1. Introduction
The prevention and treatment of pancreatic cancer is
a difficult issue worldwide, as pancreatic cancer is associated
with higher malignancy and metastatic rates from the early stage of
disease (1). More than 95% of
patients diagnosed with pancreatic cancer succumb to the disease
and half of these patients within six months after diagnosis
(2,3). Radio- and chemo-therapy do not have
major effects on the survival of pancreatic cancer patients
(2,4); thus, surgery remains the optimal
treatment method. Prognosis mainly depends on early diagnosis and
treatment. Therefore, it is of great importance to identify novel
diagnostic markers and to explore related proteins involved in
signaling pathways associated with the occurence, development and
metastasis of pancreatic cancer. S100 proteins interact with
multiple molecular targets in both a calcium-dependent and
-independent manner (5,6). They regulate multiple cellular
pathways that play key roles in pancreatic cancer progression and
metastasis (7,8). S100 proteins may thus be early
diagnostic biomarkers.
2. The S100 family
The S100 protein family, a multigene calcium-binding
family, comprises more than 20 members, each encoded by a separate
gene. At least 16 of these genes cluster on chromosome 1q21
(6), known as the epidermal
differentiation complex (7,9).
In 1965, the first member of the S100 family was purified from
bovine brain by Moore (10). Due
to its solubility in a 100% saturated solution at neutral pH with
ammonium sulfate (11,12), it was termed the ‘S100’ protein.
The S100 protein family is an acidic calcium-modulated protein
family of low molecular weight (10–12 kDa), mainly expressed in
vertebrates. It shares homology with calmodulin and other EF-hand
type calcium-modulated proteins (11). Since then the expression of S100
proteins has been demonstrated in a diverse spectrum of tissues
(5).
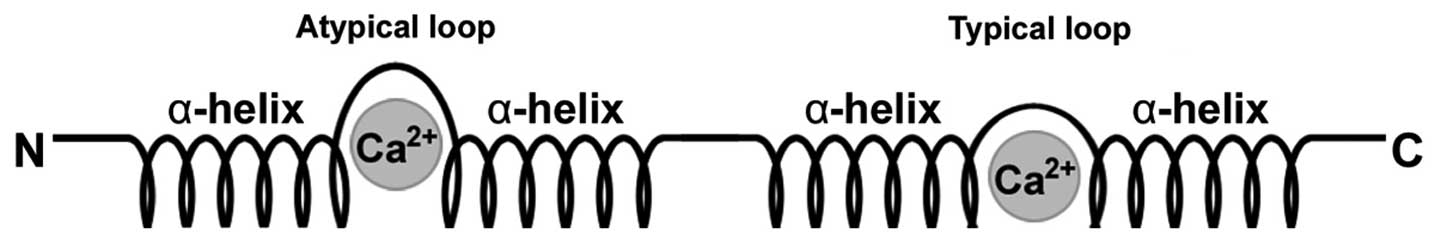
Structure of S100 proteins
S100 proteins belong to the calcium-binding EF-hand
motif superfamily and have the ability to form homodimers,
heterodimers and oligomers (6).
Each S100 protein is characterized by the presence of two
Ca2+-modulated motifs of the EF-hand type interconnected
by an intermediate region which is often referred to as the hinge
region, resulting in a helix-loop-helix arrangement (8,11,12) (Fig.
1). S100 proteins have two distinct EF-hands, one common to all
EF-hand proteins on the C-terminal portion and the other specific
to the family located at the N-terminus. Subsequent to the
C-terminal EF-hand region is a stretch of amino acids referred to
as the C-terminal extension. Between the two EF-hand domains is the
area known as the hinge (7,9).
It is the C-terminal extension and hinge areas that have the most
variability among the different proteins and hence they are
responsible for their specific biological properties (9).
Biological functions of S100
proteins
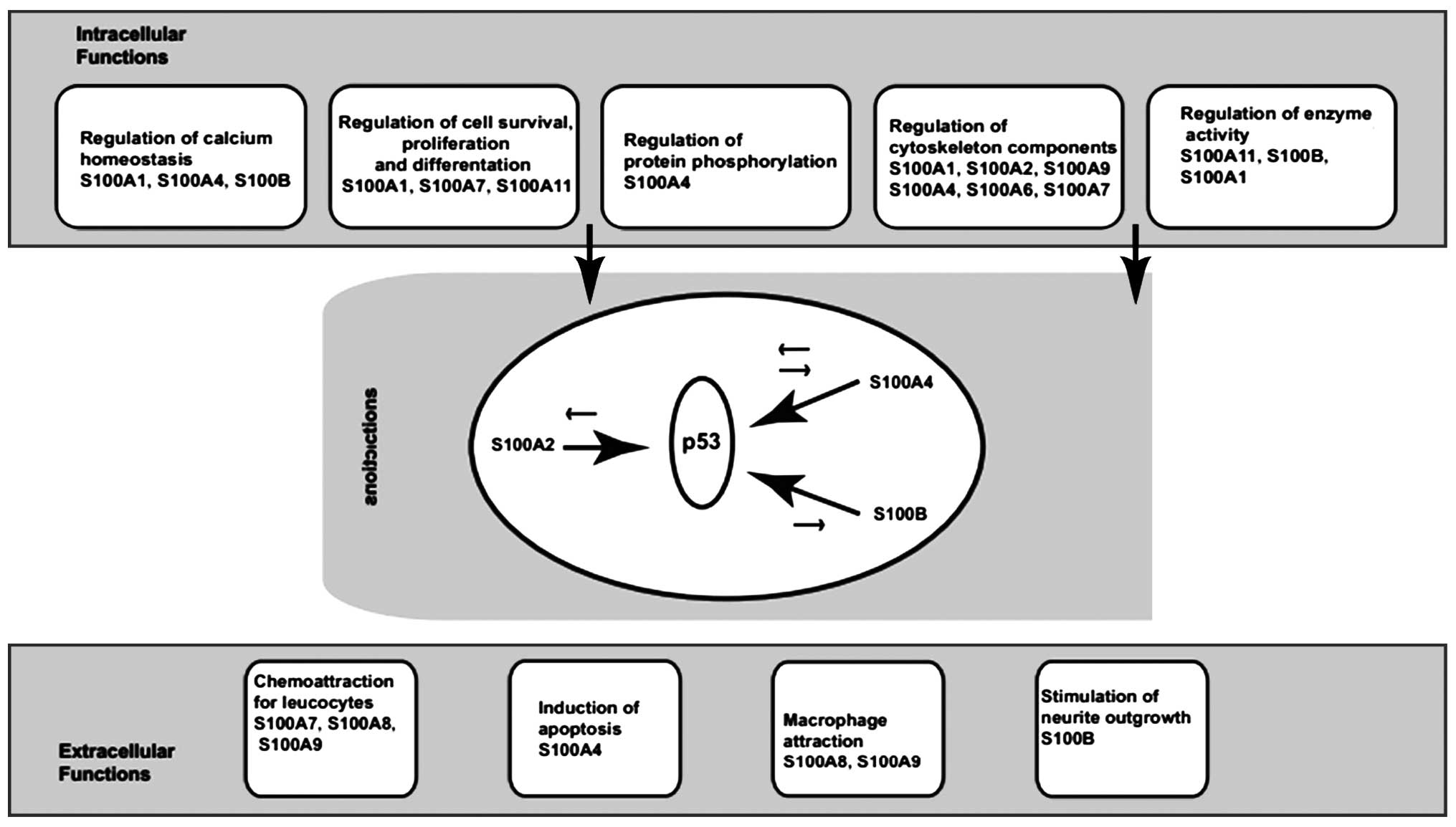
It is well documented that S100 proteins have a
broad range of intracellular and extracellular functions (9). Intracellular functions include the
regulation of enzyme activity and protein phosphorylation, calcium
homeostasis, the regulation of cytoskeletal components and the
regulation of transcriptional factors (7,9,12)
(Fig. 2).
Intracellular activities of S100
proteins
S100 proteins play a wide range of roles in cells.
The extracellular (9) and
intracellular activities of S100 proteins include the regulation of
adjusting key enzymes, calcium balance, the composition of the
cytoskeleton, protein phosphorylation and dephosphorylation, as
well as the regulation of energy metabolism (13,14), cell differentiation and the cell
cycle (8,15). Members of the S100 family interact
with p53 and these interactions with p53 produce differential
effects, depending on the activity of the protein involved
(9) (Fig. 3). Both S100A4 and S100B are
thought to inhibit p53 phosphorylation, leading to the inhibition
of its transcriptional activity, thereby compromising p53
tumor-suppressor activity (12).
By contrast, S100A2 promotes p53 transcriptional activity (7). Thus the balanced actions of
different S100 proteins within a cell determine its function. Many
of the S100 family members are involved in modulating cytoskeletal
dynamics. Again they display remarkable diversity of function,
exhibiting direct interaction with tubulins, intermediate
filaments, actin, myosin and tropomyosin. Some of these proteins
have been implicated in mediating metastasis (8), such as S100A1 and S100A11. S100
protein members also play a role in regulating proliferation. In
addition, most of the genes of the human S100 proteins are located
on human chromosome lq21 (7,9).
Once a tumor develops, the genes in this area are easily
reorganized and can interfere with the gene expression of S100
(9). Therefore, it is often
observed that S100 proteins are accompanied by an abnormal
expression in advanced cancer and metastasis. Thus, S100 proteins
are closely related to the development and metastasis of a variety
of tumors (9).
Extracellular roles of S100 proteins
S100 proteins are involved in the extracellular
stimulation of neuronal survival, differentiation and astrocyte
proliferation, resulting in neuronal death via apoptosis, and
stimulate (in some cases) or inhibit (in other cases) the activity
of inflammatory cells (9,16). S100 proteins are closely related
to a variety of human diseases, such as neurological disorders,
cancer, inflammation and heart disease (15).
3. S100 proteins as molecular targets in
pancreatic cancer
S100 proteins interact with multiple molecular
targets in pancreatic cancer. The key multiple molecular targets
include the following:
Extracellular S100 proteins: interaction
with receptor for advanced glycation end-products (RAGE)
S100 proteins form heterodimers. These complexes
display different affinities to target proteins, depending on their
oligomerization state (17). This
has been demonstrated for p53 and RAGE (8,9,18).
Structural analysis of receptor-ligand interaction has indicated
that RAGE recognizes three-dimensional structures: one ‘V-type’
domain and two ‘C-type’ domains; a short transmembrane domain and a
43-amino acid cytoplasmic tail (19,20). The V-type domain has been found to
confer ligand binding. The cytoplasmic tail is required for
intracellular signaling, and the V-type domain is responsible for
ligand binding. Multiple pathways downstream of RAGE have been
identified, such as mitogen-activated protein (MAP) kinases,
phosphatidylinositol 3-kinase (PI3K), Rho GTPases, nuclear factor
(NF)-κB and JAK/STAT (8,21,22). RAGE has been shown to transduce
the extracellular effects of S100B (22), S100A4, S100A6 (22), S100A8/A9 (23), S100A11 (24), S100A12, S100A13 and S100P
(19,20,25). The activation of RAGE by S100P
stimulates cellular signaling pathways, including the MAP kinase
and NF-κB pathways (19,22,26). Certain studies have indicated that
inhibiting S100P-RAGE interactions significantly reduces the basal
levels of NF-κB activity in pancreatic cancer and supports the
existence of an autocrine loop involving RAGE ligands and RAGE in
pancreatic cancer (2,25,26). S100B and S100A6 have been shown
not only to interact with distinct RAGE immunoglobulin domains, but
also to exert opposite effects on cell survival (27,28). At similar concentrations, S100B
increases cellular proliferation (26), whereas S100A6 triggers apoptosis
(29,30). In addition, both S100 proteins
induce the formation of reactive oxygen species (ROS); however,
S100B recruits PI3K/AKT and NF-κB (31,32), whereas S100A6 activates JNK
(30). The study by Arumugam
et al also showed that S100A4 binding to RAGE was blocked by
RAP (25). It has been reported
that S100A4 may influence the resistance of pancreatic cancer cells
to therapy (16,33).
S100 proteins bind with cytoskeletal
proteins and plasma membrane, thereby increasing cell
migration
S100 proteins regulate all three major constituents
of the cytoplasmic cytoskeleton, i.e. microtubules (MTs),
intermediate filaments (IFs) and microfilaments (MFs) (22,34), as well as tropomyosin and myosin
(8,35). S100 proteins can exert their
effects depending on their interactions with cytoskeletal proteins
and the membrane resulting in an enhancement of cell migration
(8,9). For example, S100B and S100A1
disassemble cytoplasmic MTs and induce the aggregation of vimentin
IFs as a result of in situ MT disassembly in
triton-cytoskeletons from several cell lines (20). The consequent sequence potentially
implicated in S100B binding has been identified in helix H8 of the
central portion of tubulin and in its C-terminus, regions
considered important for protofilament formation and, hence, for
the MT assembly (8,34,36). S100A4 interacts with cytoskeletal
elements, such as actin, tubulin and non-muscle tropomyosin,
establishing a direct role of S100A4 in regulating cell motility
and cytoskeletal rearrangement (16). This suggests that S100A4 plays a
possible mechanistic role in cell shape, motility and thus,
invasion (12,16). Other studies reported that S100A11
can associate with actin, β-tubulin, IFs and actin organization and
Annexin I (37,38). S100A6 interacts with tropomyosin
β, Annexin 11 and 2, and the novel binding protein, lamin B1, in
pancreatic cancer cells. Hayes et al demonstrated that
Annexin 2 was concentrated in the dynamic actin-rich protrusions of
motile cells and that the siRNA-mediated depletion of Annexin 2 led
to loss of protrusive and retractile activity (39). De Graauw et al pointed out
that the phosphorylation of Annexin 2 was a key event in the
remodelling of the actin cytoskeleton during cell spreading
(40).
Interaction with p53
p53 is subjected to complex regulation. The
biochemical activity of p53 as a transcription factor is adjusted
by phosphorylation and acetylation, as well as modulation of
protein stability, the degree of oligomerization, nuclear
translocation and interactions with other components of the
transcriptional machinery (12).
The tumor suppressor protein, p53, plays a pivotal role in the
maintenance and regulation of normal cellular functions through the
induction of cell cycle arrest, DNA repair, or apoptosis in
response to a variety of cellular stress signals and DNA damage
(8,12). In response to stress, p53 prevents
tumorigenic transformation through the induction of cell cycle
arrest or apoptosis (22,41). p53 interacts with other components
of the transcriptional machinery (22,42). In unstressed cells, the expression
level of p53 tumor suppressor is low (43,44); however, upon stress challenge, p53
is activated by post-translational modifications that increase its
stability (45,46). The regulation of protein stability
is one of the most effective mechanisms for controlling the
function of p53 (9,45). The key to this process is mouse
double minute 2 (MDM2), an E3 ligase that targets p53 for
ubiquitination. Several S100 proteins, such as S100B (46), S100A1, S100A2 (47), S100A4 (48), S100A6, S100A11 (24,32) and S100A14 (49) have been shown to interact with
MDM2. Direct protein-protein interactions between S100 proteins and
MDM2 promote the degradation of p53, as has been demonstrated for
S100B and S100A4 (12,48,50). This results in the loss of
p53-dependent tumor suppressor activities. Elucidating the
consequences of the metastasis-promoting activities of S100
proteins on p53-mediated functions, is of great importance in
understanding cancer development and metastasis (8,51).
It is noteworthy that potential p53-binding sites have been
identified in the promoter sequences of several S100 genes,
indicating that the metastasis-promoting properties of S100
proteins are not as clear-cut as has been previously suggested.
This is due to their interaction with p53-dependent apoptosis
(22). This fact may explain why
some members of the S100 family are markedly downregulated in
malignant cells, in comparison to normal cells (22). Both S100A4 and S100B are thought
to inhibit p53 phosphorylation, leading to the inhibition of its
transcriptional activity, thereby compromising p53 tumor-suppressor
activity (16,41). By contrast, S100A2 promotes p53
transcriptional activity and of note, S100A4 has also been
documented to enhance p53-dependent apoptosis (16,47,50). Thus, the balance of actions of
different S100 proteins within a cell can determine function
(8).
Interaction with p21
p21/WAF1 is also known as cyclin-dependent kinase
(CDK) inhibitor 1 or CDK-interacting protein 1. p21/WAF1 is a cell
cycle checkpoint, where cells either set about repairing themselves
or commit suicide through apoptosis (52,53). The p21/WAF1 protein functions as a
regulator of cell cycle progression at the G1 phase by inhibiting
cyclin-CDK2 or -CDK1 activity (52,54,55). The expression of its gene is
tightly controlled by p53, through which the p21/WAF1 protein
mediates p53-dependent cell cycle G1 phase arrest, in response to a
variety of stress stimuli (24,55). For example, S100A11 has been shown
to be involved since TGF-β induces S100A11 gene expression and
translocation into the nucleus, where it interacts with p21/WaF1
(56,57). S100A4 target genes comprise
p21/WAF, Bax, thrombospondin-1 and MDM2 (16,58,59).
S100 proteins: role in the degradation of
the extracellular matrix (ECM) and metastasis
Angiogenesis is a crucial step in cancer
progression, as it supplies the proliferating tumor cells with
necessary nutrients and oxygen and at the same time, it provides an
escape route for invading tumor cells (60). Matrix metalloproteinases (MMPs)
promote metastasis both by degrading the ECM and promoting and
maintaining angiogenic characteristics (16). An important factor affecting the
motility of cancer cells is the degradation of the ECM (22). S100 proteins have a variety of
molecular mechanisms. For example, the metastatic function of
S100A4 is associated with its ability to upregulate the expression
of several MMPs. S100A4 gene suppression significantly decreases
the expression of MMP-9, while the overexpression of the S100A4
gene significantly increases MMP-9 expression (12,16,18). Extracellular S100A4 binds to RAGE,
and upregulates MMP-13, MMP-2 and MMP-9 gene expression (19,61), allowing cell invasion and thus
promoting metastasis (1,16). Intracellular S100A14 promotes cell
motility and invasiveness by regulating the expression and function
of MMP-2 in a p53-dependent manner (22,62). p53 transrepresses MMP-2 gene
expression and thus enables intracellular S100A14 to effect p53
transactivity and stability, resulting in an enhancement of MMP2
gene expression (22). S100A8/A9
overexpression can also induce the upregulation of MMP-9 in HaCaT
keratinocytes. MMP9 gene induction depends on NF-κB activation and
intracellular S100A8/A9 has been shown to promote epithelial NADPH
oxidases and subsequently, NF-κB activation (63). S100P induces the expression of
cathepsin D, an aspartyl protease, which takes part in the
proteolytic degradation of the ECM. Hence it increases the invasive
potential of the tumor (2,22).
4. S100 protein expression in pancreatic
cancer
Multiple proteins of the S100 protein family are
closely related to pancreatic cancer, including the following
proteins:
S100A2
The S100A2 gene is located on the long arm of Area 2
of chromosome 1. The chromosomal stability of this section is poor,
and closely related to tumor development (47,64). S100A2 is often expressed in normal
cells and regulated by cell cycle progression and the tumor
suppressor gene, p53 (47). The
lack of S100A2 has been proven to be associated with the
development of numerous human tumors. Since it is absent in the
majority of tumor types, S100A2 is very important in normal tissue
growth and differentiation (65).
Studies have demonstrated that the lack of S100A2 functionality may
be due to the selective hypermethylation in the promoter region. In
tumor cells, a transcription factor binds to S100A2 promoter, which
leads to hypermethylation and, consequently, to the transcriptional
silencing of S100A2, thereby rendering it non-reactive with most
tumor suppressor genes (65).
Ohuchida et al microdissected invasive ductal carcinoma
(IDC), pancreatic intraepithelial neoplasia (PanIN), intraductal
papillary mucinous neoplasm (IPMN), pancreatitis-affected
epithelial (PAE) and normal ductal cells and then studied S100A2
expression by quantitative reverse transcription PCR (qRT-PCR)
(27). The analyses revealed that
IDC cells expressed higher levels of S100A2 than did IPMN, PAE or
normal cells (27). Cell lines
from metastatic sites expressed higher levels of S100A2 than those
from primary sites. PanIN cells expressed higher levels of S100A2
than normal cells. IDC cells associated with poorly differentiated
adenocarcinoma expressed higher levels of S100A2 than did IDC cells
without poorly differentiated adenocarcinoma (27). Analyses of formalin-fixed
paraffin-embedded (FFPE) samples revealed that the expression
levels of S100A2 were higher in samples from patients who survived
<1,000 days after surgery than in those from patients who
survived >1,000 days (27).
S100A2 may be a marker of tumor progression or prognosis in
pancreatic carcinogenesis and pancreatic cancer (65,66). Moreover, S100A2 has recently been
suggested to be a negative prognostic biomarker in pancreatic
cancer. Biankin et al (67) demonstrated that patients with
S100A2-negative tumors had a significant survival benefit from
pancreatectomy even in the presence of involved surgical margins or
lymph node metastasis. S100A2 expression is a good predictor of the
response to pancreatectomy for pancreatic cancer. Data suggest that
a high S100A2 expression may be a marker of a metastatic phenotype
(67,68). The prospective measurement of
S100A2 expression in diagnostic biopsy samples has potential
clinical utility as a predictive biomarker of response to
pancreatectomy and other therapies that target locoregional disease
(67,68). These data demonstrate that S100A2
is associated with tumor progression in pancreatic cancer and is a
negative prognostic biomarker in pancreatic cancer.
S100A4
The S100A4 protein consists of 101 amino acids, it
is an 11-kDa molecular weight protein and exists as non-covalent
dimers in the cell and as covalent dimers in the extracellular
domain (16,69). In normal lung, kidney, breast,
thyroid, pancreas and colon tissue cells, the expression of the
S100A4 protein is absent. Studies have shown that the expression of
S100A4 protein in pancreatic cancer is significantly higher than in
adjacent normal pancreatic tissue, and that the expression of
S100A4 in poorly differentiated pancreatic tissue and metastatic
pancreatic cancer tissue (66) is
significantly higher than that in well-differentiated and
non-metastatic pancreatic cancer tissue (16,70). The overexpression of S100A4
protein is associated with hypomethylation of the first intron of
the corresponding gene, leading to poor differentiation of
pancreatic cancer (71). S100A4
protein overexpression also plays an important role in pancreatic
cancer invasion and in the metastasis process (71). Another study demonstrated that
S100A4-silenced cells exhibited a marked decrease in migration and
invasiveness and increased adhesion, whereas overall proliferation
and apoptosis were not overtly altered (1). S100A4 and its downstream factors
play important roles in pancreatic cancer invasion. A100A4
silencing can significantly restrain the invasiveness of pancreatic
cancer (1). Studies have
suggested that the S100A4 protein is an independent prognostic
factor of pancreatic cancer which can differentiate pancreatic
cancer from lymph node metastasis (68,72,73).
S100A6
The S100A6 gene is a single-copy gene, located on
human chromosome lq21, adjacent to ski proto-oncogene (74). S100A6 consists of 90 amino acids.
The S100A6 protein may be relevant to pancreatic cancer prognosis
(30). Ohuchida et al
(75,76) analyzed the secretion of S100A6
protein expression in normal pancreatic tissue, PanIN and IDC of
the pancreas, and found that the level of S100A6 expression in
pancreatic cancer was significantly higher than that in
non-cancerous tissue. S100A6 protein expression in cancerous
pancreatic juice was significantly higher than that in normal
pancreatic juice (35).
Vimalachandran et al (77)
showed that the expression of S100A6 in pancreatic cancer cell
nuclei was significantly higher compared with the cytoplasm.
Patients with high S100A6 protein expression levels in the nucleus
presented with poor prognosis (77). However, the expression level of
cytoplasmic S100A6 had no clear association with prognosis. An
absence of S100A6 expression was observed during PanIN period.
However, as PanIN levels increased, S100A6 protein expression
levels gradually increased as well, particularly in the nucleus
(77). This indicated that even
though S100A6 protein expression in pancreatic cancer is an early
event, it is the expression of S100A6 in the nucleus that can be
used as an independent prognostic factor. Its high expression
levels often precede a poor prognosis. However, there is no
evidence to date to support a correlation between S100A6 and the
occurrence, differentiation and metastasis of pancreatic cancer
(75,76,77).
S100P
S100P is a 95-amino-acid protein whose gene is
located on chromosome 4pl6 (42).
S100P protein monomers have shown a positive correlation with
calcium-dependent binding; whereas the dissociation rate constant
was independent of calcium (2).
The dimer contact surface and the core area of the hydrophobic
amino acid mutation S100P proteins affect polymerization. S100P and
its ligand, S100P BPR, coexist in the nucleus. In situ
hybridization has confirmed the presence of the S100P BPR
transcription product in normal pancreatic islet cells and
pancreatic ductal adenocarcinoma, which was not expressed in normal
pancreatic duct cells (78,79). As shown by qRT-PCR, S100P and
S100PBPR were observed in PanIN and pancreatic cancer tissue
specimens. These results suggest that S100P and S100PBPR play a
role in early pancreatic cancer occurrence (78,79). The expression level of S100P is
positively associated with PanIN. The gradual increase in S100P
concentration is expressed as PanlN-1, PanIN-2 and PanIN-3. This
indicates that S100P plays an important role in the progression
from PanIN to invasive ductal adenocarcinoma in the pancreas
(78,79). Ohuchida et al (80), as well as others examined the
expression levels of S100P in various other pancreatic diseases.
According to organizational analyses it was found that pancreatic
cancer and IPMN tissue expressed significantly higher levels of
S100P than did tissue from non-neoplastic pancreas (80,81). Microdissection analyses revealed
that IPMN tissue expressed significantly higher levels of S100P
than did pancreatic cancer and PanIN tissue (80,81). There was no significant difference
between the expression levels of pancreatic cancer and PanIN. In
pancreatic juice analyses, S100P expression levels in patients with
pancreatic cancer and IPMN were significantly higher than those in
patients with pancreatitis (80,81). Thus, neoplastic disease can be
effectively distinguished from chronic pancreatitis. S100P has been
shown to mediate tumor growth, drug resistance and metastasis
through RAGE (26,42,80). Arumugam et al (90) demonstrated that elevated
expression levels of S100P in mice accelerated the growth rate of
pancreatic cancer cells, contrary to its decreased expression
levels which delayed cancer cell growth. These studies demonstrate
that the S100P protein plays an important role in the incidence of
pancreatic cancer. S100P favors the early diagnosis of pancreatic
cancer (82). It can be used as
an early diagnostic biomarker for pancreatic cancer by detecting
its expression levels in the pancreatic juice of the patient. The
expression of S100P has been shown to be associated with drug
resistance, metastasis and poor clinical outcome (2,83).
S100A11
S100A11 also known as S100C (55), is a member of the family of S100
proteins, and was first discovered in 1989 (55). The S100A11 protein consists of 99
amino acids, with a molecular weight of 11 kDa. Its gene is located
on chromosome 1q21 (55,56). The detection of S100A11 RNA
expression levels in different tissues has indicated that the
highest expression levels are present in the placenta, heart,
kidneys and lungs, whereas moderate expression levels are present
in skeletal muscle tissues and the lowest in the brain tissue
(55,84). Higher levels of S100A11 protein
are expressed in duct cells of different tissues, while lower
levels are detected in the epithelial cells of the digestive tract.
The S100A11 protein is mainly distributed in the nucleus, and the
remaining S100A11 protein is distributed in the cytoplasm (55,85). Sakaguchi et al (53) reported that S100A11 increases the
transcription of p21, a negative regulator of cell growth (66,85). In addition, the expression of
several known tumor suppressor genes, including p53 and p16INK4
(86), has been reported to be
increased in premalignant lesions; S100A11 expression is elevated
in non-invasive neoplasms, such as IPMA and PanIN, but decreased in
invasive cancer, such as IDC (87,88). The expression of S100A11 protein
has been shown to be significantly decreased within human
fibroblasts that have undergone malignant transformation (57). The enforced expression of S100A11
protein has been shown to significantly inhibit the growth of
malignant cells. Data suggest that S100A11 may be a tumor
suppressor gene (56). Ohuchida
et al also showed that S100A11 expression increased during
the early stages of pancreatic cancer and then decreased as cancer
progressed (57). Previous
studies have also shown that S100A11 functions as a dual cell
growth mediator as it is highly expressed in pancreatic tissue, and
its high expression in pancreatic cancer is associated with tumor
differentiation and lymph node metastasis (56,84). S100A11 is a significant tumor
marker for pancreatic cancer and an unfavorable predictor for the
prognosis of patients who have undergone surgical resection
(84,89). We consider S100A11 relevant with
the occurrence and development of pancreatic cancer. In conclusion,
S100A11 is a putative tumor suppressor gene. It can be used as an
independent prognostic indicator of pancreatic cancer, particularly
for the early diagnosis of pancreatic cancer. S100A11 analysis in
pancreatic juice may allow the early detection of pancreatic cancer
and the effective screening of patients with high-risk lesions that
may progress to pancreatic cancer, such as patients who have a
family history of pancreatic cancer or who have chronic
pancreatitis (57).
5. Conclusion
S100 proteins interact with RAGE, p53 and p21, play
a role in the degradation of the ECM and metastasis, and bind with
cytoskeletal proteins and the plasma membrane in pancreatic cancer
progression and metastasis. S100A11 and S100P are significant tumor
markers for pancreatic cancer and unfavorable predictors for the
prognosis of patients who have undergone surgical resection. S100A2
has recently been suggested to be a negative prognostic biomarker
in pancreatic cancer. The expression of S100A6 in the nucleus may
used as an independent prognostic pancreatic factor. The expression
of S100A4 and S100P is associated with drug resistance,
differentiation, metastasis and clinical outcome. The data
presented in this review suggest that S100 proteins may be used as
molecular markers for the early diagnosis, treatment and prognosis
of pancreatic cancer.
Acknowledgements
This study was supported by grants from the
Foundation for Talents in Six Fields of Jiangsu Province (no.
2012-WSN-065), the Health Project of Jiangsu Province (no. H201318)
and the Social Development Foundation of Nantong City (nos.
S2010012, HS2011004 and BK2013069).
References
|
1
|
Li N, Song MM, Chen XH, Liu LH and Li FS:
S100A4 siRNA inhibits human pancreatic cancer cell invasion in
vitro. Biomed Environ Sci. 25:465–470. 2012.PubMed/NCBI
|
|
2
|
Arumugam T and Logsdon CD: S100P: a novel
therapeutic target for cancer. Amino acids. 41:893–899. 2011.
View Article : Google Scholar
|
|
3
|
Xie L, Ni WK, Chen XD, Xiao MB, Chen BY,
He S, Lu CH, Li XY, Jiang F and Ni RZ: The expressions and clinical
significances of tissue and serum galectin-3 in pancreatic
carcinoma. J Cancer Res Clin Oncol. 138:1035–1043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Danovi SA, Wong HH and Lemoine NR:
Targeted therapies for pancreatic cancer. Br Med Bull. 87:97–130.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rezvanpour A and Shaw GS: Unique S100
target protein interactions. Gen Physiol Biophys. 28:F39–F46.
2009.
|
|
6
|
Yao R, Lopez-Beltran A, Maclennan GT,
Montironi R, Eble JN and Cheng L: Expression of S100 protein family
members in the pathogenesis of bladder tumors. Anticancer Res.
27:3051–3058. 2007.PubMed/NCBI
|
|
7
|
Salama I, Malone PS, Mihaimeed F and Jones
JL: A review of the S100 proteins in cancer. Eur J Surg Oncol.
34:357–364. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Donato R: S100: a multigenic family of
calcium-modulated proteins of the EF-hand type with intracellular
and extracellular functional roles. Int J Biochem Cell Biol.
33:637–668. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marenholz I, Heizmann CW and Fritz G: S100
proteins in mouse and man: from evolution to function and pathology
(including an update of the nomenclature). Biochem Biophys Res
Commun. 322:1111–1122. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Moore BW: A soluble protein characteristic
of the nervous system. Biochem Biophys Res Commun. 19:739–744.
1965. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zimmer DB, Cornwall EH, Landar A and Song
W: The S100 protein family: history, function, and expression.
Brain Res Bull. 37:417–429. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berge G and Mælandsmo GM: Evaluation of
potential interactions between the metastasis-associated protein
S100A4 and the tumor suppressor protein p53. Amino Acids.
41:863–873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Komatsu K, Kobune-Fujiwara Y, Andoh A, et
al: Increased expression of S100A6 at the invading fronts of the
primary lesion and liver metastasis in patients with colorectal
adenocarcinoma. Br J Cancer. 83:769–774. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Melle C, Ernst G, Schimmel B, Bleul A and
Eggeling FV: Colon-derived liver metastasis, colorectal carcinoma,
and hepatocellular carcinoma can be discriminated by the
Ca(2+)-binding proteins S100A6 and S100A11. PloS One.
3:e37672008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schäfer BW and Heizmann CW: The S100
family of EF-hand calcium-binding proteins: functions and
pathology. Trends Biochem Sci. 21:134–140. 1996.PubMed/NCBI
|
|
16
|
Mishra SK, Siddique HR and Saleem M:
S100A4 calcium-binding protein is key player in tumor progression
and metastasis: preclinical and clinical evidence. Cancer
Metastasis Rev. 31:163–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heizmann CW, Ackermann GE and Galichet A:
Pathologies involving the S100 proteins and RAGE. Subcell Biochem.
45:93–138. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saleem M, Kweon MH, Johnson JJ, et al:
S100A4 accelerates tumorigenesis and invasion of human prostate
cancer through the transcriptional regulation of matrix
metalloproteinase 9. Proc Natl Acad Sci USA. 103:14825–14830. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gross SR, Sin CG, Barraclough R and
Rudland PS: Joining S100 proteins and migration: for better or for
worse, in sickness and in health. Cell Mol Life Sci. 30:June
30–2013.(Epub ahead of print).
|
|
20
|
Sorci G, Riuzzi F, Giambanco I and Donato
R: RAGE in tissue homeostasis, repair and regeneration. Biochim
Biophys Acta. 1833:101–109. 2012. View Article : Google Scholar
|
|
21
|
Xie J, Méndez JD, Méndez-Valenzuela and
Aguilar-Hernández MM: Cellular signalling of the receptor for
advanced glycation end products (RAGE). Cell Signal. 25:2185–2197.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maletzki C, Bodammer P, Breitrück A and
Kerkhoff C: S100 proteins as diagnostic and prognostic markers in
colorectal and hepatocellular carcinoma. Hepat Mon.
12:e72402012.PubMed/NCBI
|
|
23
|
Volz HC, Laohachewin D, Seidel C, et al:
S100A8/A9 aggravates post-ischemic heart failure through activation
of RAGE-dependent NF-κB signaling. Basic Res Cardiol.
107:2502012.PubMed/NCBI
|
|
24
|
Hung KW, Chang YM and Yu C: Resonance
assignments of Ca2+-bound human S100A11. Biomol NMR
Assign. 7:211–214. 2013. View Article : Google Scholar
|
|
25
|
Arumugam T, Ramachandran V, Gomez SB,
Schmidt AM and Logsdon CD: S100P-derived RAGE antagonistic peptide
reduces tumor growth and metastasis. Clin Cancer Res. 18:4356–4364.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arumugam T, Ramachandran V, Sun D, et al:
Designing and developing S100P inhibitor 5-methyl cromolyn for
pancreatic cancer therapy. Mol Cancer Ther. 12:654–662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohuchida K, Mizumoto K, Miyasaka Y, et al:
Over-expression of S100A2 in pancreatic cancer correlates with
progression and poor prognosis. J Pathol. 213:275–282. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Leclerc E, Fritz G, Weibel M, Heizmann CW
and Calichet A: S100B and S100A6 differentially modulate cell
survival by interacting with distinct RAGE (receptor for advanced
glycation end products) immunoglobulin domains. J Biol Chem.
282:31317–31331. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leclerc E and Heizmann CW: The importance
of Ca2+/Zn2+ signaling S100 proteins and RAGE
in translational medicine. Front Biosci (Schol Ed). 3:1232–1262.
2011.
|
|
30
|
Filipek A, Michowski W and Kuznicki J:
Involvement of S100A6 (calcyclin) and its binding partners in
intracellular signaling pathways. Adv Enzyme Regul. 48:225–239.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huttunen HJ, Kuja-Panula J, Sorci G,
Agneletti AL, Donato R and Rauuala H: Coregulation of neurite
outgrowth and cell survival by amphoterin and S100 proteins through
receptor for advanced glycation end products (RAGE) activation. J
Biol Chem. 275:40096–40105. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Taguchi A, Blood DC, del Toro G, et al:
Blockade of RAGE-amphoterin signalling suppresses tumour growth and
metastases. Nature. 405:354–360. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lukanidin E and Sleeman JP: Building the
niche: the role of the S100 proteins in metastatic growth. Semin
Cancer Biol. 22:216–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen H, Fernig DG, Rudland PS, Sparks A,
Wilkinson MC and Barraclough R: Binding to intracellular targets of
the metastasis-inducing protein, S100A4 (p9Ka). Biochem Biophys Res
Commun. 286:1212–1217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kriajevska MV, Cardenas MN, Grigorian MS,
Ambartsumian NS, Georgiev GP and Lukanidin EM: Non-muscle myosin
heavy chain as a possible target for protein encoded by
metastasis-related mts-1 gene. J Biol Chem. 269:19679–19682.
1994.PubMed/NCBI
|
|
36
|
Zimmer DB and Van Eldik LJ: Analysis of
the calcium-modulated proteins, S100 and calmodulin, and their
target proteins during C6 glioma cell differentiation. J Cell Biol.
108:141–151. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhao XQ, Naka M, Muneyuki M and Tanaka T:
Ca(2+)-dependent inhibition of actin-activated myosin ATPase
activity by S100C (S100A11), a novel member of the S100 protein
family. Biochem Biophys Res Commun. 267:77–79. 2000.
|
|
38
|
Broome AM and Eckert RL:
Microtubule-dependent redistribution of a cytoplasmic cornified
envelope precursor. J Invest Dermatol. 122:29–38. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hayes MJ, Shao D, Bailly M and Moss SE:
Regulation of actin dynamics by annexin 2. EMBO J. 25:1816–1826.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
de Graauw M, Tijdens I, Smeets MB, et al:
Annexin A2 phosphorylation mediates cell scattering and branching
morphogenesis via cofilin activation. Mol Cell Biol. 28:1029–1040.
2008.PubMed/NCBI
|
|
41
|
Rust RR, Baldisseri DM and Weber DJ:
Structure of the negative regulatory domain of p53 bound to S100B
(betabeta). Nat Struct Biol. 7:570–574. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kato K, Kamada H, Fujimori T, Aritomo Y,
Ono M and Masaki T: Molecular biologic approach to the diagnosis of
pancreatic carcinoma using specimens obtained by EUS-guided fine
needle aspiration. Gastroenterol Res Pract. 2012:2435242012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sablina AA, Budanov AV, Ilyinskaya GV,
Agapova LS, Kravchenko JE and Chumakov PM: The antioxidant function
of the p53 tumor suppressor. Nat Med. 11:1306–1313. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Green ML, Pisano MM, Prough RA and Knudsen
TB: Release of targeted p53 from the mitochondrion as an early
signal during mitochondrial dysfunction. Cell Signal. 25:2383–2390.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Leśniak W, Słomnicki LP and Filipek A:
S100A6-new facts and features. Biochem Biophys Res Commun.
390:1087–1092. 2009.
|
|
46
|
Van Dieck J, Lum JK and Fersht AR: S100
proteins interact with the N-terminal domain of MDM2. FEBS Letts.
584:3269–3274. 2010.PubMed/NCBI
|
|
47
|
Wolf S, Haase-Kohn C and Pietzsch J:
S100A2 in cancerogenesis: a friend or a foe? Amino Acids.
41:849–861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nishioku T, Furusho K, Tomita A, et al:
Potential role for S100A4 in the disruption of the blood-brain
barrier in collagen-induced arthritic mice, an animal model of
rheumatoid arthritis. Neuroscience. 189:286–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sapkota D, Costea DE, Blø M, Bruland O,
Lorens JB, Vasstrand EN and Ibrahim SO: S100A14 inhibits
proliferation of oral carcinoma derived cells through G1-arrest.
Oral Oncol. 48:219–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Grigorian M, Andresen S, Tulchinsky E, et
al: Tumor suppressor p53 protein is a new target for the
metastasis-associated Mts1/S100A4 protein: functional consequences
of their interaction. J Biol Chem. 276:22699–22708. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shimamoto S, Kubota Y, Yamaguchi F,
Tokumitsu H and Kobayashi R: Ca2+/S100 proteins act as
upstream regulators of the chaperone-associated ubiquitin ligase
CHIP (C terminus of Hsc70-interacting protein). J Biol Chem.
288:7158–7168. 2013.
|
|
52
|
Romanov VS, Pospelov VA and Pospelova TV:
Cyclin-dependent kinase inhibitor p21(Waf1): contemporary view on
its role in senescence and oncogenesis. Biochemistry (Mosc).
77:575–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sakaguchi M, Miyazaki M, Takaishi M, et
al: S100C/A11 is a key mediator of Ca(2+)-induced growth
inhibition of human epidermal keratinocytes. J Cell Biol.
163:825–835. 2003.PubMed/NCBI
|
|
54
|
Li B, Wan X, Zhu Q, et al: Net expression
inhibits the growth of pancreatic ductal adenocarcinoma cell PL45
in vitro and in vivo. PloS One. 8:e578182013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sakaguchi M and Huh NH: S100A11, a dual
growth regulator of epidermal keratinocytes. Amino Acids.
41:797–807. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
He H, Li J, Weng S, LI M and Yu Y:
S100A11: diverse function and pathology corresponding to different
target proteins. Cell Biochem Biophys. 55:117–126. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ohuchida K, Mizumoto K, Ohhashi S, et al:
S100A11, a putative tumor suppressor gene, is overexpressed in
pancreatic carcinogenesis. Clin Cancer Res. 12:5417–5422. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mann K and Hainaut P: Aminothiol WR1065
induces differential gene expression in the presence of wild-type
p53. Oncogene. 24:3964–3975. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Brain JG, Robertson H, Thompson E, et al:
Biliary epithelial senescence and plasticity in acute cellular
rejection. Am J Transplant. 13:1688–1702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yang SY, Miah A, Pabari A and Winslet M:
Growth Factors and their receptors in cancer metastases. Front
Biosci (Landmark Ed). 16:531–538. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jia W, Gao XJ, Yang ZX and Zhang ZD:
S100A4 silencing suppresses proliferation, angiogenesis and
invasion of thyroid cancer cells through downregulation of MMP-9
and VEGF. Eur Rev Med Pharmacol Sci. 17:1495–1508. 2013.PubMed/NCBI
|
|
62
|
Chen H, Yuan Y, Zhang C, et al:
Involvement of S100A14 protein in cell invasion by affecting
expression and function of matrix metalloproteinase (MMP)-2 via
p53-dependent transcriptional regulation. J Biol Chem.
287:17109–17119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Gebhardt C, Németh J, Angel P and Hess J:
S100A8 and S100A9 in inflammation and cancer. Biochem Pharmacol.
72:1622–1631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hsu TC, Young MR, Cmarik J and Colburn NH:
Activator 1 (AP-1)- and nuclear factor kappaB (NF-kappaB)-dependent
transcriptional events in carcinogenesis. Free Radic Biol Med.
28:1338–48. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bachet JB, Maréchal R, Demetter P, et al:
S100A2 is a predictive biomarker of adjuvant therapy benefit in
pancreatic adenocarcinoma. Eur J Cancer. 49:2643–53. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jamieson NB, Carter CR, McKay CJ and Oien
KA: Tissue biomarkers for prognosis in pancreatic ductal
adenocarcinoma: a systematic review and meta-analysis. Clin Cancer
Res. 17:3316–3331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Biankin AV, Kench JG, Colvin EK, et al:
Expression of S100A2 calcium-binding protein predicts response to
pancreatectomy for pancreatic cancer. Gastroenterology.
137:558–568. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Russo SM, Ove R and Saif MW:
Identification of prognostic and predictive markers in pancreatic
adenocarcinoma. In: Highlights from the ‘2011 ASCO Gastrointestinal
Cancers Sympsoium’; San Francisco, CA, USA. January 20–22, 2011;
JOP. 12. pp. 92–95. 2011, PubMed/NCBI
|
|
69
|
Sekine H, Chen N, Sato K, et al: S100A4,
frequently overexpressed in various human cancers, accelerates cell
motility in pancreatic cancer cells. Biochem Biophys Res Commun.
429:214–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ilg EC, Schäfer BW and Heizmann CW:
Expression pattern of S100 calcium-binding proteins in human
tumors. Int J Cancer. 68:325–332. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Tsukamoto N, Egawa S, Akada M, et al: The
expression of S100A4 in human pancreatic cancer is associated with
invasion. Pancreas. 42:1027–1033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chang D, Colvin E, Scarlett C, et al: A
molecular prognostic nomogram for resectable pancreatic cancer. J
Clin Oncol. 29(Suppl 4): abs. 154. 2011.
|
|
73
|
Ikenaga N, Ohuchida K, Mizumoto K, et al:
S100A4 mRNA is a diagnostic and prognostic marker in pancreatic
carcinoma. J Gastrointest Surg. 13:1852–1858. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Schneider G and Filipek A: S100A6 binding
protein and Siah-1 interacting protein (CacyBP/SIP): spotlight on
properties and cellular function. Amino Acids. 41:773–780. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Ohuchida K, Mizumoto K, Ishikawa N, et al:
The role of S100A6 in pancreatic cancer development and its
clinical implication as a diagnostic marker and therapeutic target.
Clin Cancer Res. 11:7785–7793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ohuchida K, Mizumoto K, Yu J, et al:
S100A6 is increased in a stepwise manner during pancreatic
carcinogenesis: clinical value of expression analysis in 98
pancreatic juice samples. Cancer Epidemiol Biomarkers Prev.
16:649–654. 2007. View Article : Google Scholar
|
|
77
|
Vimalachandran D, Greenhalf W, Thompson C,
et al: High nuclear S100A6 (Calcyclin) is significantly associated
with poor survival in pancreatic cancer patients. Cancer Res.
65:3218–3225. 2005.PubMed/NCBI
|
|
78
|
Dowen SE, Crnogorac-Jurcevic T,
Gangeswaran R, et al: Expression of S100P and its novel binding
partner S100PBPR in early pancreatic cancer. Am J Pathol.
166:81–92. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Han H, Bearss DJ, Browne LW, Calaluce R,
Nagle RB and Von Hoff DD: Identification of differentially
expressed genes in pancreatic cancer cells using cDNA microarray.
Cancer Res. 62:2890–2896. 2002.PubMed/NCBI
|
|
80
|
Ohuchida K, Mizumoto K, Egami T, et al:
S100P is an early developmental marker of pancreatic
carcinogenesis. Clin Cancer Res. 12:5411–5416. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Nakata K, Nagai E, Ohuchida K, et al:
S100P is a novel marker to identify intraductal papillary mucinous
neoplasms. Hum Pathol. 41:824–831. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Crnogorac-Jurcevic T, Missiaglia E,
Blaveri E, et al: Molecular alterations in pancreatic carcinoma:
expression profiling shows that dysregulated expression of S100
genes is highly prevalent. J Pathol. 201:63–74. 2003. View Article : Google Scholar
|
|
83
|
Barry S, Chelala C, Lines K, et al: S100P
is a metastasis-associated gene that facilitates transendothelial
migration of pancreatic cancer cell. Clin Exp Metastasis.
30:251–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Inada H, Naka M, Tanaka T, Davey GE and
Heizmann CW: Human S100A11 exhibits differential steady-state RNA
levels in various tissues and a distinct subcellular localization.
Biochem Biophys Res Commun. 263:135–138. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Chen JH, Ni RZ, Xiao MB, Guo JG and Zhou
JW: Comparative proteomic analysis of differentially expressed
proteins in human pancreatic cancer tissue. Hepatobiliary Pancreat
Dis Int. 8:193–200. 2009.PubMed/NCBI
|
|
86
|
Memon AA, Sorensen BS, Meldgaard P, Fokdal
L, Thykjaer T and Nexo E: Down-regulation of S100C is associated
with bladder cancer progression and poor survival. Clin Cancer Res.
11:606–611. 2005.PubMed/NCBI
|
|
87
|
Nakashima T, Wang XF, Masuda M, Inokuchi A
and Komiyama S: Overexpression of p53 nuclear protein in
premalignant and malignant laryngeal lesions. Eur Arch
Otorhinolaryngol. 256:S56–S59. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Shin DM, Kim J, Ro JY, Hittelman J, Roth
JA, Hong WK and Hittelman WN: Activation of p53 gene expression in
premalignant lesions during head and neck tumorigenesis. Cancer
Res. 54:321–326. 1994.PubMed/NCBI
|
|
89
|
Xiao MB, Jiang F, Ni WK, Chen BY, Lu CH,
Li XY and Ni RZ: High expression of S100A11 in pancreatic
adenocarcinoma is an unfavorable prognostic marker. Med Oncol.
29:1886–1891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Arumugam T, Simeone DM, Van Golen K and
Logsdon CD: S100P promotes pancreatic cancer growth, survival, and
invasion. Clin Cancer Res. 11:5356–5364. 2005. View Article : Google Scholar : PubMed/NCBI
|