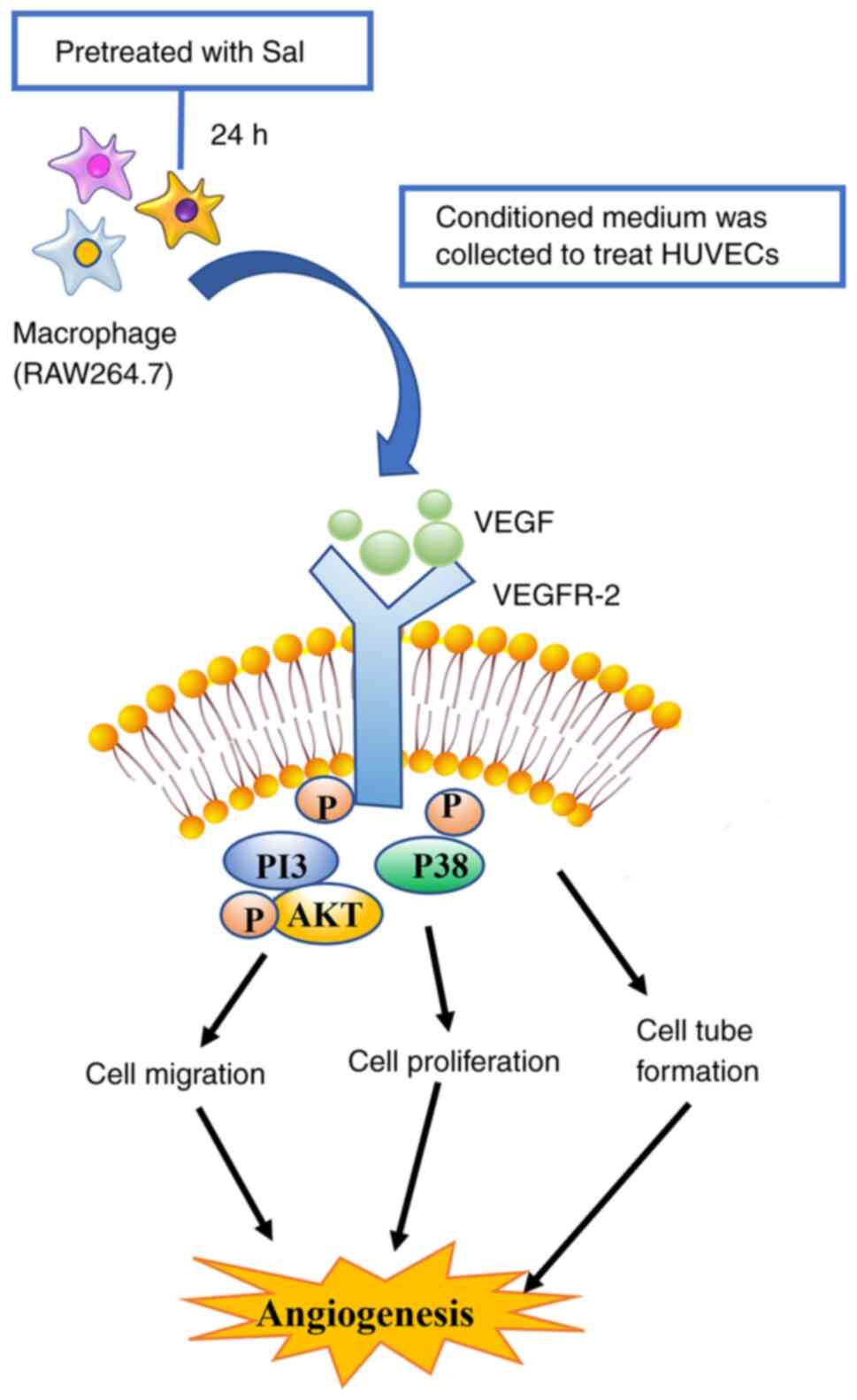
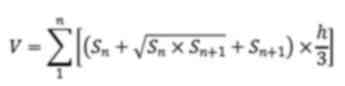Introduction
Stroke is a leading cause of long-term disability
among adults and is the third most frequent cause of mortality
worldwide. However, no effective treatments are currently available
for facilitating recovery from stroke (1,2).
Ischemic stroke, which constitutes 80% of all stroke cases, is the
most common type of stroke and results from focal cerebral ischemia
caused by the occlusion of major cerebral arteries (3). Ischemic stroke is a serious
neurological condition that occurs via a complex and varied
pathophysiological process, the fine pathophysiological mechanism
of which remains to be fully elucidated (4-6).
Therefore, it is necessary to devise a novel, safe and effective
method that can be used for early stroke treatment as well as for
the recovery of motor function lost at the latter stages of
stroke.
Angiogenesis, which involves the formation of new
blood capillaries from pre-existing blood vessels, serves an
important role in the process of tissue remodeling after ischemic
stroke (7-9).
The reconstruction of new functional microvasculature has been
documented to promote recovery from stroke, with angiogenesis being
pivotal for repair following ischemic brain injury because it
stimulates blood flow and metabolism in the ischemic boundary
(10). In addition, angiogenic
vessels secrete neurotrophic factors and chemokines, which may
create a suitable microenvironment within the damaged brain to
support the survival of newly formed neurons (11).
Damage to the brain by cerebral ischemia/reperfusion
(I/R) involves multiple reactions, including inflammatory reactions
and oxidative damage, and a large number of immune cells, such as
macrophages, are typically recruited to the site of injury
(12). These macrophages secrete
various angiogenic growth factors, including vascular endothelial
growth factor (VEGF), basic fibroblast growth factor and MMP-9 that
promote angiogenesis, which can preserve cortical blood supply and
improve neurological function during the acute phase of cerebral
I/R (13-15).
VEGF is an important promoter of post-ischemia neurovascular
remodeling (16,17). Ischemia stimulates VEGF expression
in the brain (18), thereby
promoting the formation of new cerebral blood vessels (19). Furthermore, VEGF has been found to
exert mitogenic and anti-apoptotic effects on endothelial cells,
which can increase vascular permeability and promote cell migration
(20). Among known angiogenic
signaling pathways, the VEGF/VEGF receptor-2 (VEGFR2) pathway is
especially important, since it can determine when angiogenesis is
initiated, the degree of angiogenesis and the type of blood vessels
formed. This in turn contributes to the maintenance of normal blood
vessel morphology whilst preventing endothelial cell apoptosis.
Previous studies have shown that VEGFR2 serves a leading role in
the angiogenesis mediated by VEGF (21). Following the binding of VEGF to
VEGFR2, autophosphorylation of the receptor occurs, which then
activates the downstream MAPK and PI3K/AKT signaling pathways to
regulate the migration, survival and proliferation of endothelial
cells. This in turn promotes angiogenesis (21).
Salvianolate (Sal) is a medicinal composition
derived from the principal active constituents of Danshen. It has
been shown to contain magnesium lithospermate B (≥85%), rosmarinic
acid (≥10.1%) and lithospermic acid (≥1.9%) (22-24).
Danshen is the dried root of the well-known Chinese herb Salvia
miltiorrhiza Bunge (Labiatae). Due to its ability to promote
blood circulation, it is widely used for the treatment of various
cardiovascular diseases, including coronary heart disease and
angina pectoris, in China (25-27).
In the present study, the potential effects of Sal
on endothelial cell and macrophage function and intracellular
signaling were investigated in vitro. Furthermore, the rat
transient middle cerebral artery occlusion (tMCAO) model was used
to evaluate the effects of Sal on acute cerebral ischemia in
vivo.
Materials and methods
Reagents and antibodies
Sal (batch no. 17111321) was obtained from Shanghai
Green Valley Pharmaceutical Co., Ltd. DMEM was purchased from Gibco
(Thermo Fisher Scientific, Inc.). FBS was purchased from Serana
Europe GmbH. VEGF protein (cat. no. 100-20) was purchased from
PeproTech, Inc. The rabbit monoclonal antibody against
phosphorylated (p)-VEGFR2 (cat. no. 3770), rabbit monoclonal
antibody against VEGFR2 (cat. no. 9698), mouse monoclonal antibody
against β-actin (cat. no. 3700), rabbit monoclonal antibody against
p-AKT (cat. no. 4060), rabbit monoclonal antibody against AKT (cat.
no. 4696), rabbit anti-p38 MAPK monoclonal antibody (cat. no. 8690)
and rabbit anti-p-p38 monoclonal antibody (cat. no. 4511) were
purchased from Cell Signaling Technology, Inc. The mouse anti-F4/80
(C-7) monoclonal antibody (sc-377009) and rabbit anti-VEGF
polyclonal antibody (sc-7269) were purchased from Santa Cruz
Biotechnology, Inc. The rabbit anti-CD31 polyclonal antibody
(GB12063) was purchased from Wuhan Servicebio Technology Co., Ltd.
Horseradish peroxidase (HRP)-conjugated goat anti-mouse (A0216) and
goat anti-rabbit (A0208) secondary antibodies were purchased from
Beyotime Institute of Biotechnology.
Animals
All animal protocols and procedures were approved by
the Shanghai University of Traditional Chinese Medicine
Institutional Animal Care and Use Committee (grant no.
PZSHUTCM200320004). All experiments were performed in accordance
with the guidelines described in the Regulations for the
Administration of Affairs Concerning Experimental Animals of China
enacted in 1988. A total of 97 healthy male Sprague-Dawley rats (8
weeks old; weight, 300±20 g) were purchased from Shanghai
Sino-British SIPPR/BK Lab Animal Co., Ltd. The rats were
individually caged in a climate-controlled room (20-26˚C, relative
humidity of 40-70%) housed under a 12-h dark/light cycle, and
allowed free access to water and food. The animals were fasted
without water deprivation for 12 h before the tMCAO procedure was
performed.
Cell culture
Human umbilical vein endothelial cells (HUVECs;
American Type Culture Collection HUV-EC-C cell line, cat. no.
CRL-1730) were obtained from the Cancer Research Institute of
Central South University. The murine RAW264.7 cell line was
purchased from the Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences. Both types of cells were cultured in
DMEM containing 10% FBS at 37˚C in a humidified atmosphere
comprising 5% CO2 in an incubator.
Preparation of the RAW264.7 cell
supernatant
RAW264.7 cells (~3x106 cells/ml) were
seeded into cell culture dishes (60x15 mm) and incubated for 24 h.
The cells were then incubated in medium without or with Sal (10 µM)
at 37˚C. After culturing for 24 h, the medium was collected and
centrifuged (3,500 x g) to remove cell debris. The following
solutions were then collected: i) s-control, comprising the
supernatant of RAW264.7 cells; and ii) s-Sal, comprising the
supernatant of Sal (10 µM)-treated RAW264.7 cells.
Cell treatment and groups
In total, the following five groups were designated
for the treatment of HUVECs: i) Control group, untreated HUVECs;
ii) s-control group, where the HUVECs were treated with supernatant
of RAW264.7 cells; iii) Sal group, where the HUVECs were treated
with 10 µM Sal; iv) s-Sal group, where the HUVECs were treated with
supernatant of Sal (10 µM)-treated RAW264.7 cells; and v) VEGF
group, where the HUVECs were treated with 10 ng/ml VEGF as a
positive control.
Cell viability assay
Cell viability was evaluated using the MTT assay
(Sigma-Aldrich; Merck KGaA). HUVECs (2.5x103 cells/well)
were seeded into 96-well culture plates and incubated for 24 h.
Subsequently, 100 µl complete medium containing Sal (10 µM),
s-control or s-Sal was added. VEGF (10 ng/ml) was added as the
positive control. After culturing for 24 h, 20 µl MTT (5 mg/ml) was
then added to each well for an additional 4 h, prior to the
addition of 150 µl DMSO to dissolve the formazan. The absorbance
(optical density value) at 490 nm was detected.
Wounding healing assay
HUVECs (5x105 cells/well) were seeded
into six-well culture plates to form a 90% confluent cell
monolayer. The monolayer was scratched vertically along the center
of each well with a 200-µl pipette tip and rinsed carefully with
PBS three times to remove the cell debris. The HUVECs were
incubated for 24 h with Sal (10 µM), s-control, s-Sal or VEGF (10
ng/ml; positive control) in the absence of FBS. In total, three
randomly selected views along the wound line in each well were
photographed using an inverted light microscope after incubation
for 0 and 24 h. ImageJ software 1.50i (National Institutes of
Health) was used to analyze the image migration distance and
calculate the migration rate (MR). The MR was calculated according
to the formula below: MR (%)=(1-24 h scratch distance/0 h scratch
distance) x100.
Tube formation assay
A 50-µl volume of Matrigel (BD Biosciences) was
pipetted into each well of a pre-chilled 96-well plate and allowed
to solidify at 37˚C for 30 min. Subsequently, HUVECs
(5x104 cells/well) and 100 µl culture medium without or
with Sal (10 µM), s-control, s-Sal or VEGF (10 ng/ml) were placed
into each well and incubated for another 3 h. Tube formation was
then observed and photographic images were captured using a Nikon
light microscope (Nikon Corporation) and tube formation ability was
measured by determining the number of master junctions with ImageJ
software 1.50i (National Institutes of Health).
Cell cycle
Cell cycle was detected using flow cytometry. HUVECs
(3x105 per well) were cultured in 60-mm culture plates
for 24 h and then incubated with either medium alone or medium
containing Sal (10 µM), s-control, s-Sal or VEGF (10 ng/ml) at 37˚C
for 24 h. The cell cycle analysis was performed using a Cell Cycle
Assay Kit-PI/RNase Staining (cat. no. C543) according to the
manufacturer's protocol (Dojindo Molecular Technologies, Inc.). The
cell cycle was detected via flow cytometry (CytoFLEX; Beckman
coulter, Inc.) and analysis was used ModFit software LT5 (Verity
Software House).
Reverse transcription-quantitative PCR
(RT-qPCR)
RAW264.7 cells (1x106 per well) were
cultured in 60-mm culture plates for 24 h and then incubated
without or with varying concentrations of Sal (5, 10, 50, 100 µM)
at 37˚C for 3 h. The culture medium was then removed, and total
mRNA was extracted from the cells using RNAiso Plus (cat. no. 9108;
Takara Bio, Inc.) according to the manufacturer's protocol. Reverse
transcription was performed with the PrimeScript™ RT reagent Kit
with gDNA Eraser (Perfect Real Time) (cat. no. RR047A; Takara Bio,
Inc.), and qPCR was performed using TB Green® Premix Ex
Taq™ (Tli RNaseH Plus) (cat. no. RR420A; Takara Bio, Inc.) both
according to the manufacturer's instructions. The following
thermocycling conditions were used for qPCR: Initial denaturation
at 95˚C for 30 sec, denaturation (40 cycles) at 95˚C for 5 sec, and
annealing/extending (40 cycles) at 60˚C for 30 sec; melt curve at
95˚C for 15 sec, at 60˚C for 1 min and at 95˚C for 15 sec. β-actin
was used as a reference in the experiment. Relative mRNA expression
was normalized to β-actin levels and analyzed with the
2-∆∆Cq method (28).
The mouse source primers were as follows: β-actin, forward:
5'-GTCCCTCACCCTCCCAAAAG-3' and reverse:
5'-GCTGCCTCAACACCTCAACCC-3'. Vegf, forward:
5'-TAGAGTACATCTTCAAGCCGTC-3', reverse:
5'-CTTTCTTTGGTCTGCATTCACA-3'. β-actin was used as the reference
gene.
ELISA
RAW264.7 cells (2x106 per well) were
cultured in 60-mm culture plates for 24 h and then incubated
without or with 10 µM Sal at 37˚C for 24 h. The medium was
collected and centrifuged at 3,500 x g at room temperature for 3
min to remove cell debris. The content of VEGF in the medium was
determined using a Mouse VEGF ELISA Kit (cat. no. EMC103.96;
Neobioscience Technology Co., Ltd.) according to the manufacturer's
protocol.
Western blotting
Brain tissue and cell lysates were prepared using
NP40 lysis buffer (Beyotime Institute of Biotechnology). Protein
concentrations were determined using the Enhanced BCA Protein Kit
(Beyotime Institute of Biotechnology). An equal amount of each
sample (30 µg) was separated by 10% SDS-PAGE and then transferred
onto PVDF membranes. After blocking with 5% non-fat milk for 2 h at
room temperature, the membranes were washed with TBS-10% Tween 20
three times for 10 min each and then incubated with VEGF (1:1,000
dilution), VEGFR-2 (1:1,000 dilution), p-VEGFR-2 (1:1,000
dilution), AKT (1:1,000 dilution), p-AKT (1:1,000 dilution), p38
(1:1,000 dilution) or p-p38 (1:1,000 dilution) antibodies at 4˚C
overnight. The membranes were then washed and incubated with the
appropriate HRP-conjugated secondary antibody for 1 h at room
temperature. The proteins were subsequently detected using a
Clarity™ Western ECL Substrate kit (Bio-Rad Laboratories, Inc.).
The densitometry analysis used AzureSpot software 2.0.062 (Azure
Biosystems, Inc.).
Construction of the rat model of
tMCAO
The tMCAO rat model was established using a modified
suture occlusion method. Rats were anesthetized with isoflurane (3%
for induction and 2.5% for maintenance) before an incision was made
in the neck of the rats precisely at the median position. The left
common carotid artery (CCA), external carotid artery (ECA) and
internal carotid artery (ICA) were carefully isolated. The CCA and
ECA were then ligated, before a microaneurysm clip was placed
around the ICA. A hole was cut in the CCA and the blunted tip of a
nylon suture was inserted through the hole into the ICA until mild
resistance was felt. The suture was left in place for 2 h and then
withdrawn to allow reperfusion. In the sham-operated group, the
rats were anesthetized, but only CCA and ECA ligation was
performed.
Neurological functions were evaluated with the Longa
scale (29) on the third day after
MCAO/reperfusion. The neurological deficits were assessed using the
following five-point scale: i) 0 points, no deficit; ii) 1 point,
forelimb weakness, flexion of and inability to straighten the
contralateral forelimb; iii) 2 points, circling to the affected
side; iv) 3 points, inability to bear weight on the affected side
and tilting to the contralateral side while walking; and v) 4
points, no spontaneous locomotor activity or loss of consciousness.
Animals with scores of 0 or 4 points were excluded from the
study.
Treatment and groups
Rats were randomly assigned into the following
groups: i) Sham group (n=13), in which sham-operated rats were
injected with normal saline by intraperitoneal injection; ii)
infarct group (n=28), in which tMCAO model rats were injected with
normal saline 2 h after reperfusion; iii) Sal groups, in which
tMCAO rats were intraperitoneally injected with 5 (n=5), 10 (n=5),
20 (n=28) or 40 mg/kg (n=5) Sal 2 h after reperfusion; and iv) ED
group (n=13), in which tMCAO model rats were treated with 30 mg/kg
edaravone (Zhejiang Shengtong Biotechnology Co., Ltd.) 2 h after
reperfusion as a positive control. The treatment was administered
for 3 days with a frequency of once a day. To observe the effects
of Sal (20 mg/kg) at different time periods, brain tissues were
collected after 0, 1 and 3 days from mice in the infarct and Sal
groups.
2,3,5-Triphenyltetrazolium chloride
(TTC) staining
Rats from the different groups were anesthetized
with 2% sodium pentobarbital (30 mg/kg) and then the brain tissues
were rapidly collected and cut into 2-mm slices. The brain slices
were immersed in 2% TTC (Sigma-Aldrich; Merck KGaA) solution at
37˚C for 15 min, after which they were soaked and fixed in 4%
paraformaldehyde solution at room temperature for 15 min. The
ischemic area of each brain slice was photographed and analyzed
using ImageJ software 1.50i. The infarct area is the area of viable
brain tissue in the right hemisphere minus the area of viable brain
tissue in the left hemisphere. The infarct volume was calculated
according to the following formula:
where h=2 mm, which represents the distance between
each section and S represents the infarct area (mm2) in
each brain section.
Histology and H&E staining
At the end of the experiment, the rats were
anesthetized with 2% sodium pentobarbital (30 mg/kg) and
transcardially perfused with 150 ml PBS. The brain tissues were
isolated and fixed in 4% paraformaldehyde solution at 4˚C
overnight. The cerebral hemispheres were then cut in the coronal
plane, stained with H&E at room temperature for 12 min and
examined by light microscopy.
Immunohistochemical staining
Immunohistochemical analysis was performed to detect
the expression of VEGF and VEGFR-2. After 3 days, rats were
sacrificed by deep anesthesia with 2% sodium pentobarbital (30
mg/kg) followed by transcardial perfusion with PBS, before the
brain tissues were fixed with 4% paraformaldehyde at room
temperature for 24 h. The 5-µm paraffinized brain sections were
dewaxed and dehydrated in xylene and ethanol solutions, followed by
antigen retrieval. Briefly, to deparaffinize and rehydrate, the
sections were incubated in 3 changes of xylene for 10 min each and
then dehydrate in 2 changes of pure ethanol for 5 min, followed by
dehydration in gradient ethanol (85 and 75% ethanol) for 5 min
each. The sections were then washed in distilled water. For antigen
retrieval, the slides were immersed in EDTA antigen retrieval
buffer (Wuhan Servicebio Technology Co., Ltd.) and maintained in
boiling water for 35 min before being air cooled. The slides were
then washed three times with PBS (pH 7.4) in a rocker device, for 5
min each, and blocked with 10% goat serum (Beyotime Institute of
Biotechnology) at room temperature for 15 min. All sections were
then incubated with anti-VEGF (1:100 dilution) and anti-VEGFR-2
(1:100 dilution) antibodies at 4˚C overnight. The sections were
then washed in PBS three times prior to incubation with
HRP-conjugated goat anti-rabbit IgG secondary antibodies for 10 min
at room temperature. Next, the sections were visualized using a DAB
kit and counterstained with hematoxylin at room temperature for 3
min. Using a light microscope, an investigator (FYW) blinded to the
experimental groups randomly selected three separate tissue
sections for each rat. The staining was analyzed using ImageJ
analysis software 1.50i.
Immunofluorescence staining
Paraffin-embedded brain sections were cut to a
thickness of 5 µm in the coronal plane, deparaffinized using
xylene, rehydrated with a descending ethanol gradient and washed in
distilled water, prior to blocking with 10% goat serum (Beyotime
Institute of Biotechnology) at room temperature for 1 h. The
sections were then incubated with the primary antibodies targeting
the following proteins overnight at 4˚C: VEGF (1:100 dilution) and
F4/80 (a marker of macrophage cells; 1:350 dilution), or VEGFR-2
(1:100 dilution) and CD31 (a marker of vascular endothelial cells;
1:200 dilution). The sections were then washed in PBS and incubated
with the appropriate secondary antibodies, namely anti-mouse IgG
Alexa Fluor® 488 Conjugate (cat. no. 4408; Cell
Signaling Technology, Inc.) and Cy™3-conjugated Affinipure Donkey
anti-rabbit IgG (cat no. 152679; Jackson ImmunoResearch
Laboratories, Inc.) for 2 h at room temperature in the dark. The
sections were finally mounted on coverslips with a drop of DAPI
solution (Sigma-Aldrich; Merck KGaA). The stained cells were
observed using a confocal microscope (A1; Nikon Corporation). The
staining was analyzed using ImageJ analysis software 1.50i.
Statistical analysis
The neurological score data are presented as the
median and interquartile range and were analyzed using the
Kruskal-Wallis test followed by Dunn's post-hoc tests. All other
data obtained are presented as the mean ± SD and were analyzed
statistically using one-way or two-way analysis of variance
followed by Bonferroni's multiple-comparisons tests. Statistical
analysis was performed using GraphPad 8.0 software (GraphPad
Software; Dotmatics). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of Sal on HUVEC function
mediated by macrophages in vitro
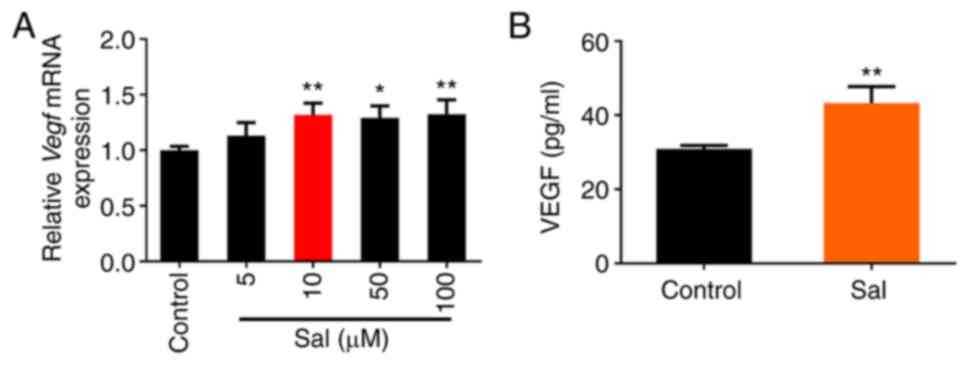
Firstly, the effect of Sal on VEGF expression and
production in macrophages at the mRNA and protein levels,
respectively, was assessed. The results showed that treatment with
≥10 µM Sal significantly promoted the expression of Vegf
mRNA in RAW264.7 cells, and 10 µM Sal increased the secretion of
VEGF protein into the supernatant of the cells (Fig. 1). The viability of HUVECs following
various treatments was then evaluated using MTT assays. As shown in
Fig. 2A, compared with that in the
control group, s-control, s-Sal and VEGF treatment all significantly
increased the proliferation of HUVECs, whilst treatment with Sal
alone did not affect the viability of HUVECs. This suggests that
Sal was unable to promote the proliferation of HUVECs directly.
However, s-Sal significantly increased the proliferation of HUVECs
compared with that in the s-control group, suggesting that Sal
indirectly promoted the proliferation of HUVECs through its effect
on macrophages. In addition, the cell cycle of the HUVECs was
detected by flow cytometry, whereby s-Sal was found to
significantly promote the number of HUVECs in the S phase compared
with that in the control and Sal groups (Fig. 2B and C).
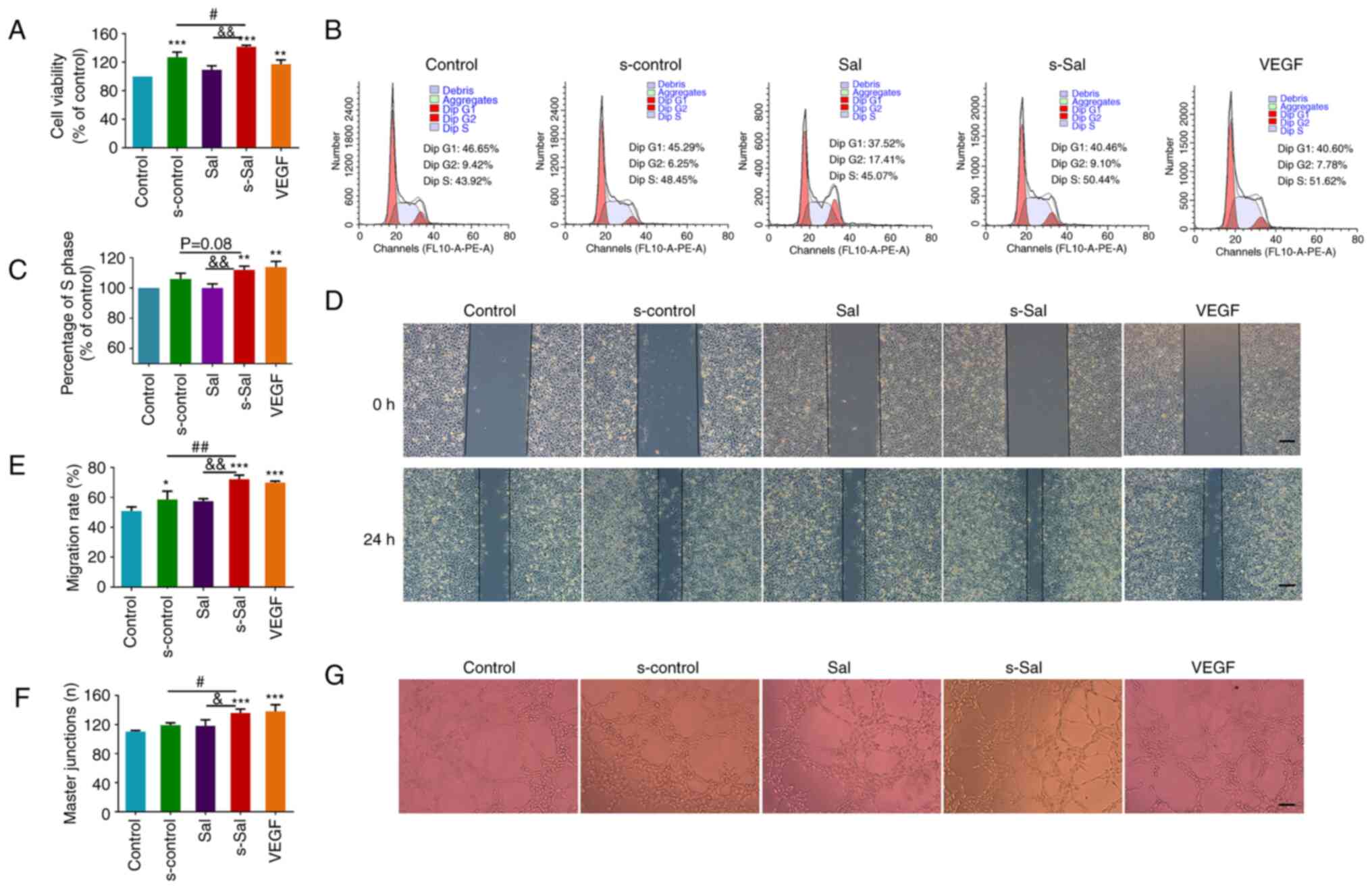 | Figure 2Effect of Sal on the viability, cell
cycle, migration and tube formation of HUVECs. (A) Effect of Sal on
HUVEC viability. (B) Cell cycle distribution of the HUVECs was
detected by flow cytometry and (C) the proportion of cells in the S
phase was quantified. (D) Representative images from a wound
healing assay of HUVECs at 0 and 24 h (scale bar, 200 µm) and (E)
quantitative analysis of cell migration to the wound. (F)
Quantitative analysis of tube formation and (G) representative
images of tube formation (scale bar, 200 µm). Values are presented
as the mean ± SD (n=3 per group); *P<0.05,
**P<0.01, ***P<0.001 vs. the control
group; #P<0.05, ##P<0.01,
&P<0.05, &&P<0.01 as
indicated. Sal, salvianolate; HUVECs, human umbilical vein
endothelial cells; VEGF, vascular endothelial growth factor.
Control group, untreated HUVECs; s-control group, HUVECs treated
with supernatant of RAW264.7 cells; Sal group, HUVECs treated with
10 µM Sal; s-Sal group, HUVECs treated with supernatant of Sal (10
µM)-treated RAW264.7 cells; VEGF group, HUVECs treated with 10
ng/ml VEGF as a positive control. |
The migration of HUVECs was next analyzed using a
wound healing assay. The s-control, s-Sal and VEGF groups all
showed increased degrees of cell migration to the wounded area
after 24 h of incubation compared with that in the control group
(Fig. 2D and E). In addition, the migration rate in the
s-Sal group was significantly higher compared with that in the Sal
and s-control groups.
To examine the effect of Sal on the tubule formation
of HUVECs, a tube formation assay was performed using Matrigel.
Images of canaliculus formation are presented in Fig. 2G, and tube formation is expressed
as the number of master junctions in Fig. 2F. No significant difference was
detected between the Sal and control groups. However, s-Sal
treatments significantly enhanced the extent of tube formation of
the HUVECs compared with that in the s-control group. Together,
these results suggest that Sal promoted endothelial cell tube
formation via its effect on macrophages.
Effect of Sal on VEGF signaling
pathways and macrophage activation in vitro
To investigate the effect of Sal on
macrophage-mediated angiogenesis and the VEGF signaling pathway
in vitro, western blotting was performed. Following VEGF
binding to VEGFR2, the proliferation and migration of endothelial
cells and their maturation into vessels are typically activated
(7). Therefore, VEGFR2 expression
levels and activation were assessed by western blotting analysis
(Fig. 3A). s-Sal significantly
promoted VEGFR2 phosphorylation at Tyr1175 compared with that in
the control, s-control and Sal groups, which indicates that s-Sal
induced the activation of this receptor (Fig. 3B). In addition, this form of VEGFR2
activation was found to be associated with the activation of AKT
and p38 MAPK signaling downstream (Fig. 3A, C and D),
as s-Sal significantly promoted the phosphorylation of AKT compared
with that in the control and Sal groups and phosphorylation of p38
proteins compared with that in the control, s-control and Sal
groups in the HUVECs. The activation of AKT and p38 MAPK is
essential for cellular responses during angiogenesis (7).
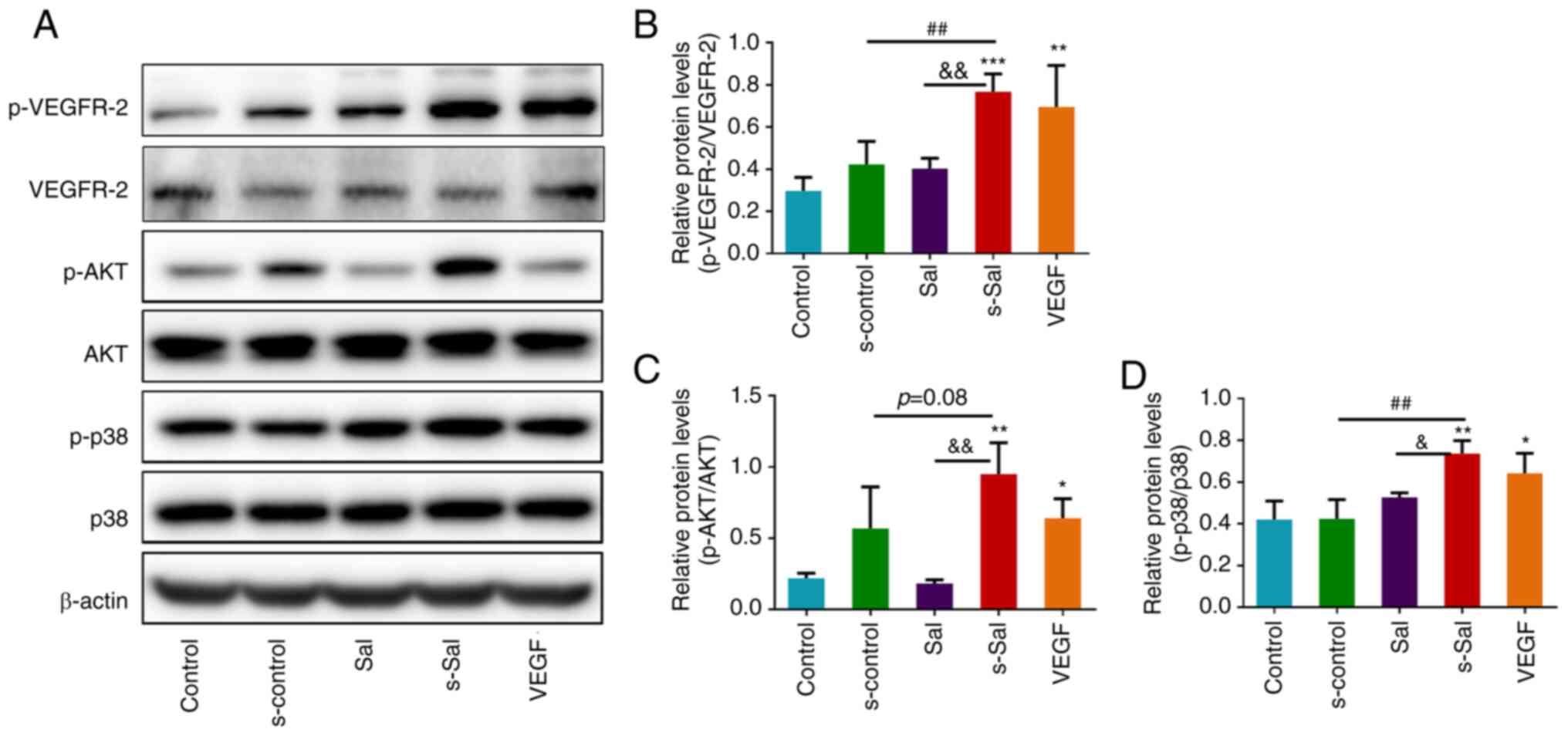 | Figure 3Effect of Sal on the VEGF signaling
pathway in vitro. (A) Representative western blots of
VEGFR-2, p-VEGFR-2, AKT, p-AKT, p38, p-p38 and β-actin.
Quantitative analysis of the relative levels of (B) p-VEGFR-2, (C)
p-AKT and (D) p-p38 normalized to those of total VEGFR-2, AKT and
p38, respectively. Values are presented as the mean ± SD (n=3 per
group); *P<0.05, **P<0.01,
***P<0.001 vs. the control group;
##P<0.01, &P<0.05,
&&P<0.01 as indicated. Sal, salvianolate;
VEGF, vascular endothelial growth factor; VEGFR-2, VEGF receptor 2;
p-, phosphorylated; Control group, untreated HUVECs; s-control
group, HUVECs treated with supernatant of RAW264.7 cells; Sal
group, HUVECs treated with 10 µM Sal; s-Sal group, HUVECs treated
with supernatant of Sal (10 µM)-treated RAW264.7 cells; VEGF group,
HUVECs treated with 10 ng/ml VEGF as a positive control. |
Effect of Sal on stroke outcomes
To investigate the possible effects of Sal in the
rat tMCAO model, tMCAO model rats were treated with Sal for 3 days
and then the neurological deficit scores of the rats were
determined. The scores are shown in Fig. 4A. The neurological deficit scores
of the infarct group were observed to be significantly increased
compared with those in the sham group. A significant reduction in
neurological deficit scores of the 10, 20 and 40 mg/kg Sal groups
and the ED group was observed compared with that in the infarct
group, indicating improved neurological recovery following Sal or
ED treatment.
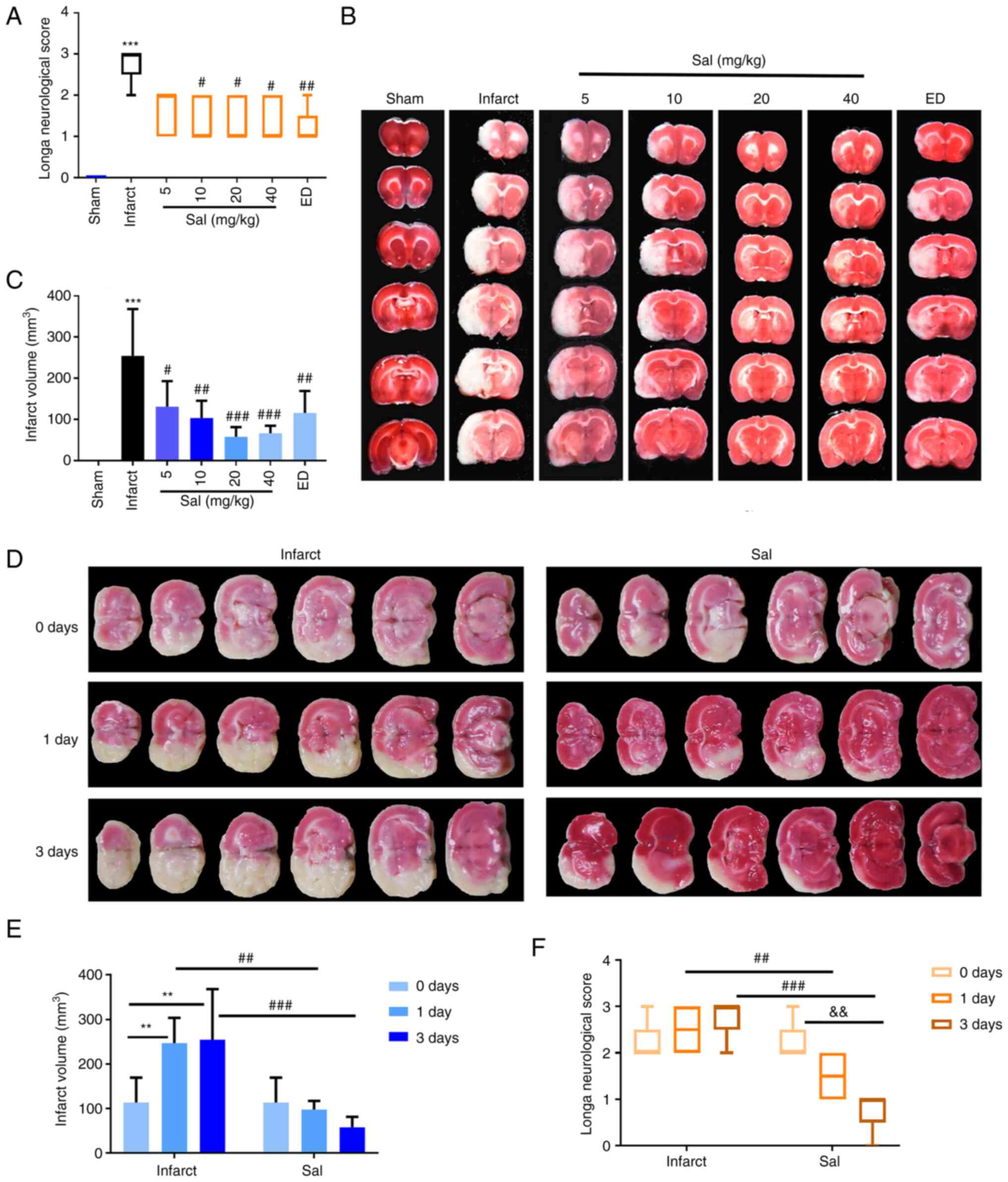 | Figure 4Sal significantly improves stroke
outcomes in a rat model of transient middle cerebral artery
occlusion. (A) Quantitative analysis of neurological deficit
scores. (B) Representative photographs of TTC-stained rat brain
slices and (C) quantitative analysis of the cerebral infarct volume
following treatment with different concentrations of Sal.
***P<0.001 vs. sham; #P<0.05,
##P<0.01, ###P<0.001 vs. the infarct
group. (D) Representative photographs of TTC-stained rat brain
slices and (E) quantitative analysis of the cerebral infarct volume
at 0, 1 and 3 days after treatment with or without 20 mg/kg Sal.
(F) Quantitative analysis of neurological deficit scores at 0, 1
and 3 days after treatment with or without 20 mg/kg Sal. Values are
presented as the median and interquartile range or mean ± SD (n=5
per group). ##P<0.01, ###P<0.001,
**P<0.01, &&P<0.01 as
indicated. Sal, salvianolate; TTC, 2,3,5-triphenyltetrazolium
chloride; ED, edaravone. |
The infarct volume was next determined by TTC
staining. The infarct volume was found to be significantly
increased in the infarct group compared with the sham group. Rats
subjected to cerebral ischemia and treated with various doses of
Sal exhibited a significantly smaller infarct volume compared with
that in the infarct group (Fig. 4B
and C). The neurological deficit
scores and infarct volume after treatment with various
concentrations of Sal indicated that 20 mg/kg was the most
effective concentration, rendering 20 mg/kg as the dose selected
for subsequent experiments. The results at the 0-, 1- and 3-day
time points for the infarct and 20 mg/kg Sal group indicated that
the infarct volume increased and nerve function deteriorated in the
infarct group. By contrast, the rats in the Sal-administered group
exhibited improvements with reductions in the extent of infarction
and nerve function impairment (Fig.
4D-F).
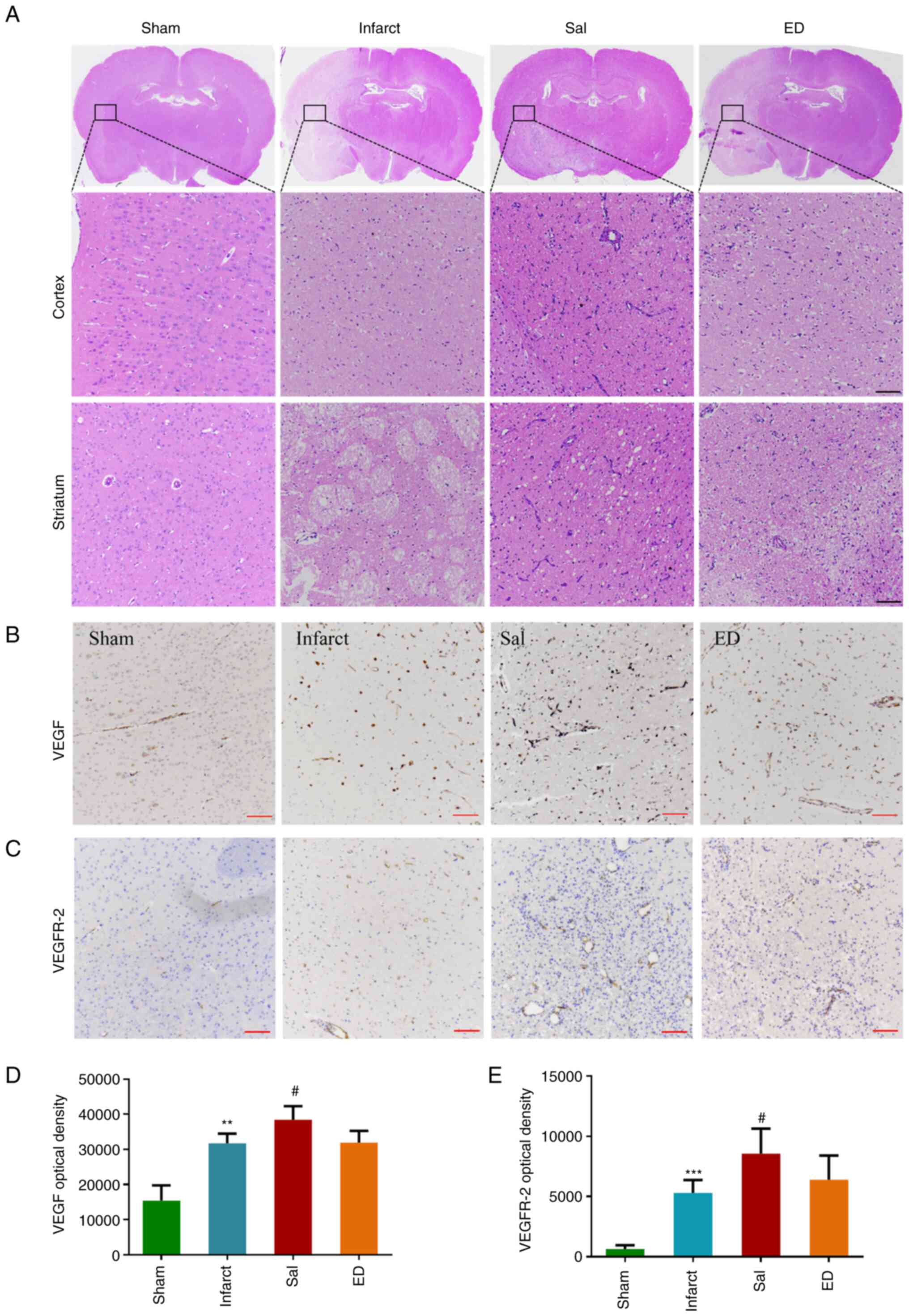
To further investigate the protective effect of Sal
against brain I/R injury, morphological changes in the brain
tissues were observed by H&E staining after 3 days of treatment
with Sal or ED. As shown in Fig.
5A, the characteristic histopathological features in the
infarct group were nuclear atrophy, cytoplasmic eosinophilia and
cellular edema. The 20 mg/kg Sal treatment group exhibited reduced
histopathological abnormalities compared with those in the infarct
group.
The effect of Sal on the VEGF signaling pathway was
investigated in vivo. Immunohistochemical staining (Fig. 5B and C) was used to detect VEGF protein
(Fig. 5B and D) and VEGFR-2 protein expression
(Fig. 5C and E). The immunohistochemical staining
intensity of VEGF and VEGFR-2 in the Sal group was found to be
significantly higher compared with that in the infarct group.
Effect of Sal on the VEGF and
downstream signaling pathways in vivo
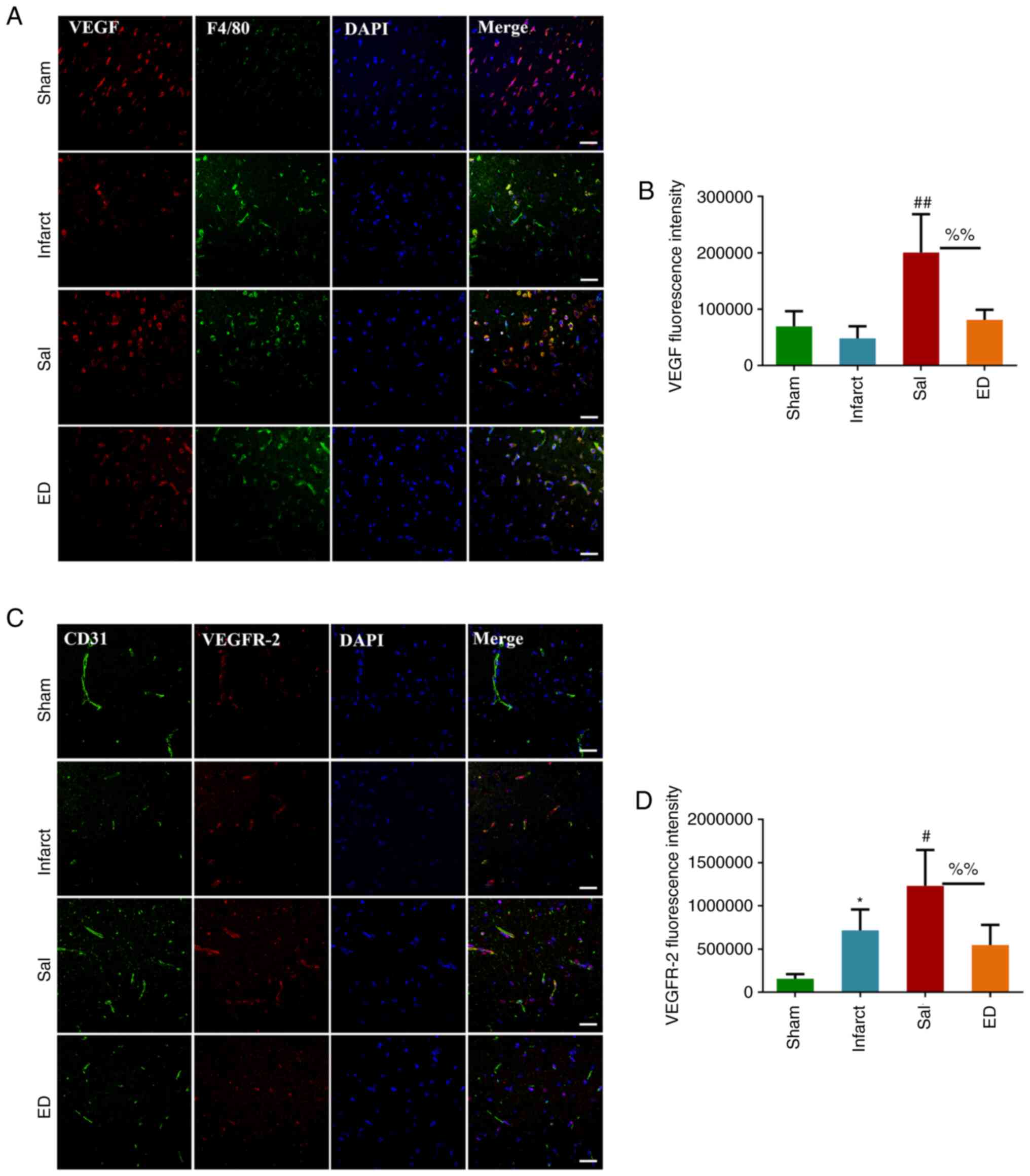
To investigate whether Sal mediates angiogenesis via
the VEGF signaling pathway in vivo, immunofluorescence
analyses were performed. After 3 days of Sal treatment, F4/80 and
VEGF double immunofluorescence staining and CD31 and VEGFR-2 double
immunofluorescence staining were used to detect the cellular
location and expression levels of VEGF and VEGFR-2 proteins in the
brain tissue (Fig. 6). The results
showed that VEGF protein was localized at sites of macrophage
accumulation, where it tended to be highly expressed. In addition,
VEGFR2 protein was found to colocalize with endothelial cells, and
the expression of VEGFR2 in the Sal group was significantly
increased compared with that in the infarct group, with higher
microvessel density in the former.
To evaluate the efficacy of Sal in upregulating VEGF
signaling activity in vivo, western blot analysis was
performed on tissues in the peri-infarct area. As shown in the
western blots in Fig. 7A, VEGF and
VEGFR2 protein expression and VEGFR2 phosphorylation in the infarct
group were lower compared with those in the sham group.
Additionally, the protein expression of VEGF (Fig. 7B) and VEGFR2 (Fig. 7C) along with VEGFR-2
phosphorylation (Fig. 7D) were
upregulated in the Sal group compared with those in the infarct
group. The phosphorylation levels of AKT and p38, which lie
downstream of the VEGF/VEGFR2 signaling pathway, were also
significantly increased by the administration of Sal (Fig. 7A, E and F).
These results suggest that Sal protected rats against acute
cerebral ischemia by promoting angiogenesis in the brain.
Specifically, the mechanism of its pro-angiogenic activity appears
to be the promotion of VEGF signaling and in turn the downstream
AKT and p38 signaling pathways.
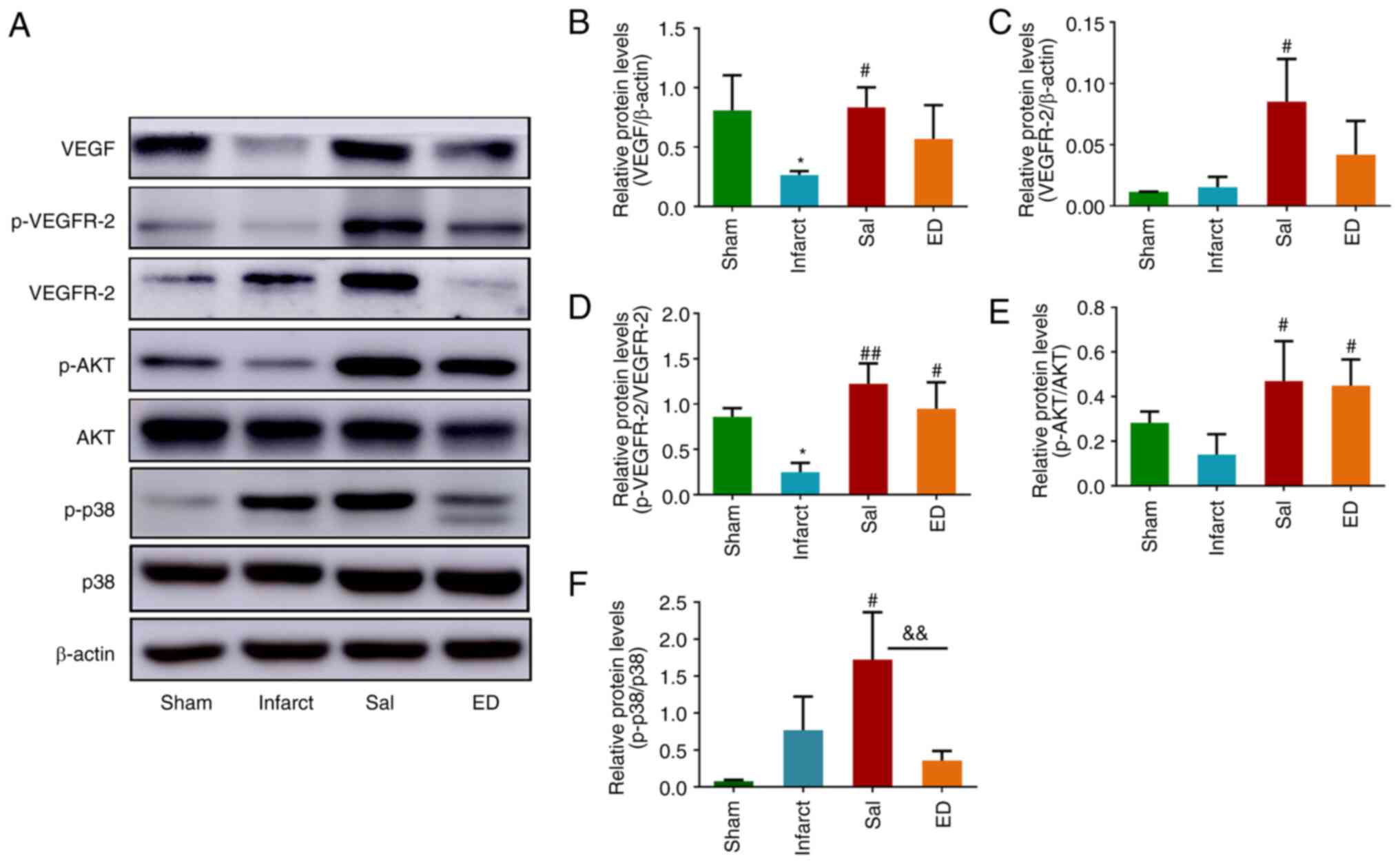 | Figure 7Effect of Sal on the VEGF signaling
pathway in a rat model of transient middle cerebral artery
occlusion. (A) Representative western blots of VEGF, VEGFR-2,
p-VEGFR-2, p-AKT, AKT, p38, p-p38 and β-actin. Quantitative
analysis of the relative levels of (B) VEGF and (C) VEGFR-2
normalized to those of β-actin, (D) p-VEGFR-2 relative to those of
total VEGFR-2, (E) p-AKT relative to those of total AKT and (F)
p-p38 relative to those of total p38. Values are presented as the
mean ± SD (n=3 per group). *P<0.05 vs. the sham
group; #P<0.05, ##P<0.01 vs. the
infarct group; &&P<0.01 as indicated. Sal,
salvianolate; VEGF, vascular endothelial growth factor; VEGFR-2,
VEGF receptor 2; p-, phosphorylated; ED, edaravone. |
Discussion
Sal can be extracted from the Chinese herb Salvia
miltiorrhiza Bunge (Labiatae) and has been widely used for the
treatment of cardiovascular diseases, including coronary heart
disease and angina pectoris in China. This is due to its reported
effect as a promotor of blood circulation. Although the present
study demonstrated that Sal exerted no significant effects on
HUVECs directly, 10 µM Sal treatment increased Vegf mRNA
expression in macrophages, which consequently enhanced the
secretion of VEGF into the macrophage supernatant. Based on these
results, it may be hypothesized that Sal mediated the activation of
RAW264.7 macrophages and secretion of VEGF to induce angiogenesis
in HUVECs. The pro-angiogenic effects of Sal-treated macrophages,
including the promotion of cell proliferation, migration and tube
formation were therefore investigated. VEGFR2 is known to regulate
vascular endothelial cell proliferation, migration, differentiation
and capillary formation (7).
Therefore, the promotion of VEGFR2 signaling represents a viable
approach for therapeutical pro-angiogenic interventions. In the
present study, Sal was found to promote VEGFR2 activation and
thereby induce AKT and p38 signaling downstream in HUVECs in
vitro, via its effect on RAW264.7 cells. Considering that Sal
was found to promote angiogenesis via VEGF and downstream signaling
pathways in vitro, an in vivo ischemic stroke model
was then established to verify the protective effects of Sal.
Ischemic stroke is a serious neurological disease,
the fine pathophysiological mechanism of which remains to be fully
elucidated. There are various proposed theories regarding the
pathological mechanism underlying I/R injury, which include
excitatory amino acids, inflammatory reactions, oxidative stress
damage, metabolic acidosis, intracellular Ca2+ overload,
mitochondrial damage, brain cell apoptosis, necrosis and autophagy
(6,30,31).
At present, there is no effective long-term treatment method for
ischemic stroke. The main treatment measure is early thrombolysis
(32). Although thrombolytic
treatment can return blood supply to the ischemic area quickly, due
to the strict time 3-h window post-ischemia during which the blood
supply must be restored and possible adverse reactions, including
reperfusion injury and increased hemorrhage risk, its use is
limited. Although neurotrophic and neurological rehabilitation are
the main treatment objectives, the clinical application of
neuroprotective agents has not achieved promising results (33). Therapeutic interventions that
involve manipulation of the cellular immune system are currently
being explored in patients with ischemic stroke (34,35).
Although several studies have previously shown that stem cell
therapy can enhance functional recovery from stroke, this treatment
is limited by the low survival rate and poor differentiation of
transplanted cells (36,37).
The current understanding of immunomodulation in the
brain is insufficient, which is largely due to potential drugs not
being successful in clinical trials. Therefore, it is necessary to
find a safe and effective method that is suitable for use in both
early stroke treatment and the recovery of motor functions after
stroke. Unfortunately, there is no one therapeutic strategy that
can effectively meet the aforementioned conditions. Therefore,
further research and development of novel agents is necessary.
Ischemic stroke can cause ischemia and hypoxia in the brain
tissues, leading to the loss of functional neurons in the
corresponding brain areas. The potentially salvageable tissue
around the ischemic core, referred to as the penumbra, is the prime
region to be targeted (38). The
penumbra is unstable with the potential to regenerate, which forms
the basis for the treatment of ischemia (1). Previous studies have found that
angiogenesis and the rapid establishment of collateral circulation
in the area of cerebral infarction and cerebral ischemia can
significantly reduce the cerebral infarct size, improve
neurobehavioral symptoms and reduce mortality in animals and
patients (1,2,39).
For the treatment of ischemic diseases, the
promotion of angiogenesis in the ischemic site and its periphery is
a promising approach for restorative therapy. In the present study,
the administration of Sal to rats following MCAO significantly
enhanced neovascularization, restored vascular function and
ameliorated neurological deficits. In addition, Sal was found to
have beneficial effects on the cortical tissue around the infarct
after MCAO. Sal was indicated to attenuate cerebral I/R injury via
upregulation of the VEGF/VEGFR2 signaling pathway. According to the
in vivo results, the expression of the VEGF signaling
pathway components VEGF and VEGFR-2 was significantly increased
after treatment with Sal. The phosphorylation of VEGFR-2 and its
downstream signaling components AKT and p38 were subsequently
measured, and the results showed that Sal promoted the
phosphorylation of VEGFR-2, AKT and p38. These results support the
hypothesis that Sal can regulate endothelial cell function and
intracellular signaling through macrophages in vitro.
The present study revealed that Sal promoted
macrophage-mediated HUVEC proliferation, migration and tube
formation. This was associated with the Sal-induced upregulation of
VEGFR2, AKT and p38 activation in HUVECs. In addition, Sal
accelerated blood vessel formation in a rat model of ischemic
stroke whilst upregulating the protein expression of VEGF and
VEGFR2, in addition to the activation of VEGFR2, AKT and p38 in
vivo. This suggests that the mechanism of its pro-angiogenic
activity is likely to mainly involve the promotion of VEGF
signaling and then AKT and p38 signaling downstream. The protective
effect of Sal on rats with acute cerebral ischemia may have been
achieved through the promotion of cerebral angiogenesis. These
results suggest that Sal is a promising candidate for the treatment
of acute cerebral ischemia. The present study has certain
limitations and further studies are required to explore
improvements. For example, although edaravone has a protective
effect against cerebral infarction in vivo, its main
mechanism of action is to reduce oxidative stress caused by
cerebral ischemic injury (40).
Drugs associated with angiogenesis will be selected as positive
controls in subsequent studies. In addition, the hypothesis that
the pro-angiogenic effect of Sal is mediated by blocking the
VEGFR-2 signaling pathway will be further verified in the
future.
In summary, the results of the present study suggest
that Sal regulated endothelial cell function through VEGF and its
downstream signaling pathways, likely in a macrophage-dependent
manner, in vitro. In addition, the protective effect of Sal
was verified further in a model of cerebral ischemia, the mechanism
of which is summarized in Fig. 8.
These findings shed light on the novel therapeutic effects of the
administration of Sal, which may provide information useful for the
development of drug leads and candidates for the treatment of
ischemic disease.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from the National
Natural Science Foundation of China (grant no. 82003800), the
Shanghai Municipal Education Commission (grant no.
2019-01-07-00-10-E00072), Shanghai Municipal Health
Commission/Shanghai Municipal Administration of Traditional Chinese
Medicine [grant no. ZY(2021-2023)-0501], Shanghai Science and
Technology Development Fund from Central Leading Local Government
(grant no. YDZX20223100001004), Science and Technology Commission
of Shanghai Municipality (grant no. 20ZR1473200), ‘Chenguang
Program’ supported by Shanghai Education Development Foundation and
Shanghai Municipal Education Commission (grant no. 21CGA51), Open
Project of National Major Scientific and Technological
Infrastructure for Translational Medicine (Shanghai) (grant no.
TMSK-2021-405) and Open Project of Shanghai Key Laboratory for
Molecular Engineering of Chiral Drugs (grant no. SMECD2022004).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, JX and PL were responsible for study conception
and design. JX, YS, YX, LJ, RL and FW performed experiments and
analyzed the data. JX and JZ interpreted the data and drafted the
manuscript. HW and YZ helped with the data analysis. JZ, YZ, JX and
HW edited the manuscript. All authors read and approved the final
version of the manuscript. JZ and YZ confirm the authenticity of
all the raw data.
Ethics approval and consent to
participate
All animal experiments were approved by the Ethics
Committee of the animal experiment center of Shanghai University of
Traditional Chinese Medicine (Shanghai, China; approval no.
PZSHUTCM200320004).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guo H, Adah D, James PB, Liu Q, Li G,
Ahmadu P, Chai L, Wang S, Liu Y and Hu L: Xueshuantong injection
(lyophilized) attenuates cerebral ischemia/reperfusion injury by
the activation of Nrf2-VEGF pathway. Neurochem Res. 43:1096–1103.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Xu H, Cao Y, Yang X, Cai P, Kang L, Zhu X,
Luo H, Lu L, Wei L, Bai X, et al: ADAMTS13 controls vascular
remodeling by modifying VWF reactivity during stroke recovery.
Blood. 130:11–22. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Yin KJ, Hamblin M and Chen YE:
Angiogenesis-regulating microRNAs and ischemic stroke. Curr Vasc
Pharmacol. 13:352–365. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Deb P, Sharma S and Hassan KM:
Pathophysiologic mechanisms of acute ischemic stroke: An overview
with emphasis on therapeutic significance beyond thrombolysis.
Pathophysiology. 17:197–218. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Feng D, Wang B, Wang L, Abraham N, Tao K,
Huang L, Shi W, Dong Y and Qu Y: Pre-ischemia melatonin treatment
alleviated acute neuronal injury after ischemic stroke by
inhibiting endoplasmic reticulum stress-dependent autophagy via
PERK and IRE1 signalings. J Pineal Res. 62:2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Puyal J, Ginet V and Clarke PG: Multiple
interacting cell death mechanisms in the mediation of
excitotoxicity and ischemic brain damage: A challenge for
neuroprotection. Prog Neurobiol. 105:24–48. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu Y, Xu J, Zong A, Wang J, Liu Y, Jia W,
Jin J, Yan G and Zhang Y: Anti-angiogenic activity and mechanism of
a chemically sulfated natural glucan from Phellinus ribis. Int J
Biol Macromol. 107:2475–2483. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Thiyagarajan M, Fernández JA, Lane SM,
Griffin JH and Zlokovic BV: Activated protein C promotes
neovascularization and neurogenesis in postischemic brain via
protease-activated receptor 1. J Neurosci. 28:12788–12797.
2008.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhao BQ, Wang S, Kim HY, Storrie H, Rosen
BR, Mooney DJ, Wang X and Lo EH: Role of matrix metalloproteinases
in delayed cortical responses after stroke. Nat Med. 12:441–445.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Zhang ZG and Chopp M: Neurorestorative
therapies for stroke: underlying mechanisms and translation to the
clinic. Lancet Neurol. 8:491–500. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cattin AL, Burden JJ, Van Emmenis L,
Mackenzie FE, Hoving JJ, Garcia Calavia N, Guo Y, McLaughlin M,
Rosenberg LH, Quereda V, et al: Macrophage-Induced blood vessels
guide schwann cell-mediated regeneration of peripheral nerves.
Cell. 162:1127–1139. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Manoonkitiwongsa PS, Schultz RL, Whitter
EF and Lyden PD: Contraindications of VEGF-based therapeutic
angiogenesis: Effects on macrophage density and histology of normal
and ischemic brains. Vascul Pharmacol. 44:316–325. 2006.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu J, Wang Y, Akamatsu Y, Lee CC, Stetler
RA, Lawton MT and Yang GY: Vascular remodeling after ischemic
stroke: Mechanisms and therapeutic potentials. Prog Neurobiol.
115:138–156. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Pedragosa J, Salas-Perdomo A, Gallizioli
M, Cugota R, Miró-Mur F, Briansó F, Justicia C, Pérez-Asensio F,
Marquez-Kisinousky L, Urra X, et al: CNS-border associated
macrophages respond to acute ischemic stroke attracting
granulocytes and promoting vascular leakage. Acta Neuropathol
Commun. 6(76)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Manoonkitiwongsa PS, Jackson-Friedman C,
McMillan PJ, Schultz RL and Lyden PD: Angiogenesis after stroke is
correlated with increased numbers of macrophages: The clean-up
hypothesis. J Cereb Blood Flow Metab. 21:1223–1231. 2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li WL, Fraser JL, Yu SP, Zhu J, Jiang YJ
and Wei L: The role of VEGF/VEGFR2 signaling in peripheral
stimulation-induced cerebral neurovascular regeneration after
ischemic stroke in mice. Exp Brain Res. 214:503–513.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee DH, Lee J, Jeon J, Kim KJ, Yun JH,
Jeong HS, Lee EH, Koh YJ and Cho CH: Oleanolic acids inhibit
vascular endothelial growth factor receptor 2 signaling in
endothelial cells: Implication for anti-angiogenic therapy. Mol
Cells. 41:771–780. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wise GE and Yao S: Expression of vascular
endothelial growth factor in the dental follicle. Crit Rev Eukaryot
Gene Expr. 13:173–180. 2003.PubMed/NCBI
|
|
19
|
Krum JM, Mani N and Rosenstein JM:
Angiogenic and astroglial responses to vascular endothelial growth
factor administration in adult rat brain. Neuroscience.
110:589–604. 2002.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Melincovici CS, Boşca AB, Şuşman S,
Mărginean M, Mihu C, Istrate M, Moldovan IM, Roman AL and Mihu CM:
Vascular endothelial growth factor (VEGF)-key factor in normal and
pathological angiogenesis. Rom J Morphol Embryol.
59(455)2018.PubMed/NCBI
|
|
21
|
Greenberg DA and Jin K: From angiogenesis
to neuropathology. Nature. 438:954–959. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Watzke A, O'Malley SJ, Bergman RG and
Ellman JA: Reassignment of the configuration of salvianolic acid B
and establishment of its identity with lithospermic acid B. J Nat
Prod. 69:1231–1233. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Han B, Zhang X, Zhang Q, Zhao G, Wei J, Ma
S, Zhu W and Wei M: Protective effects of salvianolate on
microvascular flow in a porcine model of myocardial ischaemia and
reperfusion. Arch Cardiovasc Dis. 104:313–324. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Qin CZ, Ren X, Zhou HH, Mao XY and Liu ZQ:
Inhibitory effect of salvianolate on human cytochrome P450 3A4 in
vitro involving a noncompetitive manner. Int J Clin Exp Med.
8:15549–15555. 2015.PubMed/NCBI
|
|
25
|
Li X, Xu X, Wang J, Wang X, Yang H, Xu H,
Tang S, Li Y, Yang L, Huang L, et al: A system-level investigation
into the mechanisms of Chinese traditional medicine: Compound
Danshen formula for cardiovascular disease treatment. PLoS One.
7(e43918)2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu JR, Liu S, Zhang XM and Zhang B:
Danshen injection as adjuvant treatment for unstable angina
pectoris: A systematic review and meta-analysis. Chin J Integr Med.
23:306–311. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dong P, Hu H, Guan X, Ung COL, Shi L, Han
S and Yu S: Cost-consequence analysis of salvianolate injection for
the treatment of coronary heart disease. Chin Med.
13(28)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhu Y, Yang L, Xu J, Yang X, Luan P, Cui
Q, Zhang P, Wang F, Li R, Ding X, et al: Discovery of the
anti-angiogenesis effect of eltrombopag in breast cancer through
targeting of HuR protein. Acta Pharm Sin B. 10:1414–1425.
2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Luo Y, Tang H, Li H, Zhao R, Huang Q and
Liu J: Recent advances in the development of neuroprotective agents
and therapeutic targets in the treatment of cerebral ischemia. Eur
J Med Chem. 162:132–146. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bielewicz J, Kurzepa J, Łagowska-Lenard M
and Bartosik-Psujek H: The novel views on the patomechanism of
ischemic stroke. Wiad Lek. 63:213–220. 2010.PubMed/NCBI(In Polish).
|
|
32
|
Cohen JE, Leker RR and Rabinstein A: New
strategies for endovascular recanalization of acute ischemic
stroke. Neurol Clin. 31:705–719. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Weintraub MI: Thrombolysis (tissue
plasminogen activator) in stroke: A medicolegal quagmire. Stroke.
37:1917–1922. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fu Y, Zhang N, Ren L, Yan Y, Sun N, Li YJ,
Han W, Xue R, Liu Q, Hao J, et al: Impact of an immune modulator
fingolimod on acute ischemic stroke. Proc Natl Acad Sci USA.
111:18315–18320. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fu Y, Liu Q, Anrather J and Shi FD: Immune
interventions in stroke. Nat Rev Neurol. 11:524–535.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Carmichael ST: Emergent properties of
neural repair: Elemental biology to therapeutic concepts. Ann
Neurol. 79:895–906. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Parr AM, Tator CH and Keating A: Bone
marrow-derived mesenchymal stromal cells for the repair of central
nervous system injury. Bone Marrow Transplant. 40:609–619.
2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li D, Lang W, Zhou C, Wu C, Zhang F, Liu
Q, Yang S and Hao J: Upregulation of microglial ZEB1 ameliorates
brain damage after acute ischemic stroke. Cell Rep. 22:3574–3586.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hillen F and Griffioen AW: Tumour
vascularization: Sprouting angiogenesis and beyond. Cancer
Metastasis Rev. 26:489–502. 2007.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yagi K, Kitazato KT, Uno M, Tada Y,
Kinouchi T, Shimada K and Nagahiro S: Edaravone, a free radical
scavenger, inhibits MMP-9-related brain hemorrhage in rats treated
with tissue plasminogen activator. Stroke. 40:626–631.
2009.PubMed/NCBI View Article : Google Scholar
|