Introduction
Osteoporosis (OP) is a common disease characterized
by reduced bone mass, microstructural deterioration, fragility and
consequent fragility fractures and is particularly prevalent among
the elderly population (1).
Osseous metabolic imbalance is the major pathogenic factor involved
in OP; it is affected by diet, physical activity and clinical and
hormonal states (2,3).
The aforementioned conditions associated with OP are
significantly more common in patients with type 1 and 2 diabetes
mellitus (DM) (4,5). Studies have shown that patients with
DM have fragile bones, despite normal bone mineral density (BMD)
(6,7). Type 2 DM patients have increased
circulating levels of sclerostin, an osteocyte-secreted negative
regulator of bone formation (8).
The mechanisms underlying bone fragility in DM remain to be
elucidated; however, aggregation of advanced glycation
end-products, altered collagen cross-linking and reduced osseous
turnover may serve a role (9). In
addition to the intrinsic effects of DM on bones, several
anti-diabetic drugs negatively affect bone health. For instance,
thiazolidinediones increase the risk of fractures (10).
Previous research has shown that glucagon-like
peptide-1 receptor agonists (GLP-1 RAs), which are novel incretin
therapy-based anti-diabetic drugs, increase insulin secretion,
promote insulin sensitivity and reduce gastrointestinal motility
(11). The GLP-1 RA exenatide has
positive effects on bones (12).
GLP-1 RAs may alter the bone turnover rate (13). GLP-1 indirectly inhibits bone
resorption by binding to GLP-1 receptors (GLP-1Rs) within thyroid C
cells and stimulating calcitonin production (14). In addition, mice with pancreatic
GLP-1R deletion develop cortical osteopenia and increased
calcitonin pathway-mediated bone resorption (15). A study found that GLP-1R activation
positively affects bone quality and strength, whereas
GLP-1R-deficient mice have significantly reduced bone mechanical
strength, outer diameter and cortical thickness of bones and
collagen matrix maturity (16).
Additionally, mice with double incretin receptor knock-out have
substantially altered bone strength and microarchitecture,
suggesting that incretin hormones are essential for normal bone
quality (17). The present study
evaluated the effects of GLP-1RA on fracture healing in a rat model
of variectomized OP (OVX).
Materials and methods
Animal model and pharmaceutical
treatment
The 5-month-old Sprague Dawley female rats (n=36;
weight: 280-320 g) were obtained from the Animal Center of Chinese
Academy of Sciences (Shanghai, China). The rat experiment was
performed in accordance with the guidelines of the Animal Care and
Use Committee of Hainan Medical University (approval no. 2020-56).
The animals were maintained at 25˚C in a temperature-controlled
room with 40-50% humidity and free access to water and food. The
rats were subjected to 1-week environmental acclimation under a
12-h light/dark cycle. The rats were randomly divided into the sham
operation (control), OVX and OVX + LIRA groups. After inducing
general anesthesia with an intraperitoneal injection of
pentobarbital sodium (50 mg/kg), an incision was made on the back
of mice to perform bilateral OVX. Then, the skin, abdominal cavity
and muscles were cut using a 1.5-cm incision to expose the ovaries.
Silk suture was used to ligate the oviduct. Next, bilateral OVX was
performed. The remaining rats underwent a sham operation and
examination of bilateral ovaries. The remaining steps were
completed using an identical protocol for all groups. The BMD in
the tibial metaphysis was tested to evaluate for the development of
OP. Unilateral transverse osteotomy was performed in OP model rats
in the right femur center and a 1-mm intramedullary nail [Wego
Healthcare (Shenzhen) Co., Ltd.] was used for pinning. The solution
of LIRA and saline (0.3 mg/kg/day) was injected subcutaneously
around the back wound in OVX + LIRA rats until sacrifice (18). In the remaining rats, same quantity
of saline was injected subcutaneously.
X-ray
At 3 and 6 weeks postoperatively, anteroposterior
X-ray of the rat femur was performed to evaluate fracture union and
bone formation using a small animal imaging system (12 sec; 26 kV;
MX20; Faxitron X-Ray LLC).
Histological analysis
At 3 and 6 weeks postoperatively, 4%
paraformaldehyde was added to fix the samples at 48˚C for a day.
The tissues were subjected to decalcification for 2 days,
dehydration using a graded ethanol series and embedding in
paraffin. The tissues were sliced into 5-µm sagittal sections for
hematoxylin & eosin (HE) staining. Light microscopy was used to
analyze the histological images and Image-Pro Plus Software (Media
Cybernetics, Inc.) was used for quantitative analysis. The sagittal
cross-sectional area, callus thickness and callus area on the
histological images were also assessed (19). In accordance with the
tartrate-resistant acid phosphatase (TRAP) staining reagent
protocols, the TRAP incubation solution was added to
water-deparaffinized paraffin sections for 30 min incubation at
37˚C. The sections were washed using distilled water and treated
with 2-amino-2-methyl-1,3-propane-diol-HCl solution (pH 9.4) for 10
min. Then, methyl green was added to stain the paraffin sections.
The stained sections were washed using distilled water, dried and
sealed. An inverted optical microscope (magnification, x40) was
used to select the field of view and analyze the images. ImageJ
software (Version 1.52e; National Institutes of Health) was used to
determine osteoclast (OC) number per unit area of every
TRAP-stained film. Each assay was performed three times and the
average value was recorded. The OC cytoplasm and nucleus were
stained wine red and light green, respectively.
Biomechanical examination
A three-point bending test (Instron 4302; Instron)
was performed to evaluate the biomechanical properties of fracture
healing at 3 and 6 weeks postoperatively. In brief, the femur was
mounted on two loading bars (spacing: 18 mm), whereas the central
callus was compressed using the mobile head at a compression
velocity of 2 mm/min until fracture. A material testing machine was
used to determine the ultimate load (N) and stiffness (N/mm) based
on a load-deformation curve (20).
Statistical analysis
Data are presented as means ± standard deviation
(SD) and analyzed using SPSS software (version 19.0; IBM Corp.).
Differences between groups were analyzed using one-way analysis of
variance and Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
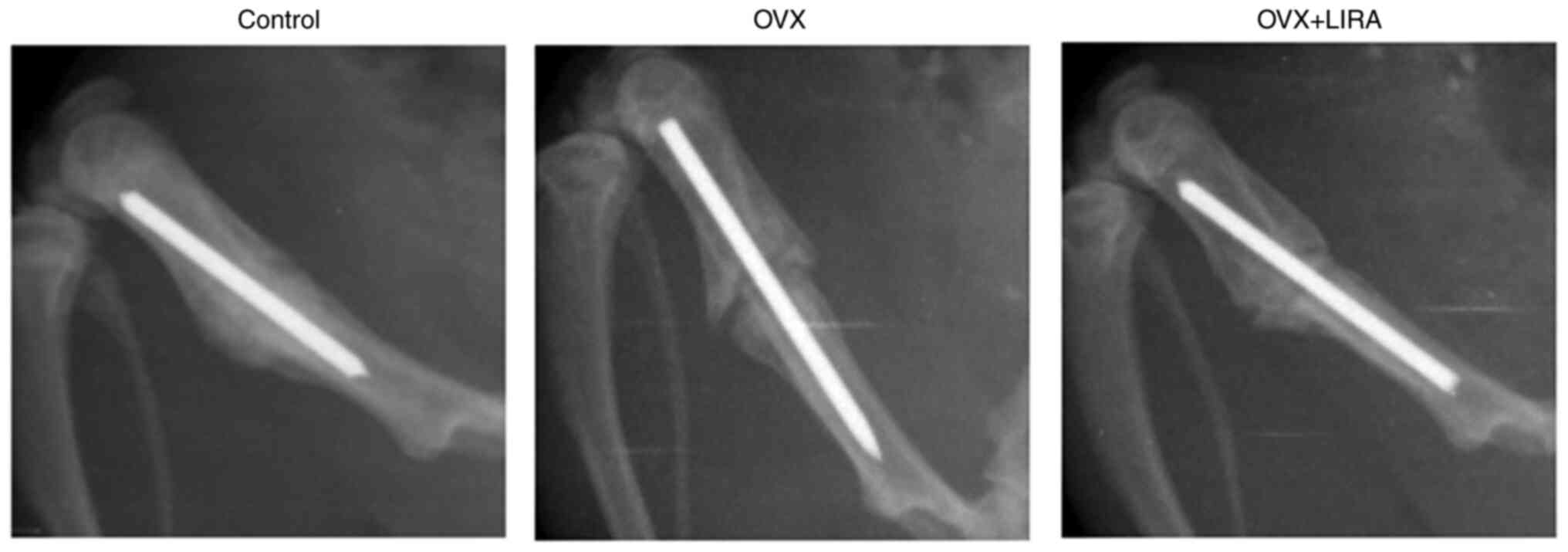
X-ray evaluation of the effects of
GLP-1R activation
X-rays were performed at 6 weeks postoperatively to
determine the effects of GLP-1R activation on callus formation and
bone union. The control group exhibited callus formation and bone
union, while the OVX group exhibited fractional callus formation
and incomplete bone union. Bone remodeling was promoted by the OVX
+ LIRA (Fig. 1).
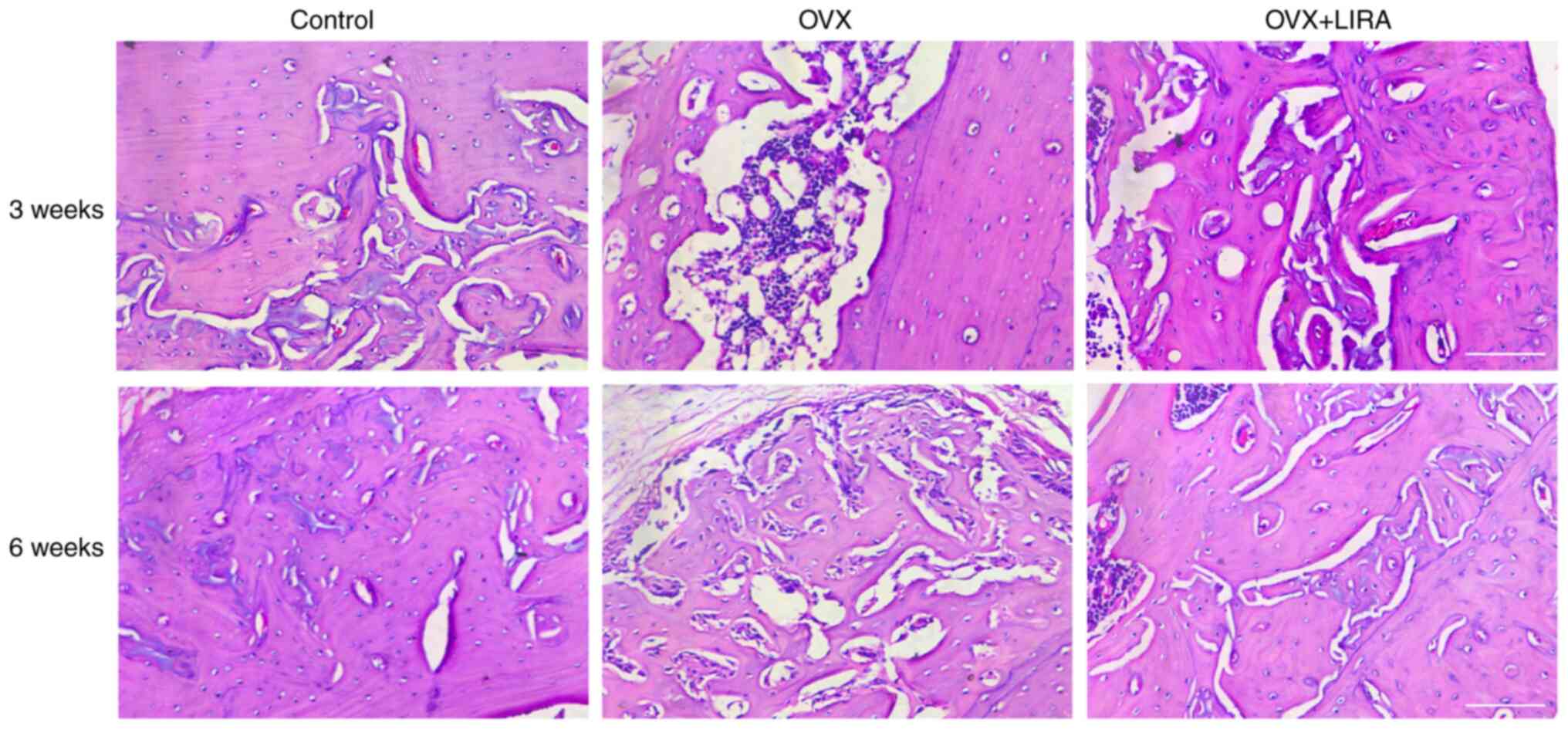
Histological analysis of the effects
of GLP-1R activation
HE staining of the histological sections was
performed at 3 and 6 weeks postoperatively to analyze callus
formation (Fig. 2). At 6 weeks
postoperatively, compared with the OVX group, the control group
exhibited greater bone cell growth and differentiation and
regularly arranged bone trabeculae and collagen fibers. Tissue
samples from the OVX + LIRA group exhibited broad bone trabeculae
and rich cement lines compared with the control group. Similar
findings were observed in the OVX group compared with the control
group.
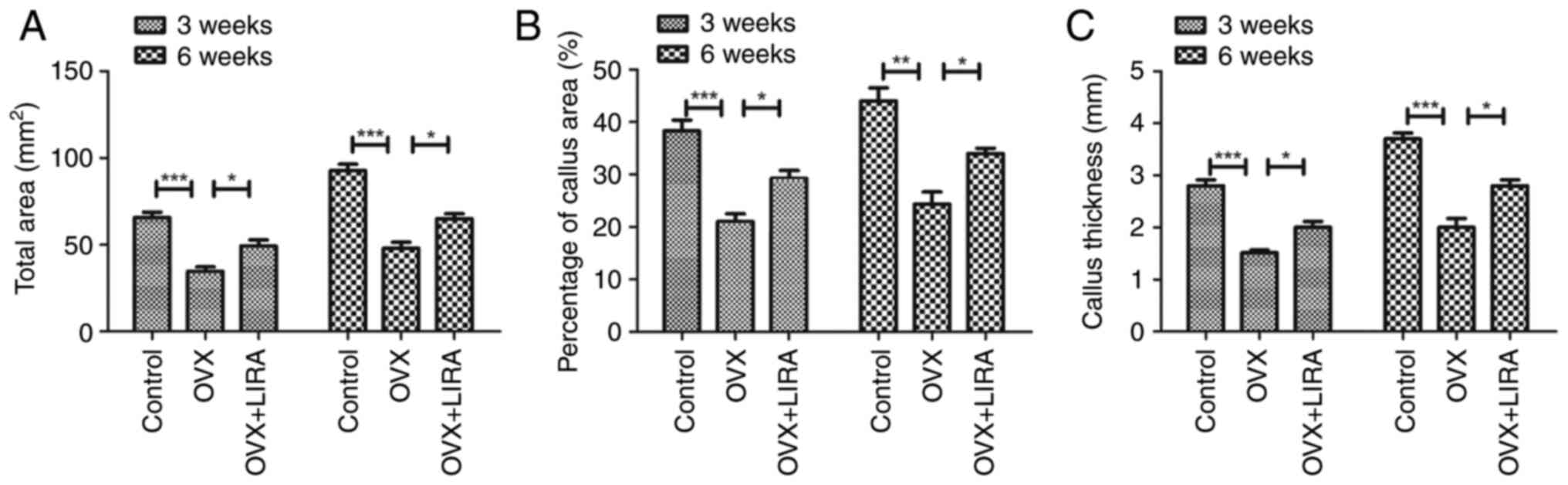
Quantitative analysis of fracture
calluses
The quantitative analysis showed that the control
group had greater sagittal femoral cross-sectional area compared
with the OVX group at 3 and 6 weeks postoperatively, whereas the
OVX + LIRA group showed a further increase in the area compared
with the other groups (Fig. 3A).
Similar findings were observed for callus area and thickness
(Fig. 3B and C).
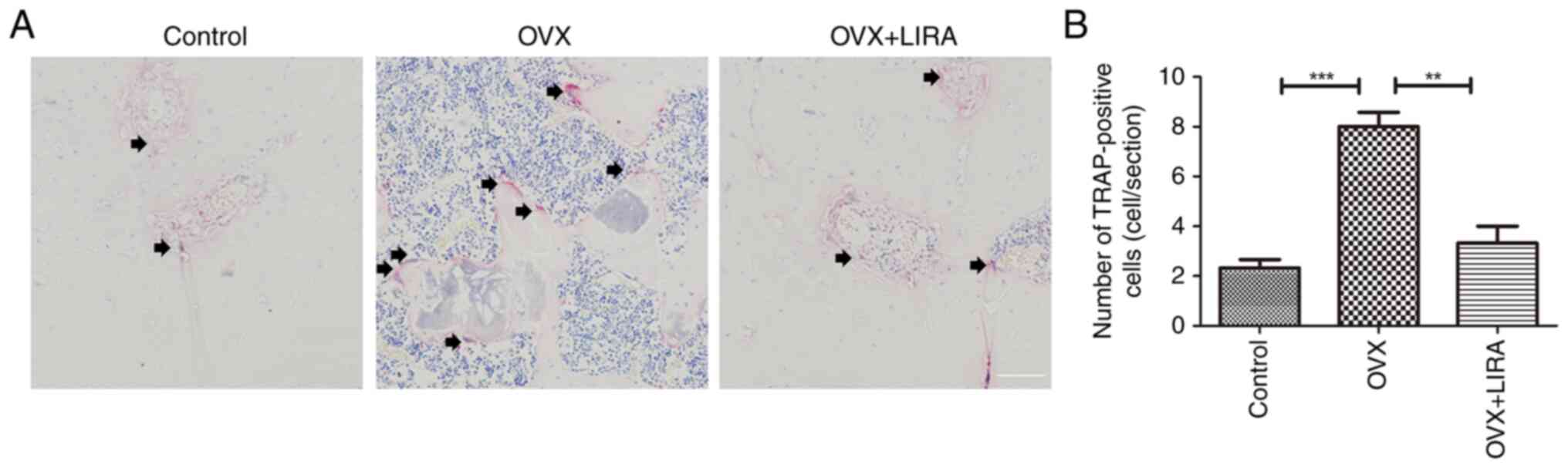
In vivo inhibition of OC generation by
LIRA
At 3 weeks postoperatively, histological sections of
the OVX group exhibited large multinucleated OCs. However, the OVX
+ LIRA group exhibited a markedly lower OC count compared with the
OVX group (Fig. 4).
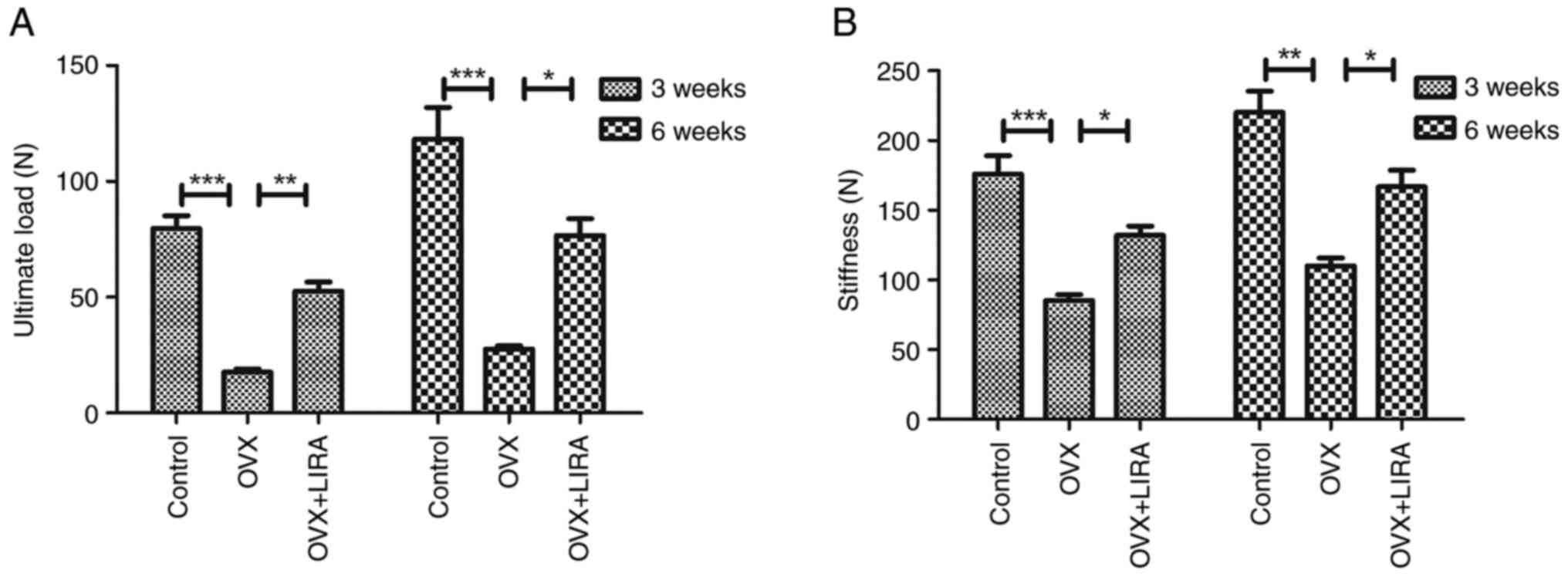
Biomechanical tests for the role of
GLP-1 Ras
The three-point bending test was performed to
evaluate the biomechanical strength of femoral cortical bone.
Compared with the OVX group, the OVX + LIRA group had greater
stiffness and ultimate load, which strengthened the femoral
diaphysis of OP fracture rats at 3 and 6 weeks postoperatively
(Fig. 5).
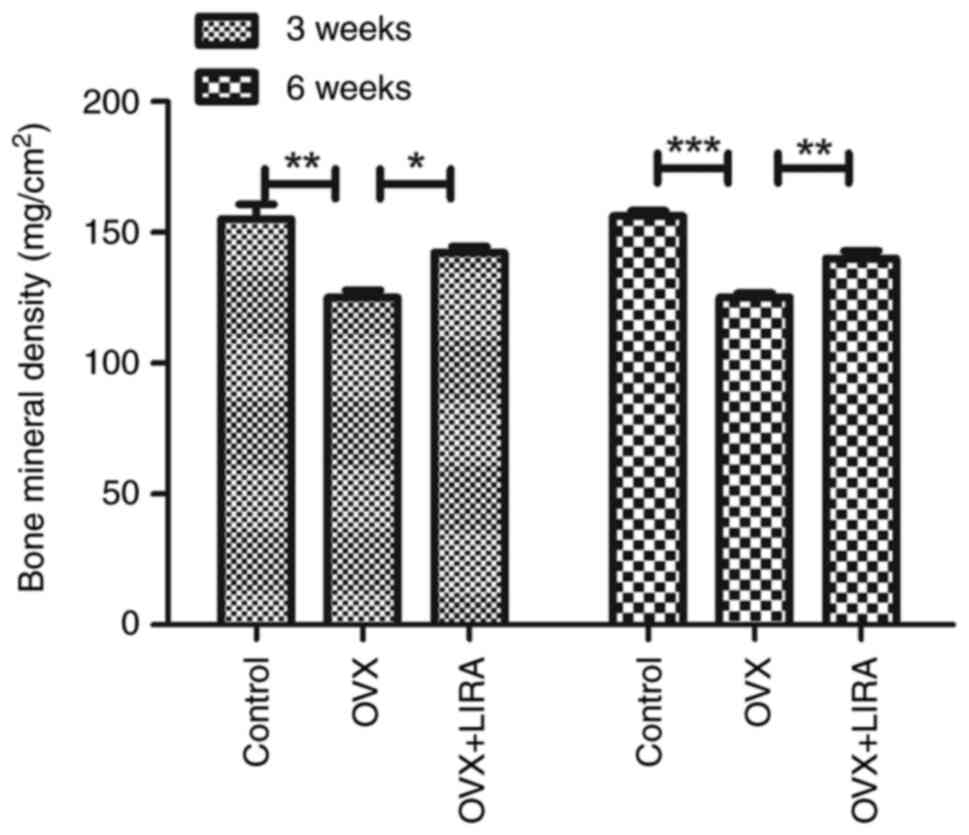
Effects of GLP-1 RAs on BMD
The present study measured the trabecular bone BMD
in dismal femur. At 3 and 6 weeks after the fracture, the OVX group
exhibited a lower trabecular BMD than the control group, whereas
LIRA substantially restored the femoral trabecular BMD Compared
with the OVX group (Fig. 6).
Discussion
OP may be caused by increased bone resorption and
decreased bone formation, which leads to abnormal bone remodeling.
It is characterized by low bone mass, increased fracture risk
(21,22) and bone fragility due to excessive
bone resorption and decreased bone formation. Abnormal bone
remodeling leads to increased fracture risk at the hip or other
bones, leading to a great economic burden (23). Fracture healing involves
reconstruction of bone tissues and restoration of their
biomechanical function. OP hinders fracture healing and leads to
pain and even physical disability. Patients with OP have reduced
metaphyseal bone mass and may need symptomatic treatment to
increase bone mass and decrease bone loss (24). Postmenopausal OP accounts for most
cases of OP in females and increases the fracture risk in women.
Estrogen therapy can substantially reduce the fracture risk among
postmenopausal women (25);
however, it may increase the risk of cardiovascular disease,
ovarian cancer and breast cancer (26). Therefore, additional treatments are
required to reduce the fracture burden in OP.
GLP-1Rs have variable effects on bones. In mouse
pancreas, MC3T3 osteoblasts express a distinct cloned GLP-1R
(27), whereas in vitro and
in vivo human osteocytes and osteoblasts express GLP-1R mRNA
(28). Conversely, GLP-1Rs are not
detected in plastic-cultured primary osteocytes (29). GLP-1 is an incretin hormone derived
from the gut and released in the bloodstream where it binds to
GLP-1Rs. It serves a role in the homeostasis of blood glucose
(30). GLP-1Rs are activated by
several GLP-1 analogues and GLP-1 RAs. GLP-1 analogues, such as
LIRA, lixisenatide and exendin-4, are approved for the treatment of
type 2 DM (31). The GLP-1 RA LIRA
is a new anti-diabetic agent that mimics the positive effects of
endogenous GLP-1 on insulin production (32). It binds to serum albumin to prevent
its proteolytic degradation via dipeptidyl peptidase-4, leading to
a longer half-life of LIRA Compared with the other GLP-1 analogues
(33).
The effects of GLP-1 on bone turnover after OP
fracture are unclear. Mice with GLP-1R homozygous deletion develop
fragile bones, cortical osteopenia, enhanced OC-mediated bone
resorption and possibly reduced thyroid calcitonin production
(15). The mechanisms underlying
the effects of GLP-1 on bone turnover are unclear; however, in
vitro studies have found that GLP-1 indirectly affects
osteoblasts through the GLP-1R (34,35),
which may or may not be similar to the mechanism of action of GLP-1
on the pancreas. In the present study, X-rays showed that the GLP-1
RA LIRA enhanced bone remodeling, leading to callus formation and
fracture healing, in the OP fracture rat model. Additionally, LIRA
substantially restored femoral trabecular BMD and femoral diaphysis
strength in rats with OP fracture by promoting bone stiffness and
ultimate load.
GLP-1R-deficient mice exhibit bone breakdown,
suggesting an important role of GLP-1R signaling in OC inhibition
and bone resorption. Therefore, GLP-1 signaling inhibits bone
resorption (15) and promotes bone
anabolic activity. GLP-1R activation promotes bone regeneration in
rats with streptozotocin-induced diabetes and fructose-triggered
insulin resistance (36). In a
previous study, reverse transcription PCR showed the expression of
GLP-1R mRNA within mouse calvaria osteoblasts, bone marrow cells
and bone after culture for 28 days (18). Mature osteoblasts express
differentiated osteoblast markers and lead to in vitro bone
nodule formation (37). GLP-1R
expression can be detected in OCs of an in vitro bone
resorption model, in which OCs are cultured on dentin disks to
stimulate cell activity and differentiation to mimic the in
vivo environment (18). The
present study assessed the effects of LIRA on in vivo OC
formation. We found that LIRA suppressed OC production in the OP
fracture rat model.
In conclusion, the GLP-1R agonist LIRA inhibited OC
production and bone resorption in the OP fracture rat model.
Furthermore, GLP-1 RA promoted trabecular bone architecture and
mass, thereby improving the biomechanical strength of bones. GLP-1
RAs may be useful for the treatment of OP fractures, particularly
in patients with DM.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Hainan Natural
Science Foundation Youth Fund Project (grant no. 820QN406).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RW and YG designed the study. HN, YZ and SC
contributed to the statistical analysis, data interpretation and
manuscript preparation. RW, YB and JY performed the experiments and
interpretation. RW and YG confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal Care
and Use Committee of Hainan Medical College (approval no.
2020-56).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lupsa BC and Insogna K: Bone health and
osteoporosis. Endocrinol Metab Clin North Am. 44:517–530.
2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gkastaris K, Goulis DG, Potoupnis M,
Anastasilakis AD and Kapetanos G: Obesity, osteoporosis and bone
metabolism. J Musculoskelet Neuronal Interact. 20:372–381.
2020.PubMed/NCBI
|
|
3
|
Li Z, Xue H, Tan G and Xu Z: Effects of
miRNAs, lncRNAs and circRNAs on osteoporosis as regulatory factors
of bone homeostasis (Review). Mol Med Rep. 24(788)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ala M, Jafari RM and Dehpour AR: Diabetes
mellitus and osteoporosis correlation: Challenges and hopes. Curr
Diabetes Rev. 16:984–1001. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kurra S, Fink DA and Siris ES:
Osteoporosis-associated fracture and diabetes. Endocrinol Metab
Clin North Am. 43:233–243. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Napoli N, Chandran M, Pierroz DD,
Abrahamsen B, Schwartz AV and Ferrari SL: IOF Bone and Diabetes
Working Group. Mechanisms of diabetes mellitus-induced bone
fragility. Nat Rev Endocrinol. 13:208–219. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Eller-Vainicher C, Cairoli E, Grassi G,
Grassi F, Catalano A, Merlotti D, Falchetti A, Gaudio A, Chiodini I
and Gennari L: Pathophysiology and management of type 2 diabetes
mellitus bone fragility. J Diabetes Res.
2020(7608964)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Garcia-Martin A, Rozas-Moreno P,
Reyes-Garcia R, Morales-Santana S, García-Fontana B, García-Salcedo
JA and Muñoz-Torres M: Circulating levels of sclerostin are
increased in patients with type 2 diabetes mellitus. J Clin
Endocrinol Metab. 97:234–241. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Balint E, Szabo P, Marshall CF and Sprague
SM: Glucose-induced inhibition of in vitro bone mineralization.
Bone. 28:21–28. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lecka-Czernik B: Bone loss in diabetes:
Use of antidiabetic thiazolidinediones and secondary osteoporosis.
Curr Osteoporos Rep. 8:178–184. 2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gallwitz B: GLP-1 receptor agonists in
type 2 diabetes and beyond-New insights 2015. Eur Endocrinol.
11:21–25. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mabilleau G, Gobron B, Bouvard B and
Chappard D: Incretin-based therapy for the treatment of bone
fragility in diabetes mellitus. Peptides. 100:108–113.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bjarnason NH, Henriksen EE, Alexandersen
P, Christgau S, Henriksen DB and Christiansen C: Mechanism of
circadian variation in bone resorption. Bone. 30:307–313.
2002.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lamari Y, Boissard C, Moukhtar MS,
Jullienne A, Rosselin G and Garel JM: Expression of glucagon-like
peptide 1 receptor in a murine C cell line: Regulation of
calcitonin gene by glucagon-like peptide 1. FEBS Lett. 393:248–252.
1996.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yamada C, Yamada Y, Tsukiyama K, Yamada K,
Udagawa N, Takahashi N, Tanaka K, Drucker DJ, Seino Y and Inagaki
N: The murine glucagon-like peptide-1 receptor is essential for
control of bone resorption. Endocrinology. 149:574–579.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mabilleau G, Mieczkowska A, Irwin N, Flatt
PR and Chappard D: Optimal bone mechanical and material properties
require a functional glucagon-like peptide-1 receptor. J
Endocrinol. 219:59–68. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mieczkowska A, Mansur S, Bouvard B, Flatt
PR, Thorens B, Irwin N, Chappard D and Mabilleau G: Double incretin
receptor knock-out (DIRKO) mice present with alterations of
trabecular and cortical micromorphology and bone strength.
Osteoporos Int. 26:209–218. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pereira M, Jeyabalan J, Jorgensen CS,
Hopkinson M, Al-Jazzar A, Roux JP, Chavassieux P, Orriss IR,
Cleasby ME and Chenu C: Chronic administration of Glucagon-like
peptide-1 receptor agonists improves trabecular bone mass and
architecture in ovariectomised mice. Bone. 81:459–467.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fu LJ, Tang TT, Hao YQ and Dai KR:
Long-term effects of alendronate on fracture healing and bone
remodeling of femoral shaft in ovariectomized rats. Acta Pharmacol
Sin. 34:387–392. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vashghani Farahani MM, Masteri Farahani R,
Mostafavinia A, Abbasian MR, Pouriran R, Noruzian M, Ghoreishi SK,
Aryan A and Bayat M: Effect of pentoxifylline administration on an
experimental rat model of femur fracture healing with
intramedullary fixation. Iran Red Crescent Med J.
17(e29513)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Barron RL, Oster G, Grauer A, Crittenden
DB and Weycker D: Determinants of imminent fracture risk in
postmenopausal women with osteoporosis. Osteoporos Int.
31:2103–2111. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kim M, Kim JH, Hong S, Kwon B, Kim EY,
Jung HS and Sohn Y: Effects of Melandrium firmum Rohrbach on
RANKL-induced osteoclast differentiation and OVX rats. Mol Med Rep.
24(610)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Mohd-Tahir NA and Li SC: Economic burden
of osteoporosis-related hip fracture in Asia: A systematic review.
Osteoporos Int. 28:2035–2044. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Renno AC, de Moura FM, dos Santos NS,
Tirico RP, Bossini PS and Parizotto NA: Effects of 830-nm laser,
used in two doses, on biomechanical properties of osteopenic rat
femora. Photomed Laser Surg. 24:202–206. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kim CS, Yea K, Morrell CN, Jeong Y and
Lowenstein CJ: Estrogen activates endothelial exocytosis. Biochem
Biophys Res Commun. 558:29–35. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ibrahim N, Mohamed N and Shuid AN: Update
on statins: Hope for osteoporotic fracture healing treatment. Curr
Drug Targets. 14:1524–1532. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nuche-Berenguer B, Portal-Nunez S, Moreno
P, González N, Acitores A, López-Herradón A, Esbrit P, Valverde I
and Villanueva-Peñacarrillo ML: Presence of a functional receptor
for GLP-1 in osteoblastic cells, independent of the cAMP-linked
GLP-1 receptor. J Cell Physiol. 225:585–592. 2010.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pacheco-Pantoja EL, Ranganath LR,
Gallagher JA, Wilson PJ and Fraser WD: Receptors and effects of gut
hormones in three osteoblastic cell lines. BMC Physiol.
11(12)2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kim JY, Lee SK, Jo KJ, Song DY, Lim DM,
Park KY, Bonewald LF and Kim BJ: Exendin-4 increases bone mineral
density in type 2 diabetic OLETF rats potentially through the
down-regulation of SOST/sclerostin in osteocytes. Life Sci.
92:533–540. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bell GI, Sanchez-Pescador R, Laybourn PJ
and Najarian RC: Exon duplication and divergence in the human
preproglucagon gene. Nature. 304:368–371. 1983.PubMed/NCBI View
Article : Google Scholar
|
|
31
|
Lovshin JA and Drucker DJ: Incretin-based
therapies for type 2 diabetes mellitus. Nat Rev Endocrinol.
5:262–269. 2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nauck MA: Incretin-based therapies for
type 2 diabetes mellitus: Properties, functions, and clinical
implications. Am J Med. 124 (1 Suppl):S3–S18. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Holst JJ: The physiology of glucagon-like
peptide 1. Physiol Rev. 87:1409–1439. 2007.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zheng Y, Xiao Y, Zhang D, Zhang S, Ouyang
J, Li L, Shi W, Zhang R, Liu H, Jin Q, et al: Geniposide
ameliorated dexamethasone-induced cholesterol accumulation in
osteoblasts by mediating the GLP-1R/ABCA1 axis. Cells.
10(3424)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee HM, Joo BS, Lee CH, Kim HY, Ock JH and
Lee YS: Effect of glucagon-like peptide-1 on the differentiation of
adipose-derived stem cells into osteoblasts and adipocytes. J
Menopausal Med. 21:93–103. 2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nuche-Berenguer B, Moreno P, Portal-Nunez
S, Dapia S, Esbrit P and Villanueva-Penacarrillo ML: Exendin-4
exerts osteogenic actions in insulin-resistant and type 2 diabetic
states. Regul Pept. 159:61–66. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Orriss IR, Taylor SE and Arnett TR: Rat
osteoblast cultures. Methods Mol Biol. 816:31–41. 2012.PubMed/NCBI View Article : Google Scholar
|