Introduction
Osteoporosis is a systemic bone metabolic disorder
characterized by the destruction of bone tissue microstructure, the
continuous reduction of the proportion of bone mineral composition
and bone matrix proportion, bone thinning, reduction of the number
of bone trabeculae, increased bone fragility and increased risk of
fracture (1). According to the
relevant statistical data of WHO, the incidence rate of
osteoporosis has been increasing yearly. Osteoporosis is
responsible for 8.9 million fractures each year. It is estimated
that by 2050, the global number of hip fracture patients induced by
osteoporosis will increase by 310% in men and 240% in women,
especially in postmenopausal women (2). In China, the prevalence rate of
osteoporosis in women >50 years old was 32.1% and that in
females >65 years old was 51.6%,with the incidence of
complicated fractures ~33% (1-4).
With increasing aging globally, osteoporosis has become a major
disease that affects the health and quality of life of the elderly,
especially in postmenopausal women. Therefore, the pathogenesis and
prevention methods of postmenopausal osteoporosis have been the
focus of academic research.
Autophagy is a phenomenon that widely exists in
eukaryotic cells to maintain self-stability. In addition to
maintaining self-protection for emergency factors (5), it also plays an important role in
bone formation (6-8).
Researchers have found that trehalose is a mTOR-independent
autophagy inducer, which can promote the degradation of toxic
proteins in cells (9). In
addition, trehalose has been approved for human use by the US Food
and Drug Administration as safe and harmless to human body and has
broad clinical application prospects.
There have been no studies, to the best of our
knowledge, on the effect of trehalose on postmenopausal
osteoporosis. Our research team found that trehalose can delay the
development of postmenopausal osteoporosis in previous studies
(10,11), but its mechanism is still unclear.
The present study further investigated whether trehalose delay
postmenopausal osteoporosis by enhancing AKT/transcription factor
EB (TFEB) pathway-dependent autophagy.
Materials and methods
Laboratory animals and modeling
The present study was approved by the Institutional
Animal Care and Use Committee of Hangzhou Hibio Animal Care
Technology Co., Ltd. (IACUC protocol number HBFM 3.68-2015) and the
animal experiments were performed at Hangzhou Hibio Animal Care
Technology Co., Ltd.
A total of 18 healthy female SD rats, each weighing
200 g and aged 6 weeks, were purchased from Shanghai Slake
Laboratory Animal Co., Ltd. Experimental animals received ad
libitum feed and water and 12/12-h of daily light/dark time.
The rats were SPF grade the supplier held license number SCXK
(Shanghai) 20170005 and certificate number 20170005021392. The
drinking water was sterilized Grade II ultrapure water and the
quality of drinking water complied with the national standard of
the People's Republic of China, Hygienic Standard for Drinking
Water (GB5749 2006). The laboratory animal room is licensed under
SYXK (Zhe) 2015 0008, with a breeding environment featuring a
temperature range of 20-25˚C and relative humidity range of 40-70%.
Prior to experimentation, the animals were acclimated to the animal
room environment for one week.
After adaptive feeding, the 18 rats were randomly
assigned to three groups: Normal group (n=6), model group (n=6) and
trehalose group (n=6). Following one week of adaptation, each rat
in the model and trehalose groups underwent anesthesia with a 2%
(w/v) pentobarbital sodium solution administered via
intraperitoneal injection. The abdominal hair of the rats was
removed using a depilatory agent and disinfected as per standard
protocol. The rats in the model group underwent a 1-2 cm incision
in the abdomen to expose the uterus, which was ligated, and the
ovaries removed followed by closure of the abdominal cavity. The
normal group were subjected to identical procedures without
additional ligation and ovariectomy. All rats were administered
penicillin injections for three consecutive days. At the 16-week
modeling mark, the trehalose group was administered gavage of 100
mg/kg trehalose with a volume of 0.2 ml/10 g, (12,13),
while an equivalent volume of drinking water was given to the model
and normal group. The normal group received no treatment. After 16
weeks, both groups were sacrificed using a massive intraperitoneal
injection of 2% (w/v) pentobarbital sodium solution and their
femurs on both sides were extracted. One side was frozen, the other
side was fixed in 4% paraformaldehyde solution at room temperature
for 24 h and then treated with decalcifying solution for 2 month at
37˚C.
Hematoxylin and eosin (H&E)
staining
Tissue sections were subjected to H&E staining
in room temperature. The paraffin sections were melted in an oven
at 65˚C for ~45 min, followed by two rounds of dewaxing with xylene
for ~10 min each time. Then, they were hydrated using a gradient
ethanol series (100, 90, 80, and 70% ethanol) and washed with PBS
before being immersed in hematoxylin solution for five minutes.
After rinsing with running water, the sections underwent acidic
differentiation and eosin staining before weak acid differentiation
and another rinse. Finally, the stained tissue sections were
dehydrated using ascending ethanol solutions starting from low
concentration to higher concentrations before sealing.
Western blotting
Protein was extracted from tissue using tissue lysis
buffer (Beyotime Institute of Biotechnoloy; cat. no. P0013B) for 1
h and centrifuge (12,000 x g, 15 min, 4˚C) to obtain the
supernatant. s to use the BCA reagent kit (Beyotime, P0009) to
determine protein concentration. The 10% SDS-PAGE precast gel
solution (Beyotime Institute of Biotechnology) was prepared
according to the manufacturer's instructions and added between two
glass plates. The electrophoresis buffer was formulated by adding
glycine (Beyotime, ST085), Tris (Beyotime, ST760-500 g) and SDS
(Beyotime Institute of Biotechnology; cat. no. ST627) in specific
proportions to ultrapure water while membrane transfer buffer was
made by mixing glycine, Tris, SDS and methanol in certain ratios.
The SDS-PAGE gel plate was secured onto the electrophoresis clamp,
followed by removal of the loading comb and addition of 15 µl cell
protein and 10 µl tissue protein per well, along with 2 µl protein
ladder at both ends. The electrophoresis process was initiated by
accurately positioning the electrodes and introducing a
preconfigured electrophoresis solution. The electrophoresis was
conducted under a constant voltage regime, whereby the protein
sample was subjected to 80 V until it migrated beyond the
separation gel and subsequently at 120 V for 55 min. The separation
of proteins with varying molecular weights was achieved through
differential migration in response to an electric field.
Electrophoresis could be terminated once the target molecular
weights were adequately resolved and membrane transfer using a 0.22
µm pore size PVDF membrane for wet transfer printing was performed.
The entire membrane was placed into the groove and immersed in
liquid to initiate film transfer. The membrane transfer was
performed at 300 mA for about 90-120 min. The specific transfer
time should be adjusted according to the molecular weight of the
target protein and the time needed for the larger molecular weight
was longer. The protein containing membrane was blocked with 5% BSA
(cat. no. ST023-50 g; Beyotime Institute of Biotechnology) solution
for 2 h at room temperature and then washed with TBST solution (5
min, 3 times; 0.1% Tween). Target molecular weight bands were
excised, with reference to the protein ladder on both sides. The
bands were incubated with preconfigured primary antibodies against
the target protein for 12-16 h on a shaker at 4˚C. The primary
antibodies used were AKT (1:1,000; Cell Signaling Technology, Inc.;
cat. no. 9272S), phosphorylated (p-)AKT (1:1,000; Cell Signaling
Technology, Inc.; cat. no. 4060S), TFEB (1:1,000; BIOSS; cat. no.
bs5137R), GAPDH (1:2,000, Multi Sciences cat. no. Mab5465 100), LC3
(1:1,000; Abcam; cat. no. ab62721) and P62 (1:1,000; Abcam; cat.
no. ab109012). Cells underwent three washes with TBST solution (5
min each) and were subsequently co-incubated with secondary
antibody solution of identical species origin for 2 h. The
secondary antibodies were horseradish peroxidase-conjugated goat
anti-rabbit IgG (1:5,000; Beyotime Institute of Biotechnology; cat.
no. A0208) and Goat anti Mouse IgG (1:5,000; Beyotime Institute of
Biotechnology; cat. no. A0216). BeyoECL Plus (cat. P0018M,
Beyotime) was used to bind secondary antibodies for
chemiluminescence. Upon conclusion of incubation, the bands
received an additional three washes in similar fashion.
Chemiluminescence imaging system was used for image acquisition and
subsequent analysis.
Micro computerized tomography (CT)
scanning
The frozen femur tissue were harvested and fixed in
4% paraformaldehyde for 48 h at room temperature. Subsequently, the
prepared femur was subjected to Micro CT scanning using a source
voltage of 70 KV and current of 200 µA with exposure time of 300
msec and resolution of 10 µm. The images were reconstructed into a
three-dimensional model using SCANCO MICROCT Evaluation V6.5-3
(SCANCO Medical AG) analysis software, from which regions of
interest were selected for quantitative analysis. The specific
analysis indices were as follows: scanning bone volume (TV) was
expressed in cubic millimeters, bone volume (BV) in the region of
interest (ROI) was expressed in cubic millimeters, bone volume
fraction (BV/TV) was expressed as a percentage, trabecular
thickness (Tb. Th) was expressed in µm, number of bone trabeculae
(Tb. N) was expressed per mm and separation degree of bone
trabeculae (Tb. SP), represented by bone mineral density (BMD), was
expressed in m g/cm3.
Transmission electron microscopy
Femoral tissue (<1 mm3) excised
following euthanasia were fixed overnight in 2.5% glutaraldehyde
PBS (12 h at room temperature); flushed with 0.1 M PBS for 15 min,
2 times; fixed with 1% osmic acid for 1 h at 37˚C, washed with 0.1
M PBS for 15 min, 2 times; stained with 2% uranium acetate solution
for 30 min at room temperature; dehydrated with 50, 70 and 90%
alcohol successively for 15 min; dehydrated with 100% alcohol for
20 min; dehydrated with 100% acetone for 20 min, 2 times; anhydrous
acetone and embedding agent were mixed in 1:1 volume to penetrate
the tissue and vibrated for 2 h at room temperature; pure embedding
agent and vibrated for 2 h at room temperature. Finally, the tissue
blocks were embedded with pure embedding agent which was
polymerized in the oven at 37˚C for 24 h, 45˚C for 24 h and 60˚C
for 48 h. The block was trimmed and sectioned at ~120 nm. Staining
was performed with 4% uranium acetate for 20 min and lead citrate
for 5 min at room temperature. The stained ultrathin section were
placed on single hole copper mesh and observation and images were
captured using a transmission electron microscope (TECNAI-10, 80
kV, Philips, magnification, x2,500 times). Digital micrograph 3.4
(Gatan, America) was used for analysis.
Statistical analysis
The data are presented as mean ± standard deviation
and statistical analysis was performed using SPSS 20.0 (IBM Corp.).
The significance of differences between two groups was determined
by two-tailed unpaired Student's t-test using GraphPad Prism 8
software (GraphPad Software Inc.; Dotmatics). One-way ANOVA was
used to determine significant differences among the three groups.
P<0.05 was considered statistically significant.
Results
H&E staining
According to the H&E staining results, the bone
trabeculae and hematopoietic tissue in the bone marrow cavity of
the femur in the normal group were evenly distributed, while the
bone trabeculae in the model group and trehalose group were small
and dense. The bone trabeculae in the model group were narrower
than those in the trehalose group. These results indicated that the
bone trabecular histological structure of the trehalose group was
superior to that of the model group (Fig. 1).
Micro CT scanning
Compared with the normal group, BV/TV, BMD, Tb. N
and Tb. Th in the model group decreased and trabecular separation
Tb. SP increased, with statistically significant differences
(P<0.05); Compared with the model group, the trehalose group had
higher BV/TV, BMD, Tb. N, Tb. Th and decreased Tb. SP, with
statistically significant differences (P<0.05). These results
indicated that the imaging structure of bone trabeculae in the
trehalose group was superior to than that in the model group
(Table I).
 | Table IQuantitative morphometric parameters
of micro computerized tomography of distal femur. |
Table I
Quantitative morphometric parameters
of micro computerized tomography of distal femur.
| Group | BV/TV(%) | Tb. Th (µm) | Tb. N | BMD
(mg/cm3) | Tb. Sp (µm) |
|---|
| Normal group | 0.276±0.034 | 0.076±0.005 | 3.898±0.336 | 909.886±10.302 | 0.170±0.017 |
| Model group | 0.190±0.014 | 0.048±0.013 | 2.503±0.326 | 869.668±22.589 | 0.277±0.020 |
| Trehalose group | 0.234±0.023 | 0.068±0.001 | 3.251±0.222 | 882.979±12.003 | 0.234±0.018 |
Western blotting
Compared with the normal group, the expression of
TFEB and LC3 protein in the model group was upregulated, while the
expression of p-AKT and P62 protein was down regulated and there
was a significant difference (P<0.05). Compared with the model
group, the expression of TFEB and LC3 protein in trehalose group
was upregulated, while the expression of p-AKT, p-AKT/AKT ratio and
P62 protein was downregulated and there was a significant
difference (P<0.05); There was no significant change in AKT
protein expression in each group (P>0.05). These results
indicated the activation of autophagic flow in trehalose group, the
autophagy degree of the trehalose group is greater than that of the
model group (Fig. 2).
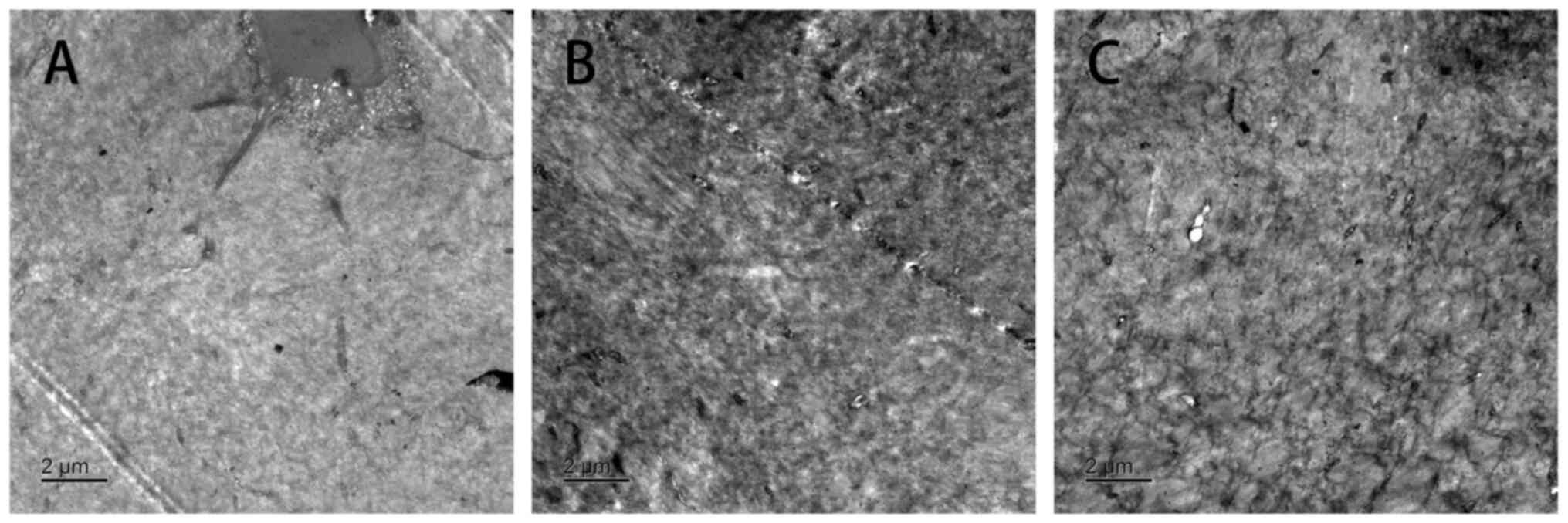
Transmission electron microscopy
At the 16th week of modeling, the number of
autophagosomes in trehalose group was the largest among the three
groups. The results of transmission electron microscopy showed that
the number of autophagosomes was trehalose group>model
group>normal group. These results indicated the autophagy degree
of the Trehalose group was greater than that of the model group
under electron microscopy (Fig.
3).
Discussion
Osteoporosis is a systemic osteopathy characterized
by low bone mass, destruction of bone tissue microstructure,
increased bone fragility and ease of fracture. With the aging of
the population, osteoporosis has become an important public health
problem in China. An epidemiological survey showed that the
prevalence of osteoporosis in the population >50 years old in
China was 20.7%, especially in postmenopausal women (4). Osteoporosis is preventable and
curable (1). The research on the
pathogenesis and prevention methods of osteoporosis has been a hot
topic in the academic community.
Autophagy is a phenomenon that widely exists in
eukaryotic cells to maintain homeostasis. Stimulated by some
factors (including ischemia, hypoxia and starvation), autophagy
precursor forms an autophagic vesicle with double membrane
structure, which gradually encapsulates proteins and organelles to
be degraded, forming autophagolysosome and then the outer membrane
of autophagolysosome fuses with lysosomes to form an autolysosome,
which then enter the lysosome cavity and is degraded by proteolytic
enzymes. The degraded components are recycled by cells (14). The process of autophagy in
vivo includes three steps: Autophagosome formation,
autolysosome fusion and autolysosome degradation. The dynamic
process of each step is called autophagy flow (15). The potential regulatory mechanism
of autophagy flow is a post transcriptional regulation. As a member
of the microphthalmia/translation Factor E (MiTF/TFE) family of
leucine zipper basic helix loop helix-leucine zipper (bHLH-LZ)
transcription factors, TFEB plays an important role in regulating a
variety of cellular processes (16,17).
The transcriptional activity of TFEB is mainly regulated by two
pathways, namely serine/threonine protein kinase AKT and mTOR1. AKT
and mTOR1 phosphorylate TFEB filament amino acid 467 (Ser467) and
211 (Ser211), respectively, to make TFEB form a complex and remain
in the cytoplasm. Once AKT and mTOR are inactivated under the
action of their respective inhibitors, TFEB complex can be
disassembled and free TFEB enters the nucleus, exerting its
transcription factor effect and finally achieving the effect of
enhanced autophagic flow.
In previous studies (10,18,19),
it was found that trehalose has the biological activity of
inhibiting AKT and can induce TFEB phosphorylation and nuclear
internalization downstream of AKT, thus leading to the enhancement
of autophagic flow in cells (18).
Nishizaki et al (17) and
Yoshizane et al (18)
studied the changes of osteoclast in ovariectomized mice after
trehalose use and proved that trehalose could inhibit the
activation of osteoclast by decreasing IL-6 and TNF-α, and that
trehalose could inhibit bone loss. However, ovariectomy results in
cancellous osteopenia and accelerated bone turnover in the mice.
There are differences between human and mice physiology regarding
the actions of estrogens and estrogen analogs such as tamoxifen.
While these species differences do not necessarily contraindicate
its use, the ovariectomized mice model should be approached with
extreme caution (19). Therefore,
the rat model is a more ideal model than a mice model for studying
postmenopausal osteoporosis. Also, Nishizaki et al (17) and Yoshizane et al (18) 20 years ago had performed relatively
simple researches on the changes of osteoclast under the trehalose
use and no further research on the mechanism. Xu et al
(20) showed that trehalose
decreased the OB-mediated osteoclastogenesis and reduced the
primary biliary cirrhosis (PBC)-related bone loss by regulating ERK
phosphorylation via autophagosome formation, proving the potential
value of trehalose in delaying osteoporosis as have we (20). However, they studied bile
duct-ligated male rats which belonging to a secondary osteoporosis
model. Bone loss was just a severe complication of PBC and their
model could not be used for postmenopausal osteoporosis. This is
why the present study chose the trehalose for further research.
In the present study, compared with the model group,
the trehalose group decreased the expression of p-AKT and P62
protein and increased the expression of LC3 and TFEB protein,
suggesting that the trehalose induced increase in TFEB expression
and autophagy level. At the same time, via histology, the
trabecular arrangement in trehalose group was more dense and
uniform than that in model group at 16 weeks, with less change.
Micro CT showed that the trabecular bone number, trabecular
structure arrangement, trabecular bone space and separation in
trehalose group were improved compared with than those in model
group at 16 weeks of modeling; In transmission electron microscopy,
autophagosome had three forms according to the different stages of
autophagy. In the early stage, it was shaped like crescent and
wrapped around the cytoplasmic components; In the middle stage, it
had a bilayer or multilayered vacuole structure, containing
cytoplasm and organelles such as ribosome or mitochondria; in the
later stage, it had a single-layer membrane structure and the
internal slurry composition had been degraded (21). The increase of autophagosomes
indicated an increase in autophagosome synthesis or inhibition of
autolysosome fusion, so it was necessary to exclude the situation
of blocked autolysosome fusion by detecting the P62 expression.
In Fig. 3, the
number of autophagosomes in the trehalose group was greater than
that in other groups. The number of autophagosomes of the trehalose
group mainly manifested as vacuoles or containing contents, similar
to the morphology of autophagosomes in the middle and late stages,
combined with the expression of autophagy digestion substrate P62,
indicating enhanced autophagy in the trehalose group. Based on the
results of the present study, it is hypothesized that after the use
of trehalose, p-AKT in rat bone tissue is inhibited and
downregulated, promoting the dissociation of TFEB complex, TFEB
nuclear translocation and enhancing autophagic flow, leading to an
increase in LC3 expression and a decrease in P62 expression,
finally alleviating postmenopausal osteoporosis (Fig. 4).
However, there are still some limitations to the
present study. First, the postmenopausal rat model was used in the
experiment and no relevant experiments were performed on other
animals. Second, in vitro experiments were not performed; In
addition, the effect of trehalose on AKT/TFEB pathway dependent
autophagy flow was only preliminarily discussed, but the specific
mechanism and the relationship between trehalose and osteoporosis
need to be further explored.
Trehalose can delay postmenopausal osteoporosis in
rats, which may be achieved by inducing and enhancing AKT/TFEB
pathway-dependent autophagy flow. The specific mechanism of its
occurrence remains to be studied. Trehalose-containing drugs have
prospects in delaying postmenopausal osteoporosis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Zhejiang Provincial
Science and Technology Department Public Welfare Technology
Research Plan/Laboratory Animal Project (grant no. LGD21H010001),
General public welfare projects of Huzhou Science and Technology
Bureau (grant no. 2020GYB16) and Public welfare research project of
Huzhou Science and Technology Bureau (grant no. 2018GYB42).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QL conceived the present study. HG constructed
figures and contributed to the analysis and interpretation of data.
XL and YW designed the study and wrote the manuscript. QL and YW
revised the manuscript. All authors read and approved the final
manuscript. YW and QL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Hangzhou Hibio Animal Care
Technology Co., Ltd. (IACUC protocol number HBFM 3.68-2015) and the
animal experiments were carried out at Hangzhou Hibio Animal Care
Technology Co., Ltd.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arceo-Mendoza RM and Camacho PM:
Postmenopausal osteoporosis: Latest guidelines. Endocrinol Metab
Clin North Am. 50:167–178. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gopinath V: Osteoporosis. Med Clin North
Am. 107:213–225. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ayers C, Kansagara D, Lazur B, Fu R, Kwon
A and Harrod C: Effectiveness and safety of treatments to prevent
fractures in people with low bone mass or primary osteoporosis: A
living systematic review and network meta-analysis for the American
college of physicians. Ann Intern Med. 176:182–195. 2023.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Zhang C, Feng J, Wang S, Gao P, Xu L, Zhu
J, Jia J, Liu L, Liu G, Wang J, et al: Incidence of and trends in
hip fracture among adults in urban China: A nationwide
retrospective cohort study. PLoS Med. 17(e1003180)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Galluzzi L, Pietrocola F, Levine B and
Kroemer G: Metabolic control of autophagy. Cell. 159:1263–1276.
2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nuschke A, Rodrigues M, Stolz DB, Chu CT,
Griffith L and Wells A: Human mesenchymal stem cells/multipotent
stromal cells consume accumulated autophagosomes early in
differentiation. Stem Cell Res Ther. 5(140)2014.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Whitehouse CA, Waters S, Marchbank K,
Horner A, McGowan NW, Jovanovic JV, Xavier GM, Kashima TG, Cobourne
MT, Richards GO, et al: Neighbor of Brcal gene (Nbrl) functions as
a negative regulator of postnatal osteoblastic bone formation and
p38 MAPK activity. Proc Natl Acad Sci USA. 107:12913–12918.
2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chang KH, Sengupta A, Nayak RC, Duran A,
Lee SJ, Pratt RG, Wellendorf AM, Hill SE, Watkins M, Gonzalez-Nieto
D, et al: p62 is required for stem cell/progenitor retention
through inhibition of IKK/NF-kappaB/Cc14 signaling at the bone
marrow macrophage-osteoblast niche. Cell Rep. 9:2084–2097.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
He Q, Koprich JB, Wang Y, Yu WB, Xiao BG,
Brotchie JM and Wang J: Treatment with trehalose prevents
behavioral and neurochemical deficits produced in an AAV
α-Synuclein rat model of Parkinson's disease. Mol Neurobiol.
53:2258–2268. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kakoty V, Sarathlal KC, Dubey SK, Yang CH
and Taliyan R: Neuroprotective effects of trehalose and sodium
butyrate on preformed fibrillar form of α-synuclein-induced rat
model of Parkinson's disease. ACS Chem Neurosci. 12:2643–2660.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qing H, Koprich JB, Wang Y, Yu WB, Xiao
BG, Brotchie JM and Wang J: Treatment with trehalose prevents
behavioral and neurochemical deficits produced in an AAV
α-synuclein rat model of Parkinson's disease. Mol Neurobiol.
53:2258–2268. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ho TT, Warr MR, Adelman ER, Lansinger OM,
Flach J, Verovskaya EV, Figueroa ME and Passegué E: Autophagy
maintains the metabolism and function of young and old stem cells.
Nature. 543:205–210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou K, Zheng Z, Li Y, Han W, Zhang J, Mao
Y, Chen H, Zhang W, Liu M, Xie L, et al: TFE3, a potential
therapeutic target for Spinal Cord Injury via augmenting autophagy
flux and alleviating ER stress. Theranostics. 10:9280–9302.
2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Williamson SR, Eble JN and Palanisamy N:
Sclerosing TFEB rearrangement renal cell carcinoma: A recurring
histologic pattern. Hum Pathol. 62:175–179. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Franco-Juárez B, Coronel-Cruz C,
Hernández-Ochoa B, Gómez-Manzo S, Cárdenas-Rodríguez N,
Arreguin-Espinosa R, Bandala C, Canseco-Ávila LM and Ortega-Cuellar
D: TFEB; Beyond its role as an autophagy and lysosomes regulator.
Cells. 11(3153)2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chen M, Dai Y, Liu S, Fan Y, Ding Z and Li
D: TFEB biology and agonists at a glance. Cells.
10(333)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Nishizaki Y, Yoshizane C, Toshimori Y,
Arai N, Akamatsu S, Hanaya T, Arai S, Ikeda M and Kurimoto M:
Disaccharide-trehalose inhibits bone resorption in ovariectomized
mice. Nutr Res. 20:653–664. 2000.
|
|
18
|
Yoshizane C, Arai N, Arai C, Yamamoto M,
Nishizaki Y, Hanaya T, Arai S, Ikeda M and Kurimoto M: Trehalose
suppresses osteoclast differentiation in ovariectomized mice:
Correlation with decreased in vitro interleukin-6 production by
bone marrow cells. Nutr Res. 20:1485–1491. 2000.
|
|
19
|
Komori T: Animal models for osteoporosis.
Eur J Pharmacol. 759:287–294. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu X, Wang R, Wu R, Yan W, Shi T, Jiang Q
and Shi D: Trehalose reduces bone loss in experimental biliary
cirrhosis rats via ERK phosphorylation regulation by enhancing
autophagosome formation. FASEB J. 34:8402–8415. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Melia TJ, Lystad AH and Simonsen A:
Autophagosome biogenesis: From membrane growth to closure. J Cell
Biol. 219(e202002085)2020.PubMed/NCBI View Article : Google Scholar
|