Introduction
Non-small cell lung cancer (NSCLC) is a major
subtype of lung cancer, accounting for 11.6% of all new cancer
cases and 18.4% of all cancer-associated mortalities in 2018
worldwide (1). Surgery,
chemotherapy, radiation therapy and targeted therapy are currently
the main options for NSCLC treatment (2). To date, platinum-based chemotherapy
remains the standard treatment for the majority of patients with
advanced NSCLC (3). Despite the
improved understanding of tumor progression and therapy, the
overall survival rate remains low, particularly for patients with
metastatic disease (4). Therefore,
it is urgent to improve cure rate and prognosis of patients with
NSCLC.
Paclitaxel, also known as Taxol/Tax, is a natural
diterpene alkaloid initially extracted from the bark of the
Taxus brevifolia tree. The mechanism of action of paclitaxel
is inducing cell cycle arrest and cell death by stabilizing
microtubules and interfering with microtubule disintegration during
cell division (5). Paclitaxel has
been approved by the Food and Drug Administration for the treatment
of several types of cancer, including ovarian, breast and lung
cancer, and Kaposi's sarcoma (6).
The combination of paclitaxel and platinum chemotherapy has also
been approved for the treatment of advanced NSCLC (7). In addition, paclitaxel as a single
agent also exhibits similar antitumor efficiency in the second-line
setting (8). However, accumulating
evidence indicates that the therapeutic efficacy of paclitaxel is
compromised by drug resistance (9).
Therefore, there is an urgent need to elucidate the underlying
mechanism in order to overcome drug resistance and improve the
efficacy of paclitaxel.
IL-1β, one of the isoforms of IL-1, is a
pro-inflammatory cytokine that is secreted in response to
inflammatory stimuli, and aggravates the tissue injury caused by
acute and chronic diseases (10,11).
IL-1β is mainly produced by myeloid cells and its expression is
increased under disease conditions (12). Previous studies have demonstrated
that IL-1β plays a key role in various inflammatory diseases,
including lung cancer (13). High
serum IL-1β level in NSCLC is associated with short overall
survival (14). In addition, IL-1β
is associated with resistance to cancer drugs, such as cisplatin
(15), 5-fluorouracil (16) and imatinib (17). However, to the best of our
knowledge, the association between IL-1β and paclitaxel resistance
in NSCLC remains largely unknown.
The aim of the present study was to investigate the
role of IL-1β in Paclitaxe resistance of NSCLC cells. The
expression of IL-1β in lung cancer tissues and cells was detected,
and the effects of IL-1β on paclitaxel sensitivity and autophagy,
as well as the effects of autophagy on paclitaxel sensitivity, were
investigated, in order to determine whether IL-1β may be used for
modulating the sensitivity of NSCLC to paclitaxel.
Materials and methods
Tissue samples
A total of 30 paired NSCLC tissues and adjacent
normal tissues after resection were collected from Xi'an Chest
Hospital (Xi'an, China) between January 2017 and June 2018. The
tissues were frozen at -80˚C. There were 19 males and 11 females,
with a mean age of 62 years (range, 41-74). The distance between
the normal and the lung cancer tissue was >3 cm. None of the
patients who participated in the present study had received any
treatment prior to surgery. Written informed consent was obtained
from each participant and the study protocol was approved by the
Ethics Committee of Xi'an Chest Hospital (Xi'an, China).
Cell culture and treatment
The human NSCLC cell lines A549, HCC827 and H1650,
and the normal epithelial cell line BEAS-2B, were purchased from
American Type Culture Collection. The cells were cultured in
RPMI-1640 medium supplemented with 10% FBS and 100 mg/ml
streptomycin and 100 U/ml penicillin (all from Gibco; Thermo Fisher
Scientific, Inc.) at 37˚C with 5% CO2. A549 cells
exposed to different treatments were used for subsequent
experiments.
Cell Counting Kit-8 (CCK-8)
The CCK-8 assay (Dojindo Molecular Technologies,
Inc.) was performed to test cell viability. Following treatment
with 8 nM paclitaxel (APeXBIO Technology LLC), 20 ng/ml recombinant
human IL-1β (Sangon Biotech Co., Ltd.), 0.1 µg/ml tunicamycin
(Beijing Solarbio Science & Technology Co., Ltd.), paclitaxel +
IL-1β, paclitaxel + tunicamycin and blank control, then
2x103 A549 cells were seeded into 96-well plates and
incubated at 37˚C with 5% CO2 for 48 h. At 0, 12, 24 and
48 h, 10 µl CCK-8 reagent was added to each well and incubated with
the cells for 2 h. Absorbance was measured at 450 nm by a
microplate reader (Thermo Fisher Scientific, Inc.).
Flow cytometry
Cell apoptosis was analyzed using an Annexin V-FITC
Apoptosis Detection kit (BD Biosciences). A549 cells in the
logarithmic growth phase were exposed to different treatments (same
as in the CCK-8 assay) at 37˚C with 5% CO2 for 48 h,
washed twice with cold PBS and resuspended in binding buffer at a
density of 1x106 cells/ml. A total of 5 µl Annexin
V-FITC and 5 µl propidium iodide were added to cells and incubated
for 15 min in the dark at room temperature. Then, 400 µl binding
buffer was added to each tube. Apoptosis was analyzed using a BD
FACSCalibur flow cytometer (Accuri™ C6 Plus; BD Biosciences) within
1 h.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from paired NSCLC and
para-carcinoma tissues and NSCLC cell lines using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) and 1 µg of total RNA was reverse-transcribed to cDNA using
PrimeScript RT reagent kit at 37˚C for 15 min (Takara Bio, Inc.).
Subsequently, qPCR was performed using SYBR Premix Ex Taq (Perfect
Real Time; Takara Bio, Inc.) and an ABI Prism 7700 Real-Time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
following thermocycling conditions were used: Initial denaturation
for 30 sec at 95˚C; 40 cycles of 5 sec at 95˚C and 30 sec at 60˚C;
and dissociation for 15 sec at 95˚C, 30 sec at 60˚C and 15 sec at
95˚C. The specific primers for IL-1β and GAPDH were synthesized by
GenScript and the following sequences were used: IL-1β forward,
5'-ATGGCAGAAGTACCTAAGCTC-3'; and reverse,
5'-TTAGGAAGACACAAATTGCATGGTGAACTCAGT-3'; GAPDH forward,
5'-TGACTTCAACAGCGACACCCA-3' and reverse,
5'-CACCCTGTTGCTGTAGCCAAA-3'. The relative expression was calculated
using the 2-ΔΔCq method and presented as fold change
(18). GAPDH was used for
normalization.
Western blot analysis
Total protein was extracted from tissues and NSCLC
cells using ProteoPrep Sample Extraction kit (Sigma-Aldrich; Merck
KGaA). The QuantiPro™ BCA Assay kit (cat. no. QPBCA; Sigma-Aldrich;
Merck KGaA) was used to measure protein concentration. Protein
samples (30 µg) were separated via SDS-PAGE (10% gel; Bio-Rad
Laboratories, Inc.) and transferred to PVDF membranes. The
membranes were then blocked with 5% non-fat milk in TBS with 0.05%
Tween-20 for 1 h at room temperature. After the membranes were
incubated with primary antibodies at 4˚C overnight, secondary
antibodies were added and incubated with the membranes at room
temperature for 1 h. The bands were visualized with an ECL Western
Blotting substrate kit (cat. no. K820; BioVision, Inc.) and the
protein expression was quantified using ImageJ v1.8.0 software
(National Institutes of Health) with GAPDH as the loading control.
The primary antibodies used included: Mouse anti-IL-1β (1:1,000;
cat. no. 12242; Cell Signaling Technology, Inc.), rabbit anti-LC3B
(1:1,000; cat. no. ab51520; Abcam), rabbit anti-P62 (1:10,000; cat.
no. ab109012; Abcam) and rabbit anti-GAPDH (1:2,500; cat. no.
ab9485; Abcam). The corresponding secondary antibodies included:
Anti-mouse IgG HRP-conjugated antibody (1;2,000; cat. no. 7076;
Cell Signaling Technology, Inc.) and goat anti-rabbit IgG
HRP-conjugated antibody (1:20,000; cat. no. ab205718; Abcam).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 5.0 (GraphPad Software, Inc.). Paired Student's
t-test was used for measuring IL-1β expression in paired tissues.
One-way ANOVA followed by Tukey's post hoc test was performed to
evaluate differences between multiple groups. Data are presented as
the mean ± standard deviation of three experimental repeats.
P<0.05 was considered to indicate a statistically significant
difference.
Results
IL-1β expression is increased in NSCLC
tissues and cell lines
In order to investigate the level of IL-1β
expression in NSCLC tissues and cell lines, RT-qPCR and western
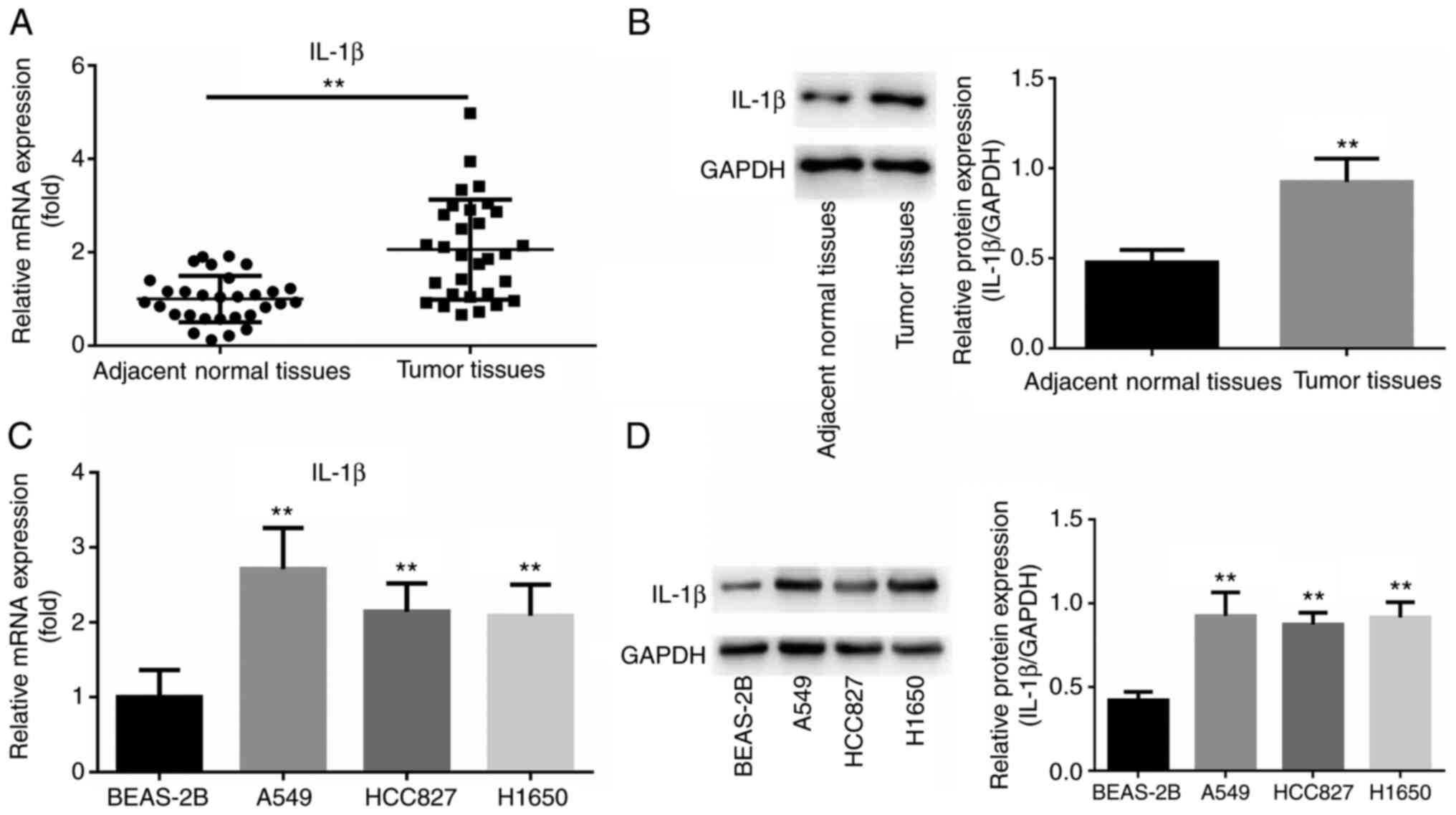
blot analyses were used. As shown in Fig. 1A, IL-1β mRNA expression was higher
in tumor tissues compared with that in corresponding non-tumor
tissues (P<0.01). Furthermore, the western blotting data also
demonstrated that the protein expression level of IL-1β was higher
in NSCLC samples than in normal lung tissue (P<0.01; Fig. 1B). In addition, the expression of
IL-1β was increased in NSCLC cell lines (A549, HCC827 and H1650),
particularly in A549 cells, compared with that in the normal
epithelial cell line BEAS-2B, at both the mRNA and protein levels
(all P<0.01; Fig. 1C and
D).
IL-1β regulates paclitaxel
chemosensitivity in NSCLC cells
A549 cells were used for further experiments. To
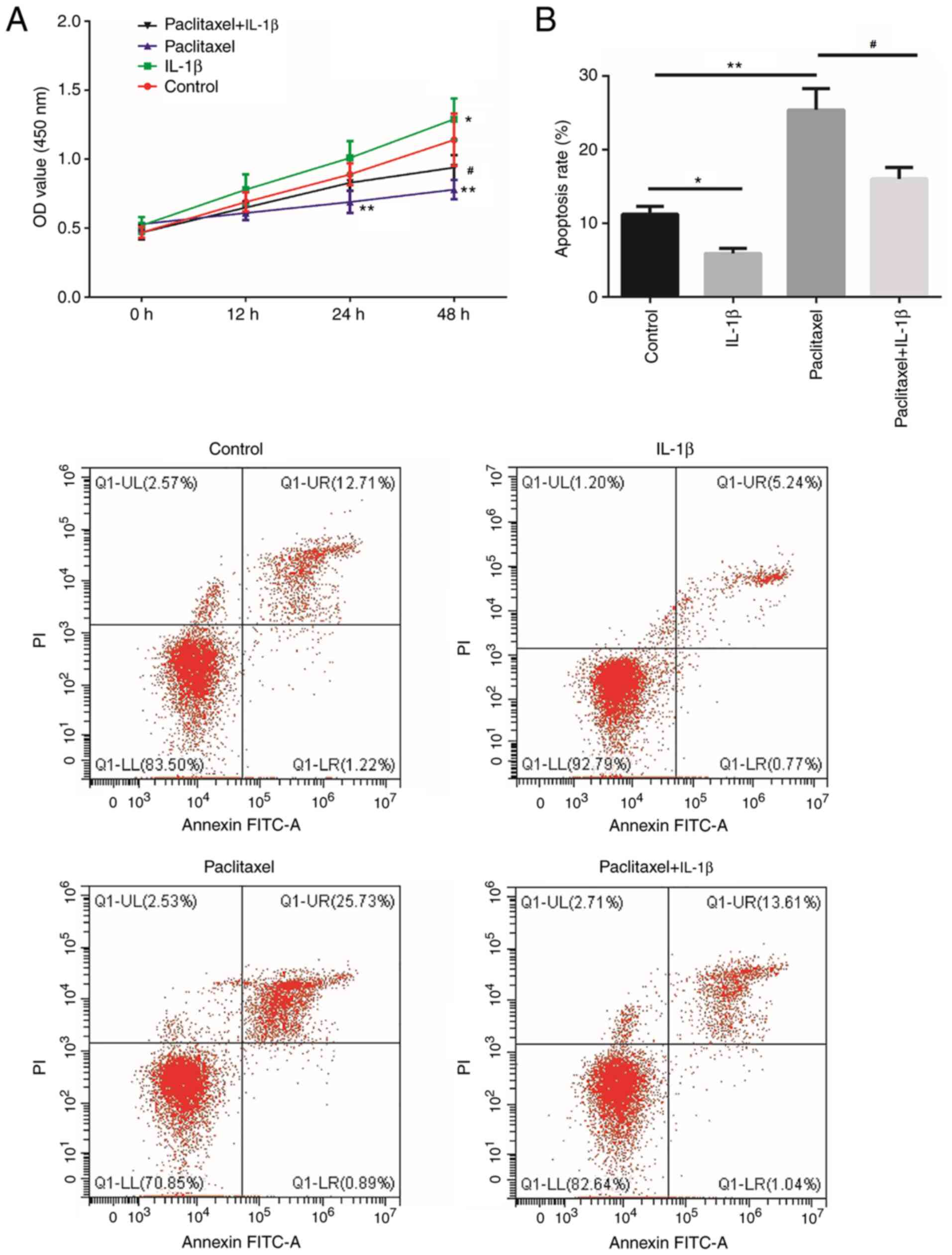
investigate the effect of IL-1β on paclitaxel sensitivity, cell
viability and apoptosis were determined. The results demonstrated
that cell viability was inhibited in the paclitaxel group, compared
with the control group at 24 and 48 h (P<0.01; Fig. 2A), and increased in the paclitaxel +
IL-1β group compared with the paclitaxel group at 48 h (P<0.05;
Fig. 2A). Compared with the control
group, cell apoptosis was induced by paclitaxel (P<0.01;
Fig. 2B) and inhibited by IL-1β
(P<0.05; Fig. 2B). Compared with
paclitaxel treatment, cell apoptosis was decreased after paclitaxel
+ IL-1β treatment (P<0.05; Fig.
2B). These results indicated that IL-1β reduced the sensitivity
of A549 cells to paclitaxel.
Association between IL-1β and
autophagy in NSCLC cells
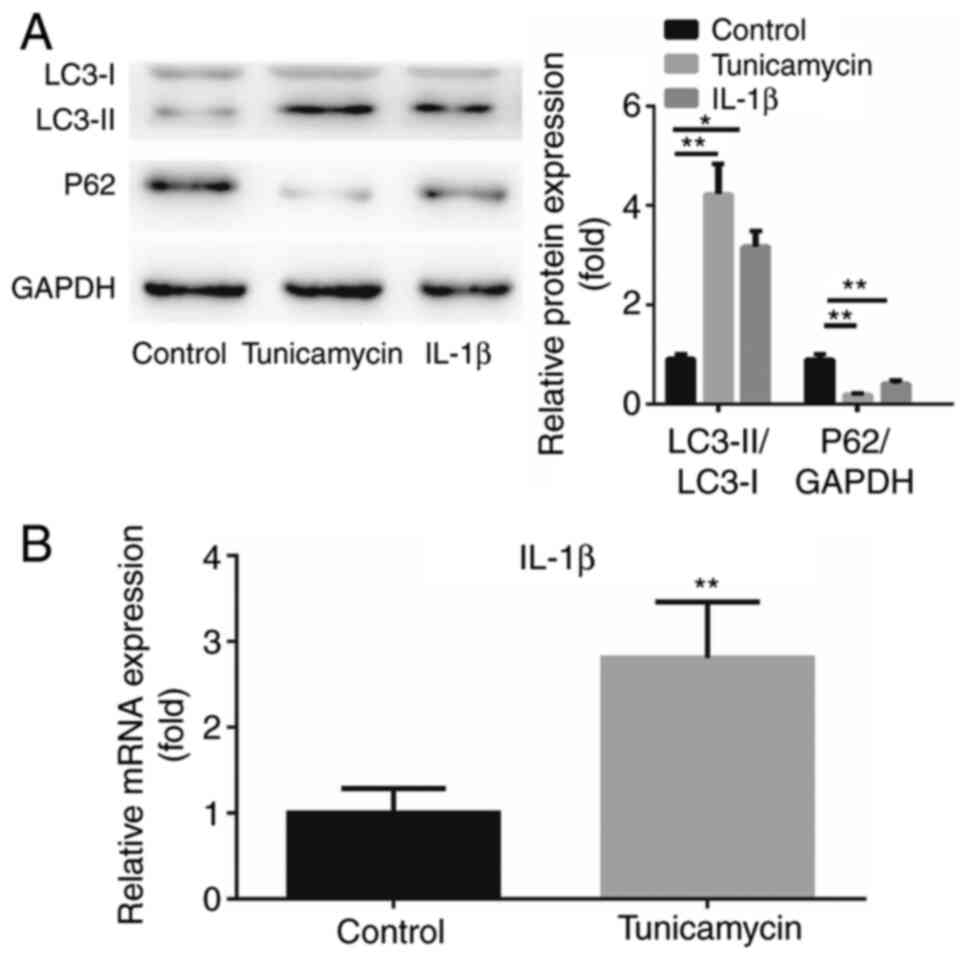
The activity of autophagy in A549 cells treated with
IL-1β was examined. Tunicamycin is known to induce autophagy
(19) and was used as a positive
control in the present study. Western blot analysis results
demonstrated that both tunicamycin and IL-1β treatment increased
the expression of LC3-II and decreased the expression of P62
(Fig. 3A). Additionally, the effect
of autophagy on the IL-1β level was investigated. The expression of
IL-1β was found to be upregulated by tunicamycin treatment compared
with the control group (P<0.01; Fig.
3B). These results suggested that IL-1β induced cell autophagy
and autophagy further enhanced IL-1β expression.
IL-1β regulates NSCLC cell sensitivity
to paclitaxel through controlling autophagy
Cell viability and apoptosis were detected by CCK-8
and flow cytometry assays in control, tunicamycin, paclitaxel and
paclitaxel + tunicamycin groups. As presented in Fig. 4A and B, compared with the control group,
tunicamycin promoted cell viability and suppressed apoptosis
(P<0.05), whereas paclitaxel inhibited cell viability and
promoted apoptosis (P<0.01). Furthermore, tunicamycin enhanced
paclitaxel-induced cell viability (P<0.05) and repressed
paclitaxel-induced apoptosis (P<0.01), as compared with the
paclitaxel alone group. These results suggested that autophagy
reduced the sensitivity of A549 cells to paclitaxel.
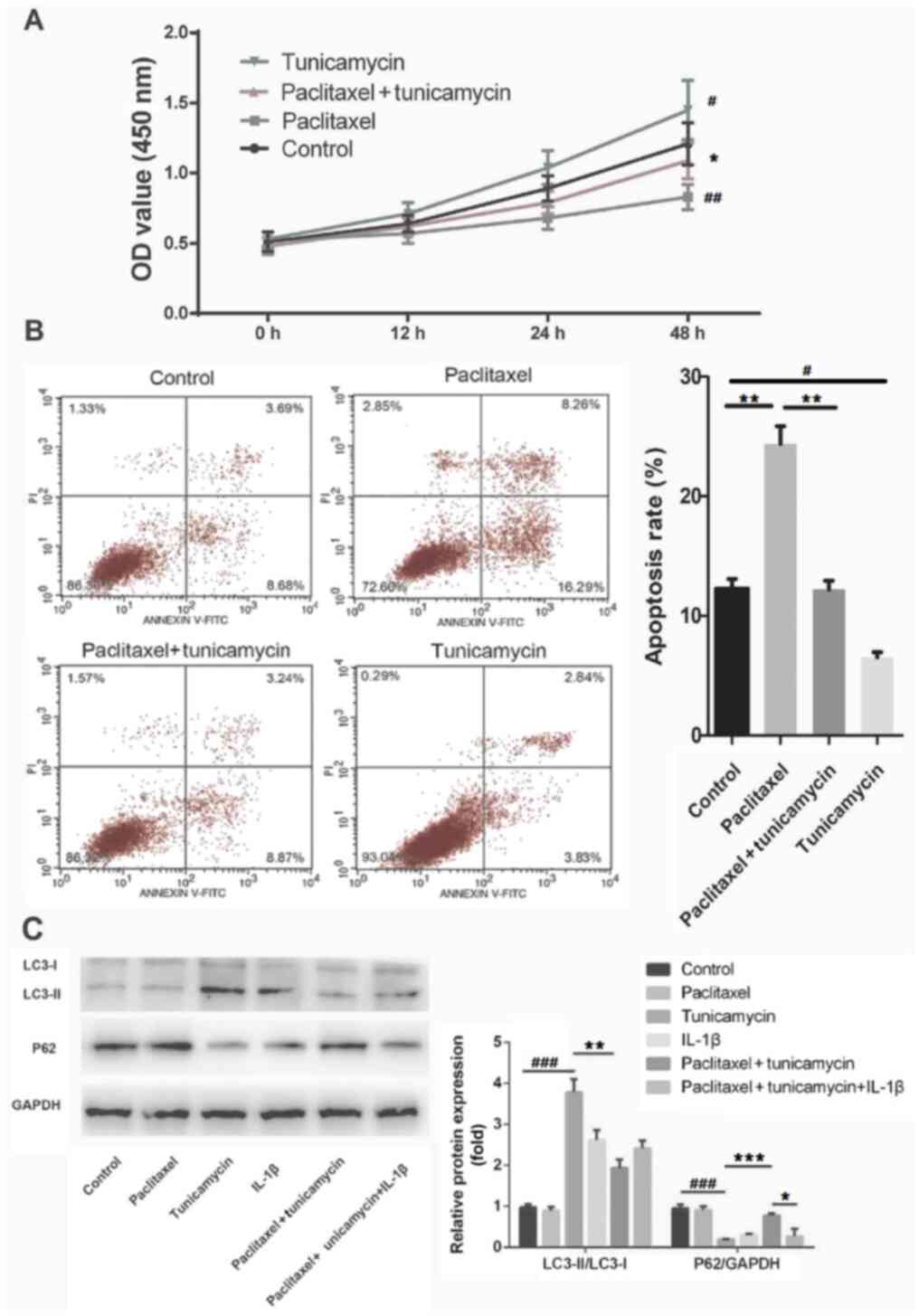 | Figure 4IL-1β modulates paclitaxel
sensitivity of NSCLC cells via regulating autophagy. A549 cells
were treated with tunicamycin, paclitaxel and tunicamycin +
paclitaxel, and the (A) cell viability and (B) apoptosis were
measured by using Cell Counting Kit-8 assay and flow cytometry,
respectively. (C) A549 cells were treated with paclitaxel alone,
tunicamycin, IL-1β alone, paclitaxel + tunicamycin and tunicamycin
+ paclitaxel + IL-1β, and western blotting was performed to test
LC3 and P62 protein expression levels. Data are presented as the
mean ± SD. #P<0.05, ##P<0.01 and
###P<0.001 vs. control; *P<0.05
**P<0.01 and ***P<0.001 vs. paclitaxel
(B); #P<0.05, **P<0.01 and
***P<0.001 vs. paclitaxel + tunicamycin. NSCLC,
non-small cell lung cancer. |
To further elucidate the association between IL-1β
and paclitaxel sensitivity, control, paclitaxel, tunicamycin,
IL-1β, paclitaxel + tunicamycin and tunicamycin + paclitaxel +
IL-1β groups were used. In Fig. 4C,
western blot analysis revealed that as an autophagy inducer,
tunicamycin significantly increased the formation of LC3-II and
decreased the expression of P62 (both P<0.001). Further analysis
revealed that paclitaxel inhibited the formation of LC3-II
(P<0.01) and increased the expression level of P62 (P<0.001)
in tunicamycin-treated A549 cells. Furthermore, IL-1β reversed the
increased expression of P62 (P<0.05) induced by paclitaxel in
tunicamycin-treated A549 cells. These data suggested that IL-1β
reduced the paclitaxel sensitivity of A549 cells by inducing
autophagy.
Discussion
The expression level of the pro-inflammatory
cytokine IL-1β was found to be elevated in various types of cancer
and tumor microenvironments, and it is associated with tumor
development (20). However, it
remains unclear whether it has beneficial or harmful effects
(21). IL-1β has been reported to
regulate malignant cell proliferation, migration, invasion and
epithelial-to-mesenchymal transition (22,23).
Furthermore, it has been shown to mediate apoptosis by regulating
caspase-3, poly ADP-ribose polymerase and inhibitor of
caspase-activated DNase (24), and
Bcl-2 protein family members (25).
In patients with lung cancer, IL-1β was found to be highly
expressed (26), and it favors the
establishment of an inflammatory microenvironment that increases
the risk of lung cancer (22),
meaning that it could be a potential biomarker of lung cancer
prognosis (27). Paclitaxel is a
well-known chemotherapeutic drug used for the treatment of NSCLC;
however, chemoresistance has been observed, which compromises
clinical efficacy and adversely affects the quality of life of the
patients (28). Therefore, it is
crucial to enhance the chemosensitivity of NSCLC cells to
paclitaxel. IL-1β is involved in drug resistance or
chemosensitivity (15-17);
however, to the best of our knowledge, there are few studies on the
association between IL-1β and paclitaxel. For example, IL-1β
expression is upregulated in macrophages as a response to
paclitaxel chemotherapy (29).
Paclitaxel treatment causes IL-1β production by LPS-stimulated
cells (30). In the present study,
the association between IL-1β and paclitaxel sensitivity in NSCLC
cells was investigated. First, it was observed that the expression
of IL-1β was increased in both NSCLC tissues and cell lines,
suggesting that IL-1β may act as a tumor promoter in NSCLC.
Subsequently, IL-1β was shown to promote paclitaxel-suppressed cell
viability and inhibit paclitaxel-induced cell apoptosis, suggesting
that IL-1β lowered paclitaxel sensitivity in NSCLC cells.
However, the underlying mechanism through which
IL-1β affects paclitaxel sensitivity remains elusive. The present
study revealed that IL-1β treatment was able to induce A549 cell
autophagy, and that the cells treated with tunicamycin exhibited
increased IL-1β levels. Autophagy is a conserved eukaryotic
cellular degradation and recycling process, which may be subdivided
into micro-autophagy, macro-autophagy and chaperone-mediated
autophagy (31). Dysfunction of the
autophagic pathway may promote aging and various diseases,
including infections, cancer, neurodegeneration and heart disease
(32). During the autophagy process
LC3-I is converted to LC3-II, while when autophagy is decreased,
the p62 level is increased (33).
Autophagy plays a key role in the immune system as a regulator of
inflammatory cytokines, particularly the release of IL-1 family
members (34). A previous study
reported that the IL-1β secretion pathway is affected by autophagy,
which may be promoted by starvation or pharmacological drugs
(35), and the results of the
present study are consistent with this conclusion.
Autophagy occurs frequently during cancer
chemotherapy, and is involved in the development of multidrug
resistance (36). Drug resistance
of cancer cells may be overcome by inhibiting autophagy, which
enhances the efficacy of anticancer therapies (37). Previous studies revealed that
autophagy mediates resistance or sensitivity to paclitaxel. For
example, autophagy inhibition was reported to sensitize endometrial
cancer cells to paclitaxel (38).
In addition, inhibition of cell autophagy promoted
paclitaxel-induced cell death, which improved the outcome of
paclitaxel in the treatment of NSCLC (39). Furthermore, certain proteins and
microRNAs may regulate paclitaxel sensitivity by inducing or
inhibiting autophagy (40,41). In the present study, it was observed
that autophagy may decrease the sensitivity of NSCLC cells to
paclitaxel. Furthermore, IL-1β decreased NSCLC cell sensitivity to
paclitaxel through inducing autophagy. Thus, it may be hypothesized
that regulation of IL-1β expression in NSCLC cells may alter the
resistance to paclitaxel. To further verify the results of the
present study, 3-methyladenine as an autophagy inhibitor will be
used to observe whether IL-1β regulates autophagy via the PI3K
pathway and reveal the unknown mechanism by which IL-1β regulates
paclitaxel sensitivity.
In conclusion, the present study suggested that
IL-1β may act as a tumor promoter in NSCLC. IL-1β decreased the
sensitivity of A549 cells to paclitaxel via inducing autophagy,
which was reflected by increased cell viability and decreased
apoptosis. The results of the present study indicated that IL-1β
may be a novel target for improving the therapeutic efficacy of
paclitaxel chemotherapy in NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Xi'an Science
and Technology Bureau's ‘Science and Technology +’ Action
Plan-Medical Research Project [grant no. 201805093YX1SF27(6)] and the corresponding supporting funds
from Xi'an Chest Hospital.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JL, TH and YF conceived and designed the study. JD
and ZL performed the experiments. JD and JX analyzed the data. JL
and TH wrote and edited the manuscript. YF and TH confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xi'an Chest Hospital (Xi'an, China) and each
participant provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Molina JR, Yang P, Cassivi SD, Schild SE
and Adjei AA: Non-small cell lung cancer: Epidemiology, risk
factors, treatment, and survivorship. Mayo Clin Proc. 83:584–594.
2008.PubMed/NCBI View
Article : Google Scholar
|
|
3
|
Rossi A and Di Maio M: Platinum-based
chemotherapy in advanced non-small-cell lung cancer: Optimal number
of treatment cycles. Expert Rev Anticancer Ther. 16:653–660.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang D, Yang R, Wang S and Dong Z:
Paclitaxel: New uses for an old drug. Drug Des Devel Ther.
8:279–284. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Weaver BA: How Taxol/paclitaxel kills
cancer cells. Mol Biol Cell. 25:2677–2681. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ramalingam S and Belani CP: Paclitaxel for
non-small cell lung cancer. Expert Opin Pharmacother. 5:1771–1780.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Joshi M, Liu X and Belani CP: Taxanes,
past, present, and future impact on non-small cell lung cancer.
Anticancer Drugs. 25:571–583. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lu C, Xie Z and Peng Q: MiRNA-107 enhances
chemosensitivity to paclitaxel by targeting antiapoptotic factor
Bcl-w in non small cell lung cancer. Am J Cancer Res. 7:1863–1873.
2017.PubMed/NCBI
|
|
10
|
Libby P: Interleukin-1 Beta as a target
for atherosclerosis therapy: Biological basis of CANTOS and beyond.
J Am Coll Cardiol. 70:2278–2289. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lopez-Castejon G and Brough D:
Understanding the mechanism of IL-1β secretion. Cytokine Growth
Factor Rev. 22:189–195. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Arranz L, Arriero MDM and Villatoro A:
Interleukin-1β as emerging therapeutic target in hematological
malignancies and potentially in their complications. Blood Rev.
31:306–317. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Azad N, Rojanasakul Y and Vallyathan V:
Inflammation and lung cancer: Roles of reactive oxygen/nitrogen
species. J Toxicol Environ Health B Crit Rev. 11:1–15.
2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kim JW, Koh Y, Kim DW, Ahn YO, Kim TM, Han
SW, Oh DY, Lee SH, Im SA, Kim TY, et al: Clinical implications of
VEGF, TGF-β1, and IL-1β in patients with advanced non-small cell
lung cancer. Cancer Res Treat. 45:325–333. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mendoza-Rodríguez MG, Ayala-Sumuano JT,
García-Morales L, Zamudio-Meza H, Pérez-Yepez EA and Meza I: IL-1β
inflammatory cytokine-induced TP63 isoform ∆NP63α signaling cascade
contributes to cisplatin resistance in human breast cancer cells.
Int J Mol Sci. 20(E270)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Feng X, Luo Q, Zhang H, Wang H, Chen W,
Meng G and Chen F: The role of NLRP3 inflammasome in 5-fluorouracil
resistance of oral squamous cell carcinoma. J Exp Clin Cancer Res.
36(81)2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee CR, Kang JA, Kim HE, Choi Y, Yang T
and Park SG: Secretion of IL-1β from imatinib-resistant chronic
myeloid leukemia cells contributes to BCR-ABL mutation-independent
imatinib resistance. FEBS Lett. 590:358–368. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang X, Srivastava R, Howell SH and
Bassham DC: Activation of autophagy by unfolded proteins during
endoplasmic reticulum stress. Plant J. 85:83–95. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mendoza-Rodríguez M, Arévalo Romero H,
Fuentes-Pananá EM, Ayala-Sumuano JT and Meza I: IL-1β induces
up-regulation of BIRC3, a gene involved in chemoresistance to
doxorubicin in breast cancer cells. Cancer Lett. 390:39–44.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bhat IA, Naykoo NA, Qasim I, Ganie FA,
Yousuf Q, Bhat BA, Rasool R, Aziz SA and Shah ZA: Association of
interleukin 1 beta (IL-1β) polymorphism with mRNA expression and
risk of non small cell lung cancer. Meta Gene. 2:123–133.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee CH, Chang JS, Syu SH, Wong TS, Chan
JY, Tang YC, Yang ZP, Yang WC, Chen CT, Lu SC, et al: IL-1β
promotes malignant transformation and tumor aggressiveness in oral
cancer. J Cell Physiol. 230:875–884. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang Q, Wang H, Mao C, Sun M, Dominah G,
Chen L and Zhuang Z: Fatty acid oxidation contributes to IL-1β
secretion in M2 macrophages and promotes macrophage-mediated tumor
cell migration. Mol Immunol. 94:27–35. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang C, Wang MW, Tashiro S, Onodera S and
Ikejima T: IL-1beta acts in synergy with endogenous IL-1beta in
A375-S2 human melanoma cell apoptosis through mitochondrial
pathway. J Korean Med Sci. 20:555–561. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Guadagno J, Swan P, Shaikh R and Cregan
SP: Microglia-derived IL-1β triggers p53-mediated cell cycle arrest
and apoptosis in neural precursor cells. Cell Death Dis.
6(e1779)2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Matanić D, Beg-Zec Z, Stojanović D,
Matakorić N, Flego V and Milevoj-Ribić F: Cytokines in patients
with lung cancer. Scand J Immunol. 57:173–178. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Vikhreva P, Petrova V, Gokbulut T,
Pestlikis I, Mancini M, Di Daniele N, Knight RA, Melino G and
Amelio I: TAp73 upregulates IL-1β in cancer cells: Potential
biomarker in lung and breast cancer? Biochem Biophys Res Commun.
482:498–505. 2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
d'Amato TA, Landreneau RJ, McKenna RJ,
Santos RS and Parker RJ: Prevalence of in vitro extreme
chemotherapy resistance in resected nonsmall-cell lung cancer. Ann
Thorac Surg. 81:440–446; discussion 446-447. 2006.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Voloshin T, Alishekevitz D, Kaneti L,
Miller V, Isakov E, Kaplanov I, Voronov E, Fremder E, Benhar M,
Machluf M, et al: Blocking IL1β Pathway following paclitaxel
chemotherapy slightly inhibits primary tumor growth but promotes
spontaneous metastasis. Mol Cancer Ther. 14:1385–1394.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wong J, Tran LT, Magun EA, Magun BE and
Wood LJ: Production of IL-1β by bone marrow-derived macrophages in
response to chemotherapeutic drugs: Synergistic effects of
doxorubicin and vincristine. Cancer Biol Ther. 15:1395–1403.
2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Parzych KR and Klionsky DJ: An overview of
autophagy: Morphology, mechanism, and regulation. Antioxid Redox
Signal. 20:460–473. 2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Tanida I and Waguri S: Measurement of
autophagy in cells and tissues. Methods Mol Biol. 648:193–214.
2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Harris J, Lang T, Thomas JPW, Sukkar MB,
Nabar NR and Kehrl JH: Autophagy and inflammasomes. Mol Immunol.
86:10–15. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Claude-Taupin A, Bissa B, Jia J, Gu Y and
Deretic V: Role of autophagy in IL-1β export and release from
cells. Semin Cell Dev Biol. 83:36–41. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li YJ, Lei YH, Yao N, Wang CR, Hu N, Ye
WC, Zhang DM and Chen ZS: Autophagy and multidrug resistance in
cancer. Chin J Cancer. 36(52)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kumar A, Singh UK and Chaudhary A:
Targeting autophagy to overcome drug resistance in cancer therapy.
Future Med Chem. 7:1535–1542. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu S and Li X: Autophagy inhibition
enhances sensitivity of endometrial carcinoma cells to paclitaxel.
Int J Oncol. 46:2399–2408. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen K and Shi W: Autophagy regulates
resistance of non-small cell lung cancer cells to paclitaxel.
Tumour Biol. 37:10539–10544. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang Z, He R, Xia H, Wei Y and Wu S:
Knockdown of STMN1 enhances osteosarcoma cell chemosensitivity
through inhibition of autophagy. Oncol Lett. 13:3465–3470.
2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ran X, Yang J, Liu C, Zhou P, Xiao L and
Zhang K: MiR-218 inhibits HMGB1-mediated autophagy in endometrial
carcinoma cells during chemotherapy. Int J Clin Exp Pathol.
8:6617–6626. 2015.PubMed/NCBI
|