Introduction
Gastric cancer (GC) is one of the most common
malignant gastrointestinal tumors and the third leading cause of
cancer deaths worldwide (1). The
majority of reported cases of GC are in East Asia, Eastern Europe
and South America, and more than half of GC cases occur in
developing countries, with the majority in China (1-3).
The global incidence rates of chronic atrophic gastritis (CAG)
range from 0-10.9% yearly (4), and
it takes several years for chronic gastritis to develop into
gastric cancer. Dysplasia and intestinal metaplasia (IM) occur
after CAG and are considered premalignant lesions of GC (5). CAG and IM greatly increase the risk of
GC, as they promote the development of dysplasia (3). Chronic Helicobacter pylori (Hp)
infection is one of the most important risk factors for GC
development. According to the anatomic site and criteria, GC is
divided into cardia GC, which arises 2-5 cm from the gastric mucosa
distal to the esophagogastric junction, and non-cardia GC which
originates from the gastric mucosa distal to the cardia (6). Hp is thought to cause 65-80% of all
gastric cancer cases (3), however,
Hp is not a risk factor for cardia GC (1,3,7-9).
Environmental factors, such as high dietary salt intake and
oncogenic agents such as methyl-N'-nitro-N-nitrosoguanidine (MNNG),
are also important risk factors, particularly under Hp
infection-free conditions (1,3,5,10).
In China, the mean sodium intake is 5,400 mg/day, which is much
higher than the World Health Organization's recommended daily
intake of 2,000 mg (11). Several
meta-analyses have shown that excess dietary salt intake is a
health hazard worldwide and is associated with CAG and IM (12-14).
Studies conducted in Japan and Korea, where residents tend to have
high-salt intakes owing to their dietary habits of eating salt-rich
traditional foods such as miso soup, found that high-salt intake
was related to an increased risk of CAG and IM (12-17).
Animal models are important for drug screening and
for studying GC, CAG and IM. Several animal models of CAG are used,
such as rats, mouse or Mongolian gerbil (18,19).
Gastric mucosal injuries, due to Hp infection, surgery, ethanol or
indomethacin, and oncogenic agents (primarily MNNG) are the main
causes of CAG with precancerous lesions (20,21).
Several studies have shown that a synergistic interaction between
Hp and a high-salt diet accelerates chronic inflammation and GC
development in Mongolian gerbils (22,23)
and a high-salt intake is also associated with CAG and IM (14). However, animal models of CAG induced
by MNNG and saturated NaCl (to simulate high-salt intake) are rare
(22,23); thus, developing a model of CAG with
IM in rats treated with oncogenic agents and saturated NaCl may be
beneficial for future research and development of drugs to treat
CAG and IM.
Chronic inflammation plays an important role in GC
development and progression. Moreover, high-salt intake and
interleukin-17 (IL-17) synergistically induce vascular endothelial
growth factor (VEGF)-A expression through nuclear factor of
activated T cells 5 (NFAT5)/signal transducers and activators of
transcription 3 (STAT3) interaction in breast cancer cells
(24). High-salt intake also
promotes inflammation in the tumor microenvironment and enhances
angiogenesis and VEGF expression (25). A previous study showed that
microcirculatory disorders existed in CAG (26). However, whether high-salt intake
with MNNG can induce microcirculatory disorders and whether the
hypoxia-inducible factor (HIF)-1α pathway is activated during the
pathological process of CAG with IM remains uncertain.
In the present study, a new rat model of CAG with IM
was developed by administration of saturated salt and MNNG by
gavage. Dynamic changes in the gastric mucosal blood
microcirculation and activation of the cyclo-oxygenase-2
(COX-2)/HIF-1α/VEGF signaling pathway during the development of CAG
with IM were investigated.
Materials and methods
Materials
MNNG (95%) and sodium chloride (99.8%) were
purchased from Sinopharm Chemical Reagent Co., Ltd. The primary
antibody against VEGF receptor (VEGFR)1 (cat. no. ab184784) was
purchased from Abcam. The primary antibodies against COX-2 (cat.
no. 12282S), VEGFR2 (cat. no. 9698S) and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH; cat. no. 5174S) were provided by Cell
Signaling Technology, Inc. The primary antibody against HIF-1α
(cat. no. 610958) was supplied by Becton-Dickinson and Company.
Animals
A total of 30 male Wistar rats (160-180 g; 5 weeks
old) were obtained from Shanghai Laboratory Animal Center of the
Chinese Academy of Science (Shanghai, China), and housed in the
Laboratory Animal Center of Shanghai University of Traditional
Chinese Medicine (Shanghai, China). All rats were housed under a
12-h light/dark cycle at room temperature (25±2˚C) and humidity
(60±2%) with free access to food and water. All animal experiments
were conducted according to protocols approved by the Animal Ethics
Committee of Shanghai University of Traditional Chinese Medicine
(approval no. SZY201703012). For euthanasia, rats were anesthetized
by an intraperitoneal injection of 3% pentobarbital sodium (30
mg/kg body weight), once fully anesthetized, rats were sacrificed
by cervical dislocation and verification of death was defined by
cessation of breathing and faded eye color.
CAG model induction
Rats were randomly divided into the control group
(n=10) and 4 model groups (n=5/group). Model rats were treated with
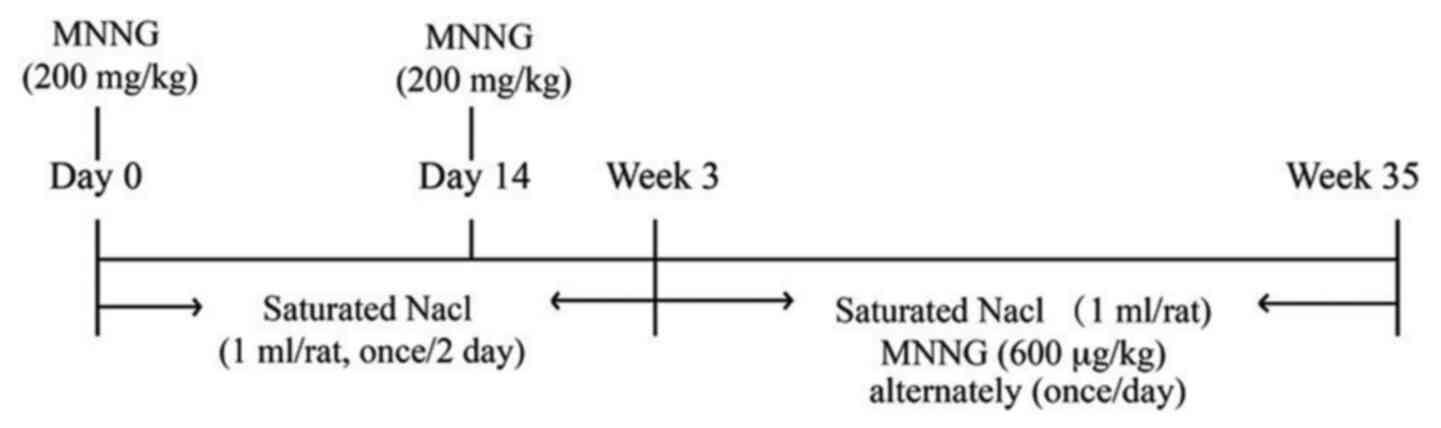
MNNG at 200 mg/kg body weight by oral gavage on days 0 and 14.
Saturated NaCl (1 ml per rat) was administered 3 times per week by
oral gavage for the first 3 weeks. MNNG (600 µg/kg) and saturated
NaCl (1 ml per rat) were then administered by gavage on alternate
days (Fig. 1). From week 5, 1 group
of 5 model rats was killed every 10 weeks (at weeks 5, 15, 25 and
35).
Measurement of gastric mucosal blood
flow (GMBF)
Rats were fasted for 24 h, then anesthetized with 3%
pentobarbital sodium (30 mg/kg body weight). After exposing the
stomach, a fiber-optic probe for laser Doppler flowmetry (Moor
Instruments Ltd.) was placed on the stomach wall of the fundus,
gastric body and antrum to measure the blood flow in the stomach.
The voltage number was recorded to represent the relative GMBF.
Specimen collection
After measuring the GMBF, anesthetized rats were
sacrificed by cervical dislocation. The stomach was then quickly
removed, cut along the greater curvature and washed with 0.9%
sodium chloride. The antral tissues were then separated into two or
three parts. A part was fixed for 24-48 h in 10% formalin at room
temperature for hematoxylin and eosin (H&E) and alcian
blue-periodic acid-Schiff (AB-PAS) staining. The other two parts
were used for western blotting and quantitative PCR analysis and
stored at -80˚C.
Morphological assay
Tissue specimens were fixed for 24-48 h with 10%
formalin at room temperature, processed, embedded in paraffin and
cut into 5-µm sections. All sections were stained with H&E and
AB-PAS (12), observed under a
light microscope and scored according to the histopathological
grading standard. The degrees of atrophy and metaplasia were
assessed according to the updated Sydney classification as: 1,
absent; 2, mild; 3, moderate; or 4, severe (12).
Western blot analysis
Stomach tissue was homogenized on ice with
CellLytic™ MT mammalian tissue lysis reagent (Sigma-Aldrich)
containing protease and phosphatase inhibitor cocktails and
centrifuged at 13,523 x g for 15 min at 4˚C. The supernatant was
then collected, and the protein concentration was determined via
BCA assay. Next, 30 µg of protein per sample was loaded and
separated by SDS-PAGE (8 for HIF-1α, VEGFR1 and VEGFR2 or 15% for
COX-2, GAPDH), then transferred onto polyvinylidene fluoride (PVDF)
membranes by a wet-transfer system (Bio-Rad Laboratories, Inc.).
The PVDF membranes were then blocked with 5% bovine serum albumin
(BSA; cat. no. G611BA0007; Sangon Biotech Co., Ltd.) in 1X
phosphate buffered saline with 0.1% Tween-20 (PBST) for 1 h. PVDF
membranes were incubated with primary antibodies (each, 1:1,000)
against COX-2, HIF-1α, VEGFR1, VEGFR2 and GAPDH at 4˚C overnight.
The membranes were further incubated with goat anti-rabbit
horseradish peroxidase-conjugated secondary antibodies (Jackson
ImmunoResearch Laboratories, Inc.; cat. no. 111-035-003; 1:1,000)
for 1 h at room temperature. Thereafter, the protein bands were
visualized using an ECL-prime kit (EMD Millipore; cat. no.
WBKLS0500) in a Tanon-5200 (Tanon Science & Technology Co.,
Ltd.). Quantification of the target proteins was normalized to that
of GAPDH within the same sample.
Reverse transcription quantitative PCR
(qPCR) analysis
Total RNA was extracted from the stomach tissues
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) as per the manufacturer's instructions. cDNA was
reverse transcribed from RNA (1 µg) using the RevertAid First
Strand cDNA Synthesis kit (cat. no. K1622; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
cDNA (1 µl) was diluted with nuclease-free water 5 times for the
qPCR reaction. RT-qPCR was performed using SYBR Premix EX Taq
(Roche Diagnostics) on the Quant Studio 6 Flex System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) under the following
conditions: 95˚C for 30 sec, followed by 40 cycles of 95˚C for 5
sec, 60˚C for 34 sec, 95˚C for 15 sec, 60˚C for 1 min and 95˚C for
15 sec. The target genes were quantified via the 2-ΔΔCq
method (27). Table I lists the sequences of primers
obtained from Shanghai GeneRay Biotech Co., Ltd. The relative
expression of the individual target genes were normalized to that
of GAPDH in the same sample.
 | Table IPrimer sequences used in the qPCR
analysis. |
Table I
Primer sequences used in the qPCR
analysis.
| Genes | Forward primer
(5'-3') | Reverse primer
(5'-3') |
|---|
| VEGF |
CCTCTCCCTACCCCACTTCCT |
CACTTTCTCTTTTCTCTGCCTCCAT |
| VEGFR1 |
TTGATGGTAGGCTGAGGGATG |
AGATGTAACTGCCGAGGATGC |
| VEGFR2 |
GAGTTGGTGGAGCATTGGGAA |
ATACAGGAAACAGGTGAGGTAGGCA |
| HIF-1α |
CCCATTCCTCATCCATCAAACATT |
CTTCTGGCTCATAACCCATCAACTC |
| COX-2 |
TGAAATATCAGGTCATCGGTGGAG |
CATACATCATCAGACCCGGCAC |
| Ang-1 |
TTGGTTACTCGTCAGACATTCATC |
TCTTCTTCTCTTTTTCCTCCCTTTA |
| Ang-2 |
AAGTCAACGCTGCCATCTTCC |
GACCTTCCCCAACTCCACAGA |
| GAPDH |
TCTCTGCTCCTCCCTGTTC |
ACACCGACCTTCACCATCT |
Statistical analysis
All data are expressed as the mean ± SEM.
Differences between two groups were analyzed using an unpaired
Student's t-test. Differences among more than two groups were
analyzed using one-way analysis of variance with Dunnett's multiple
comparison test. Analyses were performed using GraphPad Prism 6
(GraphPad Software, Inc.). Differences were considered significant
at P<0.05.
Results
Atrophic changes in the gastric
mucosa
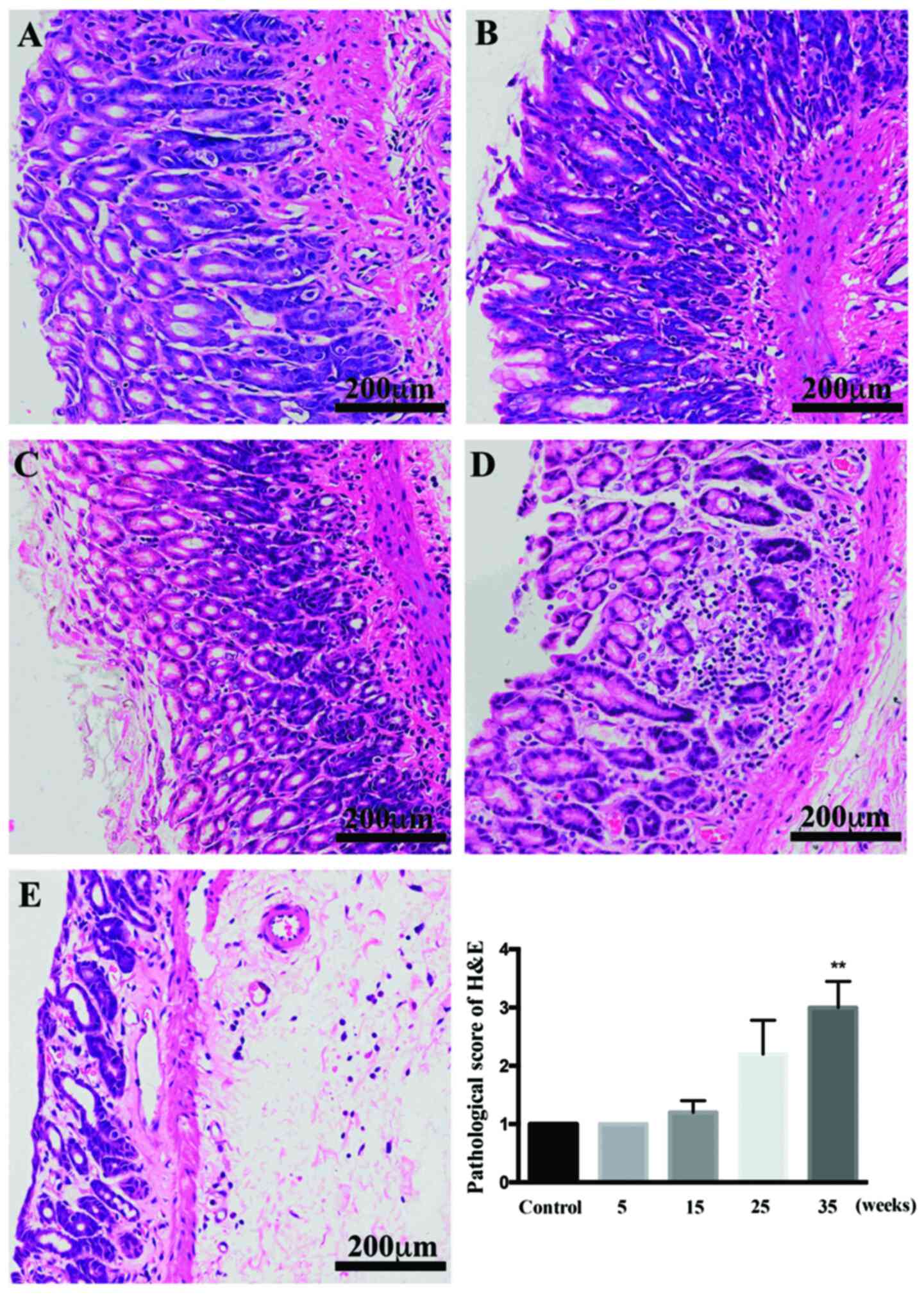
In control rats, stomach glands were arranged neatly
and were the same size. After treatment for 5 and 15 weeks, the
glands were arranged in order, and the number of glands remained
similar (Fig. 2). CAG was detected
by HE staining developed in the model rats after 25 weeks. Changes
in the glands were much more evident after 35 weeks of treatment
than at earlier weeks and compared with the control rats, the
glands were reduced in number and visibly disordered. IM, detected
by AB-PAS staining, was induced after 35 weeks of induction
(P<0.01 vs. control).
IM changes in the gastric mucosa
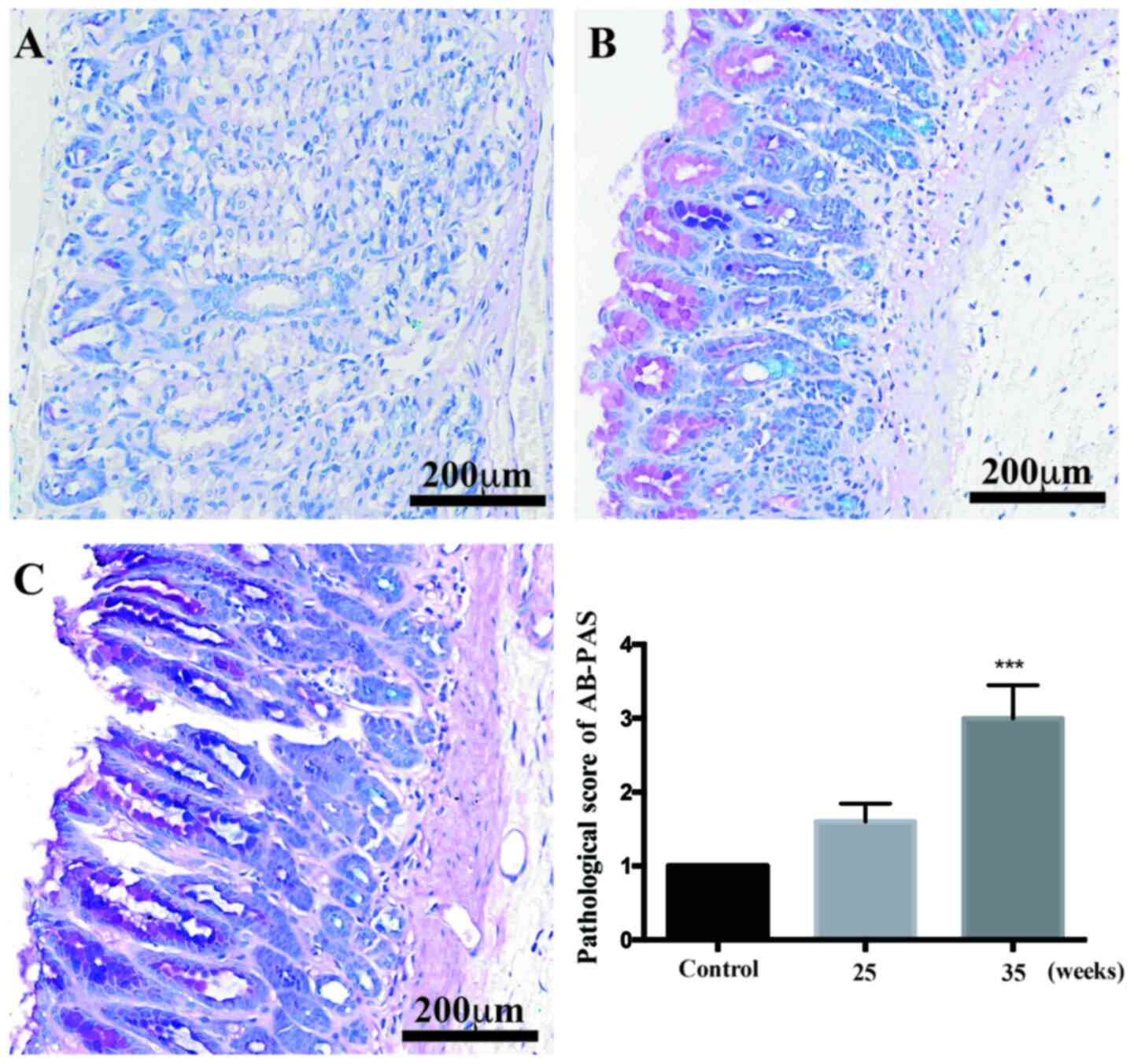
The AB-PAS staining results showed that, compared
with the control rats, glands were decreased in number, but mild IM
was not obvious at 25 weeks of treatment (Fig. 3). After 35 weeks of induction,
moderate and severe IM appeared in the antrums of the model rats
(P<0.001 vs. control).
GMBF changes at different time
points
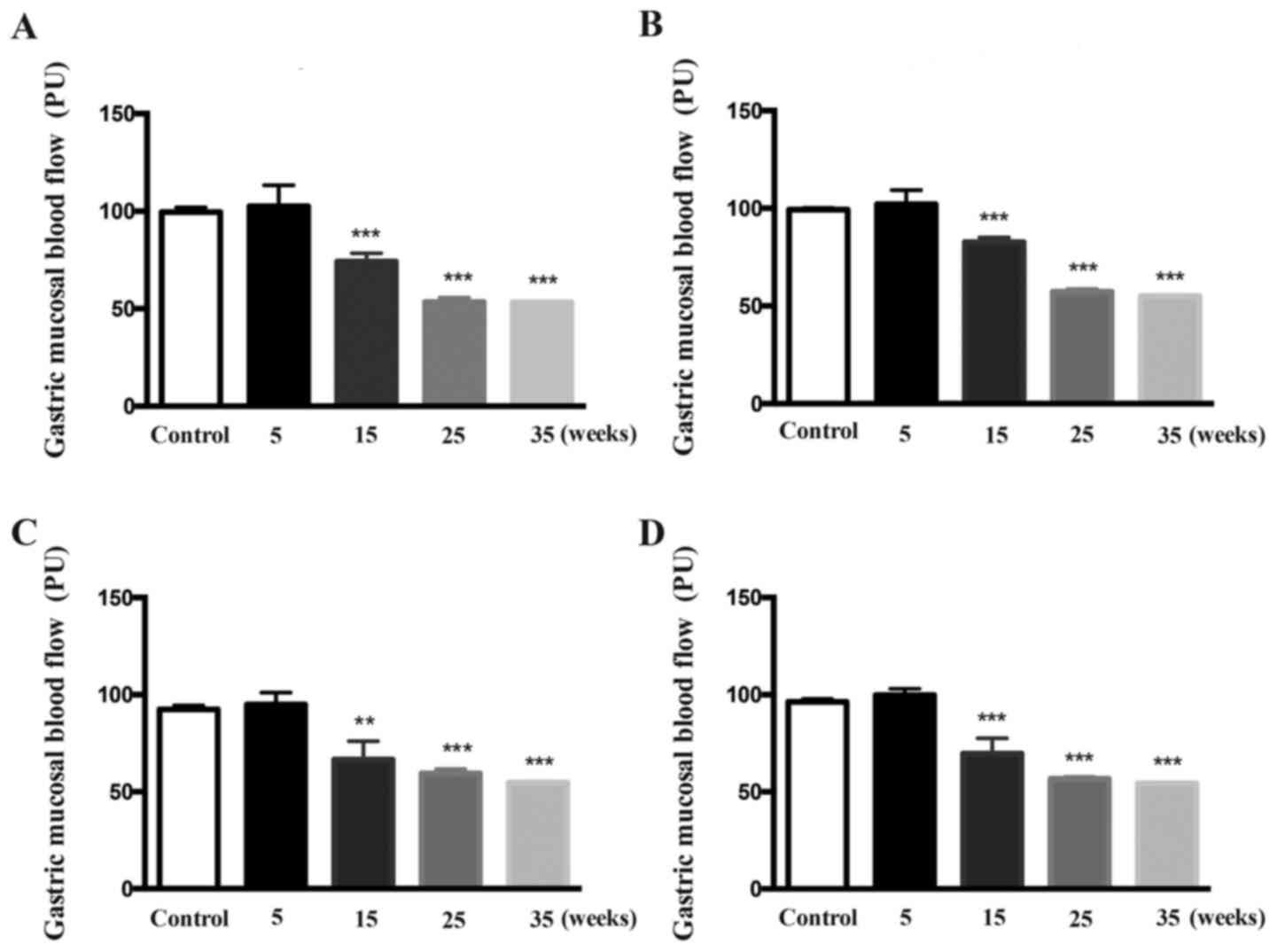
After 5 weeks of treatment, the GMBF of the fundus,
gastric body and antrum did not differ between the control and
model rats (Fig. 4). After
treatment for 15-35 weeks, the GMBF of the fundus, gastric body and
antrum in the model rats was decreased time-dependently, compared
with that of the control rats (P<0.01; P<0.001 vs.
control).
Activation of the COX-2/HIF-1α/VEGF
pathways at different time points
As early as 25 weeks after treatment, the mRNA
expression levels of HIF-1α (P<0.05 vs. control), COX-2
(P<0.01 vs. control), VEGF (P<0.001 vs. control), VEGFR1
(P<0.05 vs. control) and VEGFR2 (P<0.05 vs. control) were
increased compared with those of the control rats. However, the
mRNA expression levels of Ang-1 and Ang-2 were not obviously
affected (Fig. 5).
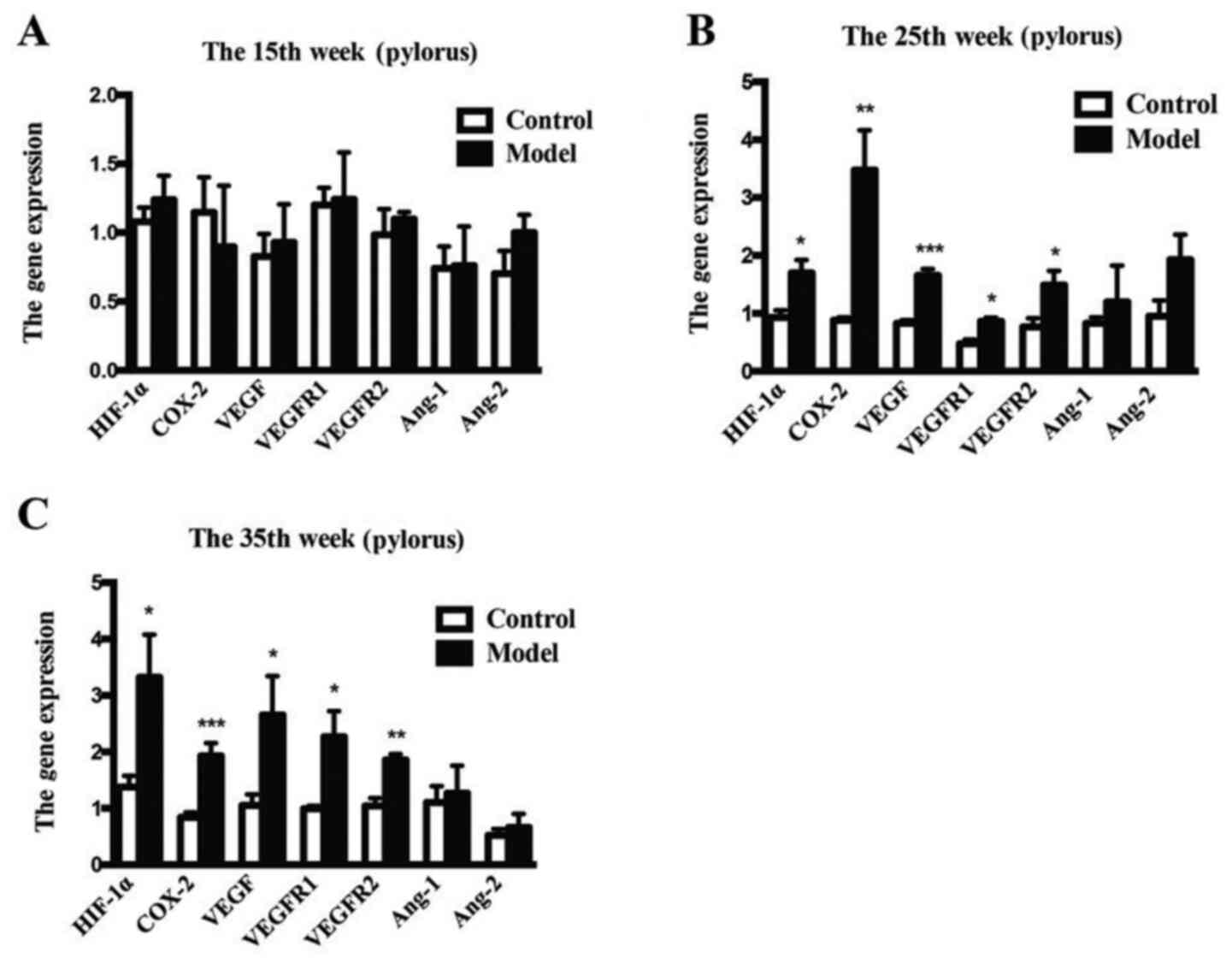 | Figure 5Gene expression levels of COX-2,
HIF-1α, VEGFR1, VEGFR2, Ang-1 and Ang-2 in the gastric tissue. (A)
Week 15, (B) week 25 and (C) week 35. Values are expressed as the
mean ± SEM (n=5/group). *P<0.05.
**P<0.01, ***P<0.001 vs. model group.
COX-2, cyclooxygenase-2; HIF, hypoxia inducible factor; VEGFR,
vascular endothelial growth factor receptor; Ang, angiotensin. |
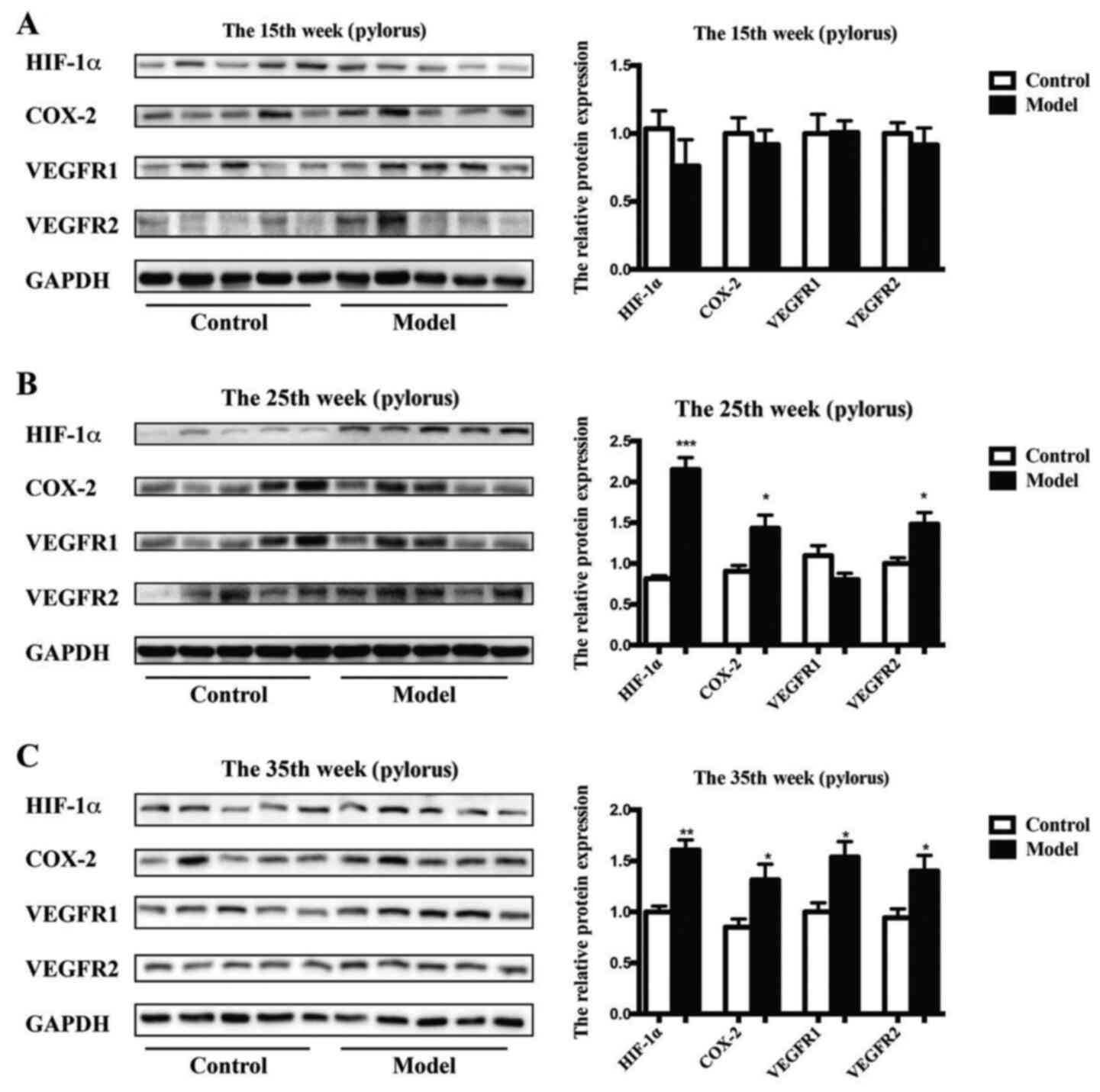
Further study demonstrated that the protein
expression levels of HIF-1α (P<0.001 vs. control), COX-2
(P<0.05 vs. control) and VEGFR2 (P<0.05 vs. control), but not
VEGFR1 were significantly enhanced in the model rats after 25 weeks
compared with those of the control rats (Fig. 6). The protein levels of HIF-1α
(P<0.01 vs. control), COX-2 (P<0.05 vs. control), VEGFR1
(P<0.05 vs. control) and VEGFR2 (P<0.05 vs. control) were
obviously upregulated in the model rats after 35 weeks compared
with those of the control rats.
Discussion
CAG with IM and dysplasia are the most significant
risk factors for GC and considered the two key types of
precancerous lesions in GC (1-3).
However, the molecular mechanisms of CAG with precancerous lesions
are unclear. Suitable animal models that are similar to clinical
patients are important for determining the underlying molecular
mechanisms of CAG and drug screening and development to treat CAG
with precancerous lesions. At present, several methods exist to
induce CAG with precancerous lesions in animals. The main methods
include Hp infection with or without MNNG (28,29) in
Mongolian gerbils, surgery with or without 150 g/l NaCl paste at
50-70˚C (30,31), and MNNG with or without the other
two or more factors (32-37)
(Table II). However, multiple
factors, complex operations, long procedure times, high costs and
high death rates have resulted in no standard model for CAG with
precancerous lesions. Oncogenic agents, such as MNNG, can easily
penetrate the pylorus and gastric mucosa of the stomach to cause
DNA damage; thus, MNNG in the drinking water is used as a specific
carcinogen to induce GC (38).
However, it takes a long time for MNNG in the drinking water to
induce precancerous lesions in animals, and the success rate is low
(35). Thus, other stimulating
factors, such as ammonia, sodium deoxycholate, salicylic acid and
ethanol, are also used to promote the development of CAG with
precancerous lesions (Table
II).
 | Table IIMethods of establishing chronic
atrophic gastritis with precancerous lesions in rats. |
Table II
Methods of establishing chronic
atrophic gastritis with precancerous lesions in rats.
| Method | |
|---|
| Gastrogavage | Water | Food | Operation | Others | Time (week) | Mortality rate
(%) | Success rate
(%) | (Refs.) |
|---|
| H.
pylori | - | - | - | - | 72 | 0 | 100 | (29) |
| H.
pylori | MNNG 20 m.m.p | - | - | - | 50 | 0 | 60 | (28) |
| - | - | - |
Gastrojejunostomy | - | 36 | 0 | 71.4 | (31) |
| 50-60 Paste
containing 150 g/l NaCl, 2 ml | - | - | Spring pyloric
implantation | - | 16 | 0 | 100 | (30) |
| - | MNNG 100 µg/ml | - | - | - | 50 | 0 | 64.3 | (35) |
| Sodium deoxycholate
20 mmol/l | | | | | | | | |
| Ethanol 60%, 8
ml/kg | | | | | | | | |
| Indomethacin 0.05%,
8 ml/kg | Ammonia
0.05-0.1% | - | - | - | 42 | 0 | 100 | (33) |
| MNNG 2 mM, 10
ml/kg | | | | | | | | |
| Ethanol 5
ml/kg | - | - | - | Fasting food Tail
clamp | 32 | 0 | 100 | (36) |
| MNNG 120 µg/ml, 10
ml/kg | Ammonia 0.1% | Ranitidine
0.03% | - | - | 28 | 30 | 70 | (32) |
| MNNG 120 µg/ml, 10
ml/kg | | | | | | | | |
| Ethanol 35%, 3
ml/rat | Salicylic acid
2% | Ranitidine
0.03% | - | - | 28 | 15.4 | 25 | (37) |
| - | MNNG 100 µg/ml | Nacl 8% | - | - | 42 | 45 | 31.3 | (34) |
High-salt diets can directly damage the gastric
mucosa and induce hypergastrinemia, leading to parietal cell loss
and GC progression (39). High-salt
diets also promote inflammatory cell infiltration and increased
COX-2 expression in Hp-infected Mongolian gerbils (40). Transgenic COX-2 expression and
high-salt intake enhance susceptibility to methylnitrosourea
(MNU)-induced GC development in mice (39,41). A
10% NaCl diet significantly increased the incidence of GC induced
by oncogenic agents, such as MNNG, in drinking water (42). These findings indicate that
high-salt intake plays an important role in GC progression. Reports
on the induction of CAG with precancerous lesions in animals using
MNNG and NaCl are rare. A previous study reported that combining
100 µg/ml MNNG in the drinking water and chow pellets with 8% NaCl
induced CAG with precancerous lesions in rats after modeling for 42
weeks. However, the mortality rate was 45% with only a 31.3%
success rate (34).
Therefore, in the present study, only MNNG and
saturated NaCl were intragastrically administered to induce CAG
with IM in rats. At week 25, the success rate reached 60% with no
deaths. At week 35, the success rate reached 100% with no deaths.
Moreover, long-term saturated NaCl is similar to high-salt intake
in clinical patients with CAG with IM (43,44).
Therefore, the protocol described in the present study of inducing
CAG with IM is simple, easy, controllable, less costly and
repeatable.
Angiogenesis and microcirculatory disorders have
been found in both CAG patients and animals (45,46).
As a transcription factor, HIF-1α directly regulates VEGF gene
expression in cancer angiogenesis, especially under hypoxia
(47). The HIF-1α signaling pathway
plays a vital role in blood microcirculatory disorders, including
ischemia, hypoxia, inflammation and tumor angiogenesis (48-51).
Gastric mucosal injury can induce upregulation of HIF-1α, VEGF and
COX-2(52). COX-2 can enhance the
expression of HIF-1α and VEGF (53). HIF-1α can also upregulate COX-2
expression in human endothelial cells (54). However, whether high-salt and MNNG
intake can induce gastric microcirculatory disturbance and
activation of the HIF-1α pathway remains uncertain. In the present
study, GMBF disturbance first appeared without significant
activation of the HIF-1α pathway at week 15. This demonstrated that
blood microcirculatory problems began to appear before the
development of HIF-1α pathway dysfunction and CAG with IM,
indicating that high-salt-induced gastric injury may be involved in
blood microcirculatory disorders of the stomach. At week 25, CAG
was successfully established, and the blood microcirculatory
disorder was much more severe. The gastric mucosal blood flow was
reduceded, indicating that the decreased speed of the GMBF induced
hypoxia in the model rats. HIF-1α and COX-2 mRNA expression levels
were significantly increased, indicating that blood
microcirculatory disorder-induced hypoxia activated the HIF-1α
pathway and COX-2. After 35 weeks, moderate or severe atrophic
gastritis with IM occurred in the model rats, indicating that
high-salt-induced gastric mucosal injury may induce
microcirculatory disorders, then microcirculatory disorder-induced
hypoxia would induce abnormal HIF-1α pathway expression and gastric
inflammation evidenced by COX-2 upregulation. This would further
enhance angiogenesis and consequently enhance the GC process
(48,50,55,56).
VEGFR1 and VEGFR2 are VEGF receptors (57). Upregulated VEGFR1 and VEGFR2
expressions in the gastric tissues of rats with CAG and IM are
consistent with overactivation of the COX-2/HIF-1α/VEGF pathway. In
this study, blood microcirculatory disorders occurred during CAG
with IM in rats. Thus, this animal model can help clarify the
molecular mechanisms of CAG with IM and help develop drugs to treat
CAG with IM.
In conclusion, a new rat model of CAG with IM was
stably and effectively established by intragastrically
administering saturated NaCl and MNNG. Activation of the HIF-1α
pathways and gastric inflammation, resulted from high-salt-induced
stomach microcirculatory disorders, might be involved in the
pathological process of CAG with IM induced by high-salt and MNNG
intake. However, the inconsistencies between replicate experiments
is a limitation of the present study, which should be improved in
future work.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81403349, 81603354,
81673626), the Construction Project of Shanghai Union of
Traditional Chinese Medicine [grant no. ZY(2018-2020)-FWTX-4018]
and the Opening Project of Shanghai Key Laboratory of Compound
Chinese Medicines (grant no. 17DZ2273300).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JYin and JYi performed experiments and drafted the
manuscript. CY, BX and JL performed experiments.HH and XW made
substantial contributions to conception and design, analysis and
interpretation of data. HS and XF designed the research and revised
the manuscript. All authors read and reviewed the final
manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted according to
the ethical guidelines of the National Guide for the Care and Use
of Laboratory Animals and were approved by the Institutional Ethics
Committee of Shanghai University of Traditional Chinese Medicine
(approval no. SZY201703012).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Forman D and Burley VJ: Gastric cancer:
Global pattern of the disease and an overview of environmental risk
factors. Best Pract Res Clin Gastroenterol. 20:633–649.
2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Cavatorta O, Scida S, Miraglia C, Barchi
A, Nouvenne A, Leandro G, Meschi T, De' Angelis GL and Di Mario F:
Epidemiology of gastric cancer and risk factors. Acta Biomed.
89:82–87. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Adamu MA, Weck MN, Gao L and Brenner H:
Incidence of chronic atrophic gastritis: Systematic review and
meta-analysis of follow-up studies. Eur J Epidemiol. 25:439–448.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Park YH and Kim N: Review of atrophic
gastritis and intestinal metaplasia as a premalignant lesion of
gastric cancer. J Cancer Prev. 20:25–40. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao J, Zhao J, Du F, Zhang Y, Shen G, Zhu
H, Ji F, Ma F, Dong L, Kan J, et al: Cardia and non-cardia gastric
cancer have similar stage-for-stage prognoses after R0 resection: A
large-scale, multicenter study in China. J Gastrointest Surg.
20:700–707. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Devesa SS, Blot WJ and Fraumeni JF Jr:
Changing patterns in the incidence of esophageal and gastric
carcinoma in the United States. Cancer. 83:2049–2053.
1998.PubMed/NCBI
|
|
8
|
Powell J and McConkey CC: Increasing
incidence of adenocarcinoma of the gastric cardia and adjacent
sites. Br J Cancer. 62:440–443. 1990.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cavaleiro-Pinto M, Peleteiro B, Lunet N
and Barros H: Helicobacter pylori infection and gastric
cardia cancer: Systematic review and meta-analysis. Cancer Causes
Control. Netherlands. 22:375–387. 2011.
|
|
10
|
Yoon H and Kim N: Diagnosis and management
of high risk group for gastric cancer. Gut Liver. 9:5–17.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Hipgrave DB, Chang S, Li X and Wu Y: Salt
and sodium intake in China. JAMA. 315:703–705. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bergin IL, Sheppard BJ and Fox JG:
Helicobacter pylori infection and high dietary salt
independently induce atrophic gastritis and intestinal metaplasia
in commercially available outbred Mongolian gerbils. Dig Dis Sci.
48:475–485. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
D'Elia L, Rossi G, Ippolito R, Cappuccio
FP and Strazzullo P: Habitual salt intake and risk of gastric
cancer: A meta-analysis of prospective studies. Clin Nutr.
31:489–498. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Song JH, Kim YS, Heo NJ, Lim JH, Yang SY,
Chung GE and Kim JS: High salt intake is associated with atrophic
gastritis with intestinal metaplasia. Cancer Epidemiol Biomarkers
Prev. 26:1133–1138. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Montani A, Sasazuki S, Inoue M, Higuchi K,
Arakawa T and Tsugane S: Food/nutrient intake and risk of atrophic
gastritis among the Helicobacter pylori-infected population
of northeastern Japan. Cancer Sci. 94:372–377. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wiseman M: The second World Cancer
Research Fund/American Institute for Cancer Research expert report
Food, nutrition, physical activity, and the prevention of cancer: A
global perspective. Proc Nutr Soc. 67:253–256. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim J, Park S and Nam BH: Gastric cancer
and salt preference: A population-based cohort study in Korea. Am J
Clin Nutr. 91:1289–1293. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ling L, Tao H, Lu L, Qianqian S and Mingyu
S: Summary and review of animal models for chronic atrophic
gastritis and precancerous lesions of gastric cancer. Chinese J Exp
Trand Med Formulae. 24:1–8. 2018.
|
|
19
|
Fuhua L, Xiaoguang Q, Junhua L and Mei L:
Research progress on animal models of chronic atrophic gastritis.
Chinese Med Moedern distance Educ China. 16:18–19. 2018.
|
|
20
|
Li Y, Xia R, Zhang B and Li C: Chronic
atrophic gastritis: A review. J Environ Pathol Toxicol Oncol.
37:241–259. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Sipponen P and Maaroos HI: Chronic
gastritis. Scand J Gastroenterol. 50:657–667. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lee JY, Kim N, Nam RH, Choi YJ, Seo JH,
Lee HS, Oh JC and Lee DH: No correlation of inflammation with
colonization of Helicobacter pylori in the stomach of mice
fed high-salt diet. J Cancer Prev. 19:144–151. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Toyoda T, Tsukamoto T, Yamamoto M, Ban H,
Saito N, Takasu S, Shi L, Saito A, Ito S, Yamamura Y, et al: Gene
expression analysis of a Helicobacter pylori-infected and
high-salt diet-treated mouse gastric tumor model: Identification of
CD177 as a novel prognostic factor in patients with gastric cancer.
BMC Gastroenterol. 13(122)2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Amara S, Alotaibi D and Tiriveedhi V:
NFAT5/STAT3 interaction mediates synergism of high salt with IL-17
towards induction of VEGF-A expression in breast cancer cells.
Oncol Lett. 12:933–943. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Amara S and Tiriveedhi V: Inflammatory
role of high salt level in tumor microenvironment (Review). Int J
Oncol. 50:1477–1481. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang Y, Li J, Zhu L and Cui W: Effect of
three methods for activating blood circulation on early stage
apoptosis in rats with chronic atrophic gastric complicated
precancerous lesion. Chinese J Integr Tradit West Med. 28:448–450.
2008.PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shimizu N, Inada KI, Tsukamoto T,
Nakanishi H, Ikehara Y, Yoshikawa A, Kaminishi M, Kuramoto S and
Tatematsu M: New animal model of glandular stomach carcinogenesis
in Mongolian gerbils infected with Helicobacter pylori and
treated with a chemical carcinogen. J Gastroenterol. 34:61–66.
1999.PubMed/NCBI
|
|
29
|
Honda S, Fujioka T, Tokieda M, Satoh R,
Nishizono A and Nasu M: Development of Helicobacter
pylori-induced gastric carcinoma in mongolian gerbils. Cancer
Res. 58:4255–4259. 1998.PubMed/NCBI
|
|
30
|
Shi XY, Zhao FZ, Dai X, Ma LS, Dong XY and
Fang J: Effect of jianpiyiwei capsule on gastric precancerous
lesions in rats. World J Gastroenterol. 8:608–612. 2002.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dong Xi, Lei D, Jun G, Ningli C, Huibin Q
and Jinyan L: Experimental study on the relationship between cell
proliferation and apoptosis and the expression of the related gene
in rats with duodenogastric reflux. J Xi'an Jiaotong Univ Sci.
25:261–265. 2004.
|
|
32
|
Wei Y, Yang J, Yang H and Song Y:
Experimental effect of Yiqi Huayu Jiedu principle on chronic
atrophic gastritis with dysplasia rats. Chinese J Gastroenterol
Hepatol. 20:916–919. 2011.
|
|
33
|
Si J, Zhou W, Wu J, Cao Q, Xiang Z, Jiang
L, Lü W and Huang H: Establishment of an animal model of chronic
atrophic gastritis and a study on the factors inducing atrophy.
Chin Med J (Engl). 114:1323–1325. 2001.PubMed/NCBI
|
|
34
|
Gu J, Hu X, Shao W, Ji T, Yang W, Zhuo H,
Jin Z, Huang H, Chen J, Huang C and Lin D: Metabolomic analysis
reveals altered metabolic pathways in a rat model of gastric
carcinogenesis. Oncotarget. 7:60053–60073. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tsukamoto H, Mizoshita T, Katano T,
Hayashi N, Ozeki K, Ebi M, Shimura T, Mori Y, Tanida S, Kataoka H,
et al: Preventive effect of rebamipide on
N-methyl-N'-nitro-N-nitrosoguanidine-induced gastric carcinogenesis
in rats. Exp Toxicol Pathol. 67:271–277. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xu J, Shen W, Pei B, Wang X, Sun D, Li Y,
Xiu L, Liu X, Lu Y, Zhang X and Yue X: Xiao Tan He Wei Decoction
reverses MNNG-induced precancerous lesions of gastric carcinoma in
vivo and vitro: Regulation of apoptosis through NF-κB pathway.
Biomed Pharmacother. 108:95–102. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ren J, Yang Y, Qu X, Ying L, Yang M and
Yan N: Evaluation of a compound modling method with
N-methyl-N'-nitro-N-nitrosoguanidine for establishing chronic
atrophic gastritis model rats. J Tradit Chinese Med. 58:1961–1964.
2017.
|
|
38
|
Tsukamoto T, Mizoshita T and Tatematsu AM:
Animal models of stomach carcinogenesis. Toxicol Pathol.
35:636–648. 2007.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang XQ, Terry PD and Yan H: Review of
salt consumption and stomach cancer risk: Epidemiological and
biological evidence. World J Gastroenterol. 15:2204–2213.
2009.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kato S, Matsukura N, Matsuda N, Yamashita
N, Naito Z and Tajiri T: How to evaluate mRNA gene expressions of
cancer related cytokines for the infectious diseases including
stomach and liver carcinogenesis and prevention. Cancer Res.
66(871)2006.
|
|
41
|
Leung WK, Wu K, Wong CYP, Cheng AS, Ching
AK, Chan AW, Chong WW, Go MY, Yu J, To KF, et al: Transgenic
cyclooxygenase-2 expression and high salt enhanced susceptibility
to chemical-induced gastric cancer development in mice.
Carcinogenesis. 29:1648–1654. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Takahashi M and Hasegawa R: Enhancing
effects of dietary salt on both initiation and promotion stages of
rat gastric carcinogenesis. Princess Takamatsu Symp. 16:169–182.
1985.PubMed/NCBI
|
|
43
|
Chen Z, Li K, Bi J and Wang B: Sodium
intake, salt taste and gastric cancer risk according to
Helicobacter pylori infection, smoking, histological type
and tumor site in China. Asian Pacific J Cancer Prev. 13:2481–2484.
2012.PubMed/NCBI View Article : Google Scholar
|
|
44
|
You W, Blot WJ, Chang YS, Ershow AG, Yang
Z, An Q, Henderson B, Xu GW, Fraumeni JF Jr and Wang TG: Diet and
high risk of stomach cancer in shandong, China. Cancer Res.
48:3518–3523. 1988.PubMed/NCBI
|
|
45
|
Yu S, E Z and Liu S: Examination of the
gastric mucosal vessels in chronic gastritis by color Doppler flow
imaging. Chinese J Ultrasound Med. 17:45–46. 2001.
|
|
46
|
Pousa ID and Gisbert JP: Gastric
angiogenesis and Helicobacter pylori infection. Rev Esp
Enferm Dig. 98:527–541. 2006.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
47
|
Mirzoeva S, Kim ND, Chiu K, Franzen CA,
Bergan RC and Pelling JC: Inhibition of HIF-1 alpha and VEGF
expression by the chemopreventive bioflavonoid apigenin is
accompanied by Akt inhibition in human prostate carcinoma PC3-M
cells. Mol Carcinog. 47:686–700. 2008.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Huang SP, Wu MS, Shun CT, Wang HP, Hsieh
CY, Kuo ML and Lin JT: Cyclooxygenase-2 increases hypoxia-inducible
factor-1 and vascular endothelial growth factor to promote
angiogenesis in gastric carcinoma. J Biomed Sci. 12:229–241.
2005.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Stoeltzing O, Liu W, Reinmuth N, Fan F,
Parikh AA, Bucana CD, Evans DB, Semenza GL and Ellis LM: Regulation
of hypoxia-inducible factor-1alpha, vascular endothelial growth
factor, and angiogenesis by an insulin-like growth factor-I
receptor autocrine loop in human pancreatic cancer. Am J Pathol.
163:1001–1011. 2003.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Liu LZ, Jing Y, Jiang LL, Jiang XE, Jiang
Y, Rojanasakul Y and Jiang BH: Acacetin inhibits VEGF expression,
tumor angiogenesis and growth through AKT/HIF-1α pathway. Biochem
Biophys Res Commun. 413:299–305. 2011.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Park SA, Jeong MS, Ha KT and Jang SB:
Structure and function of vascular endothelial growth factor and
its receptor system. BMB Rep. 51:73–78. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Liu D, He Q and Liu C: Correlations among
Helicobacter pylori infection and the expression of
cyclooxygenase-2 and vascular endothelial growth factor in gastric
mucosa with intestinal metaplasia or dysplasia. J Gastroenterol
Hepatol. 25:795–799. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Hu H, Zheng H and Lu X: Effects of
‘Weiqiyin Drink’ in reversing chronic atrophic gastritis
complicated with precancerous morbid cells. Chinese J Integr Tradit
West Med Gastro-spleen. 9:32–34. 2001.
|
|
54
|
Naomoto Y, Gunduz M, Takaoka M, Okawa T,
Gunduz E, Nobuhisa T, Kobayashi M, Shirakawa Y, Yamatsuji T, Sonoda
R, et al: Heparanase promotes angiogenesis through Cox-2 and
HIF1alpha. Med Hypotheses. 68:162–165. 2007.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Tarnawski AS, Ahluwalia A and Jones MK:
Angiogenesis in gastric mucosa: An important component of gastric
erosion and ulcer healing and its impairment in aging. J
Gastroenterol Hepatol. 29 (Suppl 4):S112–S123. 2014.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Isobe T, Aoyagi K, Koufuji K, Shirouzu K,
Kawahara A, Taira T and Kage M: Clinicopathological significance of
hypoxia-inducible factor-1 alpha (HIF-1α) expression in gastric
cancer. Int J Clin Oncol. 18:293–304. 2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Kopparapu PK, Boorjian SA, Robinson BD,
Downes M, Gudas LJ, Mongan NP and Persson JL: Expression of VEGF
and its receptors VEGFR1/VEGFR2 is associated with invasiveness of
bladder cancer. Anticancer Res. 33:2381–2390. 2013.PubMed/NCBI
|