Introduction
Rectal cancer is a common malignant tumor of the
digestive tract, and low rectal cancer accounts for approximately
70% of all rectal cancers (1).
During the previous three decades, the limits of anus preservation
have been persistently surpassed owing to the popularization and
promotion of total mesorectal excision, the development of
laparoscopic technology, the use of endoluminal cutting and
stapler, and continuous exploration and innovation of surgeons,
accompanied by marked improvements in post-surgical survival period
and quality of life for low rectal cancer. With the extensive
application of laparoscopy and double staplers in rectal cancer
surgery, low anastomosis and mechanical anastomosis are
increasingly common in rectal cancer surgery (2,3).
Dixon surgery, also known as transabdominal radical resection of
rectal cancer, belongs to the category of low anterior resection of
the rectum and is the most frequently employed radical resection
surgery for rectal cancer, primarily suitable for tumors located ≥6
cm from the anal margin (4).
Anastomotic stenosis is a common complication of Dixon surgery for
rectal cancer (5), which can be
classified as membranous or tubular stenosis. The incidence of
anastomotic stenosis following colorectal stapling surgery ranges
between 3-30%, which is challenging to manage using conventional
approaches (e.g., open surgery and colonoscopy) (6). Severe anastomotic stenosis, to a
certain extent, can lead to anastomotic closure, which is
classified as the most severe category of anastomotic stenosis,
resulting in only a small gap or complete occlusion (7,8). The
precise cause of anastomotic stenosis and occlusion remains unknown
and may be related to previous radiation therapy, improper use of
staplers, postoperative complications, such as anastomotic fistula
and local ischemia, and inflammatory bowel disease. Mild stenosis
can be treated effectively through methods such as endoscopy, stent
placement, and balloon dilation (9). However, for patients with severe
anastomotic stenosis or even occlusion, endoscopic treatment is not
effective and requires surgical intervention, resulting in
increased trauma to the patient. The present study reports three
cases of rectal anastomotic atresia where standard colonoscopy was
unsuccessful in bypassing or removing the physical obstruction.
Notably, the patients showed successful surgical outcomes using a
novel minimally invasive approach, wherein prostate resection
instrumentation was passed through the anus. All patients provided
informed consent prior to the study, and Ethical approval was
provided by the Ethics Committee of Hexi University Affiliated
Zhangye People's Hospital (Zhangye, China; approval no.
B2018-021).
Case report
Case 1
A 72-year-old male underwent Dixon surgery for
rectal cancer with a double distal ileostomy (Zhangye People's
Hospital Affiliated to Hexi University, Zhangye, China). The
patient recovered well, had appropriate stomal output, and was
subsequently discharged from the hospital. At six months after the
Dixon surgery, the patient requested reinstatement of the
anastomosis, and hence, stoma closure was performed in October
2018. At that time, the patient's stoma was unobstructed; however,
no rectal examination was performed. Despite uncomplicated
anastomosis surgery, the patient developed significant abdominal
distension on postoperative day 4 (the patient was still
hospitalized), and the patient was diagnosed with rectal
obstruction. Digital rectal examination revealed complete
obstruction 5 cm from the anal edge, reaching the blind end.
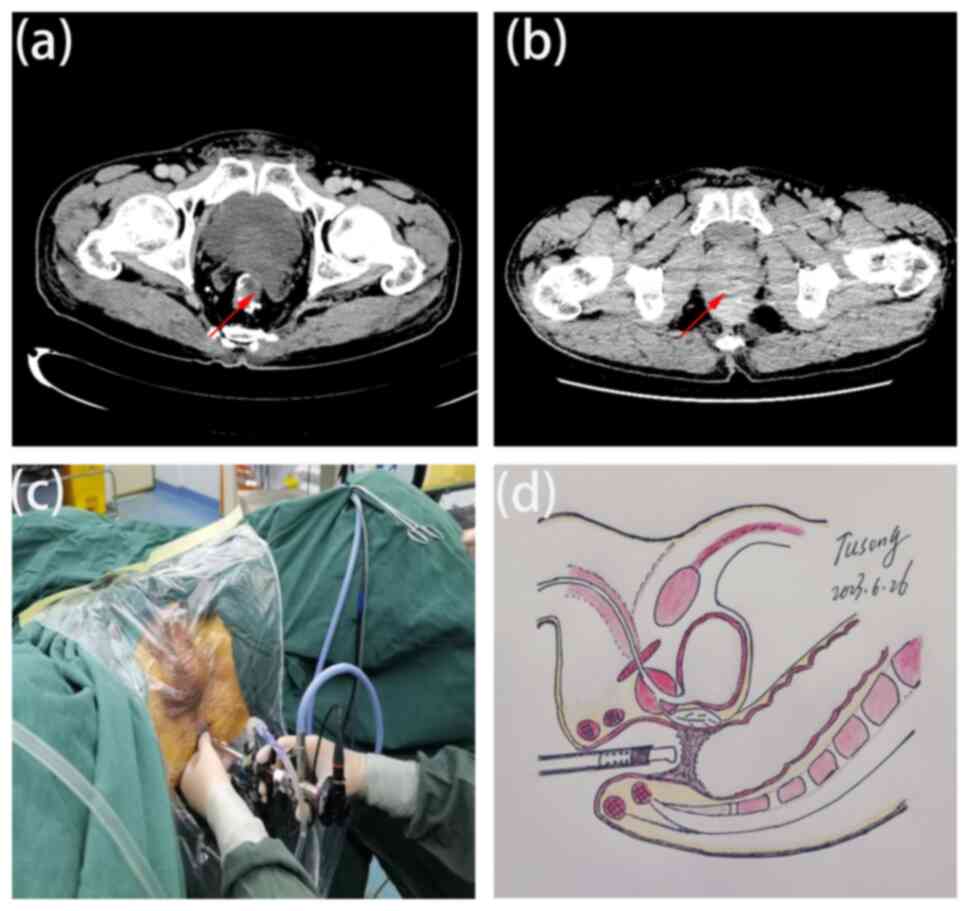
Subsequent computed tomography (CT) confirmed rectal anastomotic
atresia ~10 mm in length (Fig.
1A). However, attempts at colonoscopic evaluation and
management were unsuccessful. Examination via transurethral
prostate resection instrumentation revealed complete closure and
scar formation of the rectal anastomosis ~5 cm from the anal
margin. The central area showed staple exposure owing to the
previous anastomotic stapler without evidence of tumor
recurrence.
Under general anesthesia, the patient was placed in
the lithotomy position, and transurethral prostate resection
instrumentation was used to treat the rectal anastomotic atresia
(Fig. 1C and D) (10).
The instrument was inserted through the anus, and a tunneling
incision was made at the center of the atresia. As the sheath
traversed the opened segment, discharge of gas and feces confirmed
successful decompression. Further electrosurgical resections were
made at the scar to widen the diameter to 20 mm. To maintain
patency, weekly anastomotic dilatations were performed for 6 months
using a 20-mm dilator. Postoperatively, the patient reported the
return of normal bowel movements. The patient is followed up every
3 months and currently shows no signs of recurrence. The patient is
satisfied with the treatment effect.
Case 2
A 49-year-old man who underwent Dixon surgery
(Zhangye People's Hospital Affiliated to Hexi University, Zhangye,
China) for rectal cancer with a single distal ileostomy presented
with a 1-week history of abdominal pain, bloating and low-grade
fever at 8 months postoperatively in January 2022. Although the
ileostomy opening was unremarkable, digital rectal examination
revealed complete obstruction of the anastomosis 4 cm from the anal
edge, with the presence of blood. CT examination confirmed rectal
anastomotic atresia of ~13 mm in length (Fig. 1B). However, attempts at
colonoscopic evaluation and management were unsuccessful.
Under general anesthesia, the patient underwent
treatment for rectal anastomotic atresia via transurethral prostate
resection instrumentation (10).
The instrument was inserted through the anus, revealing scar
formation and complete atresia at 4 cm from the anal margin.
Erosion and an unevenly distributed local intestinal cavity were
observed, suggesting the possibility of local tumor recurrence. A
tunneling incision was made at the center of the closed stoma until
the lens sheath opened the closed segment. Gas and fecal liquid
were immediately ejected at high pressure, indicating successful
decompression. Further electrosurgical resections were made to
expand the diameter of the anastomotic stoma to 20 mm, and a rectal
drainage tube (Fr24 three-chamber balloon Foley catheter) was
retained. Symptoms of abdominal distension and infection after
surgery and antibiotic use were resolved postoperatively.
Pathological examination of the resected tissue revealed rectal
mucinous adenocarcinoma. The patient is followed up every 3 months
and is satisfied with the effectiveness of obstruction treatment.
At the latest follow-up in August 2023, the patient remained in
good health with a satisfactory treatment response. The patient has
refused further treatment for recurrent tumors.
Case 3
A 65-year-old man who underwent Dixon surgery with a
double distal ileostomy for rectal cancer was admitted 6 months
postoperatively for a planned ileostomy closure in May 2023.
Digital rectal examination revealed a complete obstruction 5 cm
from the anal edge, which was confirmed with a blinded probe.
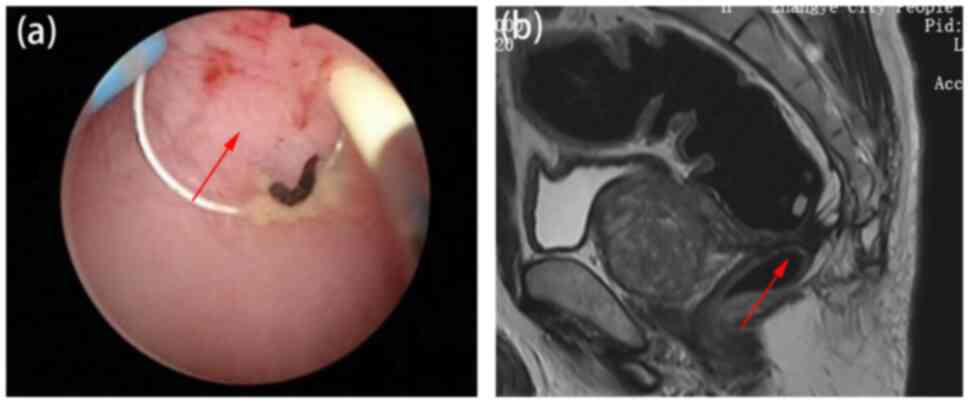
Magnetic resonance imaging (MRI) further showed rectal anastomotic
atresia ~8 mm in length (Fig. 2A
and B). However, attempts at
colonoscopic evaluation and treatment were unsuccessful. Endoscopy
using prostate resection instrumentation confirmed complete closure
and scar formation of the anastomosis at 5 cm from the anal
margin.
Under general anesthesia, the patient was placed in
the lithotomy position, and transurethral prostate resection
instrumentation was used to treat the rectal anastomotic atresia
(10). Prior to the procedure,
1,000 ml methylene blue saline was infused into the distal ileum
(only for this case) to visualize the proximal end of the atresia
and facilitate dilatation. The instrument was inserted through the
anus 30 min after, and a tunneling incision was made at the center
of anastomotic closure. As the lens sheath traversed the opened
segment, methylene blue solution and feces were discharged,
indicating surgical decompression. Further electrosurgical
resections were made to widen the anastomotic diameter to 20
mm.
In the present study, preoperative CT or MRI
examination was performed to determine the distance from the
anastomotic site to the anal margin, the length of anastomotic
closure and the degree of proximal intestinal dilation. Utilizing
the urological plasma resectoscope, which served as the primary
surgical instrument, the rectum and anastomotic site were examined
transanally. The surgical direction was determined, and an
electrode was used to excavate and open the anastomotic site.
During this procedure, 0.9% sodium chloride solution, with a set
flushing pressure of 40 cm H2O and an electrocoagulation
power of 280/180 W, was used for irrigation. The front end of the
circular electrode was angled 30˚ forward for tunneling incisions,
and an electrosurgical endoscope was inserted to evaluate the
condition of the rectum and anastomotic site, including the degree
of stenosis, tumor recurrence, inflammation, bleeding and ulcers.
To maintain patency, weekly anastomotic dilatations were performed
for 6 months. Furthermore, the patient underwent a reductive
ileostomy and was discharged without any complications. On the
latest follow-up in August 2023, the patient remained in good
health with a satisfactory treatment response.
Discussion
Anastomotic stenosis and leakage are serious
complications that negatively impact long-term prognosis and
quality of life following anterior resections of the rectum,
particularly in low anterior resections (11,12).
In cases with severe anastomotic stenosis, permanent colostomy is
usually necessitated, substantially diminishing the quality of life
(13,14). For patients with rectal cancer who
undergo Dixon surgery with a single distal ileostomy, severe
postoperative stenosis and atresia can lead to acute closed-loop
colonic obstruction, which is life-threatening and often requires
emergency treatment (15). Even
with preventive double-stoma colostomy or ileostomy, unaddressed
complications without obvious symptoms can hinder planned stoma
closure.
Anastomotic closure generally represents the most
severe form of stenosis. In particular, complete anastomotic
closure following laparoscopic low anterior resection for rectal
cancer is relatively rare in clinical practice. However, management
of this condition is often challenging, resulting in failure of
colonoscopic treatment and requiring open surgery (14). Inspired by the urological
approaches employed for urethral strictures and atresia, in the
present study, prostate resection instrumentation was adopted as a
minimally invasive approach to rectal anastomotic atresia.
Utilizing preoperative imaging and intraoperative localization of
residual anastomotic nails, three patients achieved satisfactory
treatment results with a widened diameter of >20 mm.
For cases with narrow gaps or small pore-like
channels at the anastomotic site, a guidewire was inserted and
marked under microscopic guidance. First, a tunneling incision was
made at the 6 o'clock position (lithotomy site). Once the presence
of feces and an enlarged proximal rectal cavity were visually
confirmed, the anastomotic narrow ring was radially incised at the
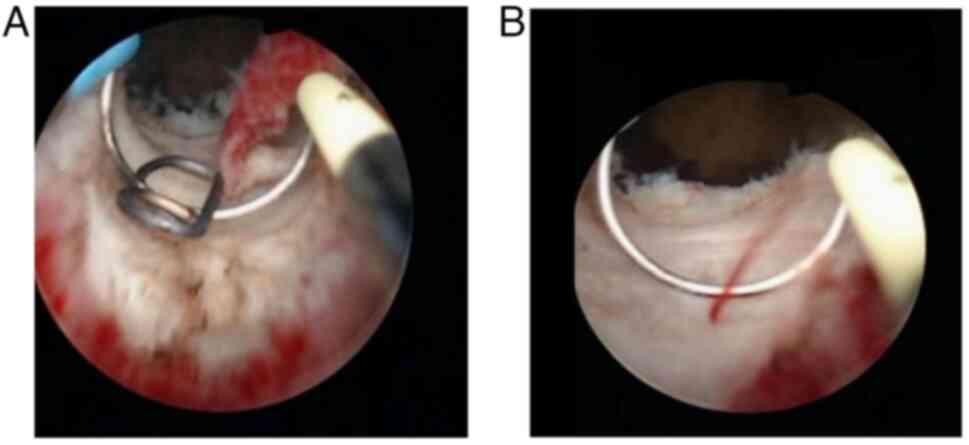
12, 3, 6 and 9 o'clock positions (four-point method). Meanwhile,
for cases of complete anastomotic closure, a tunneling incision was
made from the center of the closure under the guidance of
preoperative imaging or residual rectal staples. Electrocoagulation
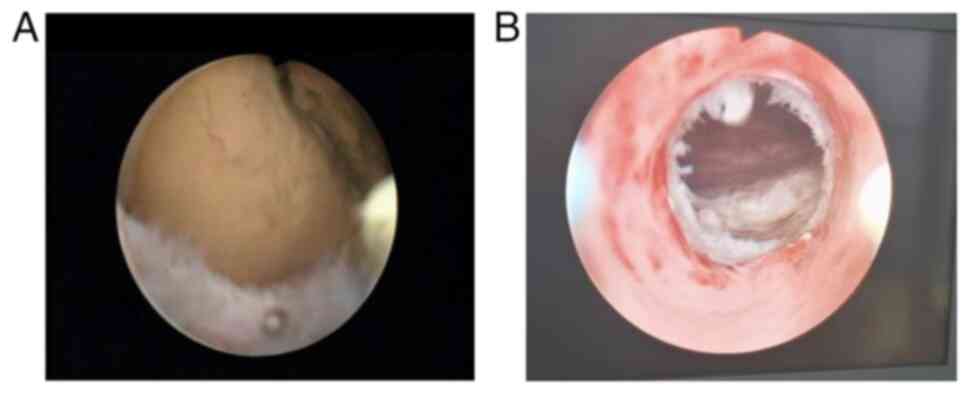
was utilized to control intraoperative bleeding (Fig. 3A and B). For all cases, the presence of stool
at the proximal rectum and an enlarged intestinal cavity (Fig. 4A) indicated a successful tunneling
incision; otherwise, a four-point radial incision method was used
in cases where the proximal cavity was not observed.
For membrane-like closures (<10 mm in length), a
single incision can create an anastomotic opening ≥20 mm deep,
allowing easy passage of two fingers. In cases of tubular closures
(length >11 mm), the anastomosis can be expanded after incision
using fingers or dilators to achieve a width of a two fingers or a
diameter >20 mm (Fig. 4B). If
primary incision and expansion fail to reach sufficient levels, a
tunnel can be passed through the lens sheath (>8 mm), and a Fr24
balloon Foley catheter is retained above the closure segment to act
as a temporary drainage tube. To prevent prolapse, a 10-ml water
injection capsule is used. After a week, a second-stage incision or
dilatation is performed to achieve surgical decompression with a
rectal anastomosis diameter >20 mm. Following satisfactory
incision and dilation, meticulous hemostasis is crucial under
electrosurgical endoscopy, involving rectal flushing, drainage of
the flushing fluid and collection of excised scar tissue for
pathological examination. Postoperative observations for fecal
drainage and bleeding are also required. Assuming no bleeding
occurs, the drainage tube is removed after 48 h. Subsequent weekly
dilations with fingers or dilators maintain the rectal anastomosis
diameter at ≥20 mm. Over 3-6 months, the dilation frequency
gradually decreases, eventually reaching monthly intervals
depending on individual responses.
Preoperative imaging examinations are crucial for
occlusion localization. Key indicators include residual anastomotic
nails and scarring of the closed anastomosis. In cases with a
single ileostomy, a closed-climbing colon obstruction occurs after
anastomosis closure. The proximal colon generally accumulates more
air and liquid content, resulting in significant distension and
enlargement on imaging. Under imaging guidance and aided by key
indicators during surgery, it is relatively easy to break through
the atresia. In cases with a double-tube ileostomy or transverse
colon ostomy, 1,000 ml methylene blue saline can be infused into
the colon through the stoma to dilate the proximal colon of the
closed anastomosis. This facilitates identification of the correct
direction and channel during the operation. For patients with a
preventive stoma, it is important to distinguish whether
anastomotic atresia or stenosis occurred before or after the
planned stoma closure. Neglecting proper assessment before closure
can lead to anastomotic rupture and acute bowel obstruction, which
would require reoperation. In case 1, anastomotic atresia occurred
prior to the planned ileostomy closure. However, due to the
surgeon's inexperience and failure to perform colonoscopy or
colorectal radiography, acute bowel obstruction developed shortly
after the operation. Timely intervention using transurethral
prostate resection instrumentation is therefore effective in
preventing serious complications.
Endoscopic anastomotic incision offers several
advantages over open surgery for digestive tract reconstruction,
including less invasiveness, faster recovery and increased
convenience (16-18).
For suitable cases, laparoscopic monitoring or guided endoscopic
incisions can be performed. However, emergency surgical preparation
should be performed simultaneously, since intestinal perforation
requires prompt management. While commonly utilized, traditional
colonoscopes make accurate cutting difficult due to their soft,
long bodies and lens instability. Bleeding or excessive intestinal
content can also obscure the visual field, which can affect the
operation. On the other hand, traditional anoscopes are relatively
short and may be insufficiently long to reach the anastomotic site
(19). Moreover, they has no
connected electrocautery system, making it impossible to perform
surgical electrocautery and precise wire-guided expansion. Thus,
selecting a stable, directional and easily operable endoscope, such
as transurethral prostate resection instrumentation, is crucial for
good outcomes. As this instrument has a rigid mirror with
directional markings, the endoscopic body is short and easily
operable without compromising directionality. Additionally, the use
of prostate resection instrumentation for anastomotic atresia is
simpler and more effective than colonoscopy. First, there is a
clear field of view, owing to the high pressure of the flushing
fluid, since prostate resection instrumentation has inlet and
outlet ports that allow continuous flushing. Second, guidewire
catheters can easily be inserted in the instrument, allowing scar
electrocautery, electrocoagulation and hemostasis. The technical
principle of prostate resection instrumentation is that the
radiofrequency electrode transmits high-energy heat to the target
tissue, causing rapid carbonization of tissue cells, water
evaporation inside the cells, and rapid decomposition and
vaporization. More specifically, the radio frequency electrode can
quickly heat the tissue to contact temperatures >400˚C,
resulting in cutting vaporization along the trajectory of the
electrode plus the hot spot. Heat penetration can produce a 2-3 mm
solidification layer on the cut tissue, resulting in good
coagulation and hemostatic effects (20).
Since prostate resection instrumentation is readily
available and commonly used in hospitals in China, its
implementation for rectal anastomotic atresia is feasible (21). Adapting its use for rectal surgery
requires no technological modifications, except a shift in the
surgery site. In this procedure, the atresic lesion is located and
incised at the distal end of the peritoneal fold. Precise incisions
and blunt dissections should therefore be performed to ensure
minimal intestinal damage and bleeding during surgery. Based on our
experience with these three cases, we consider that the treatment
of rectal anastomotic atresia with transanal prostate resection
instrumentation is a safe and effective minimally invasive
approach. The use of standard instrumentation further highlights
its potential as a novel approach to resolve the blockages.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by grants from the President Fund
Innovation Team Project of Hexi University (no. CXTD2022012) and
the Hexi University 13th Science and Technology Innovation Project
(no. 127).
Availability of data and materials
The data generated in the present study are included
in the figures of this article.
Authors' contributions
ZH, ST, JQ and JY contributed to the conception or
design of the study. ZH and YQ wrote the manuscript and acquired
and analyzed data for the study. XW and ST performed the operation.
XW, JQ, and ST agree to be accountable for all aspects of the work
in ensuring that questions related to the accuracy or integrity of
any part of the work are appropriately investigated and resolved.
ST and JY confirm the authenticity of all the raw data and
performed the manuscript review. All authors have read and approved
the manuscript.
Ethics approval and consent to
participate
Ethical approval was provided by the Ethics
Committee of Hexi University Affiliated Zhangye People's Hospital
(approval no. B2018-021). The study followed the terms set out by
the Declaration of Helsinki. The patients provided written informed
consent for participation.
Patient consent for publication
Written consent was obtained for publication of the
patients' images in this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wilkinson N: Management of rectal cancer.
Surg Clin North Am. 100:615–628. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mathew R: Radical surgery versus organ
preservation for early-stage rectal cancer. Lancet Gastroenterol
Hepatol. 6(263)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Planellas P, Farrés R, Cornejo L,
Rodríguez-Hermosa JI, Pigem A, Timoteo A, Ortega N and
Codina-Cazador A: Randomized clinical trial comparing side to end
vs end to end techniques for colorectal anastomosis. Int J Surg.
83:220–229. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zheng XC, Su JB and Zheng JJ: Risk
assessment of rectal anastomotic leakage (RAREAL) after DIXON in
non-emergency patients with rectal cancer. BMC Gastroenterol.
23(343)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hu X, Guo P, Zhang N, Guo G, Li B, Liu Y,
Niu J and Wang G: Nomogram for benign anastomotic stricture after
surgery for rectal cancer. Asian J Surg. 46:111–119.
2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Forshaw MJ, Maphosa G, Sankararajah D,
Parker MC and Stewart M: Endoscopic alternatives in managing
anastomotic strictures of the colon and rectum. Tech Coloproctol.
10:21–27. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lu G, Li J, Ren M, Ma F, Sun X, Lv Y and
He S: Endoscopy-assisted magnetic compression anastomosis for
rectal anastomotic atresia. Endoscopy. 53:E437–E439.
2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kurashima M, Joshi S, Sobrino J and
Blewett C: Rectal atresia treated via a transanal and posterior
sagittal approach: A report of two cases. Cureus.
15(e38694)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kraenzler A, Maggiori L, Pittet O, Alyami
MS, la Denise JPA and Panis Y: Anastomotic stenosis after coloanal,
colorectal and ileoanal anastomosis: What is the best management?
Colorectal Dis. 19:O90–O96. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhang Z, Hu Z, Qin Y, Qian J, Tu S and Yao
J: Application of transurethral prostate resection instrumentation
for treating low rectal anastomotic leakage: A pilot study. Cancer
Manag Res. 14:1987–1994. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lamazza A, Fiori E, Schillaci A, Sterpetti
AV and Lezoche E: Treatment of anastomotic stenosis and leakage
after colorectal resection for cancer with self-expandable metal
stents. Am J Surg. 208:465–469. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Guyton KL, Hyman NH and Alverdy JC:
Prevention of perioperative anastomotic healing complications:
Anastomotic stricture and anastomotic leak. Adv Surg. 50:129–141.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schlegel RD, Dehni N, Parc R, Caplin S and
Tiret E: Results of reoperations in colorectal anastomotic
strictures. Dis Colon Rectum. 44:1464–1468. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang B, Zhuo GZ, Tian L, Zhao K, Zhao Y,
Zhao YJ, Zhu J, Zhang T and Ding JH: Risk factors of coloanal
anastomotic stricture after laparoscopic intersphincteric resection
for low rectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi.
22:755–761. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
15
|
Sali PA, Pilania V, Sutar S, Krishna K,
Ghetla S and Shetty T: Total colectomy in a gangrenous large bowel
due to a rare double closed loop obstruction. Int J Surg Case Rep.
17:1–4. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Inoue T, Shichijo S, Yasui M, Takeuchi Y,
Michida T and Ishihara R: Endoscopic incision and balloon dilation
using the rendezvous technique for complete anastomotic obstruction
after rectal low-anterior resection. Endoscopy. 54:E90–E91.
2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang S, Wan J, Li Z, Long C, Zhang R, Luo
Y, Han Z and Yan J: Comparison of the efficacy of endoscopic radial
incision and cutting procedure and endoscopic balloon dilatation
for benign anastomotic stricture after low anterior resection
combined with preventive loop ileostomy in rectal cancer. Dis Colon
Rectum. 20:1392–1401. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou W, Xia L, Wang Z, Cao G, Chen L, Chen
E, Zhang W and Song Z: Transanal minimally invasive surgery for
rectal anastomotic stenosis after colorectal cancer surgery. Dis
Colon Rectum. 65:1062–1068. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yan P, Qin Y, Zhang Z, Xu W, Qian J, Tu S
and Yao J: Application of prostate resection endoscopy for treating
acute obstruction associated with rectal cancer. J Cancer.
13:1679–1684. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Barba M, Fastenmeier K and Hartung R:
Electrocautery: Principles and practice. J Endourol. 17:541–555.
2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zeng XT, Jin YH, Liu TZ, Chen FM, Ding DG,
Fu M, Gu XQ, Han BM, Huang X, Hou Z, et al: Clinical practice
guideline for transurethral plasmakinetic resection of prostate for
benign prostatic hyperplasia (2021 Edition). Mil Med Res.
9(14)2022.PubMed/NCBI View Article : Google Scholar
|