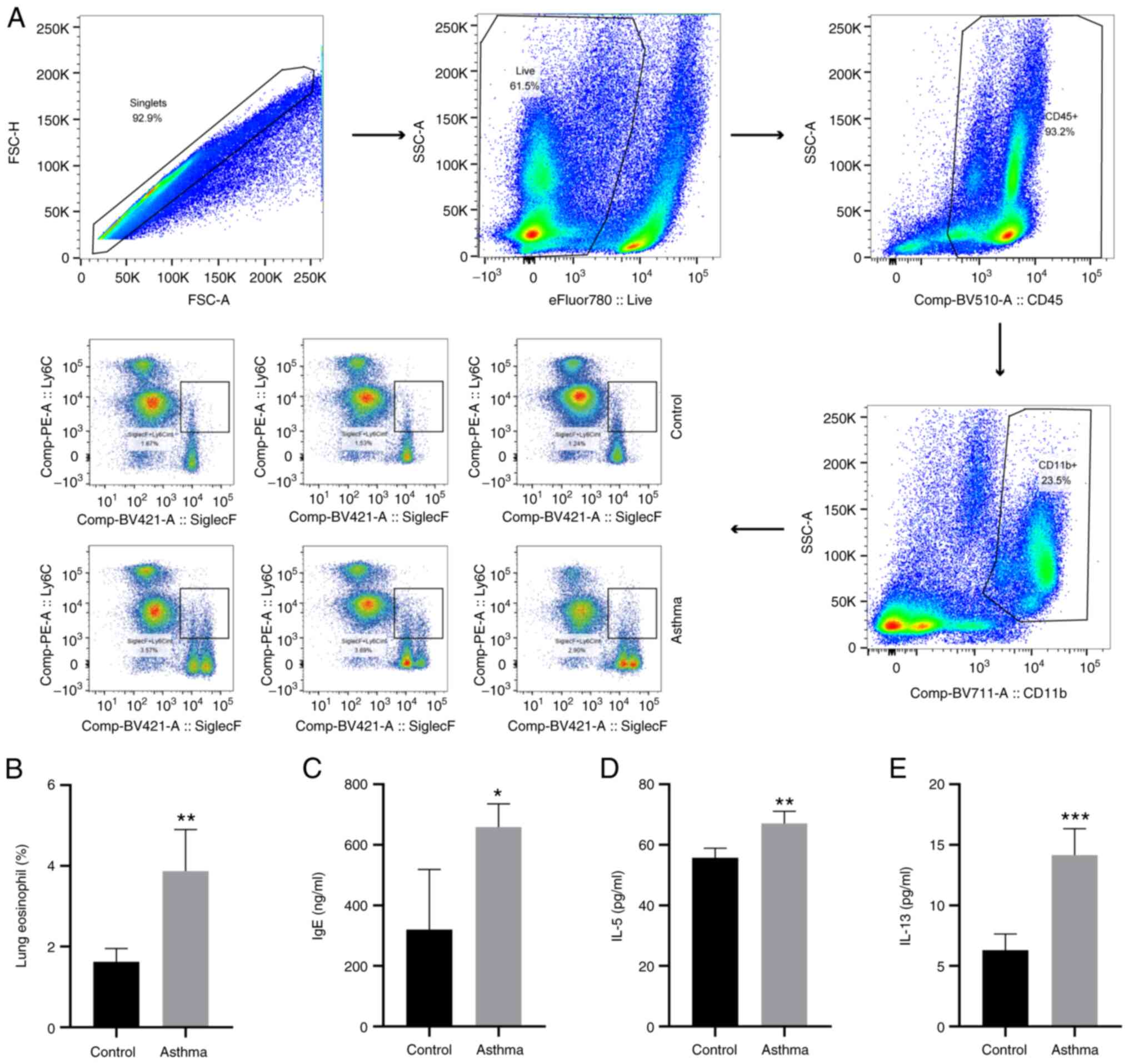
Cytokines in BALF
IgE and T helper (Th) 2 cells play a pathogenic role
in asthma (25). The levels of
IgE, along with Th2 cytokines IL-5 and IL-13, were therefore
investigated in the supernatants of BALF samples using sandwich
ELISA kits according to the manufacturer's instructions.
Flow cytometry of lung tissue
Eosinophil (Eos) has been implicated in the
pathogenesis of asthma (26). Eos
populations in lung tissue were detected using flow cytometry.
CD45+CD11b+Ly6C-SiglecF+cells were
identified as the EOS population as described by a previous study
(27). The mouse lung dissociation
kit was used to dissociate the upper right lobe of each lung to
single cells. Afterwards, single cells were stained with the
fluorescently labeled dyes or antibodies (Live eFluor780, CD45
eFluor 506, anti-mouse CD11b BV711, anti-mouse SiglecF BV421,
anti-mouse Ly6C PE) at 1x106 cells/100 µl at 4˚C for 30
min detected using an Attune NxT instrument (Thermo Fisher
Scientific, Inc.). Flow analysis was done using the FlowJo version
10 software (FlowJo, LLC).
ROS and GSH measurement
For ROS, the tissue samples were embedded with
Tissue-Tek® OCT (cat. no. 4583; Sakura Finetek USA,
Inc.) and then prepared into frozen sections. The embedded samples
were cut into 10-µm thick slices using a cryostat (CM1860; Leica
Microsystems GmbH) and transferred onto glass slide and stored at
-20˚C. They were then stained according to the protocols of the ROS
kit. For GSH sample preparation, the tissue sample was prepared
into a 10% tissue homogenate [tissue weights (g): Volume of normal
saline (ml)=1:9], and the subsequent determination was performed
according to the protocols of the GSH kit. Ferroptosis is
determined by the balance between iron accumulation-induced ROS
production and the antioxidant system that avoids lipid
peroxidation (16). In the present
study, the upper lobe of the left lung is used for ROS and part of
the lower right lung is used for GSH detection, ROS and GSH levels
were determined using commercial ROS and GSH assay kits according
to the manufacturer's instructions.
Western blot assay
GPX4, ACSL4, 15-LO1 and SLC7A11 are mediators of
lipid peroxidation and ferroptosis, and have been reported to be
involved in the ferroptosis pathway (16). The expression levels of these
proteins were assayed using western blotting. Lung tissues were
homogenized and sonicated in RIPA lysis buffer, and then
centrifuged at 13,201 x g for 10 min at 4˚C. Afterwards, the
supernatants were collected and the total protein level was
measured using a BCA protein test kit. A 12% SDS-PAGE separation
gel (30 µg per lane) was prepared for protein separation and
transferred to PVDF membranes, followed by blockage in 5% non-fat
milk at room temperature for 1 h. Afterwards, the membranes were
washed three times with Tris buffered saline 0.1% Tween-20 every 10
min. After that, the PVDF membranes were incubated with GPX4
(1:1,000), ACSL4 (1:1,000), 15-LO1 (1:1,000) and SLC7A11 (1:1,000)
antibodies at 4˚C overnight. After three washes with TBST, the PVDF
membranes were incubated with secondary antibodies for 1.5 h at
room temperature (goat anti-rabbit IgG HRP-conjugated secondary
antibody; cat. no. L3012; Signalway Antibody LLC; 1:10,000
dilution). Immobilon western chemiluminescent HRP substrate
solution was used to exhibit protein band after three washes with
TBST. β-actin (1:10,000; dilution; cat. no. HRP-60008; Proteintech
Group, Inc.) was used as the internal control for the normalization
of the data using ImageJ (v152p; National Institutes of
Health).
Statistical analysis
All the experiments were repeated ≥ three times. All
data were analyzed and graphed using GraphPad Prism 8 (GraphPad
Software, Inc.). Data are presented as the mean ± standard
deviation. Unpaired Student's t-test or Mann-Whitney U test were
used to analyze the difference between two samples. P<0.05 was
considered to indicate a statistically significant difference.
Results
HDM exposure induces an increase in
AHR following Mch treatment
Changes in AHR were investigated in the current
study. Compared with mice in the normal control group, when the Mch
dose increased, the RL increased significantly (P<0.05
or P<0.001; Fig. 1B), whereas
Cdyn decreased significantly (P<0.05 or P<0.01; Fig. 1C). There was no obvious increase in
RL between the two groups at Mch doses <50 mg/ml
(Fig. 1B). However, Mch challenge
at 50 and 100 ng/ml resulted in the prominent augmentation of
RL. At Mch doses of 12.5 (P<0.05), 50 (P<0.01) and
100 (P<0.01) mg/ml, HDM inhalation induced a significant
decrease in Cdyn compared with the control group (Fig. 1C). These data indicated that HDM
exposure could aggravate AHR.
HDM exposure promotes lung
inflammation and goblet cell hyperplasia
H&E staining was performed to evaluate the
inflammatory changes within the lung. The results revealed that
inflammatory cell infiltration around the bronchus (Fig. 2A and B) was observed in mice receiving HDM
inhalation, and that HDM exposure significantly aggravated the
pulmonary inflammatory response compared with the control
(P<0.05; Fig. 2B). Mucus
hypersecretion is a notable feature of allergic asthma (28). Therefore, PAS staining was
conducted to evaluate the degree of goblet cell metaplasia and
mucus secretion. As presented in Fig.
2A and C, mucus secretion
increased significantly compared with mice in the normal control
group. In addition, inflammatory cells in BALF were also detected,
and the results demonstrated that the total leukocytes, lymphocytes
(Lym), Eos and monocytes (Mon) increased significantly after HDM
exposure compared with the control (P<0.05; Fig. 2D). These aforementioned data
suggest that HDM exposure promoted lung inflammation and goblet
cell proliferation.
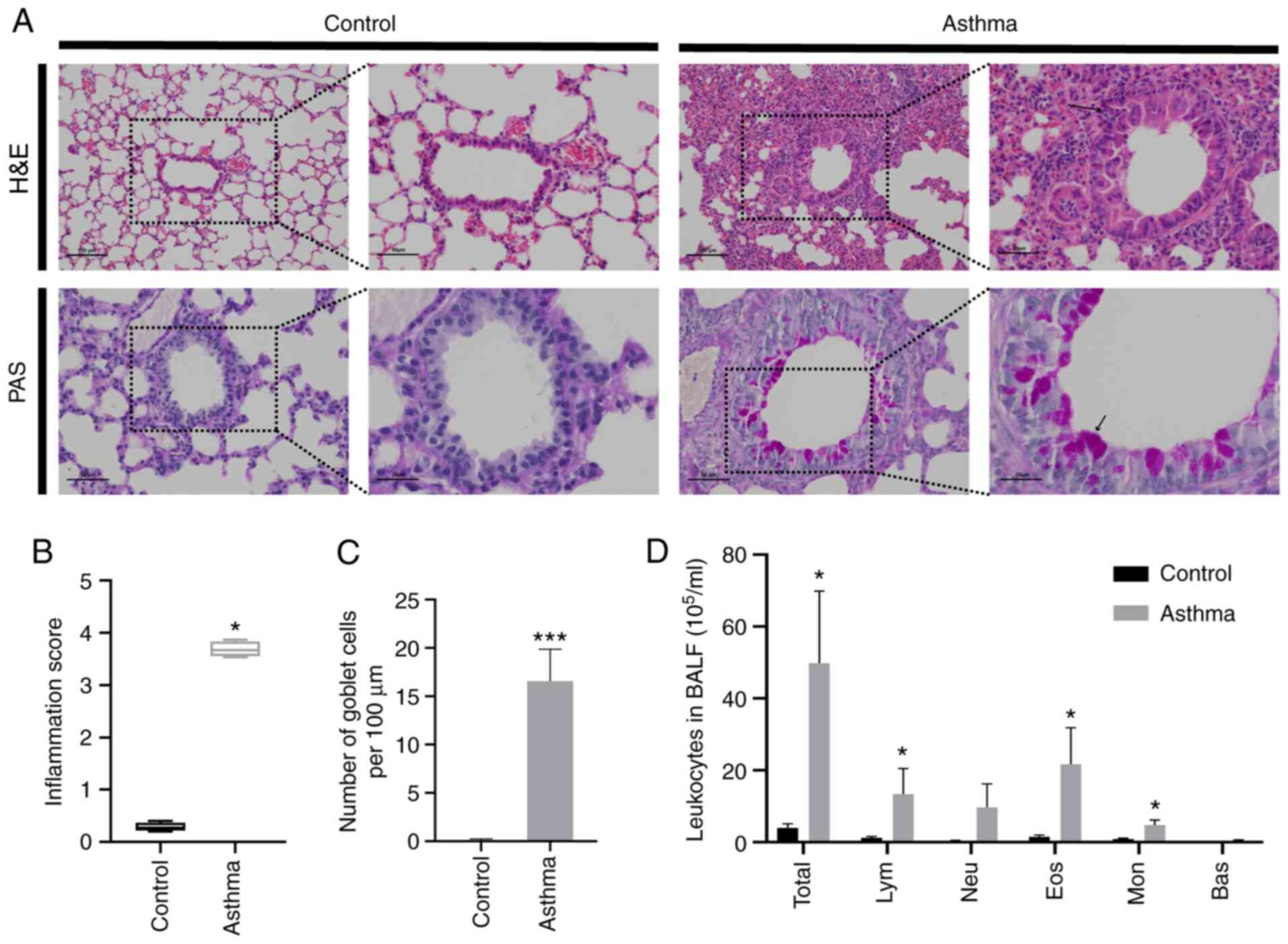 | Figure 2House dust mite exposure promotes
lung inflammation and goblet cell hyperplasia. (A) Representative
images of H&E (x100, scale bars, 100 µm; x200, scale bars, 50
µm) and PAS staining (x200, scale bars, 50 µm; x400, scale bars, 25
µm) results (arrows indicate PAS-positive cells). (B) Inflammation
score of the H&E results. (C) Changes in goblet cell counts.
(D) Classification of leukocytes in the BALF. *P<0.05
and ***P<0.001 compared with the control. H&E,
hematoxylin and eosin; PAS, periodic acid Schiff; BALF,
bronchoalveolar lavage fluid; Lym, lymphocytes; Neu, Neutrophils;
Eos, eosinophils; Mon, monocytes; Bas, basophils. |
HDM inhalation enhances airway
eosinophilic and T helper (Th)2-associated inflammation
CD45+CD11b+SiglecF+Ly6C in Eos
populations of the lung tissue were detected using flow cytometry.
Mice in the HDM-induced asthma group demonstrated significantly
increased profiles of
CD45+CD11b+SiglecF+Ly6C in the Eos
proportion compared with mice in the control group (P<0.01;
Fig. 3A and B). Furthermore, IgE, IL-5 and IL-13
levels in BALF were also investigated using ELISA. Elevated IgE,
IL-5 and IL-13 expression levels were detected compared with
control group mice (P<0.05, P<0.01 or P<0.001; Fig. 3C-E). Therefore, HDM-induced airway
inflammation was characterized by Th2-mediated eosinophilic
inflammation in the lungs.
HDM exposure induces ferroptosis in
the lung
Imbalance between ROS production and the antioxidant
system is associated with ferroptosis (16). The present study revealed that HDM
exposure resulted in significantly elevated ROS (P<0.05;
Fig. 4A and B) and reduced GSH levels (P<0.05;
Fig. 4C) in the lung tissue
compared with those in the control mice. It has been reported that
ferroptosis is associated with mitochondrial fragmentation and
cristae decrease (29). To explore
the role of HDM inhalation in ferroptosis, the morphological
changes of mitochondria were observed using TEM. Consistent with
literature reports (15,27,30),
dysmorphic small mitochondria were observed in the lung cells after
HDM inhalation. Representative results are presented in Fig. 4D. Decreased mitochondria crista,
condensed and ruptured outer membranes were also discovered in the
lung cells of mice in HDM-induced asthma group. These results
provided evidence that HDM exposure could induce ferroptosis in the
lungs.
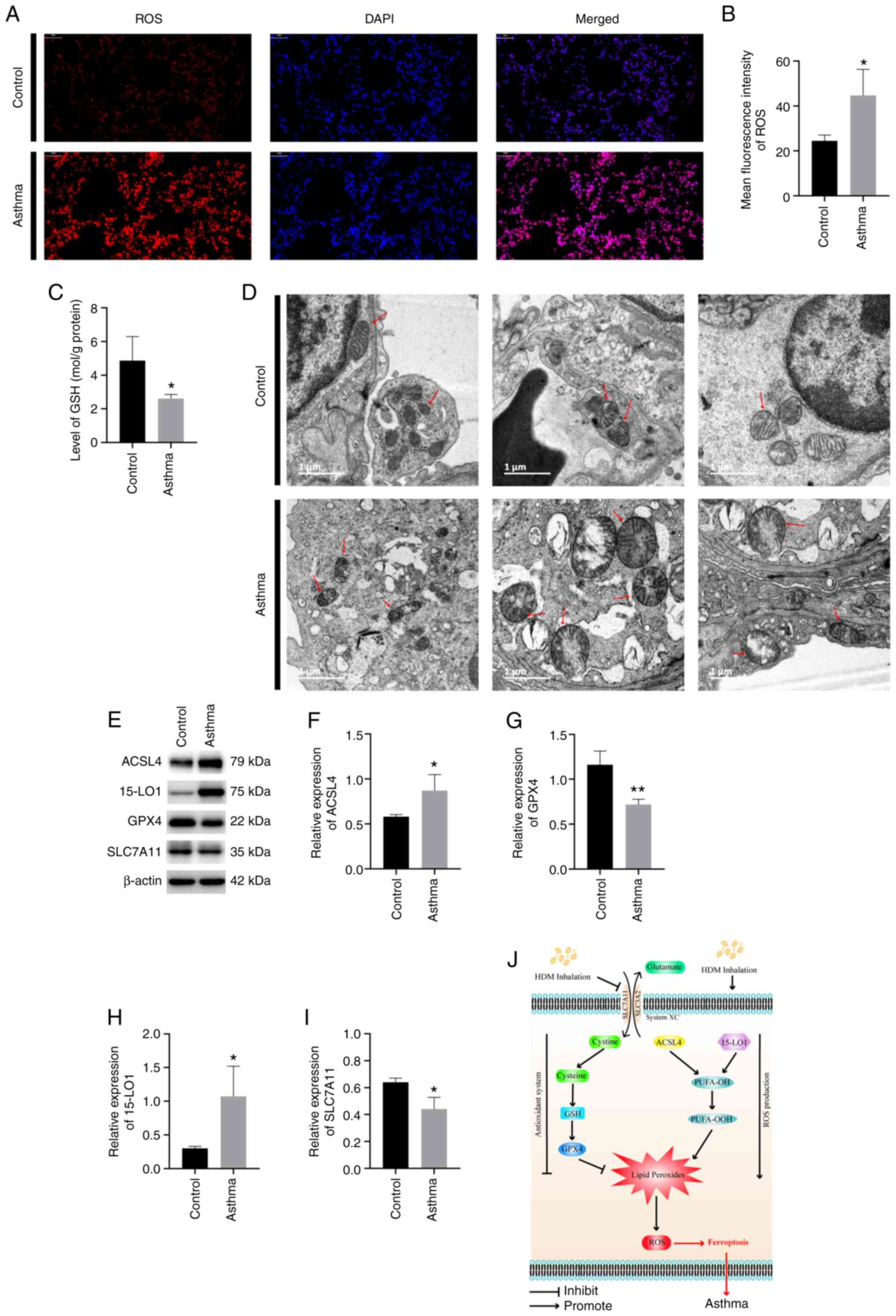 | Figure 4HDM exposure induces ferroptosis in
the lungs. (A and B) ROS production in the lungs. (C) GSH level in
the lung. (D) Morphological changes of mitochondria in ferroptosis
after HDM exposure (arrows indicate mitochondria). (E)
Representative image of western blotting of ferroptosis. Expression
levels of (F) ACSL4, (G) GPX4, (H) 15-LO1 and (I) SLC7A11 in the
lungs. (J) Pathways controlling ferroptosis in HDM-induced asthma
pathogenesis. System Xc-, a cystine-glutamate antiporter.
*P<0.05, **P<0.01. HDM, house dust
mites; ROS, reactive oxygen species; GSH, glutathione; ACSL4,
anti-acyl-CoA synthetase long-chain family member 4; GPX4,
Glutathione peroxidase 4; 15-LO1, 15 Lipoxygenase 1; SLC7A11,
solute carrier family 7 member 11; PUFA-OOH, polyunsaturated fatty
acids; -OOH, hydroperoxy radicals. |
HDM exposure induces dysregulation of
proteins associated with the ferroptosis pathway
To investigate the underlying mechanisms associated
with ferroptosis, key proteins involved in ferroptosis were
measured. The protein expression levels of GPX4, ACSL4, 15-LO1 and
SLC7A11 were detected. The quantified western blotting results
revealed that mice that received HDM inhalation had significantly
downregulated activities of GPX4 compared with the normal control
group (P<0.01; Fig. 4E and
G). Furthermore, a significant
upregulation of ACSL4 and 15-LO1 as well as downregulation of
SLC7A11 were detected in HDM-induced asthmatic mice compared with
the controls (P<0.05; Fig. 4E,
F, H and I).
These results demonstrated that GPX4, ACSL4, 15-LO1 and SLC7A11
were involved in ferroptosis in the lungs of HDM-induced asthmatic
mice.
Discussion
Asthma is a heterogeneous chronic inflammatory
disease of the airways characterized by chronic airway inflammation,
bronchoconstriction, AHR and mucus hypersecretion (31,32).
Previous studies have revealed that asthma is a heterogeneous
disease with multiple phenotypes and endotypes (33,34).
Allergic asthma is the most common form of asthma, which can be
triggered by allergens in the environment, such as HDM (7,8). As
a naturally occurring allergen in the environment, HDM is
frequently used for the preparation of allergic mouse asthma models
to uncover the key inflammatory pathways in the progress of
allergic asthma (35).
The present study used an HDM-induced mouse asthma
model to determine the effect of HDM exposure on allergic asthma
and the mechanisms that underlie this. The results demonstrated
that HDM administered intranasally increased AHR in mice compared
with mice exposed to PBS. Asthma is characterized by chronic
inflammation in the airways. In order to observe the inflammatory
changes in the airways, H&E and PAS staining of the lung
sections were performed in the current study. Inflammatory cell
infiltration around the bronchus, goblet cell metaplasia and mucus
hypersecretion were observed in murine lung tissue after HDM
inhalation. Furthermore, the levels of total leukocytes, Lym, Eos
and Mon increased significantly after HDM exposure, indicating that
lung inflammation was induced by HDM. In addition,
CD45+CD11b+SiglecF+Ly6C cells were
detected in the Eos population of the lung tissue, which was
consistent with the results of BALF leukocyte classification.
Therefore, mice in the HDM-induced asthma group demonstrated
significantly increased levels of
CD45+CD11b+SiglecF+Ly6C in the Eos
population.
IgE and Th2 cells play a pathogenic role in asthma
(36,37). Thus, the present study further
investigated the levels of IgE and Th2 cytokines IL-5 and IL-13 in
the supernatant of BALF. It has been hypothesized that the cysteine
protease activity of Der p 1 in HDM selectively enhances the IgE
response and upregulates IgE synthesis by cleaving the low-affinity
IgE receptor (CD23) from the surface of human B cell lymphocytes
(38). Allergic asthma accounts
for ~80% of asthma cases, and high levels of Th2 cytokines can be
detected in lung tissue and BALF (39). Mounting evidence has demonstrated
that IL-5 and IL-13 play a prominent role in eosinophil activation
and mucus hyperplasia (40,41).
Consistent with these former reports, the present study revealed
that mice exposed to HDM had elevated IgE, IL-5 and IL-13
expression levels compared with mice receiving PBS. Overall, the
results indicated that HDM inhalation induced AHR and type 2 airway
inflammation in asthma.
Ferroptosis is an iron-dependent cell-death modality
driven by oxidative phospholipid damage and has been implicated in
a number of diseases, including Huntington's disease and diffuse
large B cell lymphoma (42,43).
The current study investigated whether ferroptosis participated in
the pathological progression of HDM-induced asthma. Mitochondria
have been reported to be involved in cysteine-deprivation-induced
ferroptosis (15). Morphological
changes of mitochondria, which include mitochondrial fragmentation
and a decrease in cristae, are observed in ferroptosis (29). Furthermore, certain potent
ferroptosis inhibitors have been demonstrated to target
mitochondria (44). A previous
study demonstrated that lipid ROS is required for ferroptosis
(14), indicating that the
accumulation of ROS is a key feature of ferroptosis. Moreover,
mitochondria are the major organelle of cellular ROS production. A
decreased level of lipid ROS accumulation has been observed in
mitochondria-depleted cells (15),
which further confirms the involvement of mitochondria in
ferroptosis. In the present study, morphological changes of
mitochondria were observed using TEM, the results of which revealed
that decreased mitochondria crista and condensed and ruptured outer
membranes were observed in the lung cells of mice in the
HDM-induced asthma group, which was consistent with previous
results.
Polyunsaturated fatty acids (PUFAs) are the primary
targets of lipid peroxidation on cell membranes (16). The activity of multiple upstream
cascades determine the sensitivity to ferroptosis (including free
PUFAs) as well as the balance between levels of pro-oxidant factors
(ROS and lipoxygenases) and antioxidant factors (GPX4 and GSH)
(45). GSH is an anti-oxidant that
serves as a cofactor for the reduction of lipid hydroperoxides by
GPX4(46). It has been reported
that depletion of GSH by erastin inactivates GPX4, leading to the
accumulation of lipid ROS and lipid peroxidation (47). Inhibition of GSH synthesis induces
ferroptosis in certain cells. In the present study, ROS and GSH
levels in the lung were detected, and the results demonstrated that
HMD exposure induced an increase of ROS production and reduced GSH
levels in the lung tissue. This indicated that HDM exposure may
result in an imbalance between pro-oxidant and anti-oxidant factors
in the lung (26).
GPX4 is an enzyme required for the clearance of
lipid ROS. GPX4 catalyzes GSH to eliminate the production of
phospholipid hydroperoxides, which are major mediators of chain
reactions in lipoxygenases (16).
As the furthest downstream component of the ferroptosis pathway,
inactivation of GPX4 results in ferroptosis even when cellular
cysteine and GSH contents are normal (48). GPX4 activity was assessed by
western blotting in the present study. The results revealed that
mice receiving HDM inhalation had downregulated levels of GPX4
activity. ACSL4 functions to preferentially activate long PUFAs for
phospholipid biosynthesis (29).
The synthesis of long chain PUFA-CoA was inhibited in
ACSL4-/- cells, supporting the hypothesis that of ACSL4
has a function in lipid biosynthesis (29). The upregulation of ACSL4, but not
other ACSL members, is an important component for ferroptosis
(29,49). Resistance to ferroptosis was
observed in GPX4/ACSL4 double knockout cells. Previous results
indicated that ACSL4 inhibition can be used as a therapeutic
approach to prevent ferroptosis-related diseases (50).
The present study also investigated the effect of
HDM inhalation on ACSL4 expression. Upregulation of ACSL4 was
observed in HDM-induced asthma, indicating the augmentation of
lipid biosynthesis by HDM inhalation. Lipoxygenases are effective
in the oxygenation of free PUFA (51). 15LO1 is highly expressed in human
airway epithelial cells (52). A
previous study revealed that the expression of 15LO1 is increased
in Th2 inflammation observed in asthma (53). Moreover, airway epithelial cells
isolated from patients with stable, non-exacerbating asthma have an
elevated level of 15LO1, which is correlated with the fraction of
exhaled nitric oxide, one of the key markers of Th2 inflammation
(30). The present study detected
the level of 15LO1 in the lung tissues of mice exposed to HDM, and
an increase in the protein expression of 15LO1 was revealed, which
corresponded with the results of previous literature.
As a component of the cystine/glutamate antiporter
system Xc-, SLC7A11 is required for the exchange of
extracellular cystine and intracellular glutamate across the plasma
membrane, therefore providing adequate cystine within cells
(54). Cystine is further reduced
to cysteine within the cell, which is an important precursor
required for GSH synthesis (55).
Ferroptosis can be initiated by the depletion of cellular cysteine
through inhibition of cystine uptake mediated by SLC7A11 (56,57).
Previous results have demonstrated that SLC7A11 downregulation
significantly increases nascent neoplastic cell susceptibility to
ferroptosis, thus keeping tumorigenesis in check (42,43).
In the present study, downregulation of SLC7A11 was detected in
HDM-induced asthma, which is consistent with its role in
ferroptosis. These data indicated that ferroptosis was observed in
the HDM-induced mouse asthma model, and that ROS, GSH, GPX4, ACSL4,
15LO1 and SLC7A11 were involved in the ferroptosis of murine lungs
following HDM-induced asthma (Fig.
4J).
There are certain limitations to the present study.
Due to the limited sample volume and sample preparation, IL-4
levels in BALF and IgE levels in serum were not detected, which
should be detected in future follow-up experiments. Furthermore,
The effect of agonists and inhibitors of ferroptosis on airway
inflammation in HDM-induced asthma also requires further study.
In summary, the present study demonstrated that AHR,
airway inflammation, lipid peroxidation and ROS levels were
increased in a murine model of HDM-induced asthma, and that HDM
inhalation induced ferroptosis in the lungs. The results have
helped to form an improved understanding of the pathogenesis of
allergic asthma and targeted treatment strategies.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from The Young Elite
Scientists Sponsorship Program by China Association for Science and
Technology (grant no. 2018QNRC001), the National Natural Science
Foundation of China (grant no. 82174495) and Shanghai Science and
Technology Commission Project (grant no. 21S21902500).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW and JD designed the study. WT and MD analyzed the
data. WT, MD, FT, JC, XZ, WW, TW, JQ, LY and SW performed the
experiments. YW and WT wrote the manuscript. All authors read and
approved the final manuscript. YW and WT confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The experimental procedures were approved by the
Ethics Committee of Animal Experiments of Fudan University
(authorization no. 2020-10-HSYY-WY-01; Shanghai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Caraballo L, Zakzuk J, Lee BW, Acevedo N,
Soh JY, Sánchez-Borges M, Hossny E, García E, Rosario N, Ansotegui
I, et al: Particularities of allergy in the tropics. World Allergy
Organ J. 9(20)2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Abou-Hamdan M, Gharib B, Bajenoff M, Julia
V and de Reggi M: Pantethine down-regulates leukocyte recruitment
and inflammatory parameters in a mouse model of allergic airway
inflammation. Med Sci Monit Basic Res. 23:368–372. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rogliani P, Calzetta L, Matera MG, Laitano
R, Ritondo BL, Hanania NA and Cazzola M: Severe asthma and
biological therapy: When, which, and for whom? Pulm Ther. 6:47–66.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Calzetta L, Matera MG, Coppola A and
Rogliani P: Prospects for severe asthma treatment. Curr Opin
Pharmacol. 56:52–60. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Reddel HK, Bateman ED, Becker A, Boulet
LP, Cruz AA, Drazen JM, Haahtela T, Hurd SS, Inoue H, de Jongste
JC, et al: A summary of the new GINA strategy: A roadmap to asthma
control. Eur Respir J. 46:622–639. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sulaiman I, Greene G, MacHale E, Seheult
J, Mokoka M, D'Arcy S, Taylor T, Murphy DM, Hunt E, Lane SJ, et al:
A randomised clinical trial of feedback on inhaler adherence and
technique in patients with severe uncontrolled asthma. Eur Respir
J. 51(1701126)2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Caraballo L: Mite allergens. Expert Rev
Clin Immunol. 13:297–299. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yang L and Zhu R: Immunotherapy of house
dust mite allergy. Hum Vaccin Immunother. 13:2390–2396.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Custovic A, Green R, Smith A, Chapman MD
and Woodcock A: New mattresses: How fast do they become a
significant source of exposure to house dust mite allergens? Clin
Exp Allergy. 26:1243–1245. 1996.PubMed/NCBI
|
|
10
|
Fernández-Caldas E, Puerta L and Caraballo
L: Mites and allergy. Chem Immunol Allergy. 100:234–242.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li L, Qian J, Zhou Y and Cui Y: Domestic
mite-induced allergy: Causes, diagnosis, and future prospects. Int
J Immunopathol Pharmacol. 32(2058738418804095)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jacquet A: Interactions of airway
epithelium with protease allergens in the allergic response. Clin
Exp Allergy. 41:305–311. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dolma S, Lessnick SL, Hahn WC and
Stockwell BR: Identification of genotype-selective antitumor agents
using synthetic lethal chemical screening in engineered human tumor
cells. Cancer Cell. 3:285–296. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gao M, Yi J, Zhu J, Minikes AM, Monian P,
Thompson CB and Jiang X: Role of mitochondria in ferroptosis. Mol
Cell. 73:354–363.e3. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tang D, Kang R, Berghe TV, Vandenabeele P
and Kroemer G: The molecular machinery of regulated cell death.
Cell Res. 29:347–364. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Friedmann Angeli JP, Schneider M, Proneth
B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch
A, Eggenhofer E, et al: Inactivation of the ferroptosis regulator
Gpx4 triggers acute renal failure in mice. Nat Cell Biol.
16:1180–1191. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wu X, Li Y, Zhang S and Zhou X:
Ferroptosis as a novel therapeutic target for cardiovascular
disease. Theranostics. 11:3052–3059. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ghiamati Yazdi F, Zakeri A, van Ark I,
Leusink-Muis T, Braber S, Soleimanian-Zad S and Folkerts G: Crude
turmeric extract improves the suppressive effects of lactobacillus
rhamnosus GG on allergic inflammation in a murine model of house
dust mite-induced asthma. Front Immunol. 11(1092)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tibbitt CA, Stark JM, Martens L, Ma J,
Mold JE, Deswarte K, Oliynyk G, Feng X, Lambrecht BN, De Bleser P,
et al: Single-cell RNA sequencing of the T helper cell response to
house dust mites defines a distinct gene expression signature in
airway Th2 cells. Immunity. 51:169–184.e5. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wei Y, Dong M, Zhang H, Lv Y, Liu J, Wei
K, Luo Q, Sun J, Liu F, Xu F and Dong J: Acupuncture attenuated
inflammation and inhibited Th17 and treg activity in experimental
asthma. Evid Based Complement Alternat Med.
2015(340126)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wang S, Wuniqiemu T, Tang W, Teng F, Bian
Q, Yi L, Qin J, Zhu X, Wei Y and Dong J: Luteolin inhibits
autophagy in allergic asthma by activating PI3K/Akt/mTOR signaling
and inhibiting Beclin-1-PI3KC3 complex. Int Immunopharmacol.
94(107460)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Myou S, Leff AR, Myo S, Boetticher E, Tong
J, Meliton AY, Liu J, Munoz NM and Zhu X: Blockade of inflammation
and airway hyperresponsiveness in immune-sensitized mice by
dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med.
198:1573–1582. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gavino AC, Nahmod K, Bharadwaj U,
Makedonas G and Tweardy DJ: STAT3 inhibition prevents lung
inflammation, remodeling, and accumulation of Th2 and Th17 cells in
a murine asthma model. Allergy. 71:1684–1692. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Honda K, Arima M, Cheng G, Taki S, Hirata
H, Eda F, Fukushima F, Yamaguchi B, Hatano M, Tokuhisa T and Fukuda
T: Prostaglandin D2 reinforces Th2 type inflammatory responses of
airways to low-dose antigen through bronchial expression of
macrophage-derived chemokine. J Exp Med. 198:533–543.
2003.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yi S, Zhai J, Niu R, Zhu G, Wang M, Liu J,
Huang H, Wang Y, Jing X, Kang L, et al: Eosinophil recruitment is
dynamically regulated by interplay among lung dendritic cell
subsets after allergen challenge. Nat Commun.
9(3879)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fransén-Pettersson N, Duarte N, Nilsson J,
Lundholm M, Mayans S, Larefalk Å, Hannibal TD, Hansen L,
Schmidt-Christensen A, Ivars F, et al: A new mouse model that
spontaneously develops chronic liver inflammation and fibrosis.
PLoS One. 11(e0159850)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cheng D, Xue Z, Yi L, Shi H, Zhang K, Huo
X, Bonser LR, Zhao J, Xu Y, Erle DJ and Zhen G: Epithelial
interleukin-25 is a key mediator in Th2-high,
corticosteroid-responsive asthma. Am J Respir Crit Care Med.
190:639–648. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Telorack M, Meyer M, Ingold I, Conrad M,
Bloch W and Werner S: A glutathione-Nrf2-thioredoxin cross-talk
ensures keratinocyte survival and efficient wound repair. PLoS
Genet. 12(e1005800)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ish P, Malhotra N and Gupta N: GINA 2020:
What's new and why? J Asthma. 58:1273–1277. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Hogan AD and Bernstein JA: GINA updated
2019: Landmark changes recommended for asthma management. Ann
Allergy Asthma Immunol. 124:311–313. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wenzel SE: Asthma phenotypes: The
evolution from clinical to molecular approaches. Nat Med.
18:716–725. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Agache I and Akdis CA: Precision medicine
and phenotypes, endotypes, genotypes, regiotypes, and theratypes of
allergic diseases. J Clin Invest. 129:1493–1503. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ito R, Maruoka S, Soda K, Katano I, Kawai
K, Yagoto M, Hanazawa A, Takahashi T, Ogura T, Goto M, et al: A
humanized mouse model to study asthmatic airway inflammation via
the human IL-33/IL-13 axis. JCI Insight. 3(e121580)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jäger A and Kuchroo VK: Effector and
regulatory T-cell subsets in autoimmunity and tissue inflammation.
Scand J Immunol. 72:173–184. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Shang XZ, Ma KY, Radewonuk J, Li J, Song
XY, Griswold DE, Emmell E and Li L: IgE isotype switch and IgE
production are enhanced in IL-21-deficient but not
IFN-gamma-deficient mice in a Th2-biased response. Cell Immunol.
241:66–74. 2006.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gough L, Sewell HF and Shakib F: The
proteolytic activity of the major dust mite allergen Der p 1
enhances the IgE antibody response to a bystander antigen. Clin Exp
Allergy. 31:1594–1598. 2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Qiu S, Fan X, Yang Y, Dong P, Zhou W, Xu
Y, Zhou Y, Guo F, Zheng Y and Yang JQ: Schistosoma japonicum
infection downregulates house dust mite-induced allergic airway
inflammation in mice. PLoS One. 12(e0179565)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lambrecht BN, Hammad H and Fahy JV: The
cytokines of asthma. Immunity. 50:975–991. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bush A: Cytokines and chemokines as
biomarkers of future asthma. Front Pediatr. 7(72)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zou Y and Schreiber SL: Progress in
understanding ferroptosis and challenges in its targeting for
therapeutic benefit. Cell Chem Biol. 27:463–471. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Gao M and Jiang X: To eat or not to
eat-the metabolic flavor of ferroptosis. Curr Opin Cell Biol.
51:58–64. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Krainz T, Gaschler MM, Lim C, Sacher JR,
Stockwell BR and Wipf P: A mitochondrial-targeted nitroxide is a
potent inhibitor of ferroptosis. ACS Cent Sci. 2:653–659.
2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Konstorum A, Tesfay L, Paul BT, Torti FM,
Laubenbacher RC and Torti SV: Systems biology of ferroptosis: A
modeling approach. J Theor Biol. 493(110222)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lu SC: Regulation of glutathione
synthesis. Mol Aspects Med. 30:42–59. 2009.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Dixon SJ and Stockwell BR: The role of
iron and reactive oxygen species in cell death. Nat Chem Biol.
10:9–17. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ingold I, Berndt C, Schmitt S, Doll S,
Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T,
et al: Selenium utilization by GPX4 is required to prevent
hydroperoxide-induced ferroptosis. Cell. 172:409–422.e21.
2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Xu Y, Li X, Cheng Y, Yang M and Wang R:
Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary
ischemia-reperfusion. FASEB J. 34:16262–16275. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhou Y, Liao J, Mei Z, Liu X and Ge J:
Insight into crosstalk between ferroptosis and necroptosis: Novel
therapeutics in ischemic stroke. Oxid Med Cell Longev.
2021(9991001)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Nicolaou A, Mauro C, Urquhart P and
Marelli-Berg F: Polyunsaturated fatty acid-derived lipid mediators
and T cell function. Front Immunol. 5(75)2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wenzel SE, Tyurina YY, Zhao J, St Croix
CM, Dar HH, Mao G, Tyurin VA, Anthonymuthu TS, Kapralov AA,
Amoscato AA, et al: PEBP1 wardens ferroptosis by enabling
lipoxygenase generation of lipid death signals. Cell.
171:628–641.e26. 2017.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhao J, O'Donnell VB, Balzar S, St Croix
CM, Trudeau JB and Wenzel SE: 15-Lipoxygenase 1 interacts with
phosphatidylethanolamine-binding protein to regulate MAPK signaling
in human airway epithelial cells. Proc Natl Acad Sci USA.
108:14246–14251. 2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Schrier MS, Trivedi MS and Deth RC:
Redox-related epigenetic mechanisms in glioblastoma: Nuclear factor
(erythroid-derived 2)-like 2, cobalamin, and dopamine receptor
subtype 4. Front Oncol. 7(46)2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Xu X, Zhang X, Wei C, Zheng D, Lu X, Yang
Y, Luo A, Zhang K, Duan X and Wang Y: Targeting SLC7A11
specifically suppresses the progression of colorectal cancer stem
cells via inducing ferroptosis. Eur J Pharm Sci.
152(105450)2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang Y, Zhuang L and Gan B: BAP1
suppresses tumor development by inducing ferroptosis upon SLC7A11
repression. Mol Cell Oncol. 6(1536845)2018.PubMed/NCBI View Article : Google Scholar
|