Introduction
Macrophages serve a key role in host immunity
against pathogens, such as bacteria and viruses. The primary
cell-mediated immune response of macrophages is phagocytosis of
pathogens and apoptotic cells, generating phagolysosomes to
eliminate cell waste (1).
Phenotypically, macrophages are classified into two subtypes; the
classic (M1) and alternative (M2) phenotypes (2). M1 macrophages are primarily activated
by the release of lipopolysaccharide (LPS) or interferon-g
following the invasion of microorganisms (3). During the acute inflammatory phase,
activated M1 macrophages release high amounts of interleukin
(IL)-12, nitric oxide (NO) and reactive oxygen species (ROS), which
results in cytotoxic effects against the invading microorganisms,
followed by phagocytosis (4-6).
Therefore, M1 macrophages are key macrophages that are polarized
during acute inflammation (3). M2
macrophages primarily mediate anti-inflammatory reactions (3). Following activation by
anti-inflammatory cytokines, such as IL-4 and IL-13, M2 macrophages
release IL-10, which promotes tissue repair and wound healing
(4-6).
Compared with the M2 subtype, M1 macrophages express higher levels
of major histocompatibility complex (MHC) II, CD68, co-stimulatory
molecules CD80 and CD86 and inducible nitric oxide synthase (iNOS)
(2). The respiratory tract and lung
alveoli serve as first-line defense barriers against pathogens and
pollutants in the air, and the endogenous defense mechanisms of the
respiratory system are essential to maintain the integrity and
normal function of the respiratory tract and lung alveoli (6). Notable levels of macrophages are
present in alveoli and pulmonary vasculature, serving as the
front-line immunity defense barrier for the respiratory system
(7,8). However, to the best of our knowledge,
the primary subtypes of macrophages present in the lung during
basal and acute inflammation, particularly in the developing lung,
have not yet been determined. The present study analyzed the
phenotypic expression levels of macrophages in the neonatal and
adult lung and determined their functional significance in the
pulmonary immune defense during the acute phase of endotoxic
challenge.
Materials and methods
Animals
Animal studies were conducted in compliance with the
Animal Center of the National Cheng Kung University Hospital
(Tainan, Taiwan) and approved by the Institutional of Animal Care
and Use Committee (approval no. 107219). A total of 138 (7 fetal,
91 neonatal, 35 adult and 5 aged) rats were used for experimental
analysis in this study. Rats (3 days-26 weeks; BioLASCO Taiwan Co.,
Ltd.) were cared in an animal house with 13 h-light and 11 h-dark
cycles at an ambient temperature of 22-25˚C and received standard
rodent food and water ad libitum. Adult female
Sprague-Dawley rats (weight, 220-250 g) were mated with normal
males (F:M=2:1). The night after mating was considered as gestation
day 0, and the term gestation age was defined as 22 days. Fetuses
were harvested at day 21 and neonates were sacrificed within 3 days
of delivery. Adult rats were defined as 8-10 weeks old and aged
rats were those older than 26 weeks. Animals that were used for
mating were not adopted for other experiments. Neonatal, adult and
aged rats were sacrificed by intraperitoneal injection of an
overdose of pentobarbital (250 mg/kg). Blood samples were collected
by direct cardiac puncture and lung tissues were harvested
following direct thoracotomy. Plasma and tissue samples were snap
frozen at -70˚C for further experiments.
Model of pulmonary infection
Animals were anesthetized with inhaled isoflurane
(Piramal Enterprises Ltd.; 2-3 v/v% in oxygen) before induction of
pulmonary infection. LPS (20 mg/kg; Sigma-Aldrich; Merck KGaA) was
administered by intrapleural instillation into anesthetized rats to
induce pulmonary inflammation and lung injury. Animals were allowed
to recover from anesthesia on a warm blanket following LPS
injection. The activity of animals was observed following LPS
challenge and the mortality rate was recorded for up to 10 h
following administration of LPS.
Inhibition of iNOS activity
Enzymatic activity of iNOS was inhibited by
intraperitoneal injection of 1400w (Sigma-Aldrich; Merck KGaA)
(9), which was administered every 6
h for three consecutive doses (10 mg/kg for each dose); the final
dose was injected at least 1 h (range, 1-1.5 h) before
administration of LPS.
Measurement of NO metabolites and
3-nitrotyrosine (3-NT)
Lung tissue was homogenized using a Polytron
homogenizer (Thomas Scientific) with protein extraction buffer
containing sucrose (0.25 M), EDTA (1 mM), sodium azide (1 mM) and
protease inhibitors. Levels of NO in the lung homogenates, as
determined by the levels of its metabolites [nitrite/nitrate
(NOx)]. Activated macrophages release superoxide and NO, which
react to form peroxynitrite, leading to tyrosine nitration
(10). 3-NT is considered to be a
relatively specific marker of peroxynitrite-mediated oxidative
damage (11). Levels of and NOx and
3-NT in lung homogenates were determined using the Griess reagent
kit (cat. no. 780001; Cayman Chemical Company) and 3-NT ELISA kit
(cat. no. EU2560; FineTest; Wuhan Fine Biotech Co. Ltd.), according
to the manufacturer's instructions. Samples analyzed for NOx and
3-NT concentrations were performed in duplicates.
Western blotting
Lung tissue biopsies were minced and homogenized in
lysis buffer (M-PER extraction reagent; Thermo Fisher Scientific,
Inc.). Samples were centrifuged at 10,000 x g for 5 min at 4˚C and
total protein concentration was quantified with a colorimetric
assay kit based on the Bradford method (Dye Reagent Concentrate;
cat. no. 500-0006; Bio-Rad Laboratories, Inc.). Equal amounts of
total protein (50-100 µg) were loaded onto polyacrylamide gels
(9-12%). Proteins were then transferred to nitrocellulose membranes
by wet transfer. Following incubation in a commercially available
chemical-based, protein-free blocking reagent (ImmobilonÒ Block;
cat. no. WBAVDCH01; Sigma-Aldrich; Merck KGaA) for 20 min at room
temperature, the membranes were incubated overnight at 4˚C with
primary antibodies (1:1,000 dilution). The primary antibodies used
were: iNOS (mouse monoclonal antibody; cat. no. 610432; BD
Transduction Laboratories; BD Biosciences), CD86 (rabbit polyclonal
antibody; cat. no. GTX 34569; GeneTex, Inc.), arginase (Arg)-1
(rabbit polyclonal antibody; cat. no. 16001-1-AP; ProteinTech
Group, Inc.), CD206 (rabbit polyclonal antibody; cat. no.
18704-1-AP; ProteinTech Group, Inc.), NF-κB (rabbit polyclonal
antibody; cat. no. 622602; BioLegend, Inc.) and b-actin (mouse
monoclonal antibody; cat. no. MAB 1501; Sigma-Aldrich; Merck KGaA).
iNOS and CD86 were defined as cell markers for M1 macrophages;
Arg-1 and CD206 were defined as cell markers for M2 macrophages
(12,13). After three washes with PBS, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (1:2,000 dilution for goat anti-mouse
antibody, cat. no. ab205719; Abcam or 1:2,000 dilution for goat
anti-rabbit antibody cat. no. ab205718; Abcam) for 1 h at room
temperature. Bands were visualized using enhanced chemiluminescence
(Thermo Fisher Scientific, Inc.) and quantified by scanning
densitometry (ImageJ v1.48; National Institutes of Health).
Histology and immunohistostaining
Lung tissues were fixed in 10% buffered formal
saline for ≥24 h. Biopsies were processed through increasing grades
of alcohol and embedded in paraffin wax. Sections of the lung were
stained with Harris hematoxylin solution for 10 min at room
temperature, followed by counterstaining with eosin-phloxine
solution for 1 min at room temperature.
Paraffin-embedded lung tissues were sectioned for
immunohistochemical staining. Lung tissue blocks were sectioned at
5 µm thickness on a microtome and the sections were transferred
onto glass slides. After being dried overnight at room temperature,
the sections were rinsed twice with xylene and tissue sections were
dehydrated using various concentrations (100-50% v/v) of ethanol.
Endogenous peroxidase tissue activity was blocked by incubating
sections in 3% H2O2 solution in methanol at
room temperature for 10 min. The sections were incubated with
retrieval buffer (0.5 mg/ml trypsin in Tris-HCl, pH 8.0) and washed
twice with PBS. Following addition of 10% (v/v in PBS), the slides
were incubated at room temperature for 1 h. The sections were then
incubated with rabbit polyclonal anti-iNOS (1:100 dilution; cat.
no. bs-2072R; BIOSS) and CD68 (1:100 dilution; cat. no. BSB2717;
Bio SB, Inc.,) antibodies at room temperature for 1 h, followed by
incubating with polymer double stain detection system (BioTnA),
which contained horseradish peroxidase (HRP) green and
3,3'-diaminobenzidine (DAB) brown chromogen, for another 20 min at
room temperature. All procedures were performed according to the
manufacturer's instructions. The expression levels of iNOS and CD68
on the lung sections were presented in HRP Green and DAB brown.
Hematoxylin and eosin and immunohistochemically stained lung
sections were examined under a light microscope (magnification,
x100-200; Eclipse E200, Nikon Corporation).
Flow cytometry analysis
Following lysis of red blood cells with the lysis
buffer (BD FACS lysing solution; BD Biosciences), the remaining
blood cells were washed three times with PBS and resuspended in
PBS. For immunofluorescence labeling, the cells were incubated with
FITC-conjugated anti-CD86 antibody (1:100 dilution; cat. no.
130-109-180; Miltenyi Biotec) and phycoerythrin-conjugated
anti-CD68 antibody (1:100 dilution; cat. no. 130-103-363; Miltenyi
Biotec). Following incubation for 30 min at room temperature, cells
were washed three times with PBS and specificity of staining was
confirmed using equal concentrations of isotype-matched control
antibodies. Cell fluorescence was immediately measured and analyzed
using a FACSCalibur flow cytometer (BD Biosciences) and CellQuest
Pro software (version 6.0; BD Biosciences). Cells that
co-expressCD86 and CD68 were defined as M1 macrophages (14).
Statistical analysis
Results are presented as the mean ± SD. Tissue
concentrations of NOx and 3-NT were analyzed in duplicates. The
number of repeats in each experiment are presented in the figure
legends. Differences in survival rates were analyzed using
Kaplan-Meier curves and log-rank tests. Data were analyzed using an
independent t-test, or Kruskal-Wallis followed by Dunn's post hoc
test for multiple group comparisons, as appropriate. P<0.05 was
considered to indicate a statistically significant difference. All
statistical analyses were performed using the SigmaPlot v14.0
software (Systat Software, Inc.).
Results
Expression levels and enzymatic
activity of iNOS in neonatal lungs
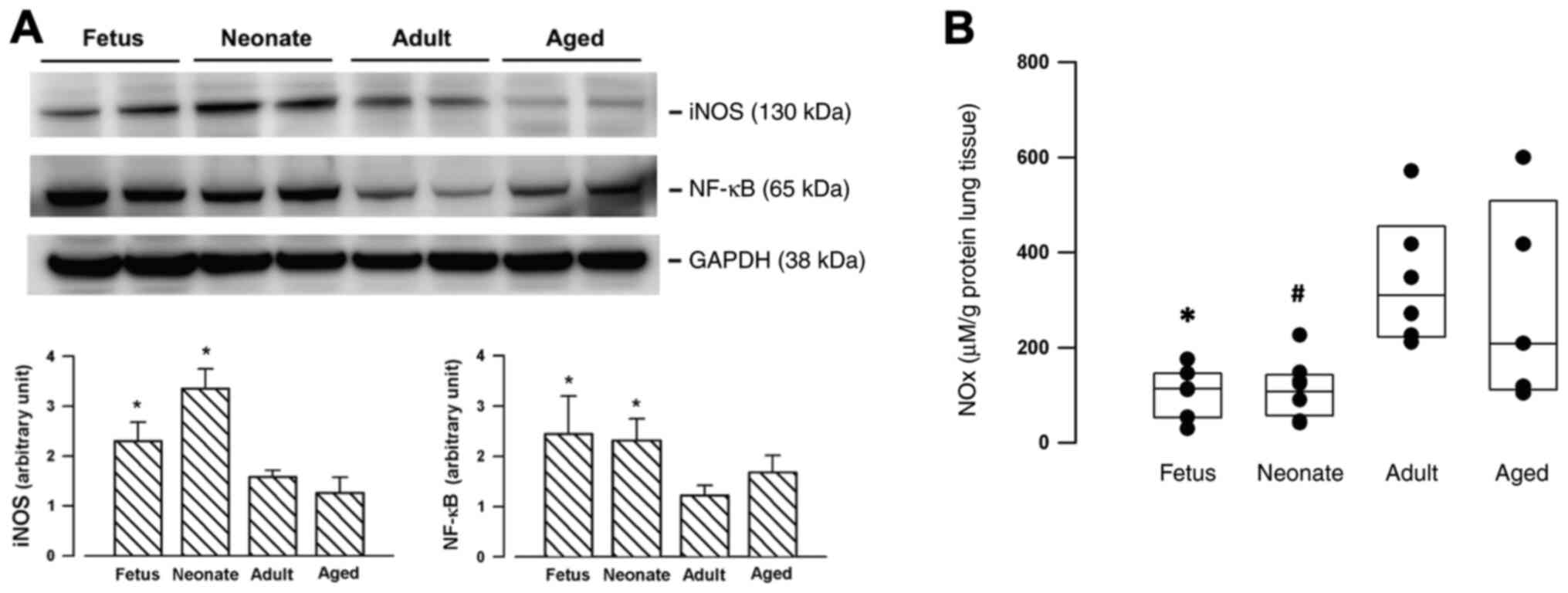
Compared with mature counterparts (adults or aged
rats), iNOS expressions were significantly enhanced in the lungs of
the younger (fetal and neonatal) rats (Fig. 1A). The transcription factor NF-κB
was also upregulated in the fetal and neonatal lungs (Fig. 1A). For the basal unstimulated
condition, the enzymatic activity of iNOS in the fetal and neonatal
lung, as determined by measuring the concentrations of NOx, was
significantly lower than the levels in the adult lung (Fig. 1B).
Characterization of phenotypical
subtypes of macrophages in neonatal rats
The phenotypical subtypes of macrophages were
characterized by the expression levels of specific cell markers on
macrophages. The total protein expression level was determined via
western blotting. Expression levels of M1 macrophage markers (i.e.
iNOS and CD86) were significantly enhanced in neonate lungs,
whereas the expression levels of markers of M2 macrophages (i.e.
Arg-1 and CD206) were decreased in neonates compared with adult and
aged rats (Fig. 2A). Flow cytometry
analysis of blood samples further confirmed that the number of
isolated cells co-expressing CD68 and CD86 (M1 phenotype) (15) was significantly increased in the
peripheral blood of neonates (Fig.
2B). Immunostaining of serial lung sections demonstrated
co-localized expression levels of CD68 and iNOS (Fig. 3), indicating the presence of M1
macrophages in the neonatal lung samples.
Functional significance of enhanced
iNOS in neonatal rats
An experimental model of pulmonary inflammation was
induced by instillation of LPS into the pleural space of neonatal
and adult rats. Compared with adult rats, the time-to-event curve
demonstrated that the mortality rate of neonates was significantly
increased up to 10 h following instillation of LPS (P<0.001;
Fig. 4A). The increase in tissue
concentrations of NOx and 3-NT in neonatal and adult lungs
following LPS instillation (Fig. 4B
and C) suggested an increase in
peroxynitrite-mediated protein nitration. A selective iNOS
inhibitor, 1400w, was used to suppress the enzymatic activity of
iNOS in the neonates. Compared with the baseline, tissue levels of
3-NT were significantly increased in neonatal lungs after LPS
challenge (2.4±0.5 vs. 20.4±6.2 ng/ml, neonatal baseline vs.
neonatal LPS; P<0.001; Fig. 4C).
Pre-treatment with 1400w significantly improved the overall
survival time of animals receiving an intrapleural injection of LPS
(P=0.02; log-rank test for Kaplan Meier curves between neonates and
neonates pretreated with 1400w; Fig.
4A). The inhibitor significantly suppressed the generation of
NOx in the lung (Fig. 4B), but the
formation of 3-NT was not decreased following inhibition of iNOS
activity (Fig. 4C). However,
neonatal lung generated high concentrations of 3-NT compared with
adult lung tissues following intrapleural sepsis (20.4±5.9 vs.
7.1±1.4 ng/ml, respectively; P<0.001; Fig. 4C).
 | Figure 4Effect of iNOS activity on the
survival following endotoxic-induced lung injury. (A)
Time-to-survival curves following intrapleural instillation of LPS
in neonatal and adult rats. Compared with adults, mortality in
neonates increased up to 10 h following pulmonary sepsis.
Pre-treatment with a selective iNOS inhibitor, 1400w, significantly
improved the survival time in neonates. *P=0.02 vs.
neonates, assessed using the log-rank test. n=9 in adults+LPS, n=14
in neonates+LPS and n=14 in neonates+LPS+1400w groups. (B)
Generation of NO following induction of pulmonary sepsis. Lung
tissue concentrations of NOx were assessed using a Griess reaction
assay kit. NOx levels were significantly decreased in lung
homogenates of neonates treated with 1400w. *P=0.043 vs.
adults; #P<0.001 vs. adults, assessed via
Kruskal-Wallis. n=9 in neonates+LPS, n=9 in neonates+LPS+1400w and
n=8 in adults+LPS groups. (C) Formation of 3-NT following induction
of pulmonary sepsis. Lung tissue concentrations of 3-NT were
measured using a 3-NT ELISA kit. *P=0.003 vs. adults;
#P<0.001 vs. adults, assessed via Kruskal-Wallis.
n=11 in neonates+LPS, n=11 in neonates+LPS+1400w and n=8 in
adults+LPS groups. Dotted lines (B and C) indicate the tissue
concentrations in the neonatal lungs at basal unstimulated
condition. LPS, lipopolysaccharide; iNOS, inducible nitric oxide
synthase; NOx, nitrite and nitrate; 3-NT, 3-nitrotyrosine. |
Discussion
Neonates and newborns are vulnerable to pulmonary
aspiration or respiratory tract infection. In the USA, respiratory
distress is the most common cause of neonatal intensive care unit
admission, accounting for the admission of 50% of term babies and
29% of late pre-term infants, with a higher incidence rate among
prematurely born infants (16).
Aspiration of meconium-stained amniotic fluid and maternal
chorionamnionitis are common risk factors for neonatal pulmonary
infection (16). The host innate
immunity in neonates is highly plastic and tolerant due to changes
in environment and exposure immediately following delivery from the
maternal uterus. This ‘quiescent mode’ of the innate immune system
is essential in neonates as their immune system encounters numerous
environmental and physical challenges during early life, and
modulates the interactions between the innate immune system and
host defense cells, such as macrophages (17).
In human adults, M1 macrophages have been
demonstrated to be activated by intracellular pathogens during the
acute phase of inflammation, and to promote Th1 polarization of CD4
cells in response to the inflammatory environment dominated by the
Toll-like receptor and interferon signaling (18). The polarization of adult macrophages
to the M1 phenotype enhances the release of proinflammatory
cytokines and C-X-C motif ligand chemokines, thereby activating the
Th1 response and complement-mediated phagocytosis (18). Although polarization and
phenotypical switching of macrophages have been well-characterized
in adults (18), the major subtypes
of macrophages in the developing lung during acute inflammation
have not been clearly determined. Therefore, the determination of
the phenotypical differences in macrophages between neonates and
adults is an important research topic, as excessive inflammation
reactions are often observed in very young subjects (19). Winterberg et al (19) proposed that phenotypical features
and functional regulation of macrophages were different in the
neonatal peritoneum with or without LPS challenge. In their
experiments, peritoneal macrophages from neonatal (age, <24 h)
and adult (age, 6 weeks) C57BL/6J mice were isolated and analyzed
by high content chipcytometry. This demonstrated that the
expression levels of F4/80, MHC-II, CD80 and CD86 (M1 markers) are
decreased in neonates, and that the transcriptomes of neonatal and
adult macrophages are different both before and after LPS (0, 1, 10
and 100 ng/ml) stimulation. Winterberg et al (19) also found that neonatal macrophages
secrete higher levels of proinflammatory cytokines following LPS
stimulation but exhibit a decreased ability to induce T-cell
proliferation. These findings demonstrated that the phenotypical
features and function of peritoneal macrophages in neonates are
distinct from those in adult mice.
In the present study, cell markers of macrophages
and circulating monocyte subtypes in the lung and peripheral blood
of fetal and neonatal rats were analyzed. The expression levels of
iNOS and CD86 were significantly enhanced, whereas the level of
Arg-1 was decreased, in the neonates, which is consistent with the
hypothesis that M1 phenotypic macrophages are predominant in the
fetal and neonatal lung. Immunohistochemical examination identified
co-expression of CD68 and CD86 in the lung parenchyma of neonatal
rats, confirming the presence of M1 macrophages in the alveoli.
Flow cytometry analysis also confirmed that circulating cells that
co-expressed CD68 and CD86 were significantly higher in neonates
compared with adults. These results demonstrated that the phenotype
of pulmonary macrophages and circulating monocytes in neonatal rats
is predominantly of the M1 subtype.
The present study revealed the biological role of
enhanced iNOS expression levels in the neonatal lung. High iNOS
expression levels in the fetal and neonatal lung were associated
with a higher protein level of inducible nuclear factor NF-κB,
indicating that iNOS was induced at the transcriptional level.
However, the bioavailability of iNOS in the basal unstimulated
condition, as measured by NOx levels, was significantly decreased
in neonates compared with in adult rats. Since iNOS is an inducible
enzyme, lower basal NOx concentrations in the neonatal lung may
indicate that neonates are exposed to fewer airborne pathogens than
adults. Following endotoxic challenge, the enzymatic activity of
iNOS was significantly upregulated in neonatal and adult rats, as
demonstrated by the increase in lung tissue levels of NOx. NO
released by activated macrophages mediates not only cytotoxic and
cytostatic effects against invading microorganisms, but also
modulates the activity of T- and B-cells, as well as the
recruitment of leukocytes (3). In
addition, activated macrophages generate superoxide and ROS via
induction of NAPDH oxidase, a process known as respiratory burst
(20,21). The reaction between NO and
superoxide anions in phagosomes generates an oxidizing
intermediate, peroxynitrite (ONOO-), which is responsible for the
microbicidal effect exhibited by macrophages (22,23).
At the cellular level, ONOO- nitrates protein-bound tyrosine at
position 3 to form 3-NT, thereby diminishing the function of
proteins (24). Therefore, the
formation of 3-NT is considered to be a sensitive in vivo
biomarker for endogenous ONOO- activity (11). In the present study, the tissue
concentrations of 3-NT in the lung were measured to determine the
degree of protein nitration in response to LPS-induced pulmonary
inflammation. Consistent with the increased production of NOx,
formation of 3-NT was also significantly enhanced in the neonatal
lung following exposure to LPS. Although the lung concentrations of
3-NT were similar in neonatal and adult rats at basal unstimulated
conditions, generation of 3-NT was more significantly increased in
neonatal lung tissues than in the adult lung tissues following
intrapleural instillation of endotoxin. Previous studies have
demonstrated that the cytotoxic response of activated macrophages
is directly derived from the generation of intra-phagosomal
ONOO-, and subsequently the development of protein
hydroxylation in the invading pathogens (25,26).
In order to clarify the functional significance of
enhanced iNOS expression levels in the neonatal lung, a selective
iNOS inhibitor, 1400w, was administrated prior to induction of lung
inflammation, and the effect on mortality following pulmonary
sepsis was recorded. Pre-treatment with 1400w suppressed the
enzymatic activity of iNOS and decreased the generation of NOx in
the lung tissue. Furthermore, the overall survival rate of neonatal
rats was improved up to 10 h following intrapulmonary LPS
challenge. The beneficial effect on survival of iNOS inhibition
during intrapulmonary sepsis in neonatal rats was consistent with
an improved survival rate in iNOS-deficient mice subjected to
intraperitoneal sepsis (27,28).
Mice with iNOS deficiency are also more responsive to microvascular
catecholamine infusion and exhibit a more stable hemodynamic
reaction during sepsis (27).
Although suppression of iNOS enzymatic activity
improved the survival outcomes of neonatal rats in the present
study, the mortality of neonates pretreated with 1400w remained
higher than that of their adult counterparts at 10 h following
pulmonary sepsis (42.8 vs. 11.1%). It is notable that the lung
tissue level of 3-NT was significantly increased in neonatal rats
pretreated with 1400w, despite the suppression of iNOS activity.
Uncontrolled or excessive production of ROS and/or ONOO-
by tissue macrophages contributes to oxidative damage and
nitrosative stress in host organs, thereby exacerbating acute
tissue injury or organ dysfunction (29). Since the generation of ROS and
reactive nitrogen species by macrophages involves the assembly and
membrane migration of NADPH oxidase subunits (23), iNOS inhibition may not be sufficient
to suppress excessive formation of ONOO- during sepsis.
Therefore, further investigations regarding the underlying
mechanisms of other macrophage-mediated cytotoxic and cytostatic
effects in the neonatal lung are essential.
The present study was limited, since the functional
role of the M2 macrophage subtype was not determined in the
neonatal lung. Since M2 macrophages are predominantly
anti-inflammatory macrophages that contribute to the late and
healing phases of inflammation (30), their function in the relatively
sterile neonatal lungs can be less significant than that in the
adults. In addition, the effect of 1400w on the survival outcome
was only observed up to 10 h following the administration of
endotoxin. The longer-term effect of iNOS suppression and the
physiological role of M2 macrophages in the late phase of
inflammation in neonates is the subject of further investigation.
In contrast to the results of the present study in neonatal lungs,
Winterberg et al (19)
demonstrated that the number of peritoneal M1 macrophages in
neonatal mice was lower than that in adults. This discrepancy may
be associated with the heterogeneity of macrophage populations in
different organs or tissues (31).
Lastly, although LPS is recognized as the primary
pathogen-associated molecule that triggers host innate immune
responses of mammalian cells to bacterial invasion, the
phenotypical modulation of macrophages in response to the challenge
of whole bacteria requires further investigation.
To the best of our knowledge, the present study was
the first to demonstrate that M1 is the predominant alveolar
macrophage subtype with enhanced expression levels of iNOS in the
neonatal lung. However, overproduction of NO and formation of 3-NT
during pulmonary sepsis increase the mortality of neonatal rats in
the acute inflammatory phase, indicating that high enzymatic
activity of iNOS in alveolar M1 macrophages may deteriorate
following endotoxin-induced lung injury.
Acknowledgements
The authors would like to thank Ms Tzu-Ting Cheng
(Department of Anesthesiology, E-Da Hospital, Kaohsiung, Taiwan)
and Mr. Young-How Lam (University of Western Australia School of
Medicine, Perth, Australia) for assistance with the preparation of
the manuscript.
Funding
Funding: The present study was funded by the Ministry of Science
and Technology of Taiwan (grant no. MOST 107-2314-b-650-004) and
institutional grants from the E-Da Hospital, Taiwan (grant no.
EDPJ107060 and EDAHP109005).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SYF performed the experiments, designed the study,
analyzed the data and contributed to manuscript preparation. JLC
was involved in animal experiments, data collection and manuscript
preparation. MHC and CCH performed western blotting and tissue
assays. MWL and CFL conceived and designed the experiments,
interpreted the results, analyzed the data and prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee (approval no. 107219) of the National
Cheng Kung University Hospital, Tainan, Taiwan.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gordon SB and Read RC: Macrophage defences
against respiratory tract infections. Br Med Bull. 61:45–61.
2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Italiani P and Boraschi D: From monocytes
to M1/M2 macrophages: Phenotypical vs. functional differentiation.
Front Immunol. 5(514)2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yang J, Zhang L, Yu C, Yang XF and Wang H:
Monocyte and macrophage differentiation: Circulation inflammatory
monocyte as biomarker for inflammatory diseases. Biomark Res.
2(1)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Arango Duque G and Descoteaux A:
Macrophage cytokines: Involvement in immunity and infectious
diseases. Front Immunol. 5(491)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mills CD, Kincaid K, Alt JM, Heilman MJ
and Hill AM: M-1/M-2 macrophages and the Th1/Th2 paradigm. J
Immunol. 164:6166–6173. 2000.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hu G and Christman JW: Editorial: Alveolar
macrophages in lung inflammation and resolution. Front Immunol.
10(2275)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Byrne AJ, Mathie SA, Gregory LG and Lloyd
CM: Pulmonary macrophages: Key players in the innate defence of the
airways. Thorax. 70:1189–1196. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Allard B, Panariti A and Martin JG:
Alveolar Macrophages in the Resolution of Inflammation, Tissue
Repair, and Tolerance to Infection. Front Immunol.
9(1777)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pang YL, Chen BS, Li SP, Huang CC, Chang
SW, Lam CF and Tsai YC: The preconditioning pulmonary protective
effect of volatile isoflurane in acute lung injury is mediated by
activation of endogenous iNOS. J Anesth. 26:822–828.
2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ferrer-Sueta G, Campolo N, Trujillo M,
Bartesaghi S, Carballal S, Romero N, Alvarez B and Radi R:
Biochemistry of peroxynitrite and protein tyrosine nitration. Chem
Rev. 118:1338–1408. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Campolo N, Issoglio FM, Estrin DA,
Bartesaghi S and Radi R: 3-Nitrotyrosine and related derivatives in
proteins: Precursors, radical intermediates and impact in function.
Essays Biochem. 64:111–133. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Jablonski KA, Amici SA, Webb LM,
Ruiz-Rosado Jde D, Popovich PG, Partida-Sanchez S and
Guerau-de-Arellano M: Novel markers to delineate murine M1 and M2
macrophages. PLoS One. 10(e0145342)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Nie H, Wang A, He Q, Yang Q, Liu L, Zhang
G, Huang Y, Ding X, Yu H and Hu S: Phenotypic switch in lung
interstitial macrophage polarization in an ovalbumin-induced mouse
model of asthma. Exp Ther Med. 14:1284–1292. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Klinder A, Markhoff J, Jonitz-Heincke A,
Sterna P, Salamon A and Bader R: Comparison of different cell
culture plates for the enrichment of non-adherent human mononuclear
cells. Exp Ther Med. 17:2004–2012. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Turtzo LC, Lescher J, Janes L, Dean DD,
Budde MD and Frank JA: Macrophagic and microglial responses after
focal traumatic brain injury in the female rat. J
Neuroinflammation. 11(82)2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Reuter S, Moser C and Baack M: Respiratory
distress in the newborn. Pediatr Rev. 35:417–428; quiz 429.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yu JC, Khodadadi H, Malik A, Davidson B,
Salles E, Bhatia J, Hale VL and Baban B: Innate immunity of
neonates and infants. Front Immunol. 9(1759)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Atri C, Guerfali FZ and Laouini D: Role of
human macrophage polarization in inflammation during infectious
diseases. Int J Mol Sci. 19(1801)2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Winterberg T, Vieten G, Meier T, Yu Y,
Busse M, Hennig C, Hansen G, Jacobs R, Ure BM and Kuebler JF:
Distinct phenotypic features of neonatal murine macrophages. Eur J
Immunol. 45:214–224. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Iles KE and Forman HJ: Macrophage
signaling and respiratory burst. Immunol Res. 26:95–105.
2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Haslund-Vinding J, McBean G, Jaquet V and
Vilhardt F: NADPH oxidases in oxidant production by microglia:
Activating receptors, pharmacology and association with disease. Br
J Pharmacol. 174:1733–1749. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wizemann TM, Gardner CR, Laskin JD,
Quinones S, Durham SK, Goller NL, Ohnishi ST and Laskin DL:
Production of nitric oxide and peroxynitrite in the lung during
acute endotoxemia. J Leukoc Biol. 56:759–768. 1994.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Prolo C, Alvarez MN and Radi R:
Peroxynitrite, a potent macrophage-derived oxidizing cytotoxin to
combat invading pathogens. Biofactors. 40:215–225. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gunaydin H and Houk KN: Mechanisms of
peroxynitrite-mediated nitration of tyrosine. Chem Res Toxicol.
22:894–898. 2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Linares E, Giorgio S, Mortara RA, Santos
CX, Yamada AT and Augusto O: Role of peroxynitrite in macrophage
microbicidal mechanisms in vivo revealed by protein nitration and
hydroxylation. Free Radic Biol Med. 30:1234–1242. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Alvarez MN, Peluffo G, Piacenza L and Radi
R: Intraphagosomal peroxynitrite as a macrophage-derived cytotoxin
against internalized Trypanosoma cruzi: Consequences for oxidative
killing and role of microbial peroxiredoxins in infectivity. J Biol
Chem. 286:6627–6640. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Hollenberg SM, Broussard M, Osman J and
Parrillo JE: Increased microvascular reactivity and improved
mortality in septic mice lacking inducible nitric oxide synthase.
Circ Res. 86:774–778. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cobb JP, Hotchkiss RS, Swanson PE, Chang
K, Qiu Y, Laubach VE, Karl IE and Buchman TG: Inducible nitric
oxide synthase (iNOS) gene deficiency increases the mortality of
sepsis in mice. Surgery. 126:438–442. 1999.PubMed/NCBI
|
|
29
|
Laskin DL: Macrophages and inflammatory
mediators in chemical toxicity: A battle of forces. Chem Res
Toxicol. 22:1376–1385. 2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gordon S and Martinez FO: Alternative
activation of macrophages: Mechanism and functions. Immunity.
32:593–604. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Liu G, Xia XP, Gong SL and Zhao Y: The
macrophage heterogeneity: Difference between mouse peritoneal
exudate and splenic F4/80+ macrophages. J Cell Physiol.
209:341–352. 2006.PubMed/NCBI View Article : Google Scholar
|