1. Introduction
Osteoarthritis (OA) is a chronic, non-specific
inflammatory disease that, under the influence of a chronic
inflammatory environment, can result in joint deterioration and
deformity, leading to pain and limited mobility (1). There are 240 million individuals
worldwide suffering from OA, and some specific populations
(elderly, women, Asian population) face higher prevalence rates
(2). This not only poses a threat
to patient health, but also imposes a significant medical burden
(3). An individual is at risk of
OA due to aging, obesity and trauma, while chronic inflammation,
metabolic disorders, and damage-associated molecular patterns
(DAMPs) are also important factors in the occurrence of OA
(4,5). As the most common joint disease, OA
affects the entire joint, causing damage to articular cartilage,
synovium, subchondral bone, and the fat pads beneath the patella
(6-8).
Pathological crosstalk among different joint tissues
actively contributes to OA development (9). Synovium-cartilage crosstalk leads to
OA symptoms and may also contribute to OA development (10). Therefore, elucidating the specific
crosstalk mechanism between synovium and cartilage is crucial for
in-depth research on the development process and pathogenic
mechanism of OA. Moreover, current OA treatments exhibit limited
efficacy in symptom relief, underscoring the need for a deeper
understanding of OA pathogenesis to establish a foundation for the
discovery of treatments.
Therefore, this article presents an exploration of
the anatomy of normal and OA joint tissues, integrating imaging,
biological information, and basic experimental evidence to
substantiate the existence of an important link between synovium
and cartilage. Additionally, it outlines the important injury
phenotypic changes associated with injury, primarily synovitis and
cartilage destruction, between cartilage and synovium. The
literature on the crosstalk between synovial cells (fibroblasts and
macrophages) and chondrocytes in OA is then collected and
summarized to outline the mechanisms of their interaction. Finally,
current and promising therapeutic modalities are presented to
provide insights into new molecular mechanisms and facilitate the
discovery of effective therapeutics.
2. Overview of joint structure and
biology
Synovial membrane
The normal synovial membrane comprises two layers,
the inner layer and the lining layer (11). Macrophages and fibroblasts are
typically located in the external layer of the synovial membrane,
jointly maintaining synovial homeostasis (12). The lining layer of the synovial
tissue contains mesenchymal stem cells (MSCs), immune cells,
heterogeneous macrophages, and fibroblasts (13). Under normal conditions, synovial
fibroblasts (SFCs) produce synovial fluid, mainly composed of
lubricants and mucins (14). Both
of these molecules play a vital role in maintaining the normal
structure of the joint cartilage by lubricating the cartilage
surface and reducing friction (15). Additionally, lubricants, mainly
lubricin, can inhibit the deposition of pathological proteins on
the joint surface (16). Since
joint cartilage lacks an inherent supply of nutrients, the synovium
also helps to provide nutrition to chondrocytes and maintain joint
soft homeostasis (17).
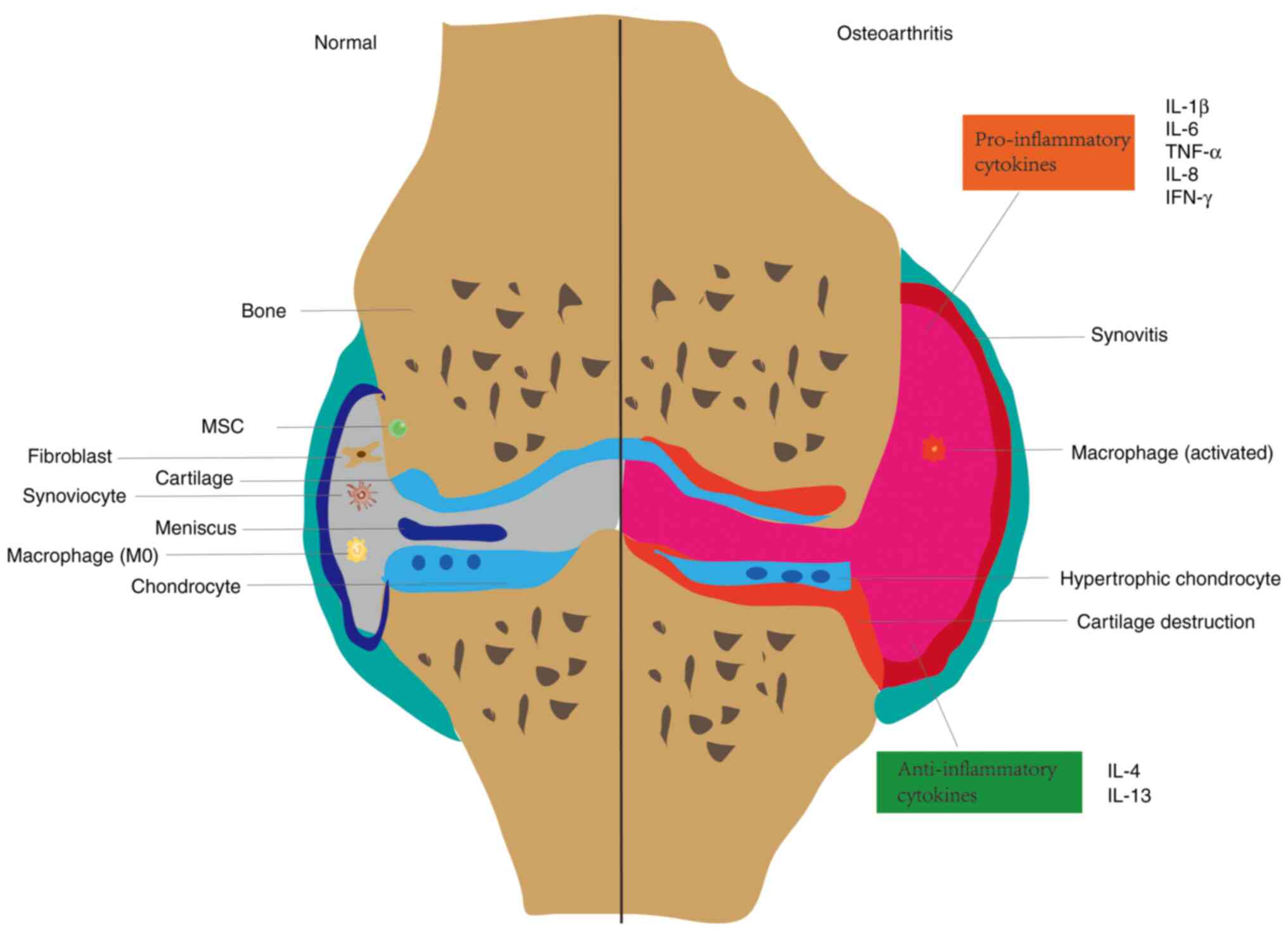
Synovial abnormalities in patients with OA are
characterized by thickening of the inner synovial layer,
inflammatory cell aggregation, interstitial vascularization, and
fibrosis of the underlying layer (18-20).
Synovitis is considered a potential precursor to the development of
OA (21). MRI and ultrasound have
often been used in the evaluation of OA (22,23),
and imaging findings have revealed hyperplasia of the synovial
lining in patients with early-stage OA (22). Synovial biopsy serves as the gold
standard in diagnosing of synovitis (10), and patients with early-stage OA
often present with synovial thickening and infiltration of
inflammatory cells (20). In
inflammatory situations, synovial lining cells are usually
proliferated and accompanied by an inflammatory cell infiltrate
consisting mainly of macrophages, and a few but significant number
of T and B cells (24). During the
OA process, synovial macrophages are involved in innate immune
response, producing pro-inflammatory cytokines that interact with
other synovial cells and chondrocytes (25). An important factor in the
pathogenesis of OA is the disturbance of cytokine balance, with an
increase in the secretion of pro-inflammatory cytokines initiating
a vicious cycle that causes damage to cartilage and other
intra-articular structures by activating catabolic enzymes, thereby
exacerbating OA progression (8).
In addition, pro-inflammatory cytokines can further activate
chemokines, such as chemokine (C-C) motif ligand (CCL)2 and CCL5,
attracting inflammatory cells to the joints, further promoting the
secretion of inflammatory factors and disease progression (9). In addition, synovitis promotes
angiogenesis (26), which may lead
to osteophyte formation and ossification, which in turn accelerates
inflammation (27). In the
advanced stages of OA, over-proliferation of fibroblasts,
differentiation into myofibroblast-like cells, and impaired
extracellular matrix (ECM) anabolism promote fibrosis of the
synovium (28). Thus, the
pathological process of the synovium is crucial for the development
of OA.
Cartilage
Articular cartilage is a connective tissue covering
the ends of bones, composed of ECM and chondrocytes, which
lubricate joints and withstand loads (29). Proteoglycans (aggregated
proteoglycans and chondroitin sulfate) are the main constituents of
the matrix and together with collagen (mainly collagen II) form the
ECM, which is involved in cartilage synthesis and degradation
(6). In a previous study,
pericellular matrix (PCM) was proposed as the matrix encasing
chondrocytes within cartilage, maintaining the mechanical load of
chondrocytes and regulating molecular interactions (30). This finding provides deeper
insights into the microstructure, physical properties, and chemical
composition of cartilage. Further exploration of ECM and PCM
facilitates a better understanding of how to regulate chondrocyte
metabolism and maintain cartilage homeostasis.
When cartilage is damaged by external forces,
chondrocytes, SFCs, and synovial macrophages produce a
desintegrin-like and metalloproteinases with thrombospondin motifs
(ADAMTS) or matrix metalloproteinases (MMPs), the former scavenging
proteoglycans (31) and the latter
cleaving collagen (32),
disrupting cartilage tissue structure and biological function. Not
only does catabolism of the ECM cause cartilage degeneration,
changes in chondrocyte status are equally important for cartilage
degeneration. Chondrocyte response patterns in OA include
proliferation and cell death, anabolic imbalance, and phenotypic
regulation (33). Early in the OA
process, chondrocytes proliferate and synthesize ECM to aid in
injury healing (34). After
sufficient catabolic signals are provided to articular
chondrocytes, this leads to their eventual phenotypic loss
(35). These phenotypes include
hypertrophy, apoptosis, autophagy and senescence, with hypertrophy
being the most common phenotype of chondrocytes. The normal bone
joint structure and OA tissue structure are depicted in Fig. 1.
Association between synovium and
cartilage
The synovium establishes a connection with cartilage
through the secretion of synovial fluid, introducing the concept of
molecular interaction across this region during OA (36). During the progression of OA, an
important network links synovitis and cartilage destruction
(1,37).
An arthroscopic score for knee OA from a
multicenter, longitudinal study showed that the intensity of
synovitis inflammation is strongly related to the severity of
cartilage destruction, which may have potential in predicting
disease progression (38). Another
study involving multicenter patients with OA demonstrated a
significantly higher frequency of cartilage loss in cases with
grade 3 or 4 effusion synovitis compared to those with grade 0 or 1
effusion synovitis (39). In OA,
bioinformatics is employed to identify the central genes and immune
environment characteristics of OA (40). By analyzing gene expression
profiles, novel diagnostic biomarkers for OA and possible
associations between key genes and infiltrating immune cells may be
identified. A transcriptomics-based study by Wang et al,
integrated data from different tissues of the knee joint in
patients with OA, clarified the relationship between synovium and
cartilage, and combined with synovial proteomics data, constructed
a crosstalk spectrum according to predicted ligand-receptor
interactions (40). Basic
experiments have shown that some secreted glycoproteins, such as
MFG-E8, whose deletion leads to progressive cartilage destruction
and synovial proliferation, are two pathological processes that may
jointly crosstalk through the NF-κB pathway (41). Notably, specific medicinal plants,
such as curcumin, a rooted plant of the ginger family, have been
shown to alleviate synovitis and cartilage damage through the NF-κB
pathway (17).
The aforementioned evidence from clinical
experiments, bioinformatics and basic research demonstrates a close
relationship between synovium and cartilage. However, the exact
mechanism remains unclear. Hence, it is particularly important to
explore the specific mechanisms of crosstalk between the two and
help discover new therapeutic modalities for OA.
3. Immune response in OA
The activation of immune cells induces changes in
the environment for cartilage survival. Factors released by immune
cells continuously influence the inflammatory microenvironment,
regulating its complex balance (42). The response of chondrocytes in the
immune response is increasingly attracting interest. Single-cell
RNA sequencing (scRNA-seq) and mass cytometry, render it possible
to investigate chondrocyte heterogeneity and intercellular
communication in detail, and to identify subgroups with different
functions and phenotypes of these cells, targeting molecular
mechanisms to understand OA (43).
In this section, the activation of different subpopulations of
chondrocytes involved in autoimmune response and subsequent
mediating of OA inflammation and structural damage, are
elucidated.
Macrophage activation
The first step in initiating the immune response
involves the recognition of foreign objects by immune cells
(44). Macrophages, recognized as
crucial players in OA, are distributed throughout the body, swiftly
moving towards inflammatory sites (45), participating in both innate and
acquired immunity (46). During
the innate immune process, macrophages express pattern recognition
receptors (PRR), including Toll-like receptors (TLR) and NOD-like
receptors (NLR) (47,48), binding to pathogen-associated
molecular patterns (PAMP) or DAMPs, mainly cartilage degradation
products (49). Further activation
of the transcription factors NF-κB and MAPK induce genes encoding
cartilage-degrading ECM enzymes and inflammatory factors,
ultimately leading to cartilage catabolism (47). The adaptive immune process in OA
involves the activation of B cells and T cells, which assist
macrophages (50). Some
stimulators (IFN-γ, TNF-α, and LPS) and transcription factors
(STATA and NF-κB) promote M1 polarization of macrophages, releasing
pro-inflammatory cytokines (51),
thereby accelerating the pathogenesis process of synovitis and
cartilage destruction (52). By
contrast, Th2 cells promote macrophage M2 polarization and mitigate
OA by releasing IL-4 and IL-13(42). In vivo experiments in mice
have shown that inhibiting synovial macrophage M1 polarization
helps reduce cartilage damage (53). In addition to immune responses,
some metabolic changes, especially glucose metabolism, as well as
energy metabolism affect macrophage processes leading to cartilage
destruction.
Regarding material metabolism, glycolysis metabolism
appears to promote OA development. Firstly, the addition of
glycolysis inhibitors under the M1 polarized cell model of
macrophages induced by alkaline calcium phosphate crystals was
revealed to effectively reverse M1 macrophage polarization
(54). This suggests that
metabolic changes, particularly increased glycolysis in synovial
macrophages, have the potential to exacerbate cartilage
destruction. Additionally, the SIRT6 inhibitor, a member of the
deacetylase protein family, has been demonstrated to exacerbate
cartilage damage by enhancing the glycolytic process (55). Introducing RNAi targeting SIRT6
into cells induced RAW264.7 to release pro-inflammatory cytokines,
promoting the M1 phenotype while inhibiting the M2 phenotype. In
in vivo experiments, a significant cartilage deficit can be
observed in mice (55). Given that
the endoplasmic reticulum (ER) is the site where various
metabolisms, such as gluconeogenesis and lipid metabolism, occur,
it is involved in regulating the progression of OA. GRP78, an ER
stress molecular chaperone and polymeric hyaluronan was revealed to
inhibit synovial inflammation and the M1 phenotype of macrophages
stimulated by IL-1β through the GRP78-NF-κB pathway. Further
investigation is needed to elucidate these findings (56).
Experiments conducted in mitochondria indicated that
GLX351322, an inhibitor of NADPH oxidase 4, reduces synovitis
cartilage destruction through the MAPK/NF-κB pathway, thus
attenuating teporomandibular joint OA (57).
In terms of epigenetic modifications, extracellular
vesicles (EVs) from human umbilical cord MSCs (hUC-MSCs) were
demonstrated to reduce NLRP3 mRNA methylation in macrophages by
releasing miR-1208 to interact with METTL3, thereby attenuating
knee OA in a mouse model (58).
In conclusion, metabolic reprogramming and
epigenetic modifications play important regulatory roles in
molecular alterations and polarization states of macrophages. The
exploration of metabolic activators or inhibitors, along with
targeted molecular epigenetic regulation, may effectively maintain
the balance of cell survival and physiological processes (56-58).
Fibroblast activation
Fibroblast-like synoviocytes (FLS) represent a
heterogeneous population of invasive fibroblasts and are considered
important in OA (59). Activated
FLS are one of the key participants in OA joint destruction,
secreting pro-inflammatory mediators including IL-1β and TNF-α
(60,61). Pro-inflammatory mediators have the
function of maintaining cartilage homeostasis (62), and chondrocytes are stimulated by
the pro-inflammatory mediators released by FLS, thereby
exacerbating inflammation (59,63).
IL-1β promotes chondrocyte synthesis of MMPs, mainly MMP1 and
MMP13, thereby shearing and disrupting ECM synthesis by articular
cartilage (64). TNF-α promotes
chondrocyte mitochondrial dysfunction and leads to death, resulting
in cartilage destruction (65). In
addition, ECM is cleaved into substances such as fibronectin and
type II collagen after the increase of the activities of MMPs and
ADAMTS, which further activates FLS as DAMPs through the integrin
and TLR pathways (66,67). A study showed that under the
stimulation of cartilage wear particles (CWP), OA-FLS cultures
increased the levels of pro-inflammatory mediators (NO, PGE2),
cytokines (IL-6, IL-8) and MMPs (MMP9, MMP10, MMP13), which modify
the inflammatory microenvironment (68,69).
Metabolic reprogramming and epigenetic modifications
have important regulatory roles in fibroblast molecular alterations
and polarization states.
Glucose metabolism appears to be crucial for
fibroblast activation. According to a study, hyperglycemia
increases AGEs expression through the HIF-1α-GLUT1 pathway, leading
to an increase in inflammatory factors in FLS and subsequent
chondrocyte degradation and OA promotion (70). Additionally, overexpression of
pyruvic acid dehydrogenase kinase can inhibit the metabolic
reprogramming of OA SFCs, thereby reducing the secretion of FLS
cytokines, which may be the mechanism to mitigate cartilage
degeneration (71).
Epigenetic modifications mainly include DNA
methylation, histone modifications, chromosome remodeling and
regulation of non-coding RNAs (72). Epigenetic modifications of FLS have
an impact on cartilage phenotype. In terms of methylation, ATG7
mRNA methylation modifications mediated by METTL3 were demonstrated
to regulate FLS cell senescence through the autophagy-GATA4 axis.
In an in vivo animal model, METTL3 knockdown ameliorated
DMM-induced cartilage destruction (73). Notably, METTL3 also has an
important role in macrophage regulation. In terms of microRNA
(miRNA or miR), miRNA expression is increased in FLS, and a
previous study revealed a protective effect in DMM-induced OA mice
using miR-34a-5p mimics (74).
However, the specific mechanisms of miRNA regulatory molecules need
to be further elucidated. In terms of histone modification, histone
modification regulates molecular expression and pathway changes in
fibroblasts. For example, spermidine inhibits TNF-α-induced
pro-inflammatory cytokine release from the NF-κB/p65 pathway in OA
by activating RIP1 deubiquitination, thereby alleviating synovitis
and cartilage degeneration (75).
Chondrocyte activation
Activation of chondrocytes arises from alterations
in the inflammatory microenvironment, characterized by cell
phenotype adjustment, molecular changes, and ECM disruption
(76), which disrupts the
anabolic-catabolic balance of chondrocytes (77), tilting this balance towards
catabolism, which in turn promotes cartilage degeneration and OA
pathological progression. Chondrocytes are the only surviving cells
in cartilage, synthesizing matrices and fibers to maintain normal
cartilage function (78).
Proliferation, viability, and secretion of chondrocytes change as
OA progresses. Various behavioral phenotypic changes are acquired,
such as cell death, and hypertrophy, which produce important
changes in OA (79). At the same
time, during the progression of OA, marked changes in metabolism
occur, with a shift to glycolysis in the glycolytic pathway, which
leads to impaired ECM synthesis and anabolic processes, in addition
to some lipid metabolism (lipid deposition and elevated
cholesterol), and oxidative stress, which also play an important
role (80).
Recent single-cell sequencing techniques have
revealed heterogeneous populations of chondrocytes induced by
different factors. Different chondrocyte heterogeneous populations
exist at different stages of disease development, have different
cartilage anatomical localizations, mediate different phenotypes
(hypertrophy, apoptosis), and perform different functions (9). The focus of the present review was on
the chondrocyte heterogeneous subpopulations associated with immune
response and fibrosis.
In a genomics study conducted by Ji et al
(81), seven articular chondrocyte
populations in human knee OA cartilage were defined for the first
time, namely proliferative chondrocytes (ProCs), pre-hypertrophic
chondrocytes (preHTCs), hypertrophic chondrocytes (HTCs),
fibrochondrocytes (FCs), effector chondrocytes (ECs), regulatory
chondrocytes (RegCs), and steady-state chondrocytes cells (HomCs).
Of these, ECs are associated with metabolic responses, which lead
to impaired ECM synthesis and anabolic processes. RegCs have
functions such as antigen-presenting and immune cell receptor
signaling, and some RegCs have high levels of immune
system-specific markers, suggesting that they are similar to immune
cells. FCs, labeled with fibroblast phenotypic markers, and with a
high proportion of genes and vascularization capacity are
associated with unfavorable OA outcomes. ECs and RegCs are
predominantly present in early OA, and FCs are predominantly a late
chondrocyte population. Functional ECs and RegCs protect cartilage
from OA, while FCs mainly destroy cartilage to exacerbate OA.
Hu et al (82) further identified chondrocyte
subtypes of patients with OA based on the research by Ji et
al (81) and explored their
immunogenicity. ECs have stronger immune reactivity and secretory
activity, mediating and recruiting immune cells in OA, mainly
through the regulation of various signaling pathways related to
tissue inflammation and exerting immune cell effects. FCs have
higher fibroblast characteristics and exhibit enhanced metabolic
activity in energy metabolism, which may mainly induce cartilage
degeneration by affecting fibrodegeneration and cartilage
repair.
Gao et al (83) processed the data from Ji et
al (81) and identified a
subset of chondrocytes accordingly. The study focused on stress
metabolism chondrocytes and ECM synthesis chondrocytes. Stress
metabolic chondrocytes may actively participate in metabolic
reprogramming (84). Chondrocytes
related to matrix synthesis may promote cell apoptosis and
neovascularization through the SLIT-ROBO pathway (85).
Articular cartilage consists of ECM and chondrocytes
with different morphologies and functions. These findings suggest
that heterogeneous subsets of chondrocytes involved in metabolic
changes as well as immune responses are important for the
progression of OA. Clarifying the classification and function of
different chondrocyte subpopulations and exploring their
communication with other cells will deeply expand knowledge in
molecular biology and OA pathology. In addition, these
aforementioned scRNA-seq studies identify the origins of crucial
synthetic and decomposed mediators in OA. Further exploration of
these mediators can help to identify targeted pathogenic cells and
molecules more centrally, and develop more effective OA
strategies.
According to Yuan et al, synovium regulates
different metabolic subgroups of cartilage in the knee joint
(86). The receptor-ligand
crosstalk action was higher in number in synovial and cartilage
inflammatory subtypes. Therefore, synovium may be a driver of these
OA subpopulations. According to Ching et al, molecular
phenotypic changes in synovial cells and chondrocytes cause
crosstalk, and a model for crosstalk has been built to explain OA
(87).
Identifying the association between chondrocyte
heterogeneity and pathogenic synovial cells contributes to the
understanding of the complexity and interactions of histopathology.
The research methods include analyzing cell surface markers, bulk
transcriptomics and single cell transcriptomics that identify a
subpopulation of chondrocytes associated with disease that may
ultimately serve as a specific target for therapy. Revealing
therapeutic targets for disease and targeting chondrocyte
subpopulations may complement therapeutic treatments.
Additional clinical studies are required to
understand how alterations in these subpopulations affect the
various clinical settings of OA, including severity of joint
damage, disease prognosis, and patient response to treatment.
Detailed phenotypic research has greatly expanded the understanding
of chondrocyte plasticity, which helps with the development of
targeted therapeutic strategies for chondrocytes. The mechanism of
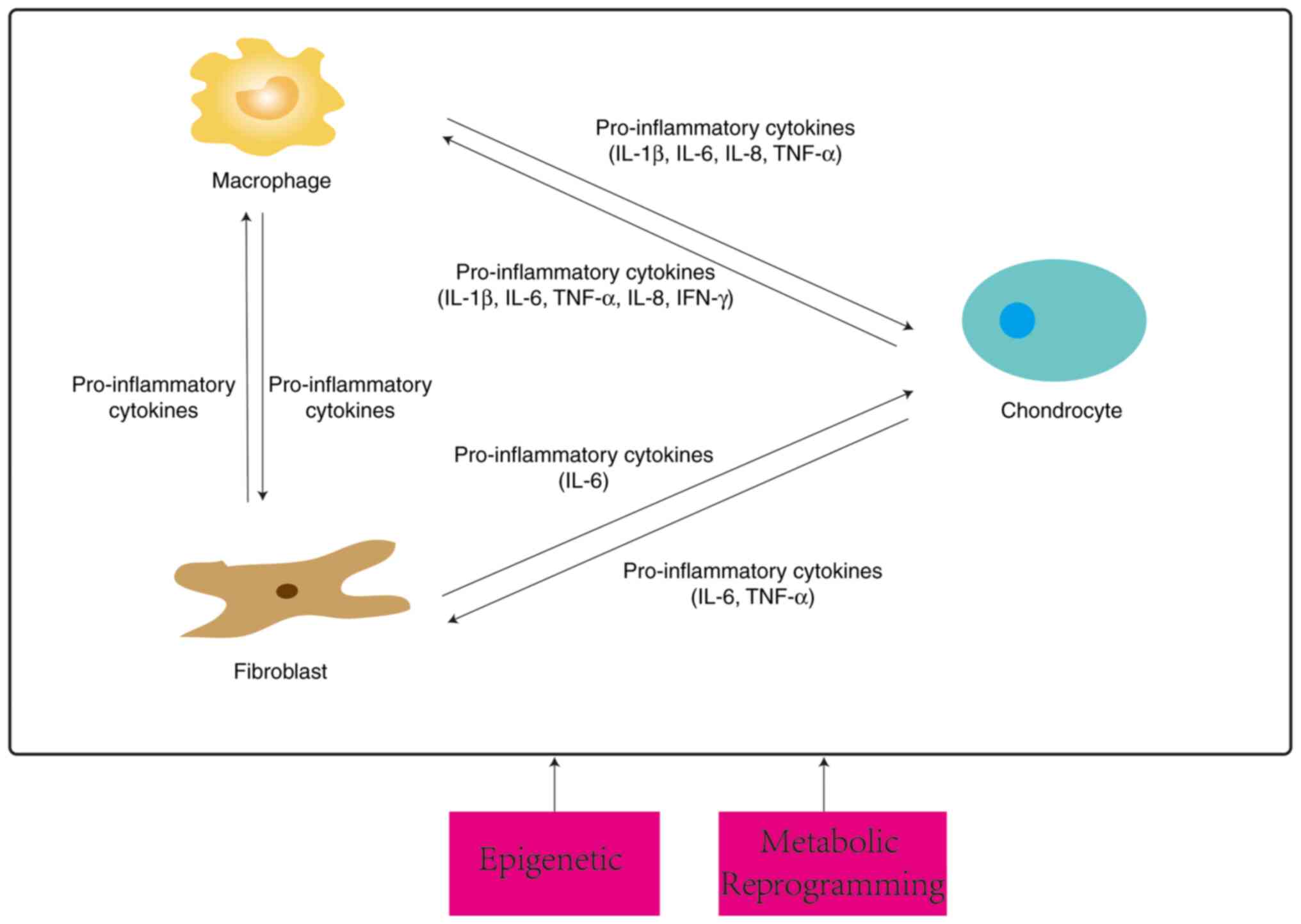
crosstalk between the three types of cells is summarized in
Fig. 2.
Crosstalk between cells
The interaction between synovium and cartilage is
ultimately between synovial cells and chondrocytes. It has been
shown that treating articular chondrocytes with EVs derived from OA
synovial fluid not only reduces cell survival, but also reduces
anabolism and increases catabolism and pro-inflammatory effects
(88,89).
Following the onset of OA, fibroblasts acquire an
invasive phenotype and interact with chondrocytes through
pro-inflammatory mediators to secrete MMPs to affect OA (37). Similarly, macrophages acquire a
polarized phenotype and induce chondrocytes to produce
pro-inflammatory mediators and phenotype changes by releasing
pro-inflammatory mediators (18,90).
At the same time, these mediators activate cartilage cells that
produce MMPs, amplify the inflammatory cascade with synovial cells,
and lead to a vicious cycle of an inflammatory microenvironment
(91).
In addition to the interaction between synovial
cells and chondrocytes, a huge network encompasses three types of
cells. SFCs have different functional properties in OA and secrete
R-spondin-2(92). R-spondin-2
activates the Wnt pathway and induces chondrocyte hypertrophy and
differentiation (93). Notably,
R-spondin-2 can also be secreted by M1 macrophages, and knockdown
of R-spondin-2 was demonstrated to exacerbate cartilage destruction
in mouse animal experiments (94,95).
This suggests an important network linking the three together.
Further research is required to identify common molecules and
pathways of action to elucidate the specific mechanisms of the
three interactions.
4. Cellular interaction
Direct evidence of the interactions between synovial
cells (mainly macrophages and fibroblasts) and chondrocytes, which
act as mutual donors and acceptor cells, has been amassed. The
interactions take place by means of cell contact, paracrine
secretion, and exocytosis. The main substances that interact are
some amino acids and peptides/proteins, pro-inflammatory cytokines,
secreted proteins, and microvesicles. Microvesicles are formed by
budding from the cell membrane, with a particle size of 150-1,000
nm. They transfer bioactive RNA [miRNA, tRNA, mRNA, and fragmented
mRNA, long stranded non-coding RNA (lncRNA), and tRNA], proteins,
lipids, and metabolites from donor cells to recipient cells, and
affect the biological characteristics of the latter (19). In terms of cellular communication,
microvesicles mediate intercellular communication and regulate the
spread of inflammation and cartilage destruction through miRNA and
lncRNA. In terms of organizational communication, microvesicles can
penetrate microcrack channels and vascular channels between bone
and cartilage interfaces, promoting bone and cartilage
communication (19).
Cellular channels
Connexins (Cxs) are subunits that form gap channels,
and Cx43 is the most prominent Cx (96). It has been confirmed that articular
chondrocytes in cartilage physically connect with distant
chondrocytes through cytoplasmic extension mainly establishing
intercellular communication through the gap junction channel
composed of Cx43.
Carpintero-Fernandez et al (96) established a co-culture system
(Transwell) to study crosstalk between synovial cells and
chondrocytes and showed the presence of a cellular channel (Cx43)
between the two cells to establish communication and exchange amino
acids and peptides/proteins, including several chaperone proteins
and cell surface proteins, which may indicate the involvement of
chaperone and cell surface proteins in the pathological process in
OA.
Paracrine
Previous research has identified the importance of
IL-6 between cellular interactions. Chondrocytes are prompted to
secrete IL-6 through IL-6R on IL-6 secretory junctions on
fibroblasts under normal conditions or after leptin stimulation,
and IL-6 secreted by FLS upregulates the expression of MMP3 and
MMP13 genes, and promotes ECM destruction (37). After IL-1β stimulates chondrocytes
to bind IL-1R, the NF-κB pathway is activated and chondrocytes
secrete IL-6 and act on IL-6R, upregulating STAT3 expression to
promote IL-6 secretion by macrophages, thus promoting IL-6 and IL-8
re-release (97). IL-6 is derived
from pro-inflammatory cytokine stimulation of chondrocytes, binding
to macrophages or IL-6R on FLS cells causes a cascade effect, which
proceeds to the facilitated expression of MMPs.
Subsequent studies have explored the types of
pro-inflammatory cytokines in greater depth. A 3D culture system
that cultured macrophages and chondrocytes found that activated
macrophages (AMs) promoted osteoarthritic chondrocytes (OAC) to
express more MMPs and ADAMTS as well as cytokines (IL-1β, IL-6,
TNF-α, IL-8 and IFN-γ). Being affected by OAC, AMs express more
IL-1β and VEGFA (98). Similarly,
findings under Transwell co-culture conditions revealed that
cartilage debris stimulated macrophages prompting them to release
pro-inflammatory mediators (TNF-α, IL-6, NO), inducing chondrocytes
to break down large amounts of pro-inflammatory metabolic factors
(MMP13, IL-6, iNOS) and exacerbating OA progression (99). In addition, M1 polarization occurs
after IL-1β stimulation of macrophages (100), which secrete pro-inflammatory
factors (IL-1β, TNF-α, IL-6), upregulating the increase of
catabolic factors (MMP13, ADATMS5) and decrease of anabolic factors
(SOX9) in chondrocytes. In turn, chondrocytes receive stimulation
to secrete PTX3, which acts on the CD32 receptor of M0 macrophages
and activates the NF-κB pathway, preventing both M2 polarization
and promoting the expression of iNOS. This also promotes the M1
phenotype (101).
Furthermore, a study revealed that synovial cells
produce one or more soluble factors that may be released into
cartilage via synovial fluid, inducing the expression of Prg4
(lubricating hormone) in surface regions, thereby inhibiting
senescent chondrocyte hypertrophy and promoting their
differentiation (102).
In conclusion, macrophages stimulated by DAMPs
secrete pro-inflammatory mediators to induce catabolism in
chondrocytes, and chondrocytes exacerbate OA by promoting further
cascade or polarization of macrophages through pro-inflammatory
cytokines or secreted proteins.
Exosomes
EVs secreted by synovial cells or chondrocytes
contains abundant miRNAs, which affects downstream pathways,
leading to the acquisition and deletion of changes in cell
phenotype and molecular expression, thereby altering the
inflammatory microenvironment and affecting OA.
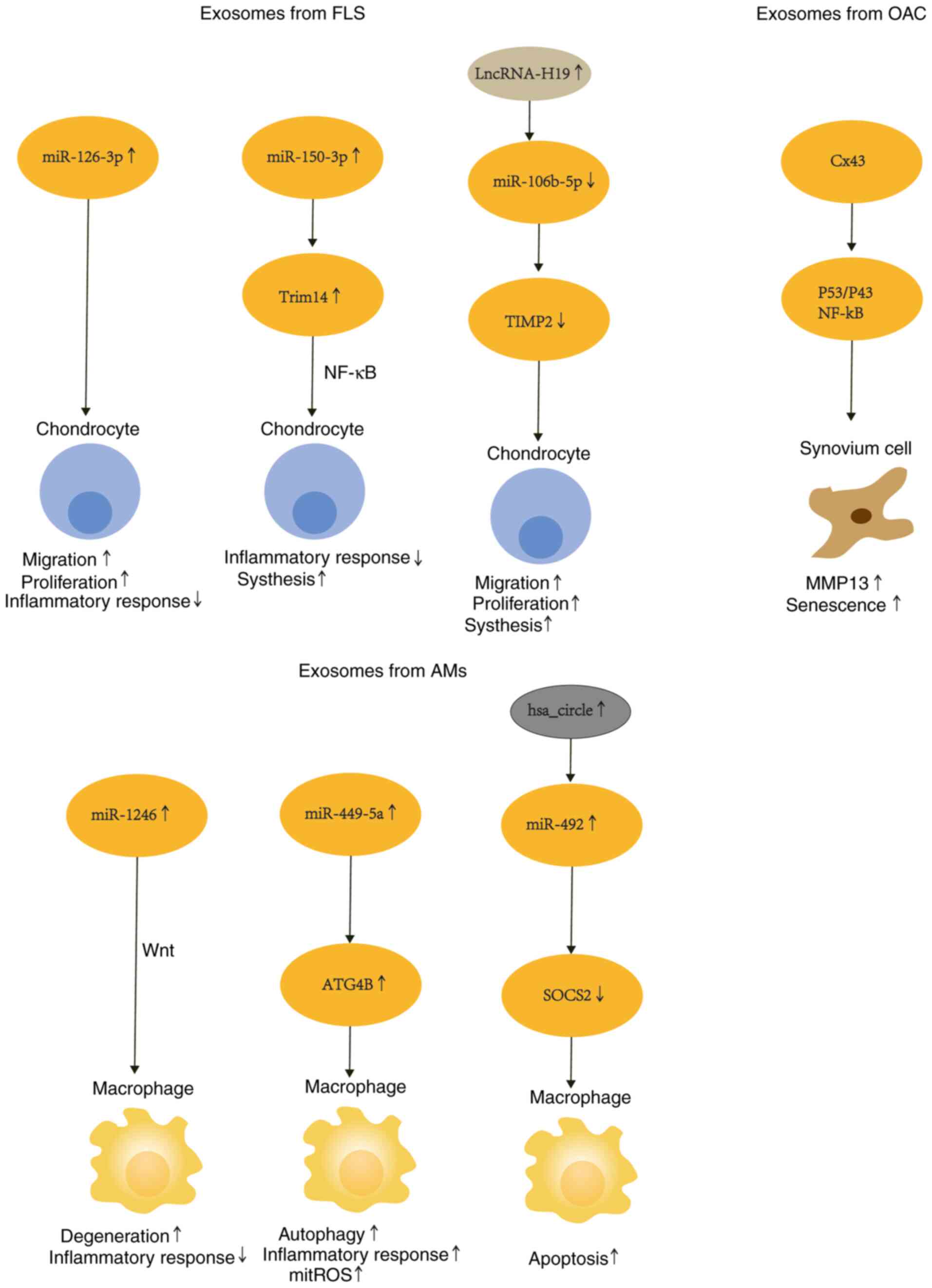
In chondrocyte-fibroblast crosstalk, it was observed
in a rat chondrocyte model that SFC secreted EVs rich in
miR-126-3p, promoted functional changes in chondrocytes, mainly
migration and proliferation, while inhibiting chondrocyte apoptosis
and expression of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α)
(103). Overexpression of
miR-150-3p was revealed to reduce the expression of Trim14 while
suppressing secretion of immune factors (NF-κB, IFN-β), leading to
a decrease in the concentration of inflammatory mediators (IL-1β,
IL-6, TNF-α), and anabolic factors (COL2, ACAN) (104). MiR-106b-5p was demonstrated to be
downregulated by lncRNA-H19 and inhibited TIMP2 expression, thereby
disrupting MMPs. In addition, in the in vitro chondrocyte
model, proliferation and migration were enhanced, and ECM synthesis
(increase in MMP13, ADAMTS5 and decrease in COL2A1, ACAN) was
weakened (61). Fibroblasts act on
chondrocytes via miRNA and regulate their secretion of ECM
degradation enzymes as well as pro-inflammatory factors by
affecting the phenotype of the cells, subsequently influencing the
progression of OA.
In macrophage and chondrocyte crosstalk, LPS and
IFN-γ stimulate macrophages after extracting EVs, and miR-1246
enriched in EV was shown to promote chondrocyte secretion of
proinflammatory mediators (IL-1β, IL-6, TNF-α) and MMPs by
inhibiting the expression of GSK3β and Axin2 through the
Wnt/β-catenin signaling pathway (105). Another study showed that LPS
stimulation of macrophage THP-1 downregulates hsa_circ_0005567
expression and upregulates miR-492 expression of chondrocytes,
inhibiting SOCS2 and promoting chondrocyte apoptosis (106). Furthermore, EVs were extracted
after IL-1β stimulation of chondrocytes and added to LPS-stimulated
macrophages, and these vesicles inhibited LPS-triggered autophagy
of macrophages by downregulating ATG4B via miR-449a-5p. Autophagy
inhibition has been revealed to exacerbate synovitis and promote OA
progression by increasing mitochondrial ROS (mitoROS) production
and promoting macrophage IL-1β secretion (100).
Macrophages stimulated to undergo M1 polarization
affect chondrocytes encoding proinflammatory factors and
ECM-degrading enzymes, as well as induce chondrocyte apoptosis and
oxidative stress via miRNA.
Notably, small extracellular vesicles derived from
OA-derived chondrocytes, containing the Cx43 protein, regulate the
epithelial-mesenchymal transition (EMT) signaling program through
NF-κB and ERK pathways, ultimately inducing the loss of a fully
differentiated phenotype and aging of synovial cells (107).
The mechanism of the interaction between exosomes
secreted by three types of cells is depicted in Fig. 3. Paracrine factors and exosomes are
summarized in Table I.
 | Table IParacrine factors and exosomes. |
Table I
Paracrine factors and exosomes.
| Phenotypic
changes | Interaction
mode | Donor cell | Recipient cell | Culture system | Mechanism | (Refs.) |
|---|
| Pro-inflammatory,
decomposing | Exosomes | Synovial
fibroblasts | Chondrocyte | Transwell
co-culture | FLS-derived
extracellular vesicle lncRNA H19 promotes cell viability and
migration, and prevents IL-1β-induced phenotypic changes in
chondrocytes by regulating miR-106b-5p and TIMP2 expression ECM
degradation in induced chondrocytes | (61) |
| Unknown | Cell contact | Chondrocyte | Synovial cells | Transwell
co-culture | The three kinds of
cells can establish intercellular connections and communicate
through gap junction channels to exchange some essential amino
acids, peptides and proteins (including calnexin, calreticulin or
CD44 antigen) and other substances | (96) |
| Pro-inflammatory,
decomposing | Paracrine | Synovial
fibroblasts | Chondrocyte | Transwell
co-culture | TNF-α produced by
chondrocytes upregulates the expression of metabolic factors in
FLS, while IL-6 derived from FLS plays an important role in
regulating MMP3 and MMP13 in chondrocytes | (37) |
| Pro-inflammatory,
decomposing | Paracrine | Chondrocyte | Macrophage | Conditional medium
cultivation | IL-1β activates
NF-κB in chondrocytes, inducing IL-6 secretion | (97) |
| Decomposing | Paracrine | Macrophage | Chondrocyte | Transwell
co-culture | Increased
production of pro-inflammatory molecules and expression of
chondrocyte catabolic factors in macrophage culture | (98) |
|
Pro-inflammatory | Paracrine | Macrophage | Chondrocyte | Transwell
co-culture | Increased release
of matrix metalloproteinases and proinflammatory mediators from
chondrocytes | (99) |
| Autophagy | Exosomes | Chondrocyte | Macrophage | Conditional
cultivation | Chondrocytes
secrete miR-449a-5p to inhibit autophagy of synovial macrophages by
inhibiting the expression of ATG4B | (100) |
| Pro-inflammatory,
M1 polarization | Paracrine | Macrophage | Chondrocyte | Conditional
cultivation | Reduced miR-224-5p
promotes the secretion of PTX3 by M1 polarized OA macrophages and
chondrocytes. The increased PTX3 promotes the polarization of M1 in
synovial macrophages and the secretion of inflammatory cytokines
that disrupt the homeostasis of chondrocytes, accelerating the
progression of OA | (101) |
| Aging and
hypertrophy | Unknown | Synovial cells | Chondrocyte | Conditional
cultivation | Synovial cells
secrete soluble factors to induce surface region Prg4
expression | (102) |
| Pro-inflammatory,
apoptosis | Exosomes | Synovial
fibroblasts | Chondrocyte | Conditional
cultivation |
SFC-miRNA-126-3p-Exos can inhibit
apoptosis, cell death, and related inflammation of
chondrocytes | (103) |
| Pro-inflammatory,
decomposing | Exosomes | Synovial
fibroblasts | Chondrocyte | Transwell
co-culture | H-FLS-EVs inhibit
the Trim14/NF-κB/IFN-β axis to regulate innate immune response,
thereby protecting chondrocyte function and maintaining joint
homeostasis | (104) |
| Pro-inflammatory,
decomposing | Exosomes | Macrophage | Chondrocyte | Conditional
cultivation | MiR-1246 inhibits
GSK3β and Axin2 expression, inducing activation of the
Wnt/β-catenin signaling pathway and promotion of the expression of
proinflammatory factors and matrix metalloproteinases in
chondrocytes | (105) |
| Apoptosis | Exosomes | Macrophage | Chondrocyte | Conditional
cultivation | Downregulation of
hsa_ circ_ 0005567 expression and upregulation of miR-492
expression in chondrocytes, induce inhibition of SOCS2 expression
and promotion of chondrocyte apoptosis | (106) |
5. Clinical application
MSCs are somatic cells that can self-renew and
differentiate in multiple directions. They are generally separated
from a variety of adult or neonatal tissues, such as bone marrow
(BM), adipose tissue (AD), placenta or umbilical cord (108). Recent evidence suggests an
‘ecological niche’ role for synovial membranes (SMs) as a rich
source of pluripotent MSCs capable of differentiating into a wide
spectrum of mature cells, including cartilage, bone, muscle, and AD
(108-110).
Through its paracrine signaling secretion, MSCs are
not only capable of differentiation into different cells, but also
have anti-inflammatory and immunosuppressive properties (111).
In OA, MSCs have a therapeutic function (112). MSCs derived from the umbilical
cord were administered intraarticularly to patients with active OA
(Phase I/II trial) by Matas et al (113). Soler et al used isolated
expanded autologous MSCs to treat knee OA, evaluating its
feasibility and effectiveness through pain scores and imaging
evidence. According to their findings, cell-based therapy exhibited
favorable tolerability, although some adverse effects were reported
(for example, mild arthralgia and gastrointestinal reactions)
(114). OA can therefore be
treated with MSCs, which secrete different factors (cytokines,
chemokines, growth factors, EVs) (115). Stem cells of different origins
have the potential to treat OA (116), and the present review describes
their mechanism of action in OA through their mode of action.
Paracrine secretion of MSCs
Proteomic analysis revealed common secreted proteins
of MSCs including BM-MSCs, AD-MSCs and SM-MSCs, classified by
function as anti-inflammatory, MMP inhibitory, ECM homeostasis, and
chondrocyte anti-death and promoting proliferation, suggesting that
secreted proteins may affect OA through several of these
aspects.
Proteomic data from BM-MSC, AD-MSC and SM-MSC
samples revealed that TSG-6 and TSP-1 proteins reduce the
concentration of inflammatory factors and MMPs by regulating the
NF-κB pathway, and enhance COL2 expression, which is involved in
cartilage repair and maintenance of cartilage homeostasis (117). Similarly, treatment with
12-Epi-Napelline, induced the metabolic secretion of
chondrocyte-repair-promoting growth factors by BM-MSCs through
modulation of the TGF-β/BMP pathway and shifted to differentiated
chondrocytes (118).
In terms of anti-inflammation and inhibition of
MMPs, intra-articularly injected AD-MSCs were revealed to inhibit
chondrocyte MMP-13 release by homing to the synovium and releasing
fluid factors with chondroprotective effects (chondrocyte
proliferation and chondrogenic matrix protection) (119). The cell mixture Nanofat is
extracted from AD, and Nanofat conditional medium treatment
increased chondrocyte viability and proliferation and reversed the
upregulation of the expression of catabolic markers and decreased
the expression of synthetic metabolic markers induced by IL-1β
(120). In addition,
intra-articular injections of SMUP cells secreted by PTX-3 induced
macrophage polarization to an M2 anti-inflammatory phenotype as
well as upregulated ARG-1 expression, attenuating osteoarthritic
destruction (121).
Placenta-derived MSCs (PDMSCs) were shown to enhance
chondrocyte proliferation and migration by paracrine means,
significantly restoring IL-1β-induced COL2, MMP13, ADAMTS4, ADAMTS5
and SOX9 aberrant gene expression, and chondrocyte COL2, MMP13 and
SOX9 aberrant protein expression (122).
Exosome secretion of MSCs
Exosomes are endocytosis-derived nanoscale vesicles
(30-140 nm) that play an important role in regenerative medicine
(123). They carry numerous
proteins and nucleic acids (124). KLF3-AS1, an exosome-derived
noncoding RNA derived from MSCs, was demonstrated to inhibit
autophagy and apoptosis in OA chondrocytes (125).
Different miRNAs have important therapeutic roles in
MSCs (126). Synovial MSC-derived
EVs contain different miRNAs. An in vivo study revealed that
overexpression of miR-31 downregulates KDM2A, increases chondrocyte
proliferation and migration, and attenuates cartilage damage and
inflammation (127). MiR-155-5p
overexpression was shown to prevent OA by increasing chondrocyte
proliferation and migration, attenuating apoptosis and targeting
Runx2(128). OA cartilage damage
was demonstrated to be ameliorated by miR-555A-26a-5p inhibition of
PTEN, which suppressed chondrocyte apoptosis and inflammation
(129). In addition, miR-130b-3p,
which originated from SM-MSCs, attenuated chondrocyte apoptosis and
ECM during OA degradation and exerted anti-inflammatory effects via
inhibition of the LRP12/AKT/β-catenin axis (130).
The EVs of BM-MSCs containing lncRNA-NEAT1 were
demonstrated to stimulate the Sesn2/Nrf2 axis through miRNA-122-5p
and stimulate chondrocyte proliferation and autophagy, but inhibit
their apoptosis (124). Notably,
parathyroid hormone (PTH) (1-32)
enhanced the treatment outcome of BM-MSC-derived exosomes on
chondrocyte regeneration by inhibiting pro-inflammatory cytokine
expression (131,132). Exosomes derived from BM-MSCs have
also been revealed to reduce OA by promoting M1 to M2 conversion of
synovial macrophages (116).
AD-MSCs act by regulating chondrocyte status and
macrophage polarization. On the one hand, exosomes secreted by
AD-MSCs promote cell proliferation and prevent chondrocyte
apoptosis via the lncRNA-KLF3-AS1/miR-206 axis (131). On the other hand, osteoarthritic
synovial fluid-treated AD-MSCs secrete factors and EV-embedded
miRNAs that play a role in M2 macrophage polarization and cartilage
repair (133).
Exosomes originated from OA chondrocytes have
significantly lower miR-92a-3p expression than those secreted by
normal chondrocytes. MiR-92a-3p was shown to inhibit the activity
of the 3'-UTR-containing reporter construct and directly target
WNT5A to suppress expression in MSCs and chondrocytes (134).
In conclusion, MSCs of different origins regulate
the pathological process of OA by promoting various phenotypic
changes in chondrocytes through exosomes or by influencing
macrophage polarization status to regulate the release of
pro-inflammatory cytokines. The mechanism of action of various MSCs
in secreting EVs is depicted Fig.
4. The mechanism of action of MSCs is summarized in Table II.
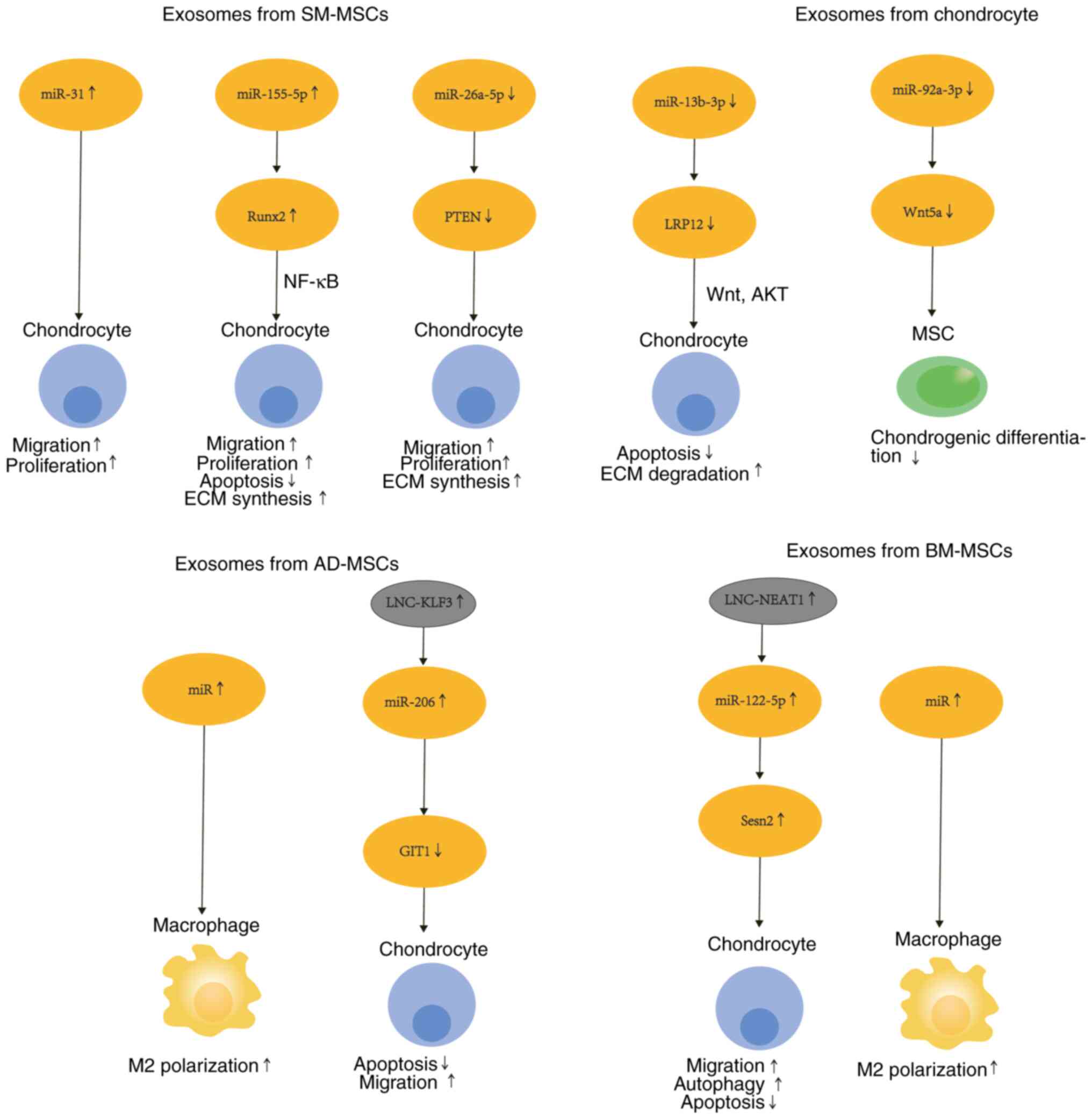 | Figure 4Mechanism of secretion of EVs by
different MSCs. EVs secreted by various MSCs contain microRNAs,
which undergo a series of changes in the phenotype of chondrocytes,
including migration, proliferation, changes in synthesis and
decomposition, apoptosis, autophagy and polarization. EVs,
extracellular vesicles; MSCs, mesenchymal stem cells; SM-MSCs, MSCs
derived from synovial membranes; AD-MSCs, MSCs derived from adipose
tissue; BM-MSCs, MSCs derived from bone marrow. |
 | Table IIMechanism of action of MSCs. |
Table II
Mechanism of action of MSCs.
| Molecule | Donor cell | Recipient cell | Phenotypic
changes | Mechanism | (Refs.) |
|---|
| Unknown | BM-MSCs | Macrophage | M2 polarization
↑ | Unknown | (116) |
| miR-122-5p | BM-MSCs | Chondrocyte | Proliferation↑
Autophagy↑ Apoptosis↓ | Sesn2/Nrf2 | (124) |
| miR-31 | SM-MSCs | Chondrocyte | Proliferation and
migration↑ |
KDM2A/E2F1/PTTG1 | (127) |
| miR-155-5p | SM-MSCs | Chondrocyte | Proliferation and
migration↑ ECM synthesis↑ Apoptosis↓ | Runx2 | (128) |
|
miR-555A-26a-5p | SM-MSCs | Chondrocyte | Apoptosis and
inflammation↓ | PTEN | (129) |
| microR-130b-3p | SM-MSCs | Chondrocyte | Apoptosis↓ ECM
degradation↓ |
LRP12/AKT/Wnt-β | (130) |
| miR-206 | AD-MSCs | Chondrocyte | Proliferation↑
Apoptosis↓ | GIT1 | (132) |
| Unknown | AD-MSCs | Macrophage | M2 polarization
↑ | Unknown | (133) |
| miR-92a-3p | Chondrocyte | MSCs | Unknown | WNT5A | (134) |
6. Conclusions
Synovial cell-chondrocyte interactions are important
in the osteoarthritic process. Functionally, the main effects are
through anti-inflammation, cell phenotype changes, ECM-cell
interactions, homeostasis and inhibition of degradation enzymes.
Therefore, it is important to further elucidate the mechanisms of
action between synoviocytes and chondrocytes and their effects on
synovitis and cartilage destruction. Immune cells begin to
activate, mainly through macrophage polarization, secreting some
pro-inflammatory factors as well as exosomes which act on
chondrocytes, thus influencing the pathological process of OA.
Fibroblasts, through invasive changes, act on chondrocytes.
Therefore, further research is required to elucidate the effects of
macrophages and fibroblasts on chondrocyte homeostasis.
scRNA-seq has confirmed the heterogeneity of cell
subsets. Analyzing the similarities and differences between
pathogenic and normal cell populations from aspects of cell
function, phenotypic changes and molecular regulation, will expand
knowledge on OA targeted therapy. In terms of treatment, it is
worth considering the restoration of joint architecture by
maintaining chondrocyte homeostasis and targeting the pathogenic
synoviocyte-chondrocyte axis. Single cell technology provides a new
means of studying the crosstalk mechanism of OA by elucidating the
communication of synoviocytes and chondrocytes. In order to combine
scRNA-seq analysis results with clinical treatment, it is necessary
to carry out in vivo experiments to verify the function
loss, such as inducing specific gene deletion in mouse OA models
and observing the pathological process of OA. It is also important
to elucidate how MSCs contribute to joint damage in OA. Integrating
electronic technology and in vivo studies may provide
thorough and comprehensive insights of the immune
cell-fibroblast-chondrocyte triad in OA, generating a molecular
basis for the development of effective therapeutic strategies aimed
at providing protection against structural damage and repair of
damaged joints.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Postgraduate
Research and Practice Innovation Program of Jiangsu Province (grant
no. SJCX22_1626).
Availability of data and materials
Not applicable.
Authors' contributions
BC wrote the original draft of the manuscript. ZC
and YS reviewed and edited the manuscript. JJ, GX, WZ and CW
created the figures, and reviewed and improved the language of this
manuscript. PX and ZC reviewed and edited the manuscript,
supervised the project and obtained funding. Data authentication is
not applicable. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sanchez-Lopez E, Coras R, Torres A, Lane
NE and Guma M: Synovial inflammation in osteoarthritis progression.
Nat Rev Rheumatol. 18:258–275. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Allen KD, Thoma LM and Golightly YM:
Epidemiology of osteoarthritis. Osteoarthritis Cartilage.
30:184–195. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Vina ER and Kwoh CK: Epidemiology of
osteoarthritis: Literature update. Curr Opin Rheumatol. 30:160–167.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nedunchezhiyan U, Varughese I, Sun AR, Wu
X, Crawford R and Prasadam I: Obesity, inflammation, and immune
system in osteoarthritis. Front Immunol. 13(907750)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang T and He C: Pro-inflammatory
cytokines: The link between obesity and osteoarthritis. Cytokine
Growth Factor Rev. 44:38–50. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang H, Cai D and Bai X: Macrophages
regulate the progression of osteoarthritis. Osteoarthritis
Cartilage. 28:555–561. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hugle T and Geurts J: What drives
osteoarthritis?-synovial versus subchondral bone pathology.
Rheumatology (Oxford). 56:1461–1471. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zeng N, Yan ZP, Chen XY and Ni GX:
Infrapatellar fat pad and knee osteoarthritis. Aging Dis.
11:1317–1328. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li Z, Huang Z and Bai L: Cell interplay in
osteoarthritis. Front Cell Dev Biol. 9(720477)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wenham CY and Conaghan PG: The role of
synovitis in osteoarthritis. Ther Adv Musculoskelet Dis. 2:349–359.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wu CL, Harasymowicz NS, Klimak MA, Collins
KH and Guilak F: The role of macrophages in osteoarthritis and
cartilage repair. Osteoarthritis Cartilage. 28:544–554.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Klein-Wieringa IR, de Lange-Brokaar BJ,
Yusuf E, Andersen SN, Kwekkeboom JC, Kroon HM, van Osch GJ,
Zuurmond AM, Stojanovic-Susulic V, Nelissen RG, et al: Inflammatory
cells in patients with endstage knee osteoarthritis: A Comparison
between the Synovium and the Infrapatellar Fat Pad. J Rheumatol.
43:771–778. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Knab K, Chambers D and Kronke G: Synovial
macrophage and fibroblast heterogeneity in joint homeostasis and
inflammation. Front Med (Lausanne). 9(862161)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Scanzello CR and Goldring SR: The role of
synovitis in osteoarthritis pathogenesis. Bone. 51:249–257.
2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hui AY, McCarty WJ, Masuda K, Firestein GS
and Sah RL: A systems biology approach to synovial joint
lubrication in health, injury, and disease. Wiley Interdiscip Rev
Syst Biol Med. 4:15–37. 2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gleason B, Chisari E and Parvizi J:
Osteoarthritis can also start in the gut: The gut-joint axis.
Indian J Orthop. 56:1150–1155. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mathiessen A and Conaghan PG: Synovitis in
osteoarthritis: Current understanding with therapeutic
implications. Arthritis Res Ther. 19(18)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sellam J and Berenbaum F: The role of
synovitis in pathophysiology and clinical symptoms of
osteoarthritis. Nat Rev Rheumatol. 6:625–635. 2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mustonen AM and Nieminen P: Extracellular
vesicles and their potential significance in the pathogenesis and
treatment of osteoarthritis. Pharmaceuticals (Basel).
14(315)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Benito MJ, Veale DJ, FitzGerald O, van den
Berg WB and Bresnihan B: Synovial tissue inflammation in early and
late osteoarthritis. Ann Rheum Dis. 64:1263–1267. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zeng C, Li YS and Lei GH: Synovitis in
knee osteoarthritis: A precursor or a concomitant feature? Ann
Rheum Dis. 74(e58)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Burke CJ, Alizai H, Beltran LS and Regatte
RR: MRI of synovitis and joint fluid. J Magn Reson Imaging.
49:1512–1527. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yoshimi R, Hama M, Takase K, Ihata A,
Kishimoto D, Terauchi K, Watanabe R, Uehara T, Samukawa S, Ueda A,
et al: Ultrasonography is a potent tool for the prediction of
progressive joint destruction during clinical remission of
rheumatoid arthritis. Mod Rheumatol. 23:456–465. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Bondeson J, Blom AB, Wainwright S, Hughes
C, Caterson B and van den Berg WB: The role of synovial macrophages
and macrophage-produced mediators in driving inflammatory and
destructive responses in osteoarthritis. Arthritis Rheum.
62:647–657. 2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sokolove J and Lepus CM: Role of
inflammation in the pathogenesis of osteoarthritis: Latest findings
and interpretations. Ther Adv Musculoskelet Dis. 5:77–94.
2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mapp PI and Walsh DA: Mechanisms and
targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev
Rheumatol. 8:390–398. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li W, Lin J, Wang Z, Ren S, Wu X, Yu F,
Weng J and Zeng H: Bevacizumab tested for treatment of knee
osteoarthritis via inhibition of synovial vascular hyperplasia in
rabbits. J Orthop Translat. 19:38–46. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Oehler S, Neureiter D, Meyer-Scholten C
and Aigner T: Subtyping of osteoarthritic synoviopathy. Clin Exp
Rheumatol. 20:633–640. 2002.PubMed/NCBI
|
|
29
|
Bhat S, Tripathi A and Kumar A:
Supermacroprous chitosan-agarose-gelatin cryogels: In vitro
characterization and in vivo assessment for cartilage tissue
engineering. J R Soc Interface. 8:540–554. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Amr M, Mallah A, Yasmeen S, Van Wie B,
Gozen A, Mendenhall J and Abu-Lail NI: From chondrocytes to
chondrons, maintenance of phenotype and matrix production in a
composite 3D hydrogel scaffold. Gels. 8(90)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The International Society for Cellular
Therapy position statement. Cytotherapy. 8:315–317. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chan CM, Macdonald CD, Litherland GJ,
Wilkinson DJ, Skelton A, Europe-Finner GN and Rowan AD:
Cytokine-induced MMP13 expression in human chondrocytes is
dependent on activating transcription factor 3 (ATF3) Regulation. J
Biol Chem. 292:1625–1636. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sandell LJ and Aigner T: Articular
cartilage and changes in arthritis. An introduction: Cell biology
of osteoarthritis. Arthritis Res. 3:107–113. 2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Otsuki S, Taniguchi N, Grogan SP, D'Lima
D, Kinoshita M and Lotz M: Expression of novel extracellular
sulfatases Sulf-1 and Sulf-2 in normal and osteoarthritic articular
cartilage. Arthritis Res Ther. 10(R61)2008.PubMed/NCBI View
Article : Google Scholar
|
|
35
|
Lian C, Wang X, Qiu X, Wu Z, Gao B, Liu L,
Liang G, Zhou H, Yang X, Peng Y, et al: Collagen type II suppresses
articular chondrocyte hypertrophy and osteoarthritis progression by
promoting integrin β1-SMAD1 interaction. Bone Res.
7(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu-Bryan R: Synovium and the innate
inflammatory network in osteoarthritis progression. Curr Rheumatol
Rep. 15(323)2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Huh YH, Lee G, Song WH, Koh JT and Ryu JH:
Crosstalk between FLS and chondrocytes is regulated by
HIF-2α-mediated cytokines in arthritis. Exp Mol Med.
47(e197)2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ayral X, Pickering EH, Woodworth TG,
Mackillop N and Dougados M: Synovitis: A potential predictive
factor of structural progression of medial tibiofemoral knee
osteoarthritis-results of a 1 year longitudinal arthroscopic study
in 422 patients. Osteoarthritis Cartilage. 13:361–367.
2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Roemer FW, Guermazi A, Felson DT, Niu J,
Nevitt MC, Crema MD, Lynch JA, Lewis CE, Torner J and Zhang Y:
Presence of MRI-detected joint effusion and synovitis increases the
risk of cartilage loss in knees without osteoarthritis at 30-month
follow-up: The MOST study. Ann Rheum Dis. 70:1804–1809.
2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang M, Tan G, Jiang H, Liu A, Wu R, Li J,
Sun Z, Lv Z, Sun W and Shi D: Molecular crosstalk between articular
cartilage, meniscus, synovium, and subchondral bone in
osteoarthritis. Bone Joint Res. 11:862–872. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lu Y, Liu L, Pan J, Luo B, Zeng H, Shao Y,
Zhang H, Guan H, Guo D, Zeng C, et al: MFG-E8 regulated by
miR-99b-5p protects against osteoarthritis by targeting chondrocyte
senescence and macrophage reprogramming via the NF-κB pathway. Cell
Death Dis. 12(533)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li M, Yin H, Yan Z, Li H, Wu J, Wang Y,
Wei F, Tian G, Ning C, Li H, et al: The immune microenvironment in
cartilage injury and repair. Acta Biomater. 140:23–42.
2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li X, Liao Z, Deng Z, Chen N and Zhao L:
Combining bulk and single-cell RNA-sequencing data to reveal gene
expression pattern of chondrocytes in the osteoarthritic knee.
Bioengineered. 12:997–1007. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Robinson WH, Lepus CM, Wang Q, Raghu H,
Mao R, Lindstrom TM and Sokolove J: Low-grade inflammation as a key
mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol.
12:580–592. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhu X, Lee CW, Xu H, Wang YF, Yung PSH,
Jiang Y and Lee OK: Phenotypic alteration of macrophages during
osteoarthritis: A systematic review. Arthritis Res Ther.
23(110)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Woodell-May JE and Sommerfeld SD: Role of
inflammation and the immune system in the progression of
osteoarthritis. J Orthop Res. 38:253–257. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Newton K and Dixit VM: Signaling in innate
immunity and inflammation. Cold Spring Harb Perspect Biol.
4(a006049)2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kawai T and Akira S: The role of
pattern-recognition receptors in innate immunity: Update on
Toll-like receptors. Nat Immunol. 11:373–384. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Estrada McDermott J, Pezzanite L, Goodrich
L, Santangelo K, Chow L, Dow S and Wheat W: Role of innate immunity
in initiation and progression of osteoarthritis, with emphasis on
horses. Animals (Basel). 11(3247)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhao X, Zhao Y, Sun X, Xing Y, Wang X and
Yang Q: Immunomodulation of MSCs and MSC-Derived extracellular
vesicles in osteoarthritis. Front Bioeng Biotechnol.
8(575057)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Davies LC and Taylor PR: Tissue-resident
macrophages: Then and now. Immunology. 144:541–548. 2015.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Fernandes TL, Gomoll AH, Lattermann C,
Hernandez AJ, Bueno DF and Amano MT: Macrophage: A potential target
on cartilage regeneration. Front Immunol. 11(111)2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang H, Zhang H, Fan K, Zhang D, Hu A,
Zeng X, Liu YL, Tan G and Wang H: Frugoside delays osteoarthritis
progression via inhibiting miR-155-modulated synovial macrophage M1
polarization. Rheumatology (Oxford). 60:4899–4909. 2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mahon OR, Kelly DJ, McCarthy GM and Dunne
A: Osteoarthritis-associated basic calcium phosphate crystals alter
immune cell metabolism and promote M1 macrophage polarization.
Osteoarthritis Cartilage. 28:603–612. 2020.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Chen J, Chen S, Cai D, Wang Q and Qin J:
The role of Sirt6 in osteoarthritis and its effect on macrophage
polarization. Bioengineered. 13:9677–9689. 2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lee CH, Chiang CF, Kuo FC, Su SC, Huang
CL, Liu JS, Lu CH, Hsieh CH, Wang CC, Lee CH and Shen PH:
High-Molecular-Weight hyaluronic acid inhibits IL-1β-Induced
synovial inflammation and macrophage polarization through the
GRP78-NF-κB signaling pathway. Int J Mol Sci.
22(11917)2021.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhen J, Chen X, Mao Y, Xie X, Chen X, Xu W
and Zhang S: GLX351322, a Novel NADPH oxidase 4 inhibitor,
attenuates TMJ osteoarthritis by inhibiting the ROS/MAPK/NF-κB
signaling pathways. Oxid Med Cell Longev.
2023(1952348)2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Zhou H, Shen X, Yan C, Xiong W, Ma Z, Tan
Z, Wang J, Li Y, Liu J, Duan A and Liu F: Extracellular vesicles
derived from human umbilical cord mesenchymal stem cells alleviate
osteoarthritis of the knee in mice model by interacting with METTL3
to reduce m6A of NLRP3 in macrophage. Stem Cell Res Ther.
13(322)2022.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Maglaviceanu A, Wu B and Kapoor M:
Fibroblast-like synoviocytes: Role in synovial fibrosis associated
with osteoarthritis. Wound Repair Regen. 29:642–649.
2021.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Bao J, Yan W, Xu K, Chen M, Chen Z, Ran J,
Xiong Y and Wu L: Oleanolic acid decreases IL-1β-Induced activation
of fibroblast-like synoviocytes via the SIRT3-NF-κB axis in
osteoarthritis. Oxid Med Cell Longev. 2020(7517219)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Tan F, Wang D and Yuan Z: The
fibroblast-like synoviocyte derived exosomal long non-coding RNA
H19 alleviates osteoarthritis progression through the
miR-106b-5p/TIMP2 Axis. Inflammation. 43:1498–1509. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Fernandes JC, Martel-Pelletier J and
Pelletier JP: The role of cytokines in osteoarthritis
pathophysiology. Biorheology. 39:237–246. 2002.PubMed/NCBI
|
|
63
|
Pap T, Dankbar B, Wehmeyer C, Korb-Pap A
and Sherwood J: Synovial fibroblasts and articular tissue
remodelling: Role and mechanisms. Semin Cell Dev Biol. 101:140–145.
2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Mehana EE, Khafaga AF and El-Blehi SS: The
role of matrix metalloproteinases in osteoarthritis pathogenesis:
An updated review. Life Sci. 234(116786)2019.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Zheng Z, Xiang S, Wang Y, Dong Y, Li Z,
Xiang Y, Bian Y, Feng B, Yang B and Weng X: NR4A1 promotes
TNF-α-induced chondrocyte death and migration injury via activating
the AMPK/Drp1/mitochondrial fission pathway. Int J Mol Med.
45:151–161. 2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Smith MD: The normal synovium. Open
Rheumatol J. 5:100–106. 2011.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Silverstein AM, Stefani RM, Sobczak E,
Tong EL, Attur MG, Shah RP, Bulinski JC, Ateshian GA and Hung CT:
Toward understanding the role of cartilage particulates in synovial
inflammation. Osteoarthritis Cartilage. 25:1353–1361.
2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Estell EG, Silverstein AM, Stefani RM, Lee
AJ, Murphy LA, Shah RP, Ateshian GA and Hung CT: Cartilage wear
particles induce an inflammatory response similar to cytokines in
human fibroblast-like synoviocytes. J Orthop Res. 37:1979–1987.
2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Cao X, Wu S, Wang X, Huang J, Zhang W and
Liang C: Receptor tyrosine kinase C-kit promotes a destructive
phenotype of FLS in osteoarthritis via intracellular EMT signaling.
Mol Med. 29(38)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Li Q, Wen Y, Wang L, Chen B, Chen J, Wang
H and Chen L: Hyperglycemia-induced accumulation of advanced
glycosylation end products in fibroblast-like synoviocytes promotes
knee osteoarthritis. Exp Mol Med. 53:1735–1747. 2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Damerau A, Kirchner M, Pfeiffenberger M,
Ehlers L, Do Nguyen DH, Mertins P, Bartek B, Maleitzke T, Palmowski
Y, Hardt S, et al: Metabolic reprogramming of synovial fibroblasts
in osteoarthritis by inhibition of pathologically overexpressed
pyruvate dehydrogenase kinases. Metab Eng. 72:116–132.
2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Han D, Fang Y, Tan X, Jiang H, Gong X,
Wang X, Hong W, Tu J and Wei W: The emerging role of
fibroblast-like synoviocytes-mediated synovitis in osteoarthritis:
An update. J Cell Mol Med. 24:9518–9532. 2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Chen X, Gong W, Shao X, Shi T, Zhang L,
Dong J, Shi Y, Shen S, Qin J, Jiang Q and Guo B: METTL3-mediated
m6A modification of ATG7 regulates autophagy-GATA4 axis
to promote cellular senescence and osteoarthritis progression. Ann
Rheum Dis. 81:87–99. 2022.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Endisha H, Datta P, Sharma A, Nakamura S,
Rossomacha E, Younan C, Ali SA, Tavallaee G, Lively S, Potla P, et
al: MicroRNA-34a-5p promotes joint destruction during
osteoarthritis. Arthritis Rheumatol. 73:426–439. 2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Chen Z, Lin CX, Song B, Li CC, Qiu JX, Li
SX, Lin SP, Luo WQ, Fu Y, Fang GB, et al: Spermidine activates RIP1
deubiquitination to inhibit TNF-α-induced NF-κB/p65 signaling
pathway in osteoarthritis. Cell Death Dis. 11(503)2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Thomson A and Hilkens CMU: Synovial
macrophages in osteoarthritis: The key to understanding
pathogenesis? Front Immunol. 12(678757)2021.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Zheng L, Zhang Z, Sheng P and Mobasheri A:
The role of metabolism in chondrocyte dysfunction and the
progression of osteoarthritis. Ageing Res Rev.
66(101249)2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Duan L, Liang Y, Xu X, Xiao Y and Wang D:
Recent progress on the role of miR-140 in cartilage matrix
remodelling and its implications for osteoarthritis treatment.
Arthritis Res Ther. 22(194)2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Charlier E, Deroyer C, Ciregia F, Malaise
O, Neuville S, Plener Z, Malaise M and de Seny D: Chondrocyte
dedifferentiation and osteoarthritis (OA). Biochem Pharmacol.
165:49–65. 2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Zhang Y, Vasheghani F, Li YH, Blati M,
Simeone K, Fahmi H, Lussier B, Roughley P, Lagares D, Pelletier JP,
et al: Cartilage-specific deletion of mTOR upregulates autophagy
and protects mice from osteoarthritis. Ann Rheum Dis. 74:1432–1440.
2015.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Ji Q, Zheng Y, Zhang G, Hu Y, Fan X, Hou
Y, Wen L, Li L, Xu Y, Wang Y and Tang F: Single-cell RNA-seq
analysis reveals the progression of human osteoarthritis. Ann Rheum
Dis. 78:100–110. 2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Hu X, Li Z, Ji M, Lin Y, Chen Y and Lu J:
Identification of cellular heterogeneity and immunogenicity of
chondrocytes via single-cell RNA sequencing technique in human
osteoarthritis. Front Pharmacol. 13(1004766)2022.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Gao H, Di J, Yin M, He T, Wu D, Chen Z, Li
S, He L and Rong L: Identification of chondrocyte subpopulations in
osteoarthritis using single-cell sequencing analysis. Gene.
852(147063)2023.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Jiang Z, Liang G, Xiao Y, Qin T, Chen X,
Wu E, Ma Q and Wang Z: Targeting the SLIT/ROBO pathway in tumor
progression: Molecular mechanisms and therapeutic perspectives.
Ther Adv Med Oncol. 11(1758835919855238)2019.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Ludin A, Sela JJ, Schroeder A, Samuni Y,
Nitzan DW and Amir G: Injection of vascular endothelial growth
factor into knee joints induces osteoarthritis in mice.
Osteoarthritis Cartilage. 21:491–497. 2013.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Yuan C, Pan Z, Zhao K, Li J, Sheng Z, Yao
X, Liu H, Zhang X, Yang Y, Yu D, et al: Classification of four
distinct osteoarthritis subtypes with a knee joint tissue
transcriptome atlas. Bone Res. 8(38)2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Chou CH, Jain V, Gibson J, Attarian DE,
Haraden CA, Yohn CB, Laberge RM, Gregory S and Kraus VB: Synovial
cell cross-talk with cartilage plays a major role in the
pathogenesis of osteoarthritis. Sci Rep. 10(10868)2020.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Li Z, Wang Y, Xiao K, Xiang S, Li Z and
Weng X: Emerging role of exosomes in the joint diseases. Cell
Physiol Biochem. 47:2008–2017. 2018.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Kolhe R, Hunter M, Liu S, Jadeja RN,
Pundkar C, Mondal AK, Mendhe B, Drewry M, Rojiani MV, Liu Y, et al:
Gender-specific differential expression of exosomal miRNA in
synovial fluid of patients with osteoarthritis. Sci Rep.
7(2029)2017.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Gordon S: Pattern recognition receptors:
Doubling up for the innate immune response. Cell. 111:927–930.
2002.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Nefla M, Holzinger D, Berenbaum F and
Jacques C: The danger from within: Alarmins in arthritis. Nat Rev
Rheumatol. 12:669–683. 2016.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Knights AJ, Farrell EC, Ellis OM, Lammlin
L, Junginger LM, Rzeczycki PM, Bergman RF, Pervez R, Cruz M, Knight
E, et al: Synovial fibroblasts assume distinct functional
identities and secrete R-spondin 2 in osteoarthritis. Ann Rheum
Dis. 82:272–282. 2023.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Takegami Y, Ohkawara B, Ito M, Masuda A,
Nakashima H, Ishiguro N and Ohno K: R-spondin 2 facilitates
differentiation of proliferating chondrocytes into hypertrophic
chondrocytes by enhancing Wnt/β-catenin signaling in endochondral
ossification. Biochem Biophys Res Commun. 473:255–264.
2016.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Sun Y, Zuo Z and Kuang Y: An emerging
target in the battle against osteoarthritis: Macrophage
polarization. Int J Mol Sci. 21(8513)2020.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Zhang H, Lin C, Zeng C, Wang Z, Wang H, Lu
J, Liu X, Shao Y, Zhao C, Pan J, et al: Synovial macrophage M1
polarisation exacerbates experimental osteoarthritis partially
through R-spondin-2. Ann Rheum Dis. 77:1524–1534. 2018.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Carpintero-Fernandez P, Gago-Fuentes R,
Wang HZ, Fonseca E, Caeiro JR, Valiunas V, Brink PR and Mayan MD:
Intercellular communication via gap junction channels between
chondrocytes and bone cells. Biochim Biophys Acta Biomembr.
1860:2499–2505. 2018.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Limagne E, Lancon A, Delmas D,
Cherkaoui-Malki M and Latruffe N: Resveratrol interferes with
IL1-β-Induced pro-inflammatory paracrine interaction between
primary chondrocytes and macrophages. Nutrients.
8(280)2016.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Samavedi S, Diaz-Rodriguez P, Erndt-Marino
JD and Hahn MS: A three-dimensional chondrocyte-macrophage
coculture system to probe inflammation in experimental
osteoarthritis. Tissue Eng Part A. 23:101–114. 2017.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Hamasaki M, Terkawi MA, Onodera T, Homan K
and Iwasaki N: A novel cartilage fragments stimulation model
revealed that macrophage inflammatory response causes an
upregulation of catabolic factors of chondrocytes in vitro.
Cartilage. 12:354–361. 2021.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Ni Z, Kuang L, Chen H, Xie Y, Zhang B,
Ouyang J, Wu J, Zhou S, Chen L, Su N, et al: The exosome-like
vesicles from osteoarthritic chondrocyte enhanced mature IL-1β
production of macrophages and aggravated synovitis in
osteoarthritis. Cell Death Dis. 10(522)2019.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Yin J, Zeng H, Fan K, Xie H, Shao Y, Lu Y,
Zhu J, Yao Z, Liu L, Zhang H, et al: Pentraxin 3 regulated by
miR-224-5p modulates macrophage reprogramming and exacerbates
osteoarthritis associated synovitis by targeting CD32. Cell Death
Dis. 13(567)2022.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Chau M, Dou Z, Baroncelli M, Landman EB,
Bendre A, Kanekiyo M, Gkourogianni A, Barnes K, Ottosson L and
Nilsson O: The synovial microenvironment suppresses chondrocyte
hypertrophy and promotes articular chondrocyte differentiation. NPJ
Regen Med. 7(51)2022.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Zhou Y, Ming J, Li Y, Li B, Deng M, Ma Y,
Chen Z, Zhang Y, Li J and Liu S: Exosomes derived from
miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte
inflammation and cartilage degradation in a rat model of
osteoarthritis. Cell Death Discov. 7(37)2021.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Wang H, Shu J, Zhang C, Wang Y, Shi R,
Yang F and Tang X: Extracellular Vesicle-Mediated miR-150-3p
delivery in joint homeostasis: A potential treatment for
osteoarthritis? Cells. 11(2766)2022.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Peng S, Yan Y, Li R, Dai H and Xu J:
Extracellular vesicles from M1-polarized macrophages promote
inflammation in the temporomandibular joint via miR-1246 activation
of the Wnt/β-catenin pathway. Ann N Y Acad Sci. 1503:48–59.
2021.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Zhang J, Cheng F, Rong G, Tang Z and Gui
B: Circular RNA hsa_circ_0005567 overexpression promotes M2 type
macrophage polarization through miR-492/SOCS2 axis to inhibit
osteoarthritis progression. Bioengineered. 12:8920–8930.
2021.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Varela-Eirin M, Carpintero-Fernandez P,
Guitian-Caamano A, Varela-Vazquez A, Garcia-Yuste A,
Sanchez-Temprano A, Bravo-Lopez SB, Yanez-Cabanas J, Fonseca E,
Largo R, et al: Extracellular vesicles enriched in connexin 43
promote a senescent phenotype in bone and synovial cells
contributing to osteoarthritis progression. Cell Death Dis.
13(681)2022.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Baboolal TG, Mastbergen SC, Jones E,
Calder SJ, Lafeber FP and McGonagle D: Synovial fluid hyaluronan
mediates MSC attachment to cartilage, a potential novel mechanism
contributing to cartilage repair in osteoarthritis using knee joint
distraction. Ann Rheum Dis. 75:908–915. 2016.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Pajarinen J, Lin T, Gibon E, Kohno Y,
Maruyama M, Nathan K, Lu L, Yao Z and Goodman SB: Mesenchymal stem
cell-macrophage crosstalk and bone healing. Biomaterials.
196:80–89. 2019.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Yang Z, Schmitt JF and Lee EH:
Immunohistochemical analysis of human mesenchymal stem cells
differentiating into chondrogenic, osteogenic, and adipogenic
lineages. Methods Mol Biol. 698:353–366. 2011.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Chang YH, Wu KC, Harn HJ, Lin SZ and Ding
DC: Exosomes and stem cells in degenerative disease diagnosis and
therapy. Cell Transplant. 27:349–363. 2018.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Maumus M, Pers YM, Ruiz M, Jorgensen C and
Noel D: Mesenchymal stem cells and regenerative medicine: Future
perspectives in osteoarthritis. Med Sci (Paris). 34:1092–1099.
2018.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
113
|
Matas J, Orrego M, Amenabar D, Infante C,
Tapia-Limonchi R, Cadiz MI, Alcayaga-Miranda F, Gonzalez PL, Muse
E, Khoury M, et al: Umbilical cord-derived mesenchymal stromal
cells (MSCs) for knee osteoarthritis: Repeated MSC Dosing Is
Superior to a Single MSC dose and to hyaluronic acid in a
controlled randomized phase I/II Trial. Stem Cells Transl Med.
8:215–224. 2019.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Soler R, Orozco L, Munar A, Huguet M,
Lopez R, Vives J, Coll R, Codinach M and Garcia-Lopez J: Final
results of a phase I-II trial using ex vivo expanded autologous
Mesenchymal Stromal Cells for the treatment of osteoarthritis of
the knee confirming safety and suggesting cartilage regeneration.
Knee. 23:647–654. 2016.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Ferraris VA: How do cells talk to each
other?: Paracrine factors secreted by mesenchymal stromal cells. J
Thorac Cardiovasc Surg. 151:849–851. 2016.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Zhang J, Rong Y, Luo C and Cui W: Bone
marrow mesenchymal stem cell-derived exosomes prevent
osteoarthritis by regulating synovial macrophage polarization.
Aging (Albany NY). 12:25138–25152. 2020.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Lee Y, Park YS, Choi NY, Kim YI and Koh
YG: Proteomic analysis reveals commonly secreted proteins of
mesenchymal stem cells derived from bone marrow, adipose tissue,
and synovial membrane to show potential for cartilage regeneration
in knee osteoarthritis. Stem Cells Int.
2021(6694299)2021.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Peng J, Mao Z, Liu Y, Tian Y, Leng Q, Gu J
and Tan R: 12-Epi-Napelline regulated TGF-β/BMP signaling pathway
mediated by BMSCs paracrine acceleration against osteoarthritis.
Int Immunopharmacol. 113(Pt A)(109307)2022.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Kuroda K, Kabata T, Hayashi K, Maeda T,
Kajino Y, Iwai S, Fujita K, Hasegawa K, Inoue D, Sugimoto N and
Tsuchiya H: The paracrine effect of adipose-derived stem cells
inhibits osteoarthritis progression. BMC Musculoskelet Disord.
16(236)2015.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Chen Z, Ge Y, Zhou L, Li T, Yan B, Chen J,
Huang J, Du W, Lv S, Tong P and Shan L: Pain relief and cartilage
repair by Nanofat against osteoarthritis: Preclinical and clinical
evidence. Stem Cell Res Ther. 12(477)2021.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Lee M, Kim GH, Kim M, Seo JM, Kim YM, Seon
MR, Um S, Choi SJ, Oh W, Song BR and Jin HJ: PTX-3 secreted by
intra-articular-injected SMUP-Cells reduces pain in an
osteoarthritis rat model. Cells. 10(2420)2021.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Fan M, Zhang J, Zhou L, Chen Z, Bao R,
Zheng L, Tong P, Ma Y and Shan L: Intra-articular injection of
placental mesenchymal stromal cells ameliorates pain and cartilage
anabolism/catabolism in knee osteoarthritis. Front Pharmacol.
13(983850)2022.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Ni Z, Zhou S, Li S, Kuang L, Chen H, Luo
X, Ouyang J, He M, Du X and Chen L: Exosomes: Roles and therapeutic
potential in osteoarthritis. Bone Res. 8(25)2020.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Zhang S and Jin Z: Bone mesenchymal stem
cell-derived extracellular vesicles containing long noncoding RNA
NEAT1 relieve osteoarthritis. Oxid Med Cell Longev.
2022(5517648)2022.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Wen C, Lin L, Zou R, Lin F and Liu Y:
Mesenchymal stem cell-derived exosome mediated long non-coding RNA
KLF3-AS1 represses autophagy and apoptosis of chondrocytes in
osteoarthritis. Cell Cycle. 21:289–303. 2022.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Bao C and He C: The role and therapeutic
potential of MSC-derived exosomes in osteoarthritis. Arch Biochem
Biophys. 710(109002)2021.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Wang K, Li F, Yuan Y, Shan L, Cui Y, Qu J
and Lian F: Synovial mesenchymal stem cell-derived EV-Packaged
miR-31 downregulates histone demethylase KDM2A to prevent knee
osteoarthritis. Mol Ther Nucleic Acids. 22:1078–1091.
2020.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Wang Z, Yan K, Ge G, Zhang D, Bai J, Guo
X, Zhou J, Xu T, Xu M, Long X, et al: Exosomes derived from
miR-155-5p-overexpressing synovial mesenchymal stem cells prevent
osteoarthritis via enhancing proliferation and migration,
attenuating apoptosis, and modulating extracellular matrix
secretion in chondrocytes. Cell Biol Toxicol. 37:85–96.
2021.PubMed/NCBI View Article : Google Scholar
|
|
129
|
Lu L, Wang J, Fan A, Wang P, Chen R, Lu L
and Yin F: Synovial mesenchymal stem cell-derived extracellular
vesicles containing microRN555A-26a-5p ameliorate cartilage damage
of osteoarthritis. J Gene Med. 23(e3379)2021.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Zeng Z, Dai Y, Deng S, Zou S, Dou T and
Wei F: Synovial mesenchymal stem cell-derived extracellular
vesicles alleviate chondrocyte damage during osteoarthritis through
microRNA-130b-3p-mediated inhibition of the LRP12/AKT/β-catenin
axis. Immunopharmacol Immunotoxicol. 44:247–260. 2022.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Shao LT, Luo L, Qiu JH and Deng DYB: PTH
(1-34) enhances the therapeutic effect of bone marrow mesenchymal
stem cell-derived exosomes by inhibiting proinflammatory cytokines
expression on OA chondrocyte repair in vitro. Arthritis Res Ther.
24(96)2022.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Liu Y, Lin L, Zou R, Wen C, Wang Z and Lin
F: MSC-derived exosomes promote proliferation and inhibit apoptosis
of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in
osteoarthritis. Cell Cycle. 17:2411–2422. 2018.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Ragni E, Colombini A, Vigano M, Libonati
F, Perucca Orfei C, Zagra L and de Girolamo L: Cartilage protective
and immunomodulatory features of osteoarthritis synovial
fluid-treated adipose-derived mesenchymal stem cells secreted
factors and extracellular vesicles-embedded miRNAs. Cells.
10(1072)2021.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Mao G, Zhang Z, Hu S, Zhang Z, Chang Z,
Huang Z, Liao W and Kang Y: Exosomes derived from
miR-92a-3p-overexpressing human mesenchymal stem cells enhance
chondrogenesis and suppress cartilage degradation via targeting
WNT5A. Stem Cell Res Ther. 9(247)2018.PubMed/NCBI View Article : Google Scholar
|