Introduction
Pheochromocytomas/paragangliomas (PPGL) are rare
neuroendocrine tumours (1). A
previous study of patients with PPGL from various countries,
including the United States, Canada, Denmark, the Netherlands,
Australia, Spain and Sweden, reported that between 1949 and 2019
the incidence of PPGL ranged from 0.04-0.95 cases per 100,000 per
year (2). Approximately 85-90% of
PPGL are localized in the adrenal glands and 10-15% are
extra-adrenal and are called paraganglioma (PGL) (1). PGL, originating from the autonomic
nervous system ganglia and its accompanying neural regions, was
first described by Fränkel in 1886 (3,4).
Most of PGLs are functional and can secrete catecholamines. Its
common clinical symptoms are headaches, heart palpitations and
sweating (1,3). Only 10% of PGL cases are clinically
silent (non-functional PGL) and are observed incidentally via
imaging (5). Non-functional PGL is
mostly located in the neck and rarely in the abdomen (6). PGL is most common in adults between
the ages of 20 and 40 years, and there is no significant difference
in sex in its occurrence (7). The
incidence of PGL has been increasing gradually, as confirmed by
Berends et al in 2018(8).
This previous study reported that the age-standardized rate for PGL
among patients from the Netherlands increased from 0.08 (95% CI:
0.06-0.10) to 0.11 (95% CI: 0.09-0.13) per 100,000 person-years in
the periods 1995-1999 and 2011-2015, respectively (8).
Once PGL is diagnosed, surgical resection is the
only curative treatment and the mortality rate of patients for PGL
resection from the United States has decreased from ~40% in the
1950s to 0-3% over the last four decades (9,10).
However, recurrence after resection has been reported to occur in
3-16% of patients (11,12). Due to the risk of local recurrence,
metastasis and a new PGL, surgical resection cannot guarantee a
complete cure (9,12). Recurrence of PGL can be difficult
to treat. Cui et al (11)
reported that 47% of patients with recurrence had multiple tumours
at the site of recurrence and 58% had metastases (11). However, as treatment significantly
decreases the risk of metastases and mortality, patients should be
treated promptly after detection of recurrence (12). Thus, long-term follow-up is
essential (13).
Case report
Patient information
The patient, a 64-year-old female, was admitted to
Binzhou Medical University Hospital (Binzhou, China) in August 2022
for ‘Epigastrium malaise for 1 year with aggravation for 7 days’.
The patient presented with upper abdominal distension accompanied
by nausea and chest tightness. Physical examination showed
abdominal tenderness that was mainly on the right side. There was a
palpable mass in the right upper quadrant of the abdomen with a
hard texture and clear boundary and other significant abnormalities
were not observed. In August 2021 the patient had abdominal
discomfort without obvious cause, mainly in the upper abdomen, with
a feeling of stuffiness and distension, accompanied by a poor
appetite. There were no abnormalities, such as nausea and vomiting,
and the patient's feeling of abdominal distension persisted, so she
went to the local hospital. After treatment with oral Chinese
medicine and infusion, the symptoms still occurred intermittently.
Approximately 7 days before admission to Binzhou Medical University
Hospital, the patient experienced aggravated abdominal distension
accompanied by nausea and chest tightness, and thus immediately
went to Huimin People's Hospital (July 2022; Binzhou, China). After
abdominal ultrasound examination, routine biochemical examinations
and gastrointestinal barium meal examinations, the patient was
diagnosed with an abdominal tumour. After 7 days, the patient was
admitted to Binzhou Medical University Hospital with an ‘abdominal
tumour’. The patient had been constipated since the onset of the
disease in August 2021 and urinated normally. In the past, the
patient had a history of tracheitis for >30 years without
regular treatments. She had a history of surgery for haemorrhoids 3
years ago.
Imaging findings
After admission, the patient underwent relevant
examinations. Abnormalities were not found in routine blood and
biochemical examinations. Other laboratory examinations showed that
the level of carcinoembryonic antigen was 7.33 ng/ml (reference
value 0-3.4 ng/ml), aldosterone was 52.81 pg/ml (reference value
70-300), methoxyepinephrine was 265.7 pg/ml (reference value 0-145
pg/ml) and norepinephrine was 212.8 pg/ml (reference value 217-1109
pg/ml). On a CT scan of the whole abdomen, a mass-like soft tissue
shadow was seen in the lower part of the gallbladder fossa with a
clear boundary, measuring ~6.5x5.4x6.6 cm. A low-density area and
punctate high-density shadow were seen in the mass against the
adjacent tissue, and the partial lesions were close to the
intestinal tract. (Fig. 1) On
whole abdominal CT enhancement, irregular masses of soft tissue
with a clear boundary were seen in the front of the kidney on the
right abdomen, and the size was ~6.5x5.4x6.6 cm. On the
contrast-enhanced CT scan, the lesions were obviously enhanced
inhomogeneously, and there were low-density areas and punctate
high-density shadows in the lesions without enhancement. Large
vessels were observed in the inside and edge of the lesion, which
pushed against the descending and horizontal parts of the duodenum.
The anterior margin of the right kidney, the head of the pancreas
and the uncinate process were compressed, and the systems of the
pancreatic duct and bile duct were slightly dilated.
Therapeutic interventions and
histopathological findings
After oral administration of phenoxybenzamine and
intravenous infusion for 1 week, the present study performed the
elective operation of laparoscopic exploration. After inserting the
laparoscope, there were no obvious abnormalities observed in the
stomach, small intestine, colon, peritoneum or pelvic cavity. Then,
the lateral membrane of the duodenum was opened and a tumour with a
hard texture and clear boundary was visible in the back of the
pancreas, the abdominal aorta and the anterior part of the inferior
vena cava. During the operation, removal of the tumour was
attempted. Because the base of the tumour was closely involved with
the abdominal aortic sheath and it was difficult to turn completely
freely under the laparoscope, we decided to perform open surgery. A
longitudinal incision around the umbilicus ~20 cm long was made in
the upper abdomen and continued to expose the retroperitoneal
tumour. Since the right margin of the tumour was free and the
peritoneum of the duodenum was fully opened, we disconnected the
tumour from the inferior vena cava after breaking off two branches
of the venous reflux. We further severed the connection between the
tumour, the abdominal aortic sheath and the tumour basilar artery.
The retroperitoneal tumour was resected. Postoperative pathology
showed that the surface of the grey-white and grey-red nodular
tumour with a volume of 8.5x7x4.5 cm was completely encapsulated.
The tangential section of the tumour was greyish red with a soft
texture, and another section was greyish white with a hard texture.
Then immunohistochemistry was performed on the tumour tissue. The
immunohistochemistry protocol included the following indicators:
Synaptophysin (Syn), Chromogranin A (CgA), CD56, S-100, epithelial
membrane antigen (EMA), cytokeratin (CK), Vimentin, Melan-A, paired
box 8 (PAX-8) and Ki-67. Immunohistochemical staining used the
following primary antibodies (ZSGB-Bio): Anti-Syn (cat. no.
ZM0246), anti-CgA (cat. no. ZM0076), anti-CD-56 (cat. no. ZM0057),
anti-S-100 (cat. no. ZM0224), anti-EMA (cat. no. ZM0095), anti-CK
(cat. no. ZM0067), anti-Vimentin (cat. no. ZM0260), anti-Melan-A
(cat. no. ZM0398), anti-PAX-8 (cat. no. ZM0468) and anti-Ki-67
(cat. no. ZM0167). The following are details of the
immunohistochemistry: Tissues were embedded after fixing with 4%
paraformaldehyde at room temperature for 48 h and the thickness of
sections was ~5-µm. Peroxidase was inactivated by incubating
sections with 3% H2O2 for 10 min at room
temperature. Normal goat serum working fluid (cat. no. ZLI9022;
OriGene Technologies, Inc.) was used to incubate the sections for
15 min at room temperature. Subsequently, the aforementioned
primary antibodies were used at a dilution of 1:100 and incubated
at 4˚C for 12 h. Next, the sections were incubated with
biotin-labelled goat anti-mouse/rabbit IgG secondary antibodies
(ZSGB-Bio; cat. no. SAP-9100) at a dilution of 1:500 for 30 min at
room temperature. After, images were captured using a fluorescent
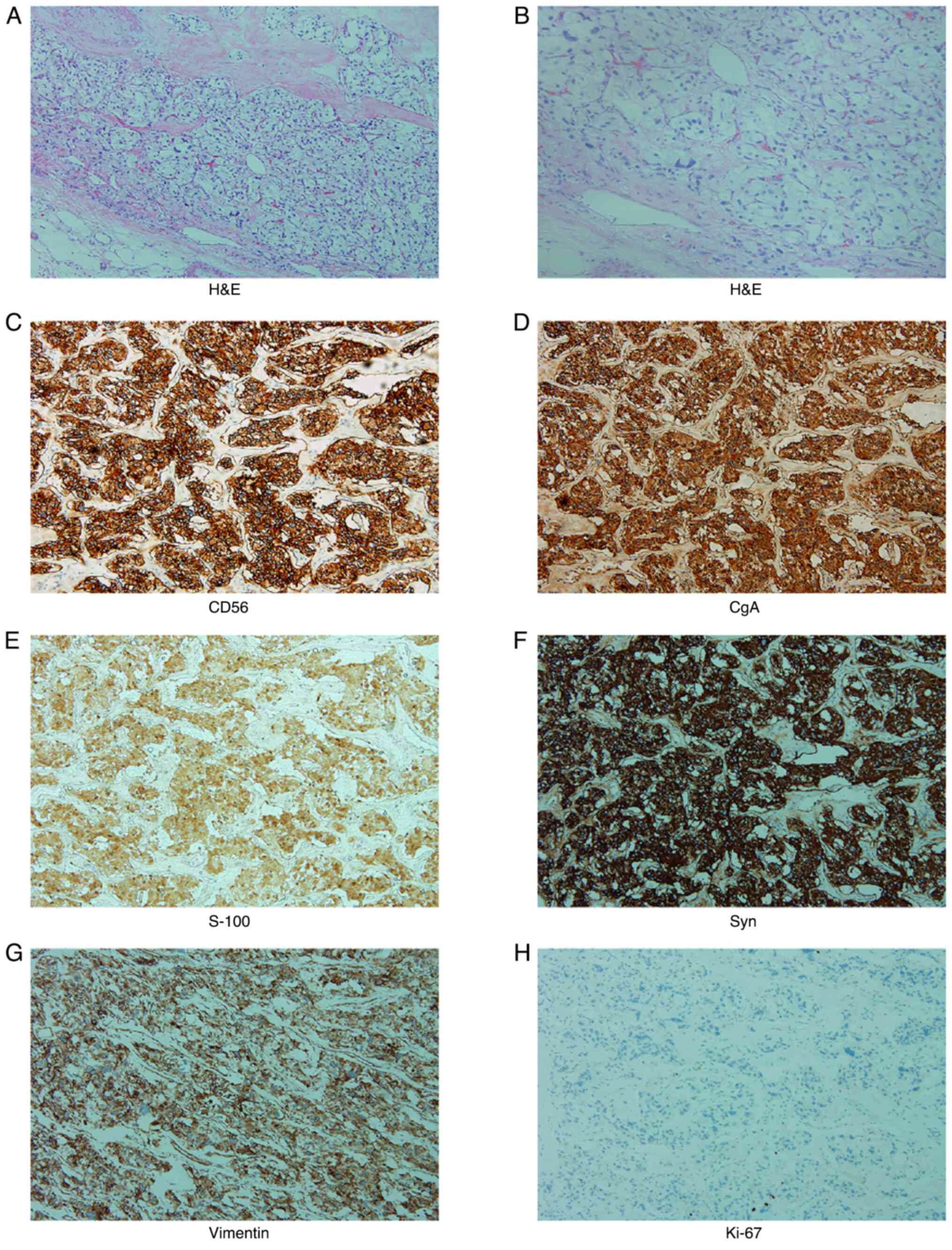
microscope. The results of H&E staining are shown in Fig. 2A and B. The immunohistochemistry results were
as follows: Syn(+), CgA(+), CD56(+), Vimentin(+), S-100(+), the
proliferation index of Ki-67 was ~1% and the others were negative
(Fig. 2C-H).
Follow-up and outcomes
After the operation, anti-infection, antithrombus
and rehydration treatment were actively performed, and the blood
pressure of the patient was monitored closely. There was no obvious
abnormality on contrast-enhanced CT after the operation. The
patient recovered successfully and was discharged. As of March
2023, the general condition of the patient during follow-up is
good.
Discussion
Predominantly located in the abdomen, PGLs are found
mostly in the confluence of the inferior vena cava and the renal
vein or in the organ of Zuckerkandl, directly above the aortic
bifurcation and near the origin of the inferior mesenteric artery
(7,14). Other rare areas are the renal
hilum, suprarenal pole, hepatic hilum, between the liver and
inferior vena cava, near the head of the pancreas, the iliac fossa
or tissues near the iliac fossa blood vessels, such as the ovary,
bladder and rectum (15,16).
Originating from the sympathetic trunk, PGL mainly
synthesizes, secretes and releases large amounts of catecholamines
(CA), such as epinephrine (E), norepinephrine (NE) and dopamine
(DA). Therefore, the clinical manifestations of PGL is mostly
similar to those of pheochromocytoma, such as the elevation of
blood pressure and metabolic changes in patients (14). Its common symptoms are persistent
or paroxysmal hypertension, and it is characterized by a triad of
headache, palpitations and hyperhidrosis or a triad of tremor,
facial pallor and dyspnoea (3,14,17).
Nausea and vomiting, abdominal pain, constipation, intestinal
obstruction and other symptoms of the digestive system are also
observed (16). However, some
patients have no typical clinical symptoms, such as patients with
non-functional PGL.
Non-functional PGLs, which are mostly located in the
neck and rarely in the abdomen, represent ~10% of all PGLs
(5,6). Non-functional tumours in abdomen are
usually large when they are found, and therefore patients usually
present with abdominal or back pain and a palpable mass (18,19).
Due to their similar symptoms, PGL is often misdiagnosed as a
gastrointestinal stromal tumour (GIST). They can be differentiated
by enhanced CT and MRI. PGL exhibits an enhanced vessel shadow on
CT and a vascular flow empty signal on T2WI of MRI, and DWI mostly
shows a high signal (20). GISTs
usually present as an exophytic growth mass with a clear boundary
on CT or MRI, uneven internal density of the tumour, and a visible
liquefied necrotic area (20).
T1WI shows a mostly heterogeneous low or equal signal, and T2WI
shows an uneven high signal (21).
The diagnosis of PGL can be missed for a lifetime,
so the most important step in diagnosing PGL is to be aware of the
possibility of the tumour (17).
When PGL is suspected, the levels of CA and its metabolite are
determined first. Preferred laboratory tests include the
concentration determination of free plasma or urine
methoxyepinephrine or methoxyadrenalin (3,8,16),
and the concentration of NE, E, DA and other metabolites such as
3-methoxytyramine, vanillylmandelic acid and homovanillic acid in
blood or urine can also be detected simultaneously to aid in
diagnosis (11). When a
qualitative diagnosis of PGL is established, CT is the preferred
imaging examination for the localization of the tumour (8,15,16);
in addition, the tumour body, which can be enhanced by contrast
medium, is shown on CT as a circular or quasicircular soft-tissue
shadow with uneven density (16,22-27).
PGL is a complicated and difficult endocrine disease
that involves numerous subjects (3,16).
It should be resected as soon as possible after localization and
qualitative diagnosis. Before surgery, the patients should first be
administered an α-receptor blocker (15,16).
At the same time, they should be treated with venous dilatation
therapy, and open surgery is recommended (28). Postoperative blood pressure and
heart rate are closely monitored (8).
After admission, the present patient underwent an
enhanced CT scan, which indicated a retroperitoneal occupying
lesion. The contrast-enhanced CT scan revealed a marked
heterogeneous enhancement of the lesion. The relevant examinations
showed that the level of methoxynorepinephrine was significantly
elevated. The diagnosis of PGL was made on the basis of the
patient's imaging findings and elevated methoxyepinephrine in
laboratory examinations. At the same time, the present study
excluded the diagnosis of GIST based on laboratory and imaging
findings. The patient, having no typical symptoms such as blood
pressure changes and only manifesting with upper abdominal
distension and accompanied by nausea and chest tightness, was
diagnosed with nonfunctional PGL. Therefore, the present study
prepared for surgery according to the diagnosis of paraganglioma.
Phenoxybenzamine was provided orally in addition to an intravenous
infusion. At 1 week later, the present study performed the
retroperitoneal tumour resection. According to the history and
relevant examination results of the patient, the final diagnosis
was non-functional PGL.
After the operation, the present patient recovered
smoothly. No significant blood pressure abnormality was observed
after the operation. The patient recovered well and was discharged
from the hospital. Based on the present case, accurate and timely
diagnosis of the disease is essential. At present, the patient's
general condition is good after 6 months of follow-up. Because of
its highly local recurrence, the short follow-up time for the
present study is a limitation and we will follow up the patient for
a longer period of time in the future.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Natural Science Fund
Project of Shandong Province (grant no. ZR2014HP028), and the
Scientific Research Foundation of Binzhou Medical University (grant
no. BY2015KYQD21).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
ZL and LK drafted the manuscript and conceived the
study. YZ provided the relevant images. XZ, ZL and YZ contributed
to collecting clinical data and confirmed the authenticity of all
the raw data. LK and ZL corrected the manuscript and prepared
histopathological results. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lima JV Júnior and Kater CE: The
pheochromocytoma/paraganglioma syndrome: An overview on mechanisms,
diagnosis and management. Int Braz J Urol. 49:307–319.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Al Subhi AR, Boyle V and Elston MS:
Systematic review: Incidence of pheochromocytoma and paraganglioma
over 70 years. J Endocr Soc. 6(bvac105)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lam AK: Update on adrenal tumours in 2017
world health organization (WHO) of endocrine tumours. Endocr
Pathol. 28:213–227. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pourian M, Mostafazadeh DB and Soltani A:
Does this patient have pheochromocytoma? A systematic review of
clinical signs and symptoms. J Diabetes Metab Disord.
15(11)2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sherwani P, Anand R, Narula MK, Siddiqui
AA and Aggarwal S: Concurrent nonfunctional paraganglioma of the
retroperitoneum and urinary bladder: A case report with literature
review. Indian J Radiol Imaging. 25:198–201. 2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Renard J, Clerici T, Licker M and Triponez
F: Pheochromocytoma and abdominal paraganglioma. J Visc Surg.
148:e409–e416. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
McNicol AM: Update on tumours of the
adrenal cortex, phaeochromocytoma and extra-adrenal paraganglioma.
Histopathology. 58:155–168. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Berends AMA, Buitenwerf E, de Krijger RR,
Veeger NJGM, van der Horst-Schrivers ANA, Links TP and Kerstens MN:
Incidence of pheochromocytoma and sympathetic paraganglioma in the
Netherlands: A nationwide study and systematic review. Eur J Intern
Med. 51:68–73. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lenders JWM, Kerstens MN, Amar L, Prejbisz
A, Robledo M, Taieb D, Pacak K, Crona J, Zelinka T, Mannelli M, et
al: Genetics, diagnosis, management and future directions of
research of phaeochromocytoma and paraganglioma: A position
statement and consensus of the working group on endocrine
hypertension of the european society of hypertension. J Hypertens.
38:1443–1456. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kinney MA, Warner ME, vanHeerden JA,
Horlocker TT, Young WF Jr, Schroeder DR, Maxson PM and Warner MA:
Perianesthetic risks and outcomes of pheochromocytoma and
paraganglioma resection. Anesth Analg. 91:1118–1123.
2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cui Y, Ma X, Gao Y, Chang X, Chen S, Lu L
and Tong A: Local-regional recurrence of
pheochromocytoma/paraganglioma: Characteristics, risk factors and
outcomes. Front Endocrinol (Lausanne). 12(762548)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Holscher I, van den Berg TJ, Dreijerink
KMA, Engelsman AF and Nieveen van Dijkum EJM: Recurrence rate of
sporadic pheochromocytomas after curative adrenalectomy: A
systematic review and meta-analysis. J Clin Endocrinol Metab.
106:588–597. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Plouin PF, Amar L, Dekkers OM, Fassnacht
M, Gimenez-Roqueplo AP, Lenders JW, Lussey-Lepoutre C and Steichen
O: Guideline Working Group. European society of endocrinology
clinical practice guideline for long-term follow-up of patients
operated on for a phaeochromocytoma or a paraganglioma. Eur J
Endocrinol. 174:G1–G10. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ayala-Ramirez M, Feng L, Johnson MM, Ejaz
S, Habra MA, Rich T, Busaidy N, Cote GJ, Perrier N, Phan A, et al:
Clinical risk factors for malignancy and overall survival in
patients with pheochromocytomas and sympathetic paragangliomas:
primary tumor size and primary tumor location as prognostic
indicators. J Clin Endocrinol Metab. 96:717–725. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Whalen RK, Althausen AF and Daniels GH:
Extra-adrenal pheochromocytoma. J Urol. 147:1–10. 1992.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chinese Society of Endocrinology: Expert
consensus on the diagnosis and treatment of pheochromocytoma and
paraganglioma. Chin J Endocrinol Metab. 36:737–750. 2020.
|
|
17
|
Fagundes GFC and Almeida MQ: Perioperative
management of pheochromocytomas and sympathetic paragangliomas. J
Endocr Soc. 6(bvac004)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Olson JR and Abell MR: Nonfunctional,
nonchromaffin paragangliomas of the retroperitoneum. Cancer.
23:1358–1367. 1969.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lack EE, Cubilla AL, Woodruff JM and
Lieberman PH: Extra-adrenal paragangliomas of the retroperitoneum:
A clinicopathologic study of 12 tumors. Am J Surg Pathol.
4:109–120. 1980.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nishino M, Hayakawa K, Minami M, Yamamoto
A, Ueda H and Takasu K: Primary retroperitoneal neoplasms: CT and
MR imaging findings with anatomic and pathologic diagnostic clues.
Radiographics. 23:45–57. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Vernuccio F, Taibbi A, Picone D, LA Grutta
L, Midiri M, Lagalla R, Lo Re G and Bartolotta TV: Imaging of
gastrointestinal stromal tumors: From diagnosis to evaluation of
therapeutic response. Anticancer Res. 36:2639–2648. 2016.PubMed/NCBI
|
|
22
|
Leung K, Stamm M, Raja A and Low G:
Pheochromocytoma: the range of appearances on ultrasound, CT, MRI,
and functional imaging. AJR Am J Roentgenol. 200:370–378.
2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Taïeb D, Timmers HJ, Hindié E, Guillet BA,
Neumann HP, Walz MK, Opocher G, de Herder WW, Boedeker CC, de
Krijger RR, et al: EANM 2012 guidelines for radionuclide imaging of
phaeochromocytoma and paraganglioma. Eur J Nucl Med Mol Imaging.
39:1977–1995. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fiebrich HB, Brouwers AH, Kerstens MN,
Pijl ME, Kema IP, de Jong JR, Jager PL, Elsinga PH, Dierckx RA, van
der Wal JE, et al: 6-[F-18]Fluoro-L-dihydroxyphenylalanine positron
emission tomography is superior to conventional imaging with
(123)I-metaiodobenzylguanidine scintigraphy, computer tomography,
and magnetic resonance imaging in localizing tumors causing
catecholamine excess. J Clin Endocrinol Metab. 94:3922–3930.
2009.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wiseman GA, Pacak K, O'Dorisio MS, Neumann
DR, Waxman AD, Mankoff DA, Heiba SI, Serafini AN, Tumeh SS,
Khutoryansky N and Jacobson AF: Usefulness of 123I-MIBG
scintigraphy in the evaluation of patients with known or suspected
primary or metastatic pheochromocytoma or paraganglioma: results
from a prospective multicenter trial. J Nucl Med. 50:1448–1454.
2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Timmers HJ, Chen CC, Carrasquillo JA,
Whatley M, Ling A, Eisenhofer G, King KS, Rao JU, Wesley RA, Adams
KT and Pacak K: Staging and functional characterization of
pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose
(18F-FDG) positron emission tomography. J Natl Cancer Inst.
104:700–708. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Janssen I, Blanchet EM, Adams K, Chen CC,
Millo CM, Herscovitch P, Taieb D, Kebebew E, Lehnert H, Fojo AT and
Pacak K: Superiority of [68Ga]-DOTATATE PET/CT to other functional
imaging modalities in the localization of SDHB-associated
metastatic pheochromocytoma and paraganglioma. Clin Cancer Res.
21:3888–3895. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Garcia-Carbonero R, Matute Teresa F,
Mercader-Cidoncha E, Mitjavila-Casanovas M, Robledo M, Tena I,
Alvarez-Escola C, Arístegui M, Bella-Cueto MR, Ferrer-Albiach C and
Hanzu FA: Multidisciplinary practice guidelines for the diagnosis,
genetic counseling and treatment of pheochromocytomas and
paragangliomas. Clin Transl Oncol. 23:1995–2019. 2021.PubMed/NCBI View Article : Google Scholar
|